Abstract
Undernourished late gestation fetuses display asymmetric growth restriction, suggestive of a redistribution of nutritional resources. The modification of fetal organ blood supply in response to acute hypoxia is well characterized, but it is not known whether similar responses occur in response to acute reductions in nutrition, or if such late gestation responses can be influenced by early gestation nutrition. In pregnant sheep, total nutrient requirements were restricted during the peri-implantation period (PI40, 40%; PI50, 50% of total, days 1–31) or in late gestation (L, 50% total, days 104–postmortem). Control animals were fed 100% nutrient requirements. Fetal organ blood flows were measured at baseline, and during acute fetal hypoglycaemia induced by maternal insulin infusion at 125 dGA. Baseline heart rate was increased in PI40 fetuses. During hypoglycaemia, an initial rise in fetal heart rate was followed by a slower fall. Fetal femoral artery blood flow decreased, and adrenal blood flow and femoral vascular resistance increased in all fetuses during hypoglycaemia. These changes were accompanied by increased fetal plasma adrenaline and cortisol, and reduced plasma insulin levels. The maximum femoral artery blood flow response to hypoglycaemia occurred earlier in PI50 and PI40 compared with control fetuses. The late gestation fetal cardiovascular response to acute hypoglycaemia was consistent with a redistribution of combined ventricular output away from the periphery and towards central organs. One element of the peripheral vascular response was modified by peri-implantation nutrient restriction, indicating that nutritional challenges early in gestation can have an enduring impact on cardiovascular control.
Disproportionate fetal growth has been linked to an increased risk of cardiovascular disease (CVD) in adult life (Barker et al. 1993; Forsen et al. 1997; Eriksson et al. 2001). The mechanisms underlying this association are likely to originate in utero in response to inadequate nutrition, since nutritional challenges in early and late gestation have been shown to affect adult CVD risk factors (Roseboom et al. 2000; Painter et al. 2005). In animals, undernutrition in early gestation (Gardner et al. 2004; Cleal et al. 2007) and even just prior to implantation (Kwong et al. 2000) has been associated with altered cardiovascular control in adulthood. The fetus may promote its immediate survival during undernutrition by sacrificing the growth of some organs to support the development of others, resulting in asymmetric growth. However, since the epidemiological associations between fetal growth and later risk of CVD relate to the normal range of fetal growth, such responses may not necessarily only confer an immediate survival advantage. They may be part of a ‘predictive adaptive response’ designed to ensure the suitability of an individual's homeostatic control settings for it's anticipated future environment (Gluckman & Hanson, 2004).
It is well known that the late gestation fetus is capable of rapid (Giussani et al. 1993; Giussani et al. 1996) and sustained (Rurak et al. 1990; Richardson et al. 1996) redistribution of organ blood supply in response to hypoxia. This response includes an increase in blood supply to the brain, heart and adrenal glands while the supply to the periphery, and organs including the liver, falls (Cohn et al. 1974; Peeters et al. 1979; Reuss & Rudolph, 1980; Itskovitz et al. 1987). Doppler ultrasound studies in humans have shown an increase in the shunting of fetal blood to the upper body via the ductus venosus in cases of intrauterine growth restriction (Richardson et al. 1996; Tchirikov et al. 1998; Bellotti et al. 2004), consistent with a protective brain-sparing response. Furthermore, late gestation fetal sheep with low levels of circulating glucose (of unknown aetiology) exhibited lower femoral artery blood flow than fetuses with normal glucose levels (Gardner et al. 2002). The cardiovascular response of the late gestation fetal sheep to hypoglycaemia, induced by fetal insulin infusion, is variable, possibly due to the concomitant hyperinsulinaemia (Milley, 1987; Stonestreet et al. 1996). To date, the fetal cardiovascular response to hypoglycaemia without fetal hyperinsulinaemia is not known. The effect of reduced late gestation maternal nutrient intake has also not been tested directly.
Fetal growth rates during early gestation are sensitive to environmental cues including maternal low-protein diet (Kwong et al. 2000) or global undernutrition (Heasman et al. 1998; Oliver et al. 2005). Moreover, early gestation maternal nutrient restriction in sheep is associated with raised basal femoral vascular resistance and augmented femoral vascular resistance response to hypoxia in late gestation (Hawkins et al. 2000a), suggestive of a reduction in peripheral blood supply. The blood supply to the upper body through the ductus venosus has been shown to be affected by maternal diet and body composition before conception (Haugen et al. 2005). However, the effect of early gestation nutrient restriction on late gestation organ blood flows, and the cardiovascular response to an acute late gestation hypoglycaemia have not previously been investigated.
The primary aim of this study was to characterize the late gestation fetal cardiovascular response to acute hypoglycaemia induced by maternal insulin infusion, in the absence of fetal hyperinsulinaemia. In addition, we aimed to determine the effect of peri-implantation and late gestation maternal nutrient restriction on this response. The intensity of the undernutrition challenges was based on previous sheep experiments (Edwards & McMillen, 2001; Gardner et al. 2004; Cleal et al. 2007) and the modest effects of the peri-implantation challenge intensity led us to increase its intensity in year 2. Previous evidence of altered blood flow through specific vessels (e.g. femoral artery; Hawkins et al. 2000b; Gardner et al. 2002) in the face of undernutrition meant that it was important to assess organ blood flows. Microsphere techniques to assess fetal organ blood flow have been advanced by the use of fluorescent microspheres which have practical and financial advantages over the radiolabelled variety. While they are still challenging technically, they have been used with success in the fetus (Tan et al. 1997) and were therefore developed by us for use in this study.
Methods
All procedures were carried out with local ethics approval and in accordance with the UK Animals (Scientific Procedures) Act 1986.
Animals and study design
Two studies were performed over two sheep-breeding seasons on singleton fetuses, as confirmed by a mid-gestation ultrasound scan. In both studies, Welsh Mountain ewes of uniform body condition score (BCS 2.0–3.0; Russel et al. 1969) and age were randomised to control or dietary restricted groups, housed individually on wheat straw and fed a complete pelleted diet with free access to water (89.2% dry matter as fed, and provided 10.7 MJ (kg dry matter)−1 (metabolisable energy) and 14.8% protein) from −16 dGA (adjusted to gestational age; AFRC, 1993). Oestrus was synchronized by withdrawal of a vaginal medoxyprogesterone acetate impregnated sponge (Veramix, Upjohn, Ltd, Crawley, UK) at −2 dGA, 14 days after insertion. One of two twin rams (randomly assigned) was introduced for 2 days, and 0 dGA was taken as the first day that an obvious raddle mark was observed. Maternal weight and BCS were assessed, and maternal blood samples (36 ml onto chilled EDTA tubes, approximately 1% blood volume; Rumball et al. 2008) were taken from the jugular vein at 29 dGA.
Study 1
Control animals (C1, n= 10) were fed 100% of nutrient requirements. Peri-implantation nutrient restricted animals (PI50, n= 9) were fed 50% of nutrient requirements from 1 to 31 dGA, and 100% at all other times.
Study 2
Control animals (C2, n= 10) were fed 100% of nutrient requirements. Peri-implantation nutrient restricted animals (PI40, n= 9) were fed 40% of nutrient requirements from 1 to 31 dGA, and 100% at all other times. Late gestation nutrient restricted animals (L, n= 6) were fed 50% of nutrient requirements from 104 dGA until postmortem, and 100% at all other times.
Surgical preparation and care
At approximately 117 dGA, anaesthesia was induced with 1 g thiopental sodium BP i.v. (10 ml, 0.1 g ml−1, Link Pharmaceuticals, UK) and maintained with 2% halothane (Concord Pharmaceuticals Laboratory Ltd, UK) in O2 (1 l min−1). Saline-filled polyvinyl catheters (Portex Ltd, Hythe, UK) were inserted into the fetal carotid and femoral artery, femoral vein, trachea, amniotic cavity and bladder (data not reported here) and the maternal jugular veins. Ultrasonic flow probes (Transonic Systems Inc., Ithaca, NY, USA) were placed around the uncatheterized carotid and femoral arteries, and stainless-steel electrodes were placed on the parietal cortex for fetal electrocorticogram (ECoG) recording. Uterine and abdominal incisions were closed and catheters were exteriorized. The vascular catheters were heparinized (fetal, 50 U ml−1; ewe, 100 U ml−1 heparin, Leo Laboratories Ltd, Princes Risborough, UK in 0.9% NaCl saline).
At surgery, antibiotics were administered to: (1) the ewe (oxytetracycline hydrochloride (Terramycin) topically to incision sites, Pfizer, Eastleigh, Northants, UK; Betamox i.m. (150 mg kg−1 amoxicillin), Norbrook Laboratories Ltd, UK; Crystapen i.v. (600 mg), Britannia Pharmaceuticals Ltd, Redhill, Surrey, UK; gentamicin i.v. (40 mg), Mayne Pharma plc, Royal Leamington Spa, UK); (2) the fetus (Crystapen i.v.; 300 mg); and (3) the amniotic cavity (Crystapen, 300 mg and gentamicin, 40 mg). Gentamicin doses were repeated on postoperative days 1 and 2, and half doses of Crystapen were administered daily for 4–5 postoperative days. Vascular and amniotic catheters were flushed daily with heparinized saline, and vascular catheters were kept patent with a continuous infusion (fetal; 0.01 ml h−1, ewe; 1 ml h−1).
Fetal monitoring
Fetal carotid artery, amniotic and tracheal pressures (Capto AS, N-3193, Horten, Norway/NL 108, Digitimer Ltd, Welwyn Garden City, UK), ECoG (NL 100/104/125) and carotid and femoral arterial blood flows (TS420, Transonic Systems Inc.) data were captured (Sampling rate 40 samples per second, Maclab/8, ADInstruments Pty Ltd, Castle Hill, Australia) and recorded (Chart, ADInstruments, Chalgrove, UK).
Experimental procedures
Fetal cardiovascular measurements and hypoglycaemia
At approximately 10.00 h, cardiovascular variables and ECoG activity were measured continuously before (approximately 60 min) and after (60 min) the onset of maternal i.v. infusion of heparinized saline vehicle (study 1 only, 125 dGA, data not shown) or insulin (125 ± 1 dGA, 130 min (30 s bolus of 0.5 iU kg−1 min−1 and 0.01 iU kg−1 min−1 thereafter), porcine, Novo Nordisk, UK; 5 iU ml−1 in heparinized saline) (Fig. 1). In addition, organ blood flows were measured with fluorescent microspheres (Molecular Probes, PoortGebouw, the Netherlands) before (just after 60 min of continuous variable recording – see above) and during (between 60 and 130 min) maternal insulin-induced fetal hypoglycaemia using techniques based on previously reported methods (Tan et al. 1997) (see below). The microsphere measurements were always made during the first available episode of high voltage ECoG activity, to control for any effect of electrocortical activity on cardiovascular parameters.
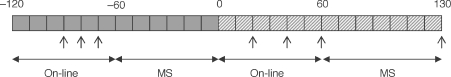
Figure 1. Late gestation maternal insulin infusion protocol.
Baseline (= 60 min, filled boxes) and maternal insulin infusion (130 min, diagonal striped boxes) periods comprised on-line fetal cardiovascular/ECoG recording, and two periods during which microspheres (MS) were administered to the fetus during episodes of high voltage ECoG activity. Maternal (5.5 ml for hormone and blood gas analysis) and fetal (0.25 ml for blood gas analysis) blood samples were taken at predetermined intervals (arrows). Fetal MS reference blood sample (7.5 ml) plasma was kept for hormone analysis. In study 2 only, an additional 4 ml fetal blood was taken immediately before the MS reference sample for additional hormone analysis.
Blood collection
Maternal (0.5 ml) and fetal femoral arterial (0.25 ml) blood was collected at predetermined intervals (Fig. 1) into cold heparinized syringes and analysed for glucose, lactate, pH, oxygen and CO2 with a blood gas analyser (ABL735, Radiometer Limited, Crawley, UK).
Maternal blood (5 ml, Fig. 1) and filtrate of the fetal blood taken for microspheres reference samples (7.9 ml, see below) was collected at predetermined intervals for hormone measurement into chilled EDTA tubes, centrifuged at 1600 g and 4°C for 10 min and plasma was aliquoted. In study 2 only, an extra 4 ml of fetal blood was taken just prior to the microspheres reference sample to provide plasma for ACTH measurement. All plasma was stored at −80°C.
Postmortem procedure
Ewes and fetuses were killed at 127 dGA with 40 ml i.v. pentobarbitone sodium (200 mg ml−1 Pentoject, Animalcare Ltd, UK), and organs/tissues were harvested immediately for microsphere blood flow analysis. Skeletal muscle samples were taken from the gastrocnemius.
Organ blood flow determination
Fetal and placental tissues and reference samples (see above) were collected into a processing unit (SPU, Gaiser Kunststoff und Metallprodukte, Kappel-Grafenhausen, Germany) and digested (4 m aqueous KOH with 2% Tween-80, covered with a 1.5 ml layer of isopropanol) (Thein et al. 2002). The microspheres were then washed with phosphate buffer, dissolved with 4 ml cellosolve® acetate (Sigma-Aldrich, Inc., UK), and analysed (LS-55 luminescence spectrometer, PerkinElmer, Inc., UK). Organ blood flows were calculated with the formula:
where blood flow is in ml min−1 (100 g tissue)−1, Wtsample is weight of the tissue sample, Wdref is withdrawal rate of the reference sample, Fsample is fluorescence intensity of the tissue sample and Fref is fluorescence intensity of the reference sample (Heymann et al. 1977).
Plasma hormone analysis
Cortisol was determined in 10 μl plasma by a solid-phase competitive chemiluminescence enzyme immunoassay (DPC Immulite analyzer (LKC05), Siemens Healthcare Diagnostics Ltd., Camberley, UK). The lower limit of the assay was 0.2 μg dl−1 and the interassay coefficient of variation was 8.2% at 3.0 μg dl−1.
ACTH was determined in 75 μl plasma by a sequential immunometric assay (automated DPC Immulite system (LKAC5)). The lower limit of the assay was 9 pg ml−1 and the interassay coefficient of variation was 3.2% at 105 pg ml−1.
Insulin was measured in 25 μl plasma by ELISA (DRG Sheep Insulin; ImmunoDiagnostic Systems Ltd, Boldon, UK). The range of the assay was 0.1–2.5 μg l−1 and the inter assay coefficient of variation was 6% at 0.12 μg l−1.
Adrenaline and noradrenaline were measured using a combined 125I radioimmunoassay (2 Cat RIA, Labor Diagnostika Nord GmBH & Co, Nordham, Germany). For noradrenaline, the sensitivity of the assay was 12.5 pg ml−1 and the interassay coefficient of variation was 15.5% at 225 pg ml−1 and 15.3% at 1125 pg ml−1. For adrenaline, the sensitivity of the assay was 2.5 pg ml−1 and the interassay coefficient of variation was 23.6% at 1125 pg ml−1 and 14.9% at 5625 pg ml−1.
Data analysis and statistics
Fetal ECoG state was assessed by visual discrimination of traces (Walker & Pratt, 1998; El Haddad et al. 1999). Carotid and femoral artery vascular resistance were calculated (arterial blood flow/mean arterial pressure) (Green et al. 1998), during high and low voltage ECoG activity.
Baseline cardiovascular parameters are presented as an average over the 1 h preceding the onset of maternal vehicle or insulin infusion. Within individual studies (i.e. study 1 and study 2), variables including baseline measurements were compared between groups by Student's t test (study 1) or one-way ANOVA (study 2) (SPSS version 12.0, Chicago, IL, USA), and Bonferroni's post hoc test were performed where significant results (P < 0.05) were found. In both studies together, changes in variables over time in response to the hypoglycaemic challenge were analysed by comparing the baseline average with 10 min averages during the infusion period. This analysis was performed using a repeated-measures two-way ANOVA with Bonferroni post hoc tests (using dietary group as a between-subject factor). Within the individual studies, differences between groups in responses to maternal insulin infusion were assessed by one-way ANOVA of the area under/over the curve of the response and the time to maximum response (Matthews et al. 1990), or by repeated-measures two-way ANOVA where only two time points were available (i.e. fetal organ blood flow, hormone and ECoG data). Significance was accepted when P < 0.05, and data are expressed as means ±s.e.m.
Results
There was no change in the variables measured with vehicle infusion (study 1 only, data not shown).
Fetal weights
There was no difference among groups in fetal body weight (C1, 3.0 ± 0.1 kg; PI50, 3.1 ± 0.1 kg; C2, 2.9 ± 0.1Kg; PI40, 2.9 ± 0.1 kg; L, 2.8 ± 0.2 kg) or in individual organ weights (data reported elsewhere).
Nutrients and metabolites
Baseline
During the baseline period there was no difference between the C1/C2 and PI50/PI40 or L groups in maternal or fetal blood glucose or lactate, or in fetal blood gases (Table 1).
Table 1.
Basal measurements
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| C1 | PI50 | C2 | PI40 | L | |
| Maternal | |||||
| Glucose (mmol l−1) | 3.76 ± 0.11 | 3.44 ± 0.20 | 3.42 ± 0.13 | 3.58 ± 0.20 | 3.22 ± 0.13 |
| Lactate (mmol l−1) | 0.60 ± 0.1 | 0.32 ± 0.05 | 0.55 ± 0.08 | 0.65 ± 0.07 | 0.47 ± 0.06 |
| Insulin (μg l−1) | 1.5 ± 0.3 | 0.8 ± 0.1 | 1.1 ± 0.2 | 1.4 ± 0.4 | 0.6 ± 0.1 |
| Cortisol (μg dl−1) | 1.8 ± 0.4 | 1.5 ± 0.3 | 0.9 ± 0.2 | 1.3 ± 0.2 | 0.9 ± 0.1 |
| ACTH (pg ml−1) | 16 ± 2 | 13 ± 1 | 22 ± 5 | 39 ± 21 | 17 ± 3 |
| Adrenaline (pg ml−1) | 10 ± 1 | 17 ± 3 | 24 ± 5 | 29 ± 5 | 19 ± 4 |
| Noradrenaline (pg ml−1) | 115 ± 15 | 140 ± 12 | 482 ± 140 | 511 ± 150 | 312 ± 84 |
| Fetal | |||||
| Glucose (mmol l−1) | 0.84 ± 0.06 | 0.81 ± 0.06 | 0.82 ± 0.04 | 0.81 ± 0.03 | 0.73 ± 0.03 |
| Lactate (mmol l−1) | 0.92 ± 0.06 | 0.90 ± 0.06 | 0.94 ± 0.05 | 1.02 ± 0.09 | 1.13 ± 0.11 |
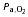 (mmHg) (mmHg) |
19.6 ± 0.7 | 18.4 ± 0.9 | 20.2 ± 0.7 | 18.6 ± 1.3 | 17.7 ± 0.8 |
| pH | 7.34 ± 0.00 | 7.34 ± 0.01 | 7.35 ± 0.01 | 7.34 ± 0.01 | 7.34 ± 0.01 |
| K+ (mmol l−1) | 2.7 ± 0.1 | 2.6 ± 0.3 | 2.8 ± 0.1 | 3.1 ± 0.2 | 2.9 ± 0.1 |
| MAP (mmHg) | 43 ± 1 | 43 ± 1 | 43 ± 1 | 45 ± 2 | 42 ± 2 |
| Heart rate (BPM) | 173 ± 6 | 174 ± 5 | 168 ± 4 | 183 ± 3† | 169 ± 3 |
| FA flow (ml min−1) | 39 ± 6 | 33 ± 5 | 32 ± 4 | 37 ± 4 | 36 ± 6 |
| CA flow (ml min−1) | 83 ± 12 | 77 ± 8 | 81 ± 5 | 89 ± 10 | 79 ± 11 |
| FVR (mmHg min ml−1) | 1.44 ± 0.30 | 1.92 ± 0.58 | 1.35 ± 0.11 | 1.49 ± 0.32 | 1.09 ± 0.12 |
| CVR (mmHg min ml−1) | 0.91 ± 0.36 | 0.62 ± 0.06 | 0.60 ± 0.05 | 0.57 ± 0.06 | 0.53 ± 0.04 |
Values are mean ±s.e.m. C1, n= 10; PI50, n= 9; C2, n= 10; PI40, n= 9; L, n= 6. MAP, mean arterial pressure; FA, femoral artery; CA, carotid artery; FVR, femoral vascular resistance; CVR, carotid vascular resistance. †P < 0.01 versus C2.
Response to maternal insulin infusion
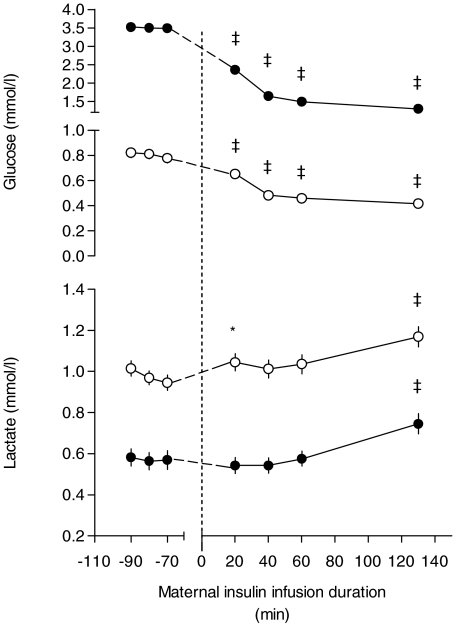
Maternal glucose fell during insulin infusion (P < 0.001, Fig. 2) to a similar extent in the C1/C2, and PI50/PI40 and L groups (Table 2), and maternal lactate increased significantly by 130 min of insulin infusion (Fig. 2). Fetal glucose fell by 20 min of maternal insulin infusion (P < 0.001, Fig. 2) to a similar extent in the C1/C2, PI50/PI40 and L groups (Table 2). Fetal lactate rose above baseline at 20 and 130 min of maternal insulin infusion (P < 0.05 and 0.001, respectively, Fig. 2).
Figure 2.
Maternal and fetal blood glucose and lactate during baseline and maternal insulin infusion Values are means ±s.e.m. (error bars are hidden within symbols if not visible) for all ewes (•) and fetuses (○), both n= 44. *P < 0.05, ‡P < 0.001 versus baseline average.
Table 2.
Glucose and maternal hormones during hypoglycaemia: dietary group comparison
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| C1 | PI50 | C2 | PI40 | L | |
| Area over curve | |||||
| Maternal glucose (mmol min l−1) | 241.8 ± 9 | 213.6 ± 27 | 230.9 ± 16 | 239.2 ± 22 | 199.0 ± 7 |
| Fetal glucose (mmol min l−1) | 39.5 ± 5 | 31.5 ± 3 | 45.5 ± 4 | 35.1 ± 4 | 32.8 ± 3 |
| Area under curve | |||||
| Insulin (μg min l−1) | 9699 ± 610 | 12033 ± 2300 | 9569 ± 1000 | 10115 ± 590 | 9733 ± 530 |
| Cortisol (μg min dl−1) | 530 ± 130 | 843 ± 160 | 283 ± 82 | 391 ± 120 | 433 ± 170 |
| ACTH (pg min ml−1) | 14439 ± 3900 | 32010 ± 14000 | 14711 ± 7100 | 22443 ± 8200 | 11896 ± 6100 |
| Adrenaline (pg min ml−1) | 12418 ± 2500 | 33455 ± 9200 | 11701 ± 4500 | 22324 ± 6300 | 8169 ± 3500 |
| Noradrenaline (pg min ml−1) | 21478 ± 12000 | 19209 ± 4000 | 35258 ± 12000 | 79578 ± 26000 | 13552 ± 4100 |
Values are mean ±s.e.m. area over- or under- the curve. C1, n= 10; PI50, n= 9; C2, n= 10; PI40, n= 9; L, n= 6.
There was no significant change in fetal 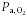 (baseline, 18.8 ± 0.4 mmHg; 130 min, 18.2 ± 0.6 mmHg; P > 0.05) or pH (baseline, 7.34 ± 0.0; 130 min, 7.35 ± 0.0; P > 0.05) during the maternal insulin infusion. There was a significant increase in fetal arterial K+ levels by 130 min of maternal insulin infusion (baseline, 2.8 ± 0.1 mmol l−1; 130 min, 3.1 ± 0.1 mmol l−1, P < 0.001) to a similar extent in dietary groups.
(baseline, 18.8 ± 0.4 mmHg; 130 min, 18.2 ± 0.6 mmHg; P > 0.05) or pH (baseline, 7.34 ± 0.0; 130 min, 7.35 ± 0.0; P > 0.05) during the maternal insulin infusion. There was a significant increase in fetal arterial K+ levels by 130 min of maternal insulin infusion (baseline, 2.8 ± 0.1 mmol l−1; 130 min, 3.1 ± 0.1 mmol l−1, P < 0.001) to a similar extent in dietary groups.
Hormones
Baseline
At 29 dGA (the end of the peri-implantation dietary restriction period), maternal plasma cortisol concentration was not different between the C1 and PI50 groups in study 1 (C1, 2.7 ± 0.5; PI50, 1.6 ± 0.2 μg dl−1), nor among the C2, PI40 and L groups in study 2 (C2, 2.6 ± 0.6; PI40, 1.5 ± 0.4; L, 2.1 ± 0.6 μg dl−1).
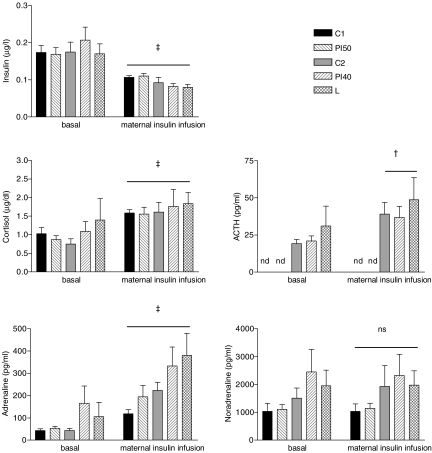
At 125 ± 1 dGA (after recovery from surgery) maternal basal plasma insulin, cortisol, ACTH and catecholamine concentrations were not different between the control and maternal dietary restricted groups (Table 1).
There was no significant difference among the dietary groups in fetal basal plasma concentration of insulin, cortisol, ACTH or catecholamines (Fig. 3).
Figure 3.
Fetal plasma hormones during baseline and maternal insulin infusion Values are means ±s.e.m. C1, n= 10; PI50, n= 9; C2, n= 10; PI40, n= 9; L, n= 6. ns, no significant difference; nd, not determined. †P < 0.01, ‡P < 0.001 versus baseline for all groups together. Measurements were made on the filtrate of microsphere reference fetal blood samples during baseline and maternal insulin infusion.
Response to maternal insulin infusion
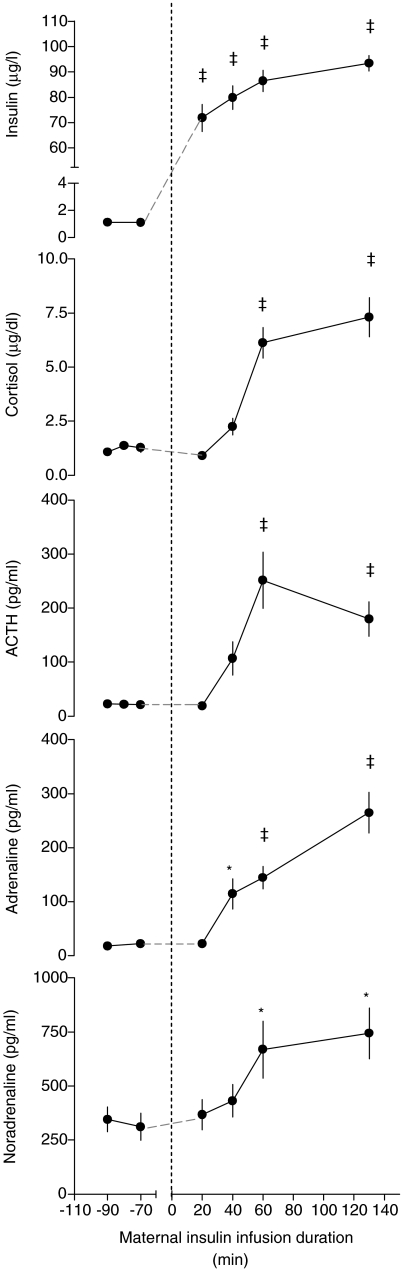
Maternal plasma insulin concentration was increased by 20 min of maternal insulin infusion (P < 0.001, Fig. 4). This was associated with an increase in maternal plasma adrenaline (P < 0.05) by 40 min, and in cortisol (P < 0.001), ACTH (P < 0.001) and noradrenaline (P < 0.05) levels by 60 min of maternal insulin infusion (Fig. 4). There was no difference between the control and maternal dietary restricted groups in the AUC of these hormone responses (Table 2).
Figure 4.
Maternal plasma hormones during baseline and maternal insulin infusion Values are means ±s.e.m. for all ewes (n= 44). *P < 0.05, ‡P < 0.001 versus baseline average.
Fetal plasma insulin decreased (P < 0.001, Fig. 3) during maternal insulin infusion. Fetal plasma cortisol (P < 0.001), ACTH (P < 0.01, study 2 only) and adrenaline levels increased (P < 0.001), and noradrenaline levels were unchanged during maternal insulin infusion (Fig. 3). These hormone changes were not significantly different among the dietary groups (Table 2).
Cardiovascular variables and ECoG state
Baseline
The time spent in high voltage (HV) fetal ECoG activity was not different among the C1/C2, PI50/PI40 and L groups during the baseline period (Table 3).
Table 3.
Fetal organ blood flows and ECoG state
| Baseline | Hypoglycaemia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | PI50 | C2 | PI40 | L | All | C1 | PI50 | C2 | PI40 | L | All | |
| Organ blood flow (mL min−1 (100 g)−1) | ||||||||||||
| Pancreas | 89.6 | 90.2 | 57.8 | 74.3 | 81.9 | 78.0 | 84.6 | 72.7 | 47.2 | 94.1 | 62.6 | 72.3 |
| ± 8.5 | ± 19.7 | ± 7.2 | ± 8.4 | ± 16.7 | ± 5.7 | ± 18.3 | ± 14.1 | ± 4.4 | ± 14.1 | ± 13.2 | ± 6.3 | |
| Right adrenal | 493.8 | 438.0 | 393.4 | 711.1 | 527.3 | 510.1 | 488.6 | 479.9 | 487.7 | 1004.4 | 616.9 | 625.6 |
| ± 70.5 | ± 79.8 | ± 73.8 | ± 182.0 | ± 139.3 | ± 56.2 | ± 82.3 | ± 85.6 | ± 62.8 | ±280.5 | ± 130.4 | ± 78.8† | |
| Right kidney | 185.1 | 216.2 | 200.7 | 231.7 | 240.7 | 212.3 | 177.6 | 196.1 | 194.8 | 257.4 | 203.6 | 206.8 |
| ± 8.7 | ± 34.1 | ± 18.5 | ± 21.5 | ± 33.1 | ± 10.5 | ± 15.6 | ± 33.3 | ± 10.1 | ± 26.9 | ± 16.7 | ± 10.6 | |
| Left liver | 6.0 | 6.2 | 2.8 | 3.9 | 5.0 | 4.7 | 4.9 | 4.9 | 2.6 | 3.3 | 3.4 | 3.7 |
| ± 1.2 | ± 1.4 | ± 0.6 | ± 0.7 | ± 1.6 | ± 0.5 | ± 1.1 | ± 1.3 | ± 0.5 | ± 0.2 | ± 0.8 | ± 0.4† | |
| Right liver | 5.8 | 4.9 | 2.9 | 4.3 | 4.8 | 4.6 | 5.3 | 4.6 | 2.9 | 4.2 | 3.5 | 4.0 |
| ± 1.6 | ± 1.2 | ± 0.5 | ± 0.7 | ± 1.9 | ± 0.5 | ± 1.1 | ± 1.3 | ± 0.4 | ± 0.6 | ± 0.9 | ± 0.4 | |
| Left heart | 243.9 | 276.5 | 227.1 | 273.3 | 290.2 | 263.1 | 235.1 | 224.7 | 237.0 | 326.0 | 257.9 | 260.3 |
| ± 29.8 | ± 30.2 | ± 8.8 | ± 30.3 | ± 47.9 | ± 11.9 | ± 12.9 | ± 38.2 | ± 14.5 | ± 51.8 | ± 24.9 | ± 14.2 | |
| Right heart | 286.9 | 369.3 | 290.9 | 357.2 | 385.1 | 334.0 | 278.7 | 329.2 | 301.5 | 445.7 | 338.4 | 345.6 |
| ± 28.8 | ± 39.5 | ± 15.0 | ± 51.1 | ± 82.5 | ± 19.0 | ± 25.0 | ± 68.9 | ± 22.0 | ± 76.7 | ± 32.1 | ± 23.7 | |
| Left lung | 126.9 | 161.8 | 145.0 | 96.2 | 105.7 | 127.6 | 145.2 | 177.4 | 132.7 | 166.9 | 127.2 | 150.5 |
| ± 21.6 | ± 44.4 | ± 24.7 | ± 27.5 | ± 38.4 | ± 14.0 | ± 25.7 | ± 57.9 | ± 37.2 | ± 47.3 | ± 39.4 | ± 18.3 | |
| Right lung | 142.6 | 106.9 | 119.7 | 91.7 | 100.3 | 111.5 | 132.6 | 226.8 | 164.2 | 160.1 | 147.5 | 167.0 |
| ± 23.9 | ± 18.8 | ± 19.7 | ± 27.1 | ± 39.2 | ± 11.1 | ± 20.0 | ± 57.4 | ± 63.0 | ± 41.4 | ± 37.5 | ± 21.8 | |
| Skeletal Muscle | 18.31 | 20.2 | 17.4 | 12.7 | 14.1 | 16.4 | 14.1 | 17.4 | 16.9 | 20.7 | 16.0 | 17.2 |
| ± 3.4 | ± 5.0 | ± 4.2 | ± 1.5 | ± 3.0 | ± 1.7 | ± 3.3 | ± 2.4 | ± 3.4 | ± 3.8 | ± 2.9 | ± 1.5 | |
| Brain stem | 213.5 | 191.9 | 198.0 | 199.8 | 166.7 | 200.0 | 207.5 | 229.5 | 195.5 | 217.1 | 153.9 | 202.9 |
| ± 20.6 | ± 15.8 | ± 48.0 | ± 29.9 | ± 29.6 | ± 14.1 | ± 12.5 | ± 29.8 | ± 64.6 | ± 34.0 | ± 39.2 | ± 17.7 | |
| Brain cortex | 162.0 | 161.6 | 132.7 | 159.4 | 176.2 | 155.1 | 147.2 | 149.0 | 129.7 | 148.1 | 133.9 | 141.2 |
| ± 17.5 | ± 19.3 | ± 7.8 | ± 18.9 | ± 65.8 | ± 9.8 | ± 7.0 | ± 19.5 | ± 14.6 | ± 11.3 | ± 25.9 | ± 6.7 | |
| Skin | 75.3 | 83.1 | 55.7 | 55.6 | 57.7 | 63.8 | 61.5 | 59.1 | 49.5 | 61.4 | 55.2 | 57.0 |
| ± 6.6 | ± 14.0 | ± 3.7 | ± 7.9 | ± 2.0 | ± 3.7 | ± 7.1 | ± 6.4 | ± 5.0 | ± 6.9 | ± 8.4 | ± 2.9 | |
| Gut | 125.9 | 129.0 | 85.3 | 95.9 | 124.0 | 110.3 | 117.8 | 126.6 | 90.1 | 127.7 | 122.7 | 115.8 |
| ± 10.7 | ± 27.3 | ± 5.1 | ± 10.0 | ± 12.6§ | ± 6.8 | ± 11.5 | ± 19.6 | ± 6.2 | ± 13.6 | ± 12.2 | ± 6.1 | |
| Placenta | 185.1 | 157.2 | 168.6 | 178.7 | 167.7 | 172.5 | 164.1 | 158.4 | 179.7 | 200.9 | 180.6 | 177.1 |
| ± 21.6 | ± 29.9 | ± 7.5 | ± 15.4 | ± 24.8 | ± 8.9 | ± 25.1 | ± 23.1 | ± 10.2 | ± 15.9 | ± 17.9 | ± 8.5 | |
| ECoG state (min hr−1) | ||||||||||||
| HV | 27 | 27 | 26 | 29 | 24 | 27 | 29 | 35 | 28 | 38 | 27 | 35 |
| ± 4 | ± 3 | ± 3 | ± 1 | ± 4 | ± 1 | ± 2 | ± 4 | ± 2 | ± 3 | ± 3 | ± 1* | |
Values are mean ±s.e.m. HV; high voltage ECoG activity. *P < 0.05 versus baseline (all animals), †P < 0.01 versus baseline (all animals), §P < 0.05 versus C2.
Basal fetal heart rate, mean arterial pressure and femoral and carotid arterial blood flows were not different between the C1 and PI50 groups in study 1. In study 2, fetal heart rate was significantly higher in PI40 fetuses than in C2 fetuses (P < 0.01, Table 1), but there was no effect of maternal dietary group on basal mean arterial pressure and femoral and carotid arterial blood flows (Table 1).
Basal organ blood flows did not differ between the C1 and PI50 groups in study 1 (Table 3). In study 2, basal gut blood flow only was higher in L than C fetuses (P < 0.05).
Response to maternal insulin infusion
The prevalence of the HV fetal ECoG state increased during maternal insulin infusion (P < 0.05, Table 3). There was no effect of maternal dietary group on the prevalence of fetal HV ECoG state (Table 3).
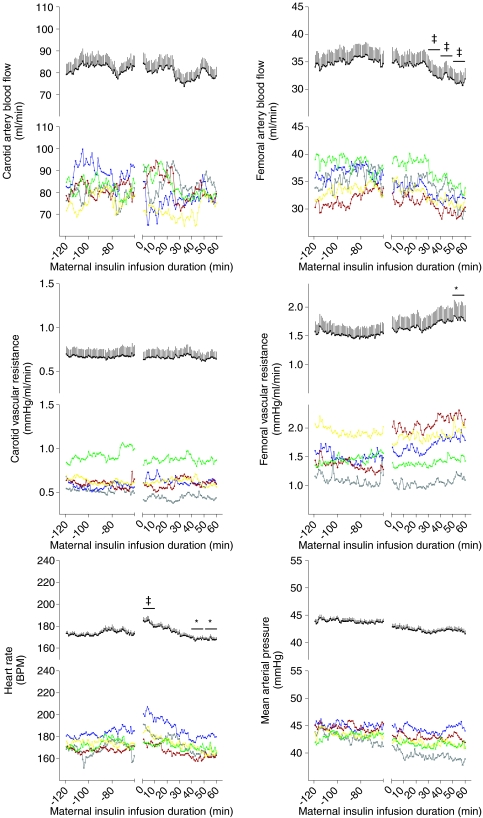
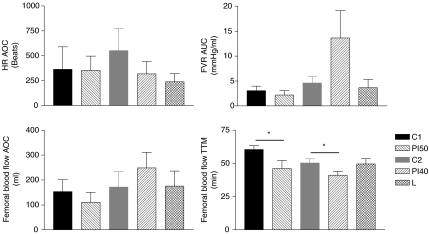
In all animals, fetal femoral artery vascular resistance increased and femoral artery blood flow fell below baseline during the maternal insulin infusion (Fig. 5). There was a transient rise, and then fall, compared to baseline in fetal heart rate during maternal insulin infusion (Fig. 5). Fetal mean arterial pressure, carotid blood flow and carotid vascular resistance did not change from baseline during maternal insulin infusion (Fig. 5). The magnitude of the fall in femoral artery blood flow and heart rate were not different among maternal dietary groups (Fig. 6). However, the time to maximum fall in femoral artery flow was significantly less in PI50 than C1 fetuses (P < 0.05, Fig. 6), and in PI40 than C2 fetuses (P < 0.05, Fig. 6). At the time of maximum fall in femoral blood flow, 51% of fetuses were in HV and of 49% fetuses (all groups together) were in LV, and there was no significant difference among groups (HV versus LV: C1, 33%versus 67%; PI50, 63%versus 38%; C2, 60%versus 40%; PI40, 56%versus 44%; L, 40%versus 60%).
Figure 5.
Fetal cardiovascular response to maternal insulin infusion For each variable, values are displayed as individual groups (lower panel, C1, green; PI50, yellow; C2, red; PI40, blue; L, grey) and mean +s.e.m. for all fetuses (upper panel, n= 44). *P < 0.05, ‡P < 0.001 versus baseline average. BPM, beats per minute.
Figure 6.
Fetal cardiovascular responses to maternal insulin infusion: dietary group comparison Values are means ±s.e.m. C1, n= 10; PI50, n= 9; C2, n= 10; PI40, n= 9; L, n= 6. HR, heart rate; FVR, femoral vascular resistance; AOC, area over curve; AUC, area under curve; TTM, time to maximum response. *P < 0.05.
In all animals, adrenal (right) blood flow increased (P < 0.01, Table 3) and left liver arterial blood flow decreased (P= 0.01, Table 3) during maternal insulin infusion. There was also a trend towards a fall in skin blood flow (P= 0.051, Table 3). In both study 1 and study 2, there were no differences among the dietary groups in organ blood flows during maternal insulin infusion (Table 3). The time between the onset of maternal insulin infusion and the injection of microspheres was not significantly different among groups (C1, 80 ± 3 min; PI50, 77 ± 3 min; C2, 78 ± 3 min; PI40, 84 ± 4 min; L, 77 ± 2 min).
Discussion
This study has demonstrated for the first time the ability of the late gestation fetus to alter the distribution of its combined ventricular output in response to acute hypoglycaemia. The response is characterized by the diversion of blood supply away from the periphery, an increase in adrenal blood flow and hormone output, and a gradual fall in fetal heart rate. Although peri-implantation nutrient restriction did not lead to a sustained redistribution of organ blood supply in late gestation, it did alter the response of the fetus to a subsequent late gestation episode of acute hypoglycaemia.
Our finding of reduced femoral artery and hepatic blood flows and increased adrenal blood flow during maternal insulin infusion-induced fetal hypoglycaemia is consistent with the previous association of low fetal blood glucose levels with low peripheral blood flow in late gestation sheep (Gardner et al. 2002), and suggests that the fetus is capable of redistributing its combined ventricular output away from the periphery and towards more vital organs in response to hypoglycaemia. Our observation of no effect of hypoglycaemia on kidney blood flow is consistent with recent findings that chronic fetal hypoglycaemia does not alter kidney growth or function (Boyce et al. 2007). Our findings are quite different from previous work in which hypoglycaemia was induced by intrafetal insulin infusion. This approach increased blood flow to the fetal heart and carcass (Milley, 1987; Stonestreet et al. 1996), and to the gut, adrenal glands, kidney and hepatic artery (Stonestreet et al. 1996), probably via the high circulating fetal insulin levels with or without low fetal oxygen levels (Milley, 1987). In the present study maternal insulin infusion did not reduce fetal oxygen levels and in fact reduced fetal plasma insulin levels.
Our observation of augmented adrenal blood flow, combined with raised plasma adrenaline and cortisol, in fetuses during hypoglycaemia is consistent with a central role for the adrenal glands in mediating the fetal cardiovascular response to hypoglycaemia (Harwell et al. 1990; Cohen et al. 1991). Our finding that noradrenaline levels did not change in the hypoglycaemic fetuses is similar to previous observations of no change (Cohen et al. 1991), or only a small increase (Harwell et al. 1990), in fetal noradrenline levels during maternal insulin infusion. Transfer of maternal catecholamines and cortisol to the fetus seems unlikely since these hormones do not readily cross the placenta in sheep (Jones & Robinson, 1975; Hennessy et al. 1982; Gu & Jones, 1986). Moreover, fetal femoral artery blood flow began to fall before maternal stress hormones were significantly elevated. In addition fetal heart rate fell during the maternal insulin infusion as observed clinically with severe maternal hypoglycaemia (Kramer et al. 1995) and therefore additional mechanisms, such as neural efferent pathways, may be involved (Boddy et al. 1974; Giussani et al. 1993). The initial rise in fetal heart rate at the onset of maternal insulin infusion is consistent with observations of increased fetal heart rate accelerations in humans during maternal hypoglycaemia possibly through increased sympathoadrenal activity (Bjorklund et al. 1996).
A coordinated cardiovascular response to a stimulus necessitates a detector. Indeed our finding of a decrease in femoral blood flow, increase in femoral vascular resistance (calculated parameter), and decrease in heart rate (second phase) during maternal insulin infusion, without a change in mean arterial pressure, seems to support this concept. This combination of outcomes appears to rule out a direct causal link between altered heart rate and femoral blood flow. The role of the carotid body is well established in the rapid cardiovascular response to hypoxia (Giussani et al. 1993; Giussani et al. 1996), and has also been shown to respond to variations in glucose levels (Alvarez-Buylla & Alvarez-Buylla, 1988; Pardal & Lopez-Barneo, 2002; Nurse, 2005), and to participate in glucoregulation (Alvarez-Buylla & de Alvarez-Buylla, 1994; Koyama et al. 2000) in adult animals. In the fetus, the carotid body has been implicated in the control of differential organ growth during undernutrition (Burrage et al. 2008). We therefore speculate that a carotid body-mediated chemoreflex may be involved in the cardiovascular response to hypoglycaemia. The fall in femoral blood flow and heart rate observed here are slower than that which occurs during hypoxia (Cohn et al. 1974; Boddy et al. 1974; Giussani et al. 1993), although comparison between different stimuli are problematic. It may be that glucose levels must reach a threshold before femoral blood flow or heart rate begin to fall or that other potential glucose detectors such as the pancreas, brain (Mizuno & Oomura, 1984; Niijima et al. 1988) or adrenal glands (Khalil et al. 1986) may be involved.
Moderate (50%) late gestation maternal nutrient restriction (104–127 dGA) did not produce any significant blood glucose or cardiovascular response in the fetus at 125–26 dGA. In Border–Leicester cross Merino sheep 50% undernutrition between 115 and 145 dGA reduced fetal glucose levels (Edwards et al. 2001; Edwards & McMillen, 2001) and elevated blood pressure measured (115–125 and 125–145 dGA; Edwards & McMillen, 2001). Thus, while our challenge overlapped with a time period during which Edwards & McMillen (2001) observed a rise in blood pressure (115–125 dGA), it is possible that fetal adaptations made to undernutrition prior to this period in the present study (i.e. 104–115 dGA) prevented a rise in blood pressure at 125 dGA. This suggests that aside from breed differences, the timing of the undernutrition challenge within late gestation itself is important. Further work will be required to determine whether this might be due to the timing of the challenge in relation to the maturity of key endocrine axes (e.g. the hypothalamo-pituitary-adrenal axis) or linked to the maternal cortisol response to undernutrition. Here, we observed no difference between groups in maternal cortisol at 125 dGA, while Edwards & McMillen (2001) observed elevated maternal cortisol between 115 and 125 but not 125 and 145 dGA. Our observation of unaltered fetal plasma glucose and unaltered fetal cardiovascular control during the late gestation challenge indirectly supports the idea that fetal hypoglycaemia per se is the signal that triggers such cardiovascular responses (Edwards & McMillen, 2001); however, this cannot be confirmed by the current study.
In the more severe (PI40) peri-implantation group, fetal heart rate was elevated compared with the control group. This is unlikely to be accounted for by increased fetal body or breathing movements since there was no difference between groups in the prevalence of HV-ECoG. Elevated heart rate could reflect increased sympathetic activity and/or altered vagal tone, although there was no difference between groups in circulating catecholamine levels. Peri-implantation gestation maternal nutrient restriction did not alter organ blood flows in late gestation. However, these fetuses appeared to be more responsive to the late gestation hypoglycaemic challenge, since the fall in femoral artery blood flow during hypoglycaemia occurred more quickly in both the PI50 and PI40 fetuses. Accordingly, previous studies observed augmented cardiovascular responses to hypoxia (Hawkins et al. 2000a) and altered baroreflex control (Hawkins et al. 2000b) in late gestation fetuses following a milder (15%) preconception to mid gestation maternal nutrient restriction. In these studies, as in ours, the cardiovascular effects were not associated with a change in fetal size. It has been postulated that organisms can make predictive adaptations, which do not necessarily confer immediate advantage, but which nonetheless are advantageous in the anticipated future environment (Gluckman & Hanson, 2004). Our study highlights one parameter but together with previous work (Hawkins et al. 2000b), it suggests that the so-called ‘predictive adaptive’ concept may operate within fetal life as well as between pre- and postnatal life. In this instance, a better diversion of late gestation blood flow away from the hindlimb skeletal muscle potentially prioritizes delivery of nutrients to other more vital organs. Sheep exposed to the same peri-implantation undernutrition, but born into an adequate postnatal environment, exhibited altered cardiovascular control in adulthood (Gardner et al. 2004; Cleal et al. 2007). This effect can be minimized when the nutritional mismatch between pre- and postnatal life is minimized (Cleal et al. 2007). While not investigated here, potential underlying mechanisms include epigenetic modification of genes involved in nutrient detector or effector mechanisms in the cardiovascular system, or specifically in vascular development.
In conclusion, this study supports a role for an acute cardiovascular response to hypoglycaemia in the late gestation fetuses, involving reduced blood supply to the periphery and augmented supply to the adrenal glands, and mediated in part by fetal adrenaline and cortisol output. While targeted towards maximizing short-term survival in poor intrauterine conditions this response may alter organ (including vascular) growth and development with important consequences for adult cardiovascular and metabolic control. Our novel findings are extremely important in understanding how the fetus responds to undernutrition in utero and may inform future clinical diagnostic measures (e.g. ultrasound measurements of tissue perfusion in human fetuses) aimed at identifying those at risk from cardiovascular disease in later life.
Acknowledgments
This work was funded by a Biotechnology and Biosciences Research Council project grant (D17858) to L.R.G. and M.A.H. M.A.H. is supported by the British Heart Foundation. We are grateful to staff at the Biological Services Unit, Royal Veterinary College and the Biological Research Facility, University of Southampton for their expert animal care. We thank the Endocrine Unit, Southampton General Hospital, for carrying out the catecholamine assays.
References
- AFRC. An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients. Wallingford, UK: CAB International; 1993. Energy and protein requirements of ruminants. [Google Scholar]
- Alvarez-Buylla R, Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Respir Physiol. 1988;72:347–359. doi: 10.1016/0034-5687(88)90093-x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla R, de Alvarez-Buylla E. Changes in blood glucose concentration in the carotid body-sinus modify brain glucose retention. Brain Res. 1994;654:167–170. doi: 10.1016/0006-8993(94)91585-7. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellotti M, Pennati G, De Gasperi C, Bozzo M, Battaglia FC, Ferrazzi E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. Am J Obstet Gynecol. 2004;190:1347–1358. doi: 10.1016/j.ajog.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Bjorklund AO, Adamson UK, Almstrom NH, Enocksson EA, Gennser GM, Lins PE, Westgren LM. Effects of hypoglycaemia on fetal heart activity and umbilical artery Doppler velocity waveforms in pregnant women with insulin-dependent diabetes mellitus. Br J Obstet Gynaecol. 1996;103:413–420. doi: 10.1111/j.1471-0528.1996.tb09766.x. [DOI] [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Fisher R, Pinter S, Robinson JS. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974;243:599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce AC, Gibson KJ, Wintour EM, Koukoulas I, Gatford KL, Owens JA, Lumbers ER. The kidney is resistant to chronic hypoglycaemia in late-gestation fetal sheep. Can J Physiol Pharmacol. 2007;85:597–605. doi: 10.1139/y07-047. [DOI] [PubMed] [Google Scholar]
- Burrage D, Green LR, Moss TJ, Sloboda DM, Nitsos I, Newnham JP, Hanson MA. The carotid bodies influence growth responses to moderate maternal undernutrition in late-gestation fetal sheep. BJOG. 2008;115:261–268. doi: 10.1111/j.1471-0528.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, Torrens C, Newman JP, Poston L, Noakes DE, Hanson MA, Green LR. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci U S A. 2007;104:9529–9533. doi: 10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen WR, Piasecki GJ, Cohn HE, Susa JB, Jackson BT. Sympathoadrenal responses during hypoglycemia, hyperinsulinemia, and hypoxemia in the ovine fetus. Am J Physiol Endocrinol Metab. 1991;261:E95–E102. doi: 10.1152/ajpendo.1991.261.1.E95. [DOI] [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol. 2001;533:561–570. doi: 10.1111/j.1469-7793.2001.0561a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Symonds ME, Warnes KE, Owens JA, Butler TG, Jurisevic A, McMillen IC. Responses of the fetal pituitary-adrenal axis to acute and chronic hypoglycemia during late gestation in the sheep. Endocrinology. 2001;142:1778–1785. doi: 10.1210/endo.142.5.8143. [DOI] [PubMed] [Google Scholar]
- El Haddad MA, Chao CR, Ma SX, Ross MG. Nitric oxide modulates spontaneous swallowing behavior in near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 1999;277:R981–R986. doi: 10.1152/ajpregu.1999.277.4.R981. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsen T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315:837–840. doi: 10.1136/bmj.315.7112.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS, Fletcher AJ, Bloomfield MR, Fowden AL, Giussani DA. Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J Physiol. 2002;540:351–366. doi: 10.1113/jphysiol.2001.013434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS, Pearce S, Dandrea J, Walker R, Ramsay MM, Stephenson T, Symonds ME. Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension. 2004;43:1290–1296. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Moraga FA, McGarrigle HH, Gaete CR, Sanhueza EM, Hanson MA, Llanos AJ. Chemoreflex and endocrine components of cardiovascular responses to acute hypoxemia in the llama fetus. Am J Physiol Regul Integr Comp Physiol. 1996;271:R73–R83. doi: 10.1152/ajpregu.1996.271.1.R73. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Green LR, McGarrigle HH, Bennet L, Hanson MA. Angiotensin II and cardiovascular chemoreflex responses to acute hypoxia in late gestation fetal sheep. J Physiol. 1998;507:857–867. doi: 10.1111/j.1469-7793.1998.857bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Jones CT. The effect of elevation of maternal plasma catecholamines on the fetus and placenta of the pregnant sheep. J Dev Physiol. 1986;8:173–186. [PubMed] [Google Scholar]
- Harwell CM, Padbury JF, Anand RS, Martinez AM, Ipp E, Thio SL, Burnell EE. Fetal catecholamine responses to maternal hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 1990;259:R1126–R1130. doi: 10.1152/ajpregu.1990.259.6.R1126. [DOI] [PubMed] [Google Scholar]
- Haugen G, Hanson M, Kiserud T, Crozier S, Inskip H, Godfrey KM. Fetal liver-sparing cardiovascular adaptations linked to mother's slimness and diet. Circ Res. 2005;96:12–14. doi: 10.1161/01.RES.0000152391.45273.A2. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, McGarrigle HH, Calder NA, Saito T, Stratford LL, Noakes DE, Hanson MA. Cardiovascular and hypothalamic-pituitary-adrenal axis development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. Reprod Fertil Dev. 2000a;12:443–456. doi: 10.1071/rd99071. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, Ozaki T, Saito T, Noakes DE, Hanson MA. Effect of maternal undernutrition in early gestation on ovine fetal blood pressure and cardiovascular reflexes. Am J Physiol Regul Integr Comp Physiol. 2000b;279:R340–R348. doi: 10.1152/ajpregu.2000.279.1.R340. [DOI] [PubMed] [Google Scholar]
- Heasman L, Clarke L, Firth K, Stephenson T, Symonds ME. Influence of restricted maternal nutrition in early to mid gestation on placental and fetal development at term in sheep. Pediatr Res. 1998;44:546–551. doi: 10.1203/00006450-199810000-00013. [DOI] [PubMed] [Google Scholar]
- Hennessy DP, Coghlan JP, Hardy KJ, Scoggins BA, Wintour EM. The origin of cortisol in the blood of fetal sheep. J Endocrinol. 1982;95:71–79. doi: 10.1677/joe.0.0950071. [DOI] [PubMed] [Google Scholar]
- Heymann MA, Payne BD, Hoffman JI, Rudolph AM. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977;20:55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Itskovitz J, LaGamma EF, Rudolph AM. Effects of cord compression on fetal blood flow distribution and O2 delivery. Am J Physiol Heart Circ Physiol. 1987;252:H100–H109. doi: 10.1152/ajpheart.1987.252.1.H100. [DOI] [PubMed] [Google Scholar]
- Jones CT, Robinson RO. Plasma catecholamines in foetal and adult sheep. J Physiol. 1975;248:15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil Z, Marley PD, Livett BG. Elevation in plasma catecholamines in response to insulin stress is under both neuronal and nonneuronal control. Endocrinology. 1986;119:159–167. doi: 10.1210/endo-119-1-159. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- Kramer DC, Fleischer FS, Marx GF. Fetal bradycardia resulting from maternal hypoglycemia. A report of two cases. J Reprod Med. 1995;40:394–396. [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milley JR. Effect of insulin on the distribution of cardiac output in the fetal lamb. Pediatr Res. 1987;22:168–172. doi: 10.1203/00006450-198708000-00014. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984;307:109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- Niijima A, Kannan H, Yamashita H. Neural control of blood glucose homeostasis; effect of microinjection of glucose into hypothalamic nuclei on efferent activity of pancreatic branch of vagus nerve in the rat. Brain Res Bull. 1988;20:811–815. doi: 10.1016/0361-9230(88)90096-2. [DOI] [PubMed] [Google Scholar]
- Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci. 2005;120:1–9. doi: 10.1016/j.autneu.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Hawkins P, Harding JE. Periconceptional undernutrition alters growth trajectory and metabolic and endocrine responses to fasting in late-gestation fetal sheep. Pediatr Res. 2005;57:591–598. doi: 10.1203/01.PDR.0000155942.18096.9C. [DOI] [PubMed] [Google Scholar]
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Peeters LL, Sheldon RE, Jones MD, Jr, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135:637–646. doi: 10.1016/s0002-9378(16)32989-1. [DOI] [PubMed] [Google Scholar]
- Reuss ML, Rudolph AM. Distribution and recirculation of umbilical and systemic venous blood flow in fetal lambs during hypoxia. J Dev Physiol. 1980;2:71–84. [PubMed] [Google Scholar]
- Richardson B, Korkola S, Asano H, Challis J, Polk D, Fraser M. Regional blood flow and the endocrine response to sustained hypoxemia in the preterm ovine fetus. Pediatr Res. 1996;40:337–343. doi: 10.1203/00006450-199608000-00024. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, Van Der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, van Montfrans GA, Michels RP, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumball CWZP, Rutland MD, Bloomfield FH, Harding JE. A method for assessment of blood volume parameters in pregnant sheep using fluorescein-labelled dextran. Placenta. 2008;29:15–19. doi: 10.1016/j.placenta.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Rurak DW, Richardson BS, Patrick JE, Carmichael L, Homan J. Blood flow and oxygen delivery to fetal organs and tissues during sustained hypoxemia. Am J Physiol Regul Integr Comp Physiol. 1990;258:R1116–R1122. doi: 10.1152/ajpregu.1990.258.5.R1116. [DOI] [PubMed] [Google Scholar]
- Russel A, Doney J, Gunn R. Subjective assessment of body fat in live sheep. J Agric Sci. 1969;72:451–454. [Google Scholar]
- Stonestreet BS, Boyle LD, Papparella A, Berard DJ. Circulatory and metabolic effects of α-adrenergic blockade in the hyperinsulinemic ovine fetus. J Soc Gynecol Invest. 1996;3:241–249. doi: 10.1016/s1071-5576(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Tan W, Riggs KW, Thies RL, Rurak DW. Use of an automated fluorescent microsphere method to measure regional blood flow in the fetal lamb. Can J Physiol Pharmacol. 1997;75:959–968. [PubMed] [Google Scholar]
- Tchirikov M, Rybakowski C, Huneke B, Schroder HJ. Blood flow through the ductus venosus in singleton and multifetal pregnancies and in fetuses with intrauterine growth retardation. Am J Obstet Gynecol. 1998;178:943–949. doi: 10.1016/s0002-9378(98)70528-9. [DOI] [PubMed] [Google Scholar]
- Thein E, Raab S, Harris AG, Kleen M, Habler O, Meisner F, Messmer K. Comparison of regional blood flow values measured by radioactive and fluorescent microspheres. Eur Surg Res. 2002;34:215–223. doi: 10.1159/000063392. [DOI] [PubMed] [Google Scholar]
- Walker DW, Pratt N. Effect of probenecid on breathing movements and cerebral clearance of prostaglandin E2 in fetal sheep. J Physiol. 1998;506:253–262. doi: 10.1111/j.1469-7793.1998.253bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]