Abstract
Sympathetic nerve activity (SNA) is elevated in established hypertension. We tested the hypothesis that SNA is elevated in neonate and juvenile spontaneously hypertensive (SH) rats prior to the development of hypertension, and that this may be due to augmented respiratory–sympathetic coupling. Using the working heart–brainstem preparation, perfusion pressure, phrenic nerve activity and thoracic (T8) SNA were recorded in male SH rats and normotensive Wistar–Kyoto (WKY) rats at three ages: neonates (postnatal day 9–16), 3 weeks old and 5 weeks old. Perfusion pressure was higher in SH rats at all ages reflecting higher vascular resistance. The amplitude of respiratory-related bursts of SNA was greater in SH rats at all ages (P < 0.05). This was reflected in larger Traube–Hering pressure waves in SH rats (1.4 ± 0.8 versus 9.8 ± 1.5 mmHg WKY versus SH rat, 5 weeks old, n= 5 per group, P < 0.01). Recovery from hypocapnic-induced apnoea and reinstatement of Traube–Hering waves produced a significantly greater increase in perfusion pressure in SH rats (P < 0.05). Differences in respiratory–sympathetic coupling in the SH rat were not secondary to changes in central or peripheral chemoreflex sensitivity, nor were they related to altered arterial baroreflex function. We have shown that increased SNA is already present in SH rats in early postnatal life as revealed by augmented respiratory modulation of SNA. This is reflected in an increased magnitude of Traube–Hering waves resulting in elevated perfusion pressure in the SH rat. We suggest that the amplified respiratory-related bursts of SNA seen in the neonate and juvenile SH rat may be causal in the development of their hypertension.
The ontogeny of essential hypertension is not fully understood, although there is accumulating evidence indicating an association with altered sympathetic nerve activity (SNA) in both humans and animal models of hypertension (Guyenet, 2006). In man, measures of sympathetic function, using microneurography or noradrenaline spill-over, have shown increased activity levels in young hypertensive patients and populations at risk of developing hypertension (Julius et al. 1991; Grassi, 1998; Esler, 2000; Schlaich et al. 2004). These findings are echoed in studies of adult spontaneously hypertensive (SH) rats which have elevated SNA and increased noradrenaline release (Judy & Farrell, 1979; Lundin et al. 1984). Furthermore, neonatal sympathectomy prevents SH rats from developing hypertension and also limits vessel and cardiac hypertrophy (Cabassi et al. 1998). The forgoing supports the view that altered SNA plays a significant role in the generation of high blood pressure in essential hypertension. Interestingly, there are some suggestions from noradrenaline measurements in 4- to 5-week-old SH rats (Korner et al. 1993) that changes in SNA may occur prior to the onset of hypertension. However, as yet this has not been demonstrated with direct recordings of SNA in young SH rats. Without such recordings it is difficult to determine what drives these changes in SNA and whether they are a cause or a consequence of hypertension.
Suggested causes of the sympathetic nervous system dysfunction in hypertension include increased density of sympathetic innervation, increased single fibre firing rates and changes in noradrenergic transmission and reuptake (Adams et al. 1989; Cabassi et al. 1998). Additionally, hypertension is associated with attenuated arterial baroreflex function (Struyker-Boudier et al. 1982; Head, 1995; Esler et al. 2001) and thus altered central control of SNA, which may also contribute to the changes in the sympathetic outflow. However, because these baroreflex changes manifest as the animals age they cannot account for the initial elevated level of sympathetic activity (Judy & Farrell, 1979).
An alternative possible mechanism is that there is a change in the pattern and/or synchronization of the vasomotor sympathetic output (Gilbey, 2001). It has long been known that sympathetic activity shows respiratory modulation that arises as a result of central coupling (Adrian et al. 1932) and this is prominent in sympathetic nerve recordings in humans (Eckberg et al. 1985; Badra et al. 2001; Dempsey et al. 2002), rats (Numao et al. 1987; Habler et al. 1996; Dick et al. 2004) and cats (Boczek-Funcke et al. 1992). The exact pattern of respiratory modulation of sympathetic activity is both species and target organ specific (see review by Habler et al. 1994b). The key determinants of arterial pressure are the ‘muscle vasoconstrictor-type’ sympathetic neurones and in adult rats these typically show a pattern of respiratory modulation with inhibition during mid-inspiration, a peak of activity seen during the postinspiratory phase and sometimes a smaller peak in late expiration (Darnall & Guyenet, 1990; Habler et al. 1994a). This respiratory modulation of the vasomotor sympathetic outflow produces phasic changes in arteriolar smooth muscle tone thus generating Traube–Hering arterial pressure waves. Importantly, a change in the pattern of respiratory modulation has previously been described in the adult SH rat where the peak of sympathetic activity is time-shifted to earlier in the respiratory cycle (Czyzyk-Krzeska & Trzebski, 1990).
By employing the working heart–brainstem preparation (Paton, 1996) we have been able for the first time to make direct recordings of SNA and phrenic nerve activity (PNA) in age- and sex-matched SH and WKY rats from postnatal day 9 to 5 weeks of age and tested the hypothesis that the respiratory modulation of SNA is altered in prehypertensive SH rats.
Methods
Ethical approval
All experiments were performed in accordance with the Australian National Health and Medical Research Council's AustralianCode of Practice for the Care and Use of Animals for Scientific Purposes and were approved by our Institutional Animal Experimentation Ethics and Biosafety committees.
Working heart–brainstem preparation
SH and WKY rats were divided into three age groups: neonatal (postnatal day (P) 9–16, n= 5 for each strain), 3 weeks old (n= 5 for each strain) and 5 weeks old (n= 11 WKY, n= 16 SH rats). For the working heart–brainstem preparation (WHBP; Paton, 1996) rats were anaesthetized deeply with isoflurane until loss of paw withdrawal reflex. Animals were bisected below the diaphragm, exsanguinated, cooled in Ringer solution on ice (composition in mm: 125 NaCl, 24 NaHCO3, 5 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4 and 10 dextrose, pH 7.3 after carbogenation (5% CO2, 95% O2)) and decerebrated precollicularly. All chemicals were purchased from Sigma-Aldrich, Australia. Lungs were removed and the descending aorta was isolated and cleaned. Retrograde perfusion of the thorax and head was achieved via a double-lumen catheter (ø 1.25 mm, DLR-4, Braintree Scientific, Braintree, MA, USA) inserted into the descending aorta. The perfusate was Ringer solution containing Ficoll (1.25%) warmed to 31°C and gassed with carbogen. The second lumen of the cannula was connected to a transducer to monitor perfusion pressure (PP) in the aorta. Neuromuscular blockade was established using vecuronium bromide added to the perfusate (2–4 μg ml−1, Organon Teknika, Cambridge, UK). Simultaneous recordings of PNA and thoracic sympathetic nerve activity (tSNA) were obtained using glass suction electrodes, amplified (20 kHz, Neurolog), filtered (50–1500 kHz, Neurolog), digitized (CED, Cambridge, UK) and recorded to hard disk using Spike2 (CED). Heart rate was derived by using a window discriminator to trigger from the R-wave of the electrocardiogram (ECG) recorded simultaneously through the phrenic nerve suction electrode. Respiratory sinus arrhythmia was measured offline as the average peak to trough of heart rate.
Flow was adjusted until an augmenting (i.e. eupnoeic) pattern of phrenic nerve activity was achieved. For neonates eupnoea was stable at pressures of 20–35 mmHg; for 3-week-olds at 30–50 mmHg and for 5-week-olds at 50–70 mmHg. The average perfusate flow was 10 ± 0.2 ml min−1 for neonates (19 ± 1 g), 13 ± 0.6 ml min−1 for 3-week-olds (44 ± 2 g) and 19 ± 0.8 ml min−1 for 5-week-olds (94 ± 7 g). Perfusate flows were not different between the matched WKY and SH rat age groups (Fig. 1B, P= 0.1) and weights were similar across age groups with the exception that 5-week-old SH rats were heavier (P < 0.05) than WKY (P= 0.1). In 5-week-old preparations vasopressin was added to the perfusate (final concentration 1.25–2.5 nm) to increase vascular resistance and maintain PP, as previously described (Pickering & Paton, 2006).
Figure 1. Perfusion pressure in SH rats is higher than WKY at comparable flow.
A, baseline perfusion pressure (PP; mmHg; mean ±s.e.m.) at similar flow rates for each of the age groups. This shows the expected trend of increasing pressure with age. Notably, at all ages, the SH rats had significantly higher perfusion pressures than age matched WKY. B, mean phrenic burst duration was not different between strains at each age (n= 5 per group, P= 0.06, P= 0.12, P= 0.06 for neonate, 3-week-old and 5-week-old groups, respectively). Representative examples of phrenic bursts for each group show the eupnoeic pattern required, indicating adequate flow to the preparation. C, perfusate flow values for each age group showing similar flows were required to achieve eupnoea in both WKY and SHR at each age (n= 5 per group, ns). D, vascular resistance was significantly higher in SH rats in the neonate and 5-week-old groups (n= 5 per group). Student's t test, *P < 0.05 compared to WKY.
All sympathetic nerve activity was recorded at T8–T10 under similar conditions from the sympathetic chain using a bipolar suction electrode. Baseline tSNA was recorded for 10 min followed by baroreceptor and peripheral chemoreceptor reflex testing.
Cardio-respiratory reflexes
Baroreceptor reflex
The baroreceptor reflex was stimulated by intra-arterial administration of phenylephrine (10 μg bolus; i.a.) to increase PP and produce reflex sympathoinhibition and bradycardia. The non-cardiac sympathetic component of the baroreflex was quantified as percentage inhibition/Δpressure (% mmHg−1). The degree of sympathoinhibition was calculated by ratioing the average integrated tSNA during the peak of the PP increase against the average activity from two preceding equivalent time periods, at equivalent stages of the respiratory cycle. This was performed using a custom written Spike2 script as previously described (Simms et al. 2007).
Peripheral chemoreceptor reflex
Peripheral chemoreceptors were stimulated using sodium cyanide (CN; 0.05% solution; 100 μl bolus) injected into the aorta. The peripheral chemoreceptor reflex consisted of an increase in central respiratory drive accompanied by bradycardia, an increase in tSNA and an associated pressor response. The chemoreflex was quantified in three ways: increase in central respiratory rate; maximum bradycardia; and the percentage increase in tSNA during a 5 s period at the peak of the chemoreflex response as compared to an equivalent control period.
Barodenervation
Acute barodenervations were performed in the WHBP (as previously described; Pickering et al. 2008) to identify a possible role of altered baroreceptor function in mediating the changes in tSNA seen in SH rats. After decerebration, while the preparation was in cold Ringer solution the proximal vagal and glossopharyngeal nerves were identified in the region of the bifurcation of the common carotid arteries and looped with thread. The preparation was then cannulated and perfused as described above. After recording baseline tSNA and cardio-respiratory reflexes, the vagal and glossopharyngeal nerves were cut to interrupt all baroreceptor inputs running in the aortic depressor nerve and carotid sinus nerve, respectively. Arterial baroreflex and peripheral chemoreflex function was tested before and after denervation to confirm successful baro- and peripheral chemoreceptor denervation. Continuous recordings of tSNA were obtained prior to and post barodenervation in 5-week-old SH rats (n= 5).
Hypo- and hypercapnia protocol
To examine the influence of respiratory drive on tSNA bursts, WHBP were exposed to varying levels of CO2 (5-week-old animals, WKY and SHR n= 5 per group). The perfusate source (5% CO2: 95% O2) was switched to a second reservoir pre-equilibrated with a known ratio of CO2: O2 (7.5%: 92.5%; 10%: 90%; 12%: 88% and 3%: 97%). Changes in respiratory pattern were monitored from PNA and measurements were taken once a new stable equilibrium had been reached (typically after 5 min). After each perfusate switch the preparation was allowed to re-equilibrate and recover for a minimum of 10 min at 5% CO2. The size of phrenic bursts was calculated as the average peak–trough amplitude from 20 phrenic cycles.
The effect of re-establishment of eupnoea (from an apnoeic state) on perfusion pressure was examined in an additional group of animals. WHBP (5-week-old animals, WKY and SHR n= 6 per group) were rendered transiently apnoeic by exposure to a hypocapnic perfusate (2% CO2, 98% O2) for approximately 1 min. The perfusate was immediately switched back to a normocapnic mixture (5% CO2) which triggered the re-establishment of a eupnoeic rhythm on the PNA. In two animals from each group concurrent recordings of tSNA were also obtained as eupnoea was re-established.
Data analysis
The noise levels for tSNA recordings were determined after application of xylocaine (0.5%) to the nerve and were comparable across all preparations (3.6 ± 0.3 μV; n= 30). For all analysis of tSNA, the signal was rectified and integrated with a time constant (Tc) of 100 ms and the noise level subtracted.
Phrenic triggered averaging of tSNA
Phrenic triggered averaging of integrated tSNA (across 20 phrenic cycles) was carried out offline for each experiment. This allowed the mean peak to trough amplitude of the respiratory burst of tSNA to be measured. We examined the temporal relationship of tSNA with the central respiratory cycle across preparations. To account for variations in cycle length between preparations, the mean phrenic inspiratory burst duration (from onset to the end of the augmenting phase) in each preparation was used as a time base to divide the respiratory cycle. Therefore, one time unit corresponded to the duration of inspiration. The tSNA was averaged across consecutive respiratory cycles aligned to the start of inspiration for a time period of 4 times the phrenic burst length (always less than the length of the respiratory cycle). The first period preceded inspiration (late expiration), followed by inspiration, postinspiration and mid-expiration. Thus, the mean level of tSNA during each respiratory period could be calculated and pooled across preparations.
Significance of data was assessed using Student's two-tailed t test, two-way ANOVA with Bonferroni post hoc tests, Kruskal–Wallis non-parametric test with Dunn's multiple comparison test or repeated measures ANOVA (GraphPad Prism). All values quoted are the mean ±s.e.m. and differences were considered significant at the 95% confidence limit.
Results
Baseline cardiorespiratory parameters
The PP in SH rats was higher than WKY rats at all ages (Fig. 1A). Given that there was no difference between the strains in the perfusate flow required to produce eupnoea (Fig. 1B and C) this corresponds to an increased vascular resistance in the SH rat (Fig. 1D). There was no significant difference in baseline heart rate nor in the degree of respiratory sinus arrhythmia between SH and WKY rats at any age (P > 0.1). The basal respiratory parameters for WKY and SH rats at each age were similar with no significant differences between strains in phrenic burst amplitude or duration (P > 0.05).
Thoracic sympathetic nerve activity
In all preparations, tSNA showed characteristic bursting, respiratory-modulated activity, consistent with previous reports in the WHBP (Boscan et al. 2001; Pickering et al. 2003; Simms et al. 2007). The mean level of tSNA measured over a 2 min period of basal activity was higher in neonatal (postnatal day 9–16) SH compared to WKY rats (9.6 ± 0.8 μV versus 7.1 ± 0.9 μV; P < 0.05, Fig. 2). In contrast, mean tSNA levels were not significantly different between SH and WKY rats at 3 and 5 weeks of age (Fig. 2A). However, we observed that tSNA in the SH rat had a more pronounced respiratory-related bursting pattern at all ages when compared to WKY rats (Figs 2B and C and 3Aa and Ba).
Figure 2. Mean levels of tSNA are elevated in neonatal SH rats.
A, mean (±s.e.m.) integrated level of thoracic sympathetic nerve activity (∫tSNA) in each age group of SH and WKY rats. A significantly higher level was observed in the neonatal SH rats compared to age-matched WKY rats, but no significant difference between SH and WKY rats at 3 and 5 weeks old. (*P < 0.05 compared to WKY (2-way ANOVA), n= 5 per group). B and C, traces of raw and integrated (∫tSNA; Tc 100 ms) tSNA and perfusion pressure (PP) in neonate WKY (B) and SH rat (C).
Figure 3. Respiratory driven bursts of SNA are larger in SH rats than WKY at all ages.
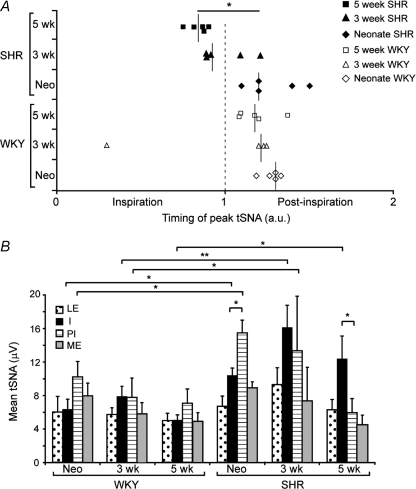
Aa and Ba, traces from 5 week old WKY (Aa) and SH (Ba) rats showing raw and integrated tSNA (∫tSNA) over a period of 20 phrenic cycles demonstrating the augmented pattern of respiratory modulation of sympathetic activity seen in the SH rat. Ab and Bb, phrenic triggered averaging of the integrated tSNA signal clearly shows the larger respiratory related burst amplitude in the SH rat. C, bar graph of pooled average respiratory-related sympathetic burst amplitude (peak–trough) from all animals at each age group showing significantly larger bursts in SH rats. *P= 0.05, 2-way ANOVA, n= 5 per group. LE, late expiration; I, inspiration; PI, postinspiration; ME, mid-expiration.
Phrenic-triggered averaging of tSNA showed SH rats exhibited larger amplitude respiratory-related bursts of sympathetic activity compared to WKY rats at all ages (Fig. 3C). The timing of the tSNA burst relative to the phase of the respiratory cycle was different between strains during development. In WKY rats, the peak of tSNA occurred during postinspiration at all ages (Fig. 4A). In contrast, in SH rats there was a progressive temporal shift with age of the tSNA burst to earlier in the respiratory cycle; the peak of tSNA shifted from postinspiration in the neonate to the inspiratory period in 3- and 5-week-old SH rats (Fig. 4A, P < 0.05). To analyse this further, the mean levels of integrated tSNA during inspiration, postinspiration, mid-expiration and late-expiration were pooled by strain of rat in each age group. This showed that compared to WKY rats, SH rats had a significantly larger sympathetic burst during inspiration at all ages and also during postinspiration in the neonatal and 3-week-old age groups (Fig. 4B). This also demonstrated a significant shift in the peak amplitude of tSNA from postinspiration in the neonate SH rat to the inspiratory phase by 5 weeks of age (P < 0.05, Fig. 4B).
Figure 4. Respiratory–sympathetic burst phase shifts with increasing age in SH rats but not in WKY.
A, scatterplot of the timing of the peak of tSNA relative to the start of inspiration (time 0) for each animal. The inspiratory period is represented by time 0–1, postinspiration by time 1–2. The median point is marked for each age and strain group. The timing of the peak of tSNA in 5-week-old SH rats was significantly different from neonate SH rats (*P < 0.05, Kruskal–Wallis non-parametric test). B, bar graph of integrated tSNA (∫tSNA; mean ±s.e.m.) during each phase of the respiratory cycle pooled across all groups of WKY and SHR. SH rats have a significantly larger sympathetic burst during inspiration at all ages and during postinspiration up to 3 weeks of age as compared to WKY. With age the peak of sympathetic activity in SH rats shifts from postinspiration in the neonates (PI > I) to inspiration by 5 weeks of age (I > PI). *P < 0.05, 2-way ANOVA with Bonferroni post tests, n= 5 per group. LE, late expiration; I, inspiration; PI postinspiration; ME, mid-expiration.
Traube–Hering waves
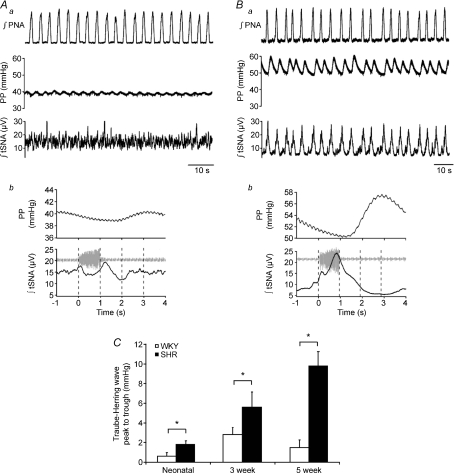
In addition to having a higher baseline PP, it was apparent that SH rats also had more prominent Traube–Hering waves (Fig. 5). The relationship between the phrenic cycle, tSNA and the subsequent change in PP was investigated using phrenic cycle triggered averaging (Fig. 5Ab and Bb). This demonstrated a relatively constant latency between the peak of sympathetic activity and the following peak in PP of 2–3 s. Pooling the data across preparations showed that SH rats had significantly larger Traube–Hering waves at all ages compared with age-matched WKY rats (Fig. 5C).
Figure 5. Sympathetically mediated Traube–Hering waves are larger in SH rats.
Aa and Ba, traces showing simultaneous recordings of integrated phrenic (∫PNA), tSNA (∫tSNA) and perfusion pressure (PP) from 5 week old WKY (A) and SH (B) rats illustrating the striking difference in Traube–Hering wave amplitude. Ab and Bb, phrenic triggered averages of integrated tSNA (∫tSNA, bottom) and PP (middle) across 20 cycles with a representative phrenic burst (PNA). LE, late expiration; I, inspiration; PI postinspiration; ME, mid-expiration. C, histogram showing mean ±s.e.m., from all rats, of Traube–Hering wave size (peak–trough). Significantly larger waves occur in SH rats at all ages. *P < 0.05, Student's t test, n= 5 per group.
Influence of central respiratory drive
Effect of altering central respiratory drive on sympathetic activity
To examine the effect of altered central respiratory drive on tSNA across strains (at 5 weeks of age, n= 5 per group) we increased the level of CO2 in the perfusate. This caused an increase in both PNA amplitude and PNA frequency in WKY and SH rats, which first reached significance at 10% and persisted at 12% CO2 (P < 0.05 compared to 5%, Fig. 6A and B). The respiratory activation by hypercapnia was considered maximal at 10% CO2 since there was no further significant increase in amplitude or frequency of PNA above that seen at 10%. There was a large variation in the frequency response to hypocapnia (3% CO2) but both strains showed a significant fall in the amplitude of PNA (P < 0.05, Fig. 6B). Exposure to 2% CO2 produced apnoea with a loss of PNA in all animals. No significant differences were seen between the rat strains in their respiratory response to the altered CO2 levels in the perfusate. The mean level of tSNA increased with hypercapnia in both WKY and SHR and reached significance at 12% CO2 (P < 0.05 compared to 5% CO2). Under conditions of hypocapnia (3% CO2) mean tSNA levels were not different between WKY and SHR rats (P= 0.5). At all levels of CO2 (3–12%) the respiratory related tSNA bursts were significantly larger in SH compared to WKY rats (Fig. 6C). The respiratory phase relationship of tSNA in WKY and SH rats was unaltered by hypercapnia such that the peak in SH rats was concentrated in the inspiratory period whereas in WKY rats it occurred in postinspiration.
Figure 6. Respiratory and sympathetic responses to changes in central CO2 levels in WKY and SH rats.
A, phrenic nerve frequency–CO2 response curve. Hypercapnia produced equivalent increases in respiratory frequency in both strains. Hypocapnia to 3% CO2 produced both increases and decreases in frequency across preparations. CO2 at 2% led to apnoea (data from separate set of experiments; see Fig. 7). B, phrenic nerve amplitude–CO2 response curve. No differences were seen in the PNA amplitude response to CO2 across the two strains. Note the consistently large decrease in amplitude at 3% CO2 when compared to the frequency response. C, bar graph of average respiratory-related sympathetic burst amplitude (peak–trough) at each CO2 level showing significantly larger bursts in SH rats at all levels. *P < 0.05 compared to same strain at 5% CO2; #P < 0.05 compared to WKY at the same percentage CO2, repeated measures ANOVA; n= 5 rats aged 5 weeks per group.
Importance of respiratory–sympathetic coupling for PP
To determine if enhanced respiratory–sympathetic coupling in SH rats was a causal factor in generating higher perfusion pressures, central respiration was transiently halted (2–4 min) using a hypocapnic perfusate (2% CO2) to induce apnoea. On restoration of eupnoea the increase in PP was significantly greater in SH rats (14.8 ± 1.3 mmHg, n= 6) than in WKY rats (4.5 ± 1.7 mmHg, n= 6, P < 0.05, Table 1 and Fig. 7). The rise in PP occurred only with the onset of bursting PNA (shown in Fig. 7A and B) and coincided with the reinstatement of the TH waves, which were clearly seen to summate in the SH rats. Recording of tSNA during this protocol (n= 2 animals of each group) revealed that the TH waves and increase in PP only occurred when the respiratory–sympathetic coupling resumed (Fig. 7E). In WKY rats, respiratory–sympathetic coupling took longer to resume after re-establishment of eupnoea (data not shown) and consequently PP took longer to increase than in SH rats after hypocapnia-induced apnoea (Fig. 7A and B).
Perfusionpressure and magnitude of Traube–Hering (TH) waves before, during and after apnoea in 5-week-old WKY and SH rats
| Baseline |
Apnoea |
Recovery |
|||||
|---|---|---|---|---|---|---|---|
| PP | TH | PP | PP fall | PP | TH | PP rise | |
| WKY | 65.2 ± 1.2 | 2.1 ± 0.8 | 60.6 ± 2.2 | 4.6 ± 1.5 | 67.1 ± 2.3 | 2.1 ± 0.7 | 4.5 ± 1.7 |
| SHR | 82.7 ± 5.1* | 4.7 ± 1.8 | 75.3 ± 2.8* | 7.3 ± 3.6 | 90.1 ± 6.3* | 3.6 ± 1.4 | 14.8 ± 4.4* |
Data are in mmHg.
P < 0.05 compared to WKY, Student's t test, n= 6 per group.
Figure 7. The greater increase in perfusion pressure in SH rats after reinstatement of phrenic nerve activity following apnoea is due to enhanced respiratory–sympathetic coupling.
A and B, a period of apnoea, induced with a hypocapnic perfusate (2%CO2; grey), and the return of PNA to eupnoea in a WKY (A) and a SH (B) rat (5-week-old animals). Expanded time scales below show PNA and PP before, during (grey) and after apnoea. Note the TH waves disappear with apnoea and recover with eupnoea in both WKY and SH rats. C, increase in pressure with the recovery of eupnoea. *P < 0.05, Student's t test, WKY n= 6, SHR n= 6. D, example showing the change in tSNA activity in an SH rat during re-establishment of eupnoea. Note the TH waves recover and summate to cause the PP rise with the onset of eupnoea as a consequence of the respiratory–sympathetic coupling being re-engaged (n= 2 WKY, n= 2 SHR).
Influence of peripheral cardio-respiratory reflexes
The arterial baroreceptor reflex was activated by the pressor response to an intra-arterial (i.a.) bolus injection of phenylephrine (10 μg). The PE pressor effect and non-cardiac sympathetic baroreflex gain were not different between WKY and SH rats at each age (Table 2).
Table 2.
Phenylephrine induced baroreflex responses
| Pressor response (%SI mmHg−1) |
Sympathetic gain (mmHg) |
|||
|---|---|---|---|---|
| WKY | SHR | WKY | SHR | |
| Neonates | 19.7 ± 2.9 | 28.6 ± 5.8 | 4.3 ± 1.3 | 5.8 ± 2.8 |
| 3 weeks | 18.2 ± 2.2 | 20.9 ± 3.8 | 2.8 ± 0.7 | 3.8 ± 0.8 |
| 5 weeks | 39.4 ± 9.4# | 28.3 ± 1.8 | 2.2 ± 0.6 | 1.6 ± 0.1# |
Mean (±s.e.m.) pressor response and degree of baroreflex evoked sympathoinhibition (sympathetic gain) in response to phenylephrine (10 μg, i.a.). #P < 0.05 compared to both neonate and 3-week-old of same strain (Student's t test), n= 5 per group.
The respiratory, sympathoexcitatory and pressor components of the response to peripheral chemoreceptor activation were similar between rat strains at each age with the exception that neonatal SH rats showed a significantly greater pressor response compared to their WKY counterparts (Table 3).
Table 3.
Peripheral chemoreflex responses
| Δ respiratory rate (%) |
Sympathoexcitation (%) |
Pressor (mmHg) |
||||
|---|---|---|---|---|---|---|
| WKY | SHR | WKY | SHR | WKY | SHR | |
| Neonates | 44.8 ± 12 | 79.2 ± 24 | 45.6 ± 10 | 38.5 ± 4 | 3.7 ± 0.8 | 8.2 ± 1.6* |
| 3 weeks | 43.8 ± 8 | 52.9 ± 14 | 26.8 ± 5 | 48.6 ± 10 | 5.6 ± 0.9 | 10.8 ± 2.7 |
| 5 weeks | 36.7 ± 11 | 66.4 ± 16 | 49.3 ± 19 | 36.5 ± 5 | 8.0 ± 1.4 | 6.6 ± 2.5 |
Change in respiratory rate, activation of tSNA and pressor response following stimulation of peripheral chemoreceptors with sodium cyanide (mean ±s.e.m.).
P < 0.05 compared to WKY of same age, Student's t test, n= 5 per group.
After baroreceptor denervation in 5-week-old SH rats (n= 5) neither the amplitude, nor the pattern nor the timing of respiratory related bursts was altered, nor was there any effect on the TH waves (Fig. 8). The peak tSNA activity remained during inspiration as seen in baro-intact 5-week-old SH rats. The associated loss of peripheral chemoreceptor input did not affect the respiratory or sympathetic response to increased CO2 (up to 12%) indicating that these effects were mediated through the central chemoreceptors (data not shown).
Figure 8. Respiratory modulation of tSNA does not change after arterial baroreceptor denervation in SH rats.
A and B, traces of integrated phrenic nerve activity (∫PNA), heart rate (HR) and integrated thoracic sympathetic nerve activity (∫tSNA) during a baroreflex challenge (A) and a chemoreflex challenge (B) before (top) and after (bottom) sectioning the glossopharyngeal and vagal nerves in an SH rat. A, a bolus of 10 μg phenylephrine (PE, i.a.) together with an increase in flow to increase perfusion pressure (PP) evoked sympathoinhibition in the intact state but this response was lost after denervation (lower panel). Phrenic-triggered averages of tSNA (A') show that the pattern of respiratory modulation is not changed with barodenervation in SH rats; the peak of tSNA remains in the inspiratory period as observed in intact 5 week old SH rat. B, a bolus of 50 μl sodium cyanide (NaCN, 0.05%, i.a.) evoked sympathoexcitation and an increased respiratory rate in the intact state. This peripheral chemoreflex response was also lost after sectioning the glossopharyngeal and vagal nerves.
Discussion
This study examines the development, patterning and control of sympathetic activity in young SH rats compared with age-matched WKY control rats. Using the WHBP, we have directly measured SNA in these young animals for the first time. We have shown that from the earliest ages studied (postnatal day 9 onwards) SH rats have higher vascular resistance and enhanced respiratory-related bursts of sympathetic activity compared to WKY rats. Additionally there is a developmental change in the SH rats in the timing of these sympathetic bursts within the respiratory cycle (not seen in WKY rats). These changes in respiratory–sympathetic coupling do not appear to be a consequence of altered central or peripheral chemoreflex nor arterial baroreflex function. The increase in the amplitude of the sympathetic bursts produced larger Traube–Hering arterial pressure waves in the SH rat. Our finding that arterial pressure increases significantly more in SH rats with the return of eupnoea after a short period of apnoea indicates that the augmented respiratory–sympathetic coupling is a causal factor in producing the increased vascular resistance seen in this animal model. Thus increased respiratory-related bursts of SNA may contribute to the generation of the increased arterial pressure seen in the spontaneously hypertensive rat strain.
Considerable attention has focused on the role of the sympathetic nervous system in the generation of hypertension in the SH rat. Several studies have shown convincingly that there is elevated sympathetic drive to the vasculature in the adult animal and have proposed that this is important in the maintenance of hypertension (Judy & Farrell, 1979; Lundin et al. 1984; Cabassi et al. 1998). It appears that this increase in sympathetic output is not initially a consequence of alterations in either arterial baroreflex (Judy & Farrell, 1979) or peripheral chemoreflex function (Grisk et al. 1996; Hayward et al. 1999) but rather is a product of an alteration in the central neural circuitry responsible for generating the sympathetic output.
It has also been advocated that the sympathetic nervous system plays an important role in the development of hypertension, based on the observation that sympathectomy in the neonatal period prevents the development of the hypertensive phenotype and also ameliorates the vascular and cardiac hypertrophy seen in SH rats (see Korner et al. 1993; and review by Zicha & Kunes, 1999). Our study provides substantive evidence for this hypothesis with an elevated level of sympathetic activity already present in the neonatal SH rat. More importantly, we observed a striking difference in the pattern of sympathetic activity at all ages in the SH rat. This manifested initially as an increase in the amplitude of the respiratory modulated bursts of SNA but developed, by 5 weeks of age, into a temporally shifted pattern with the peak of the sympathetic burst occurring earlier in the respiratory cycle. It should be noted that this pattern of augmented activity is all the more striking as it was observed in SH rats with higher perfusion pressures. Through baroreflex compensatory activity it might be expected that SH rats would actually exhibit relatively lower levels of sympathetic vasomotor activity. However, we found that in the absence of arterial baroreceptor and peripheral chemoreceptor input the pattern of respiratory–sympathetic activity in SH rats was unchanged suggesting it to be a centrally generated phenomenon.
Altered respiratory–sympathetic coupling has previously been reported in the adult SH rat (Czyzyk-Krzeska & Trzebski, 1990). In anaesthetized, vagotomised, ventilated rats, the peak of sympathetic activity in SH rats occurs during late inspiration rather than during postinspiration as seen in the WKY. Although that study did not report any change in the amplitude of the respiratory-related bursts of sympathetic activity, their report is strikingly similar to the pattern of SNA seen in our study of SH rats at 5 weeks of age. This temporal shift of the sympathetic peak to earlier in the respiratory cycle seems to evolve with age in the SH rat. The SH neonate shows an amplitude augmented version of the respiratory modulation seen in the WKY; however, by 3 weeks there is a spread of excitation into the inspiratory phase and the adult pattern is established by 5 weeks of age. This may reflect plasticity in the nervous system such that the enhanced respiratory coupling represents recruitment of inspiratory related excitatory synaptic drive. This observation has some parallels with the recently reported (Zoccal et al. 2008) changes in respiratory modulation of sympathetic activity seen in the intermittent hypoxic model of hypertension (although an enhancement of late expiratory activity was seen in this instance).
The respiratory periodicity observed in the sympathetic outflow in many species, including rats and humans, is considered to be a product of a central interaction between the brainstem respiratory network and the sympathetic neural circuitry (Habler et al. 1994b; Malpas, 1998). Although there is ongoing debate about the fundamental configuration of these two interlinked systems, with several proposed models (Barman & Gebber, 1976; Richter & Spyer, 1990), we can make some inferences about the site of generation of the pathological changes seen in the SH rat. The increase in the amplitude of respiratory modulation could be generated by changes within either the respiratory or the sympathetic neural circuits or by an alteration in the strength of the coupling between the systems (Habler et al. 1994b; Malpas, 1998; Janig, 2006). Based on our findings of similar respiratory-CO2 response curves in both WKY and SH rats (which were comparable to those previously reported in the WHBP; St-John & Paton, 2000; Day & Wilson, 2007) we can infer that central chemoreflex function is similar across the strains. We also found no difference in the strength of the peripheral chemoreflex response between the two strains, in agreement with some (Grisk et al. 1996; Hayward et al. 1999), but not all published reports (Czyzyk-Krzeska & Trzebski, 1990). Thus it would seem, on the basis of the literature and our current findings, that the changes in respiratory–sympathetic modulation in the neonate and juvenille SH rat are less likely to be a consequence of grossly altered respiratory function. The evidence suggests that SH rats have augmented respiratory–sympathetic coupling rather than altered central respiratory drive.
It should be noted that our experiments (and those of Czyzyk-Krzeska & Trzebski, 1990) were performed in the absence of any pulmonary stretch receptor feedback that would be expected to inhibit sympathetic activity towards the end of inspiration. However, this condition was the same for both rat strains. It has previously been shown that in conscious juvenile SH rats, the power distribution of prazosin-sensitive blood pressure fluctuations in the respiratory frequency range (0.35–1 Hz) were augmented compared to WKY (Almog et al. 1998), implying the presence of similar differences in the respiratory modulation of sympathetic activity in the presence of intact pulmonary afferent feedback to those seen in the present study.
The possible sites at which enhancement of the respiratory sympathetic coupling could occur in the SHR are not addressed in this study. Centrally generated respiratory modulation has been observed in (i) the rostral ventrolateral medulla (RVLM; Haselton & Guyenet, 1989; Pilowsky et al. 1994), (ii) the antecedent inhibitory centre in the caudal ventrolateral medulla (Mandel & Schreihofer, 2006) and from direct respiratory bulbospinal projections, and (iii) sympathetic preganglionic neurones (Richter & Spyer, 1990). The strengthening of synaptic transmission at any of these sites and/or an alteration in the intrinsic excitability of sympathetic preganglionic neurones in the SH rat could account for our observations. That such a mechanism could produce the selective changes in SNA we describe has been indicated by studies employing pharmacological disinhibition of the RVLM with a GABAA antagonist which selectively augmented the postinspiratory component of SNA and this produced a sustained increase in blood pressure and larger Traube–Hering waves in a normotensive rat strain (Miyawaki et al. 2002). Further elucidation of the specific mechanisms by which SH rats may be programmed to have higher sympathetic activity will require cellular recordings at each of these loci to examine the alterations in patterns of respiratory modulation, synaptic strengths and spike outputs.
The increased sympathetic burst amplitude drives the production of larger TH waves in the SH rat. The loss of respiratory drive, and thus TH waves, during hypocapnia-induced apnoea leads to a fall in PP. Importantly the subsequent increase in PP, with the return of eupnoeic PNA and the recovery of both TH waves and respiratory–sympathetic coupling, is greater in SH rats than WKY. Crucially this provides evidence of a causal link between the augmented respiratory–sympathetic coupling and increased vascular resistance in the SH rats. Indeed in the SH rat the TH waves were clearly seen to summate during the recovery from apnoea, and thus drive the mean level of PP upwards. It is also worthy of note that the alteration in the amplitude of TH waves does not appear to be secondary to alterations in the vascular responsiveness to α-adrenoceptor agonists as there was no difference between SH and WKY rats in the pressor response to a standard dose of phenylephrine. It is also not just dependent upon the mean level of SNA, as this was not different between 3- and 5-week-old SH and WKY rats. The increased TH wave amplitude and higher mean PP is a clear demonstration that the end organ response, evident as vascular smooth muscle tone, is dependent on both the pattern of sympathetic discharge (bursting better than tonic) and the mean level of activity.
Increased respiratory-related bursts of SNA will not only lead directly to increased vasomotor tone but also lead directly and indirectly to vascular hypertrophy. Bursting patterns of sympathetic discharge can increase the efficacy of vascular neuro-effector coupling through increased noradrenaline release (Hardebo, 1992). Several studies support a direct, pressure-independent effect of noradrenaline on cardiac and vascular hypertrophy in both animal models and humans (Mancia et al. 1999; Grassi, 2006; Strand et al. 2006). In addition, stretch-induced hypertrophy, as would be produced by the increased TH waves, has been described (Grassi, 2006). We suggest therefore that the increased respiratory-related bursts of sympathetic activity in SH rats are responsible for greater blood pressure variability in the SH rat and that this, in concert with increased noradrenaline release, will induce vascular and cardiac hypertrophy.
There is greater awareness that increased blood pressure variability also plays an important role in determining the degree of end-organ damage seen in hypertension. Studies in both animal models and patients have shown increased BP lability to be an independent risk factor for poor outcome (Miao et al. 2006; Tatasciore et al. 2007). Our demonstration of larger TH waves contributing to an increase in blood pressure variability moves amplified respiratory–sympathetic coupling to centre stage as a component of the cardiovascular pathogenesis of the SH rat. This combination of increased respiratory-related bursts of sympathetic activity reaching the arterial system during postnatal development and the increased lability of arterial pressure may play an important role in the development of arterial hypertrophy and altered vascular compliance which is known to predate the development of hypertension in the SH rat.
Summary
We have shown that SH rats have augmented respiratory–sympathetic coupling compared to normotensive controls at all ages. The pattern of sympathetic bursting alters with development in the SH rat such that they occur earlier in the respiratory cycle and peak in inspiration. This augmented respiratory–sympathetic coupling in the SH rat is preserved under conditions of altered central respiratory drive. The sympathetically mediated TH arterial pressure waves of SH rats were larger at all ages (compared with WKY) showing that the augmented respiratory–sympathetic coupling has effects on vascular tone even before these animals develop hypertension. Furthermore, we have shown that at 5 weeks old the increased respiratory–sympathetic coupling is a causal factor in producing the increased vascular resistance in SH rats. We speculate that the summation of TH waves may contribute to the development and maintenance of hypertension, increased arterial pressure lability and end-organ damage that characterizes the SH rat.
Acknowledgments
Australian National Health and Medical Research Council (project grant number 454432), National Heart Foundation of Australia. AEP is a Wellcome Advanced Fellow and JFRP is in receipt of a Wolfson Merit Award.
References
- Adams MA, Bobik A, Korner PI. Differential development of vascular and cardiac hypertrophy in genetic hypertension. Relation to sympathetic function. Hypertension. 1989;14:191–202. doi: 10.1161/01.hyp.14.2.191. [DOI] [PubMed] [Google Scholar]
- Adrian E, Bronk D, Philips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932;74:115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog Y, Eldor S, Oz O, Akselrod S. Beat-to-beat fluctuations in the BP related signals in rats: can it contribute to the understanding of the development of hypertension? J Auton Nerv Syst. 1998;69:39–48. doi: 10.1016/s0165-1838(98)00006-x. [DOI] [PubMed] [Google Scholar]
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280:H2674–H2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Basis for synchronization of sympathetic and phrenic nerve discharges. Am J Physiol. 1976;231:1601–1607. doi: 10.1152/ajplegacy.1976.231.5.1601. [DOI] [PubMed] [Google Scholar]
- Boczek-Funcke A, Habler HJ, Janig W, Michaelis M. Respiratory modulation of the activity in sympathetic neurones supplying muscle, skin and pelvic organs in the cat. J Physiol. 1992;449:333–361. doi: 10.1113/jphysiol.1992.sp019089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscan P, Allen AM, Paton JF. Baroreflex inhibition of cardiac sympathetic outflow is attenuated by angiotensin II in the nucleus of the solitary tract. Neuroscience. 2001;103:153–160. doi: 10.1016/s0306-4522(00)00559-5. [DOI] [PubMed] [Google Scholar]
- Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in pre-hypertensive phase of spontaneously hypertensive rats. Life Sci. 1998;62:1111–1118. doi: 10.1016/s0024-3205(98)00034-4. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol. 1990;426:355–368. doi: 10.1113/jphysiol.1990.sp018142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Guyenet P. Respiratory modulation of pre- and postganglionic lumbar vasomotor sympathetic neurons in the rat. Neurosci Lett. 1990;119:148–152. doi: 10.1016/0304-3940(90)90820-y. [DOI] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. Brainstem PCO2 modulates phrenic responses to specific carotid body hypoxia in an in situ dual perfused rat preparation. J Physiol. 2007;578:843–857. doi: 10.1113/jphysiol.2006.119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Resp Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1121–R1128. doi: 10.1152/ajpregu.00485.2003. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol. 1985;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Esler M, Rumantir M, Kaye D, Jennings G, Hastings J, Socratous F, Lambert G. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:986–989. doi: 10.1046/j.1440-1681.2001.03566.x. [DOI] [PubMed] [Google Scholar]
- Gilbey MP. Multiple oscillators, dynamic synchronization and sympathetic control. Clin Exp Pharmacol Physiol. 2001;28:130–137. doi: 10.1046/j.1440-1681.2001.03414.x. [DOI] [PubMed] [Google Scholar]
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- Grassi G. Sympathetic overdrive as an independent predictor of left ventricular hypertrophy: prospective evidence. J Hypertens. 2006;24:815–817. doi: 10.1097/01.hjh.0000222748.37078.2d. [DOI] [PubMed] [Google Scholar]
- Grisk O, Exner J, Schmidt M, Honig A. Effects of acute hypoxia and hyperoxia on ventilation in spontaneously hypertensive and normotensive rat. J Auton Nerv Syst. 1996;57:177–180. doi: 10.1016/0165-1838(95)00079-8. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Bartsch T, Janig W. Two distinct mechanisms generate the respiratory modulation in fibre activity of the rat cervical sympathetic trunk. J Auton Nerv Syst. 1996;61:116–122. doi: 10.1016/s0165-1838(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Krummel M, Peters OA. Reflex patterns in postganglionic neurons supplying skin and skeletal muscle of the rat hindlimb. J Neurophysiol. 1994a;72:2222–2236. doi: 10.1152/jn.1994.72.5.2222. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Prog Neurobiol. 1994b;43:567–606. doi: 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Hardebo JE. Influence of impulse pattern on noradrenaline release from sympathetic nerves in cerebral and some peripheral vessels. Acta Physiol Scand. 1992;144:333–339. doi: 10.1111/j.1748-1716.1992.tb09302.x. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol. 1989;256:R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Johnson AK, Felder RB. Arterial chemoreflex in conscious normotensive and hypertensive adult rats. Am J Physiol Heart Circ Physiol. 1999;276:H1215–H1222. doi: 10.1152/ajpheart.1999.276.4.H1215. [DOI] [PubMed] [Google Scholar]
- Head GA. Baroreflexes and cardiovascular regulation in hypertension. J Cardiovasc Pharmacol. 1995;26(Suppl. 2):S7–S16. [PubMed] [Google Scholar]
- Janig W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- Judy WV, Farrell SK. Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension. 1979;1:605–614. doi: 10.1161/01.hyp.1.6.605. [DOI] [PubMed] [Google Scholar]
- Julius S, Krause L, Schork NJ, Mejia AD, Jones KA, van de Ven C, Johnson EH, Sekkarie MA, Kjeldsen SE, Petrin J, et al. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens. 1991;9:77–84. doi: 10.1097/00004872-199101000-00012. [DOI] [PubMed] [Google Scholar]
- Korner P, Bobik A, Oddie C, Friberg P. Sympathoadrenal system is critical for structural changes in genetic hypertension. Hypertension. 1993;22:243–252. doi: 10.1161/01.hyp.22.2.243. [DOI] [PubMed] [Google Scholar]
- Lundin S, Ricksten SE, Thoren P. Renal sympathetic activity in spontaneously hypertensive rats and normotensive controls, as studied by three different methods. Acta Physiol Scand. 1984;120:265–272. doi: 10.1111/j.1748-1716.1984.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Malpas SC. The rhythmicity of sympathetic nerve activity. Prog Neurobiol. 1998;56:65–96. doi: 10.1016/s0301-0082(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol. 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of end-organ damage in rats. J Hypertens. 2006;24:1125–1135. doi: 10.1097/01.hjh.0000226203.57818.88. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. Evidence for a tonic GABA-ergic inhibition of excitatory respiratory-related afferents to presympathetic neurons in the rostral ventrolateral medulla. Brain Res. 2002;924:56–62. doi: 10.1016/s0006-8993(01)03025-6. [DOI] [PubMed] [Google Scholar]
- Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: the regional differences. Neurosci Lett. 1987;81:279–284. doi: 10.1016/0304-3940(87)90396-x. [DOI] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Boscan P, Paton JF. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol. 2003;551:589–599. doi: 10.1113/jphysiol.2003.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AE, Paton JF. A decerebrate, artificially-perfused in situ preparation of rat: utility for the study of autonomic and nociceptive processing. J Neurosci Methods. 2006;155:260–271. doi: 10.1016/j.jneumeth.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Simms AE, Paton JF. Dominant role of aortic baroreceptors in the cardiac baroreflex of the rat in situ. Auton Neurosci. 2008;142:32–39. doi: 10.1016/j.autneu.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Pilowsky P, Llewellyn-Smith IJ, Lipski J, Minson J, Arnolda L, Chalmers J. Projections from inspiratory neurons of the ventral respiratory group to the subretrofacial nucleus of the cat. Brain Res. 1994;633:63–71. doi: 10.1016/0006-8993(94)91522-9. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Cardiorespiratory control. In: Loewy AM, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 189–207. [Google Scholar]
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE. Hierarchical recruitment of the sympathetic and parasympathetic limbs of the baroreflex in normotensive and spontaneously hypertensive rats. J Physiol. 2007;579:473–486. doi: 10.1113/jphysiol.2006.124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Resp Physiol. 2000;123:201–213. doi: 10.1016/s0034-5687(00)00177-8. [DOI] [PubMed] [Google Scholar]
- Strand AH, Gudmundsdottir H, Os I, Smith G, Westheim AS, Bjornerheim R, Kjeldsen SE. Arterial plasma noradrenaline predicts left ventricular mass independently of blood pressure and body build in men who develop hypertension over 20 years. J Hypertens. 2006;24:905–913. doi: 10.1097/01.hjh.0000222761.07477.7b. [DOI] [PubMed] [Google Scholar]
- Struyker-Boudier HA, Evenwel RT, Smits JF, Van Essen H. Baroreflex sensitivity during the development of spontaneous hypertension in rats. Clin Sci (Lond) 1982;62:589–594. doi: 10.1042/cs0620589. [DOI] [PubMed] [Google Scholar]
- Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50:325–332. doi: 10.1161/HYPERTENSIONAHA.107.090084. [DOI] [PubMed] [Google Scholar]
- Zicha J, Kunes J. Ontogenetic aspects of hypertension development: analysis in the rat. Physiol Rev. 1999;79:1227–1282. doi: 10.1152/physrev.1999.79.4.1227. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]