Abstract
Total parenteral nutrition (TPN) leads a loss of epithelial barrier function, decline in epithelial cell (EC) proliferation, and decreased expression of E-cadherin. As a large portion of intracellular β-catenin is tightly associated with E-cadherin, we hypothesized that the loss of E-cadherin would result in a redistribution of intracellular β-catenin, and could be a contributing mechanism for this TPN-associated loss of EC proliferation. An assessment of small bowel epithelium was performed in mice given either enteral nutrition (Control) or intravenous nutrition (TPN). TPN significantly down-regulated E-cadherin and β-catenin expression, and resulted in a loss of a colocalization of these factors. TPN also up-regulated phosphorylated (p)-β-catenin (Ser31/33,Thr41) and down-regulated (p)-β-catenin(Ser552) expression. To further address mechanisms driving this, we observed a significant decrease in the abundance of p-AKT and p-GSK3β expression, and an associated decline in tcf-4 transcription factors (cyclin D1, c-myc and Axin2), as well as a loss of EC proliferation by 7 days. To address whether the mechanism driving these changes was the absence of nutritional factors, glutamine was added to the TPN solution. This resulted in a partial restoration of β-catenin expression and EC proliferation, suggesting that an alteration in nutrient delivery may affect many of these changes. Based on these findings, the loss of EC proliferation with TPN may well be due to a loss of total β-catenin, as well as a concomintant change in the differential expression of β-catenin phosphorylation sites, and a reduction in β-catenin mediated tcf-4 transcription. This potential pathway may well explain many of the findings of mucosal atrophy associated with TPN.
Total parenteral nutrition (TPN), or the removal of all enteral nutrition, is vital for the nutritional support of patients who cannot tolerate enteral nutrients. Despite its advantages, TPN is associated with a decline in epithelial cell (EC) proliferation, leading to profound atrophy of the small intestinal mucosa (Peterson et al. 1996; Yang et al. 2004). TPN has also been shown to contribute to a loss of epithelial barrier function (EBF) (Illig et al. 1992; D'Antiga et al. 1999; Yang et al. 2003b; Ziegler et al. 2008). The implications of these changes are significant in that they may lead to an increase in septic episodes in a clinical setting, which is associated with a substantially poorer outcome (Moore et al. 1989, 1992; Frost & Bihari, 1997; Ziegler et al. 2008). Some of the mechanisms which account for these TPN-associated mucosal changes have been studied (Li et al. 1995; Fukatsu et al. 2001; Wildhaber et al. 2002, 2005; Yang et al. 2002; Yang et al. 2003b). Intestinal mucosal homeostasis depends on a balance between cell proliferation and cell death (Foligne et al. 2001; Tang et al. 2006). Previous work by our group has shown that TPN administration leads to a loss of EBF including a decreased expression of E-cadherin (Sun et al. 2008). However, it remains to be determined how this loss of E-cadherin might affect EC physiology in this TPN model.
Interestingly, a large portion of β-catenin is tightly associated with the intracellular segment of E-cadherin, and functions to support and maintain intercellular adhesion (Huber & Weis, 2001). The intracellular tail of E-cadherin complexes with both β-catenin and α-catenin (Itoh et al. 1997; Huber & Weis, 2001), and this complex in turns binds to actin, and helps to maintain the integrity of this adherence protein (Rimm et al. 1995; Perez-Moreno, 2003). The intracellular distribution of β-catenin is carefully balanced between this subcellular membrane population, as well as a second population which is responsible for Wnt-mediated activation of tcf/lef nuclear transcription(He et al. 2007). Recent evidence argues that the signalling and adhesive forms of β-catenin may tightly interact with each other, and be integrally dependent (Daugherty & Gottardi, 2007). Investigators have put forth the contention that the cadherin is a favourable inhibitor of β-catenin nuclear signalling, not only because it sequesters β-catenin away from the nucleus, but also because it can compete directly with tcf for β-catenin binding (Gottardi Cara & Gumbiner, 2001). Although this excess of free β-catenin may accelerate cell proliferation in some states, the loss of E-cadherin may lead to a ubiquination of the free cytoplasmic β-catenin, and thus lead to a decline in cell proliferation. The precise site of β-catenin phosphorylation (p-β-catenin) is an important mechanism which directs these two pathways. Phosphorylation sites at serine 33 and 37 and threonine 41 promote binding to the Axin/APC degradation complex (Hart et al. 1999), whereas phosphorylation at serine 552 has been shown to both release cell–cell contact and promote entrance into the nucleus for tcf-4 transcription (Fang et al. 2007). More recently this control via p-β-catenin has been shown to have relevance to intestinal EC growth and development of polyposis (He et al. 2007).
Based on our observed loss of E-cadherin with TPN administration, we hypothesized that the loss of E-cadherin would lead to a dissociation of E-cadherin and β-catenin. We further hypothesized that this rapid increase in intracellular, cytoplasmic β-catenin would then lead to its phosphorylation and subsequent ubiquination. Finally, glutamine, a semiessential amino acid, which is not routinely added to TPN solutions has been observed to improve intestinal EC proliferation and may well be mediated via the p-Akt signalling pathway (Seth et al. 2004; Evans et al. 2005; Larson et al. 2007). We thus further hypothesized that adding glutamine to TPN would reverse the loss of β-catenin expression, increase p-Akt signalling and improve EC proliferation. Such a process may well provide for a mechanism by which EC proliferation is significantly decreased with TPN administration.
Methods
Animals
C57BL/6J male, specific-pathogen-free mice (8–10 weeks old; Jackson Laboratory, Bar Harbour, ME, USA) were maintained under temperature-, humidity- and light-controlled conditions. Mice were initially fed ad libitum with standard mouse chow and water, and allowed to acclimate for 1 week prior to surgery. During the administration of intravenous solutions, mice were housed in metabolic cages to prevent coprophagia. The studies were approved by our University Animal Use Committee (No. 7703).
Operative procedures and TPN
Cannulation and administration of TPN was identical to that previously described (Kiristioglu et al. 2002). After 24 h, mice were randomized to a control (enterally fed), TPN, or TPN plus glutamine (TPN + GLU) group. For the TPN + GLU group, 2 g glutamine (Sigma-Aldrich) was added to each 100 ml TPN solution. Nitrogen delivery was controlled between each group (iso-nitrogenous). Caloric delivery was also kept isocaloric between groups (Kiristioglu & Teitelbaum, 1998). All animals were killed at 7 days using CO2.
Real-time PCR
Muscosal scrapings were placed in TRIzol, homogenized, RNA extracted and purified as previously described (Yang et al. 2003c). Oligomers were designed using an optimization program Primer Premier (http://www.premierbiosoft.com), and sequences of specific primers are described in Table 1. Real-time PCR (RT-PCR) was performed using a Rotor-Gene 6000 (Corbett Life Science, Sydney, Australia) and β-actin was used as an internal control for normalization. Fold changes of target genes were calculated using comparative quantification to β-actin.
Table 1.
Primers used in this experiment
| Gene | GenBank accession | Forward | Reverse | Size |
|---|---|---|---|---|
| Axin2 | NM_015732 | TGACTCTCCTTCCAGATCCCA | TGCCCACACTAGGCTGACA | 105 |
| c-Myc | NM_023326 | AACCGCGACCTGGACTATGA | TGGAGAAGCTATCACCGTCGT | 238 |
| Cyclin D1 | NM_007631 | GCGTACCCTGACACCAATCTC | CTCCTCTTCGCACTTCTGCTC | 183 |
| E-Cadherin | NM_009864 | CAGCCTTCTTTTCGGAAGACT | GGTAGACAGCTCCCTATGACTG | 140 |
| β-Catenin | NM_007614 | ATGGAGCCGGACAGAAAAGC | CTTGCCACTCAGGGAAGGA | 108 |
Western immunoblotting
Intestinal epithelial cells (EC) were prepared by using a modification of Grossmann's technique (Grossmann et al. 1998; Schwarz et al. 2007) and these extracts included crypts and villi Western blotting. Because of the observed loss of membranous E-cadherin with TPN administration, using another group of isolated EC samples, a fractionation of membrane bound and cytosol proteins was determined by previously described methods (Basuroy et al. 2005; Suzuki et al. 2007). This yielded a Triton soluable fraction (non-cytoskeletal fraction) and a Triton insoluable fraction (cytoskeltetal fraction). Antibodies (Ab) used included: mouse anti-E-cadherin Ab (BD Transduction Laboratories; clone IgG2a), mouse anti-β-catenin Ab (BD Transduction Laboratories, clone IgG1), rabbit anti-α-catenin Ab (Sigma, St Louis, MO, USA), rabbit anti-phosphorylated β-catenin Ab (ser31/33, Thr41, Cell Signaling Technology, Inc., Beverly, MA, USA), rabbit anti-phosphorylated β-catenin Ab (ser552, Cell Signaling), rabbit anti-phosphorylated GSK3α/β (Ser21/9, Cell Signaling), rabbit anti-phosphorylated Akt1/2/3 (Ser 473, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and mouse anti-PCNA (Cell Signaling). Secondary antibody was either the corresponding goat anti-mouse-HRP (Santa Cruz) or goat anti-rabbit antibody (Santa Cruz).
Confocal and immunofluorescent microscopy
A 0.5 cm section of fresh jejunum from each mouse was immediately cut and embedded in an optimum cutting temperature compound (OCT Tissue-Tek, Sakura Finetechnical, Tokyo, Japan) by freezing the tissue in liquid nitrogen. Cryosections of 5 μm thickness were prepared and fixed with 2% formalin for 15 min at room temperature (RT). After washing with PBS, sections were blocked in 10% goat serum for 1 h and incubated with the primary antibodies (mouse monoclonal anti-E-Cadherin 1: 500 and mouse monoclonal anti-β-catenin 1: 250, or rabbit anti-α-catenin 1: 3000) overnight at 4°C. After overnight incubation, the sections were washed again 3 times, corresponding secondary antibodies were incubated for an additional 1 h at RT, and slides were mounted with anti-fade permanent mounting gel (Invitrogen). Fluorescence was analysed by using an Olympus BX-51 upright light microscope and Zeiss LSM 510-META laser scanning confocal microscope; images were stacked by using Zeiss proprietary image software and processed by using Adobe Photoshop CS2.
Immunohistochemistry
Techniques were similar to that previously described (Yang et al. 2004), and used anti-p-AKT (1: 100, Santa Cruz).
Intestinal morphology assessment
Morphology
Intestinal tissue sections and staining were performed as previously described (Sun et al. 2006). Five different fields of each specimen were observed and digital images were recorded with computer-aided video microscopy. Villus length and crypt depth for each specimen were measured and analysed using commercially available quantitative digital image analysis software (Media Cybernetics Inc., Bethesda, MD, USA).
BrdU Staining
Techniques were as previously described (Haxhija et al. 2008). An index of the crypt cell proliferation rate was calculated by the ratio of the number of BrdU positive crypt cells to the total number of crypt cells.
Data analysis
Data are expressed as the mean ± standard deviation (s.d.). Statistical analysis employed Student's t test for comparison of two means, and a one-way ANOVA for comparison of multiple groups (with Bonferoni's post hoc analysis to assess statistical differences between groups). The chi-square test was used for categorical data (Prism software; GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was defined as P < 0.05.
Results
E-Cadherin, α-catenin and β-catenin expression are decreased with TPN administration
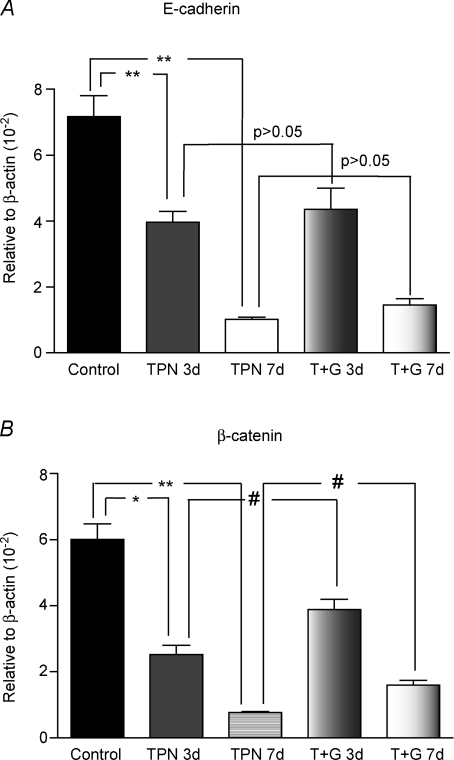
TPN administration resulted in significantly decreased E-cadherin and β-catenin expression in jejunal epithelium. Compared with controls, there was a 7-fold decline in mRNA expression (7.17 ± 1.28 versus 1.03 ± 0.12, P < 0.01) in E-cadherin and an 8-fold decline (6.01 ± 0.94 versus 0.77 ± 0.08, P < 0.01) in β-catenin at 7 days (Fig. 1A and B). To confirm these observations, Western immunoblots were performed. Similar to the real-time PCR results, a 4-fold decline in protein expression (0.33 ± 0.19 versus 0.078 ± 0.016, P < 0.01) for E-cadherin was observed. β-Catenin was almost non-detectable in our TPN group (Fig. 1C–E).
Figure 1. mRNA and protein expression of E-cadherin and β-catenin from intestinal EC measured by real-time PCR (A and B) and western immunoblots (C–E).
A and B, results are expressed as the mean fold increase in target mRNA relative to β-actin mRNA. Compared with controls, mRNA expression of E-cadherin (A) and β-catenin (B) was significantly decreased after 7 days of TPN administration. C, representative gels for both E-cadherin and β-catenin. D and E, results of E-cadherin and β-catenin protein expression are shown. Both factors decreased significantly with TPN administration compared with controls. Note that glutamine administration partially prevented the TPN-associated decline in β-catenin expression; however, glutamine did not affect E-cadherin expression at either 3 or 7 days. *P < 0.01, **P < 0.001, #P < 0.05. Abbreviations: TPN, total parenteral nutrition; n= 5–6 for each group; **P < 0.001.
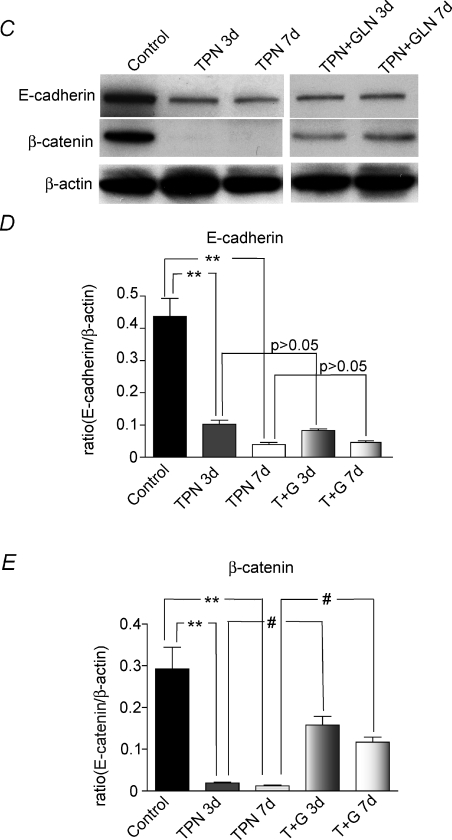
To help account for the loss of both E-cadherin and β-catenin, we next assessed the intracellular distribution of E-cadherin as well as α-catenin, as α-catenin is known to be tightly linked to both E-cadherin and β-catenin. The presence of E-cadherin and α-catenin was examined in both the cytoskeletal-associated fraction and the non-cytoskeletal fraction by assessing the amount measured in the Triton soluble (non-cytoskeletal) and Triton insoluble (cytoskeletal fraction) fractions, respectively. This was not done for β-catenin as it was weakly expressed following TPN administration. The expression of both E-cadherin and α-catenin was markedly decreased in the insoluble fraction and increased in the soluble fraction after TPN administration at 7 days (Fig. 2). This suggested a dissociation of these molecules from the cell membrane and actin cytoskeleton. We next investigated the intracellular distribution of β-catenin.
Figure 2. Expression of E-cadherin and α-catenin redistribution with the administration of TPN.
A, representative gels for the Triton insoluble and Triton soluble fractions of E-cadherin and α-catenin protein expression. B and C, immunoblot expression results for E-cadherin (B) and α-catenin (C) relative to the expression of β-actin. Note that for both factors there was a decrease in the Triton insoluble fraction and an increased expression of the Triton soluble fraction. This suggests that both adherens junctional proteins moved from the cytoskeletal to the non-cytoskeletal regions of the epithelial cell. n= 6 per group; *P < 0.01.
Expression of E-cadherin and β-catenin decreased in both villi and crypts following TPN
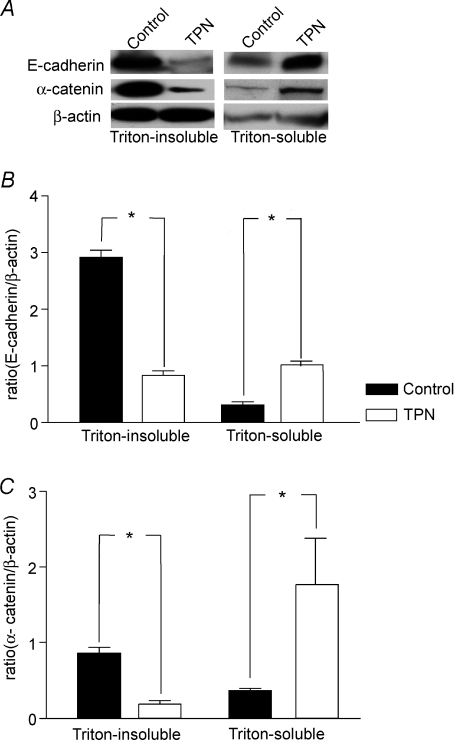
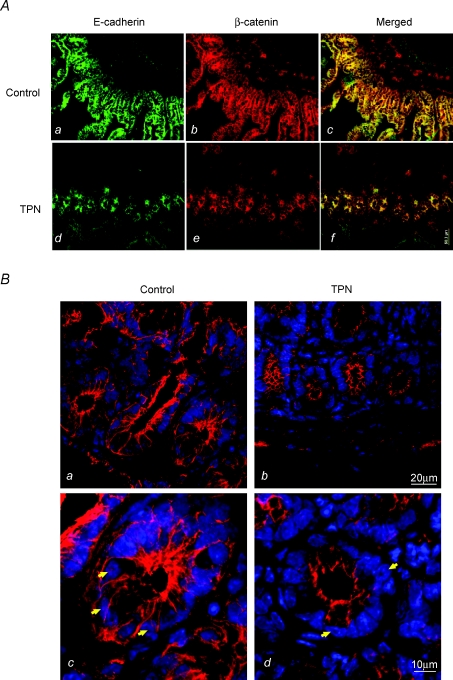
The pattern of E-cadherin and β-catenin expression was analysed along the crypt–villus axis to further address the loss of these factors with TPN administration using immunofluorescence staining. The fluorescence intensity for E-cadherin and β-catenin along the villus portion of the jejunum was consistently weaker in the TPN group than in controls (Fig. 3A and B). Compared to the control group, TPN administration also resulted in a redistribution of E-cadherin and β-catenin. Interestingly, we noted an internalization of E-cadherin from the cell membrane into the cytoplasm of TPN mice. Further, the appearance of a sharp mesh like grid, and a tight colocalization of these two factors, observed in controls, was markedly diminished with TPN.
Figure 3. Representative immunofluoresence staining for E-cadherin and β-catenin in the villus region.
A, note that there was a marked decline in intensity for both E-cadherin and β-catenin with TPN administration along the villi. B, at higher magnification, compared to controls, TPN administration led to a marked loss of β-catenin in the EC cytoplasm as well as a nearly total loss along the cellular membrane. Additionally, TPN led to a redistribution of E-cadherin, with internalization from cell membrane into cytoplasm (note the asterick (*), marks where there is internalization for the TPN group, and that this is distinctly absent in the control group). E-Cadherin and β-catenin expression had the appearance of a sharp mesh-like grid in controls (marked by arrows), and this was absent in TPN mice (a versus d). The strong colocalization of β-catenin to E-cadherin (c) in controls also diminished with TPN (d).
A similar pattern was observed in the crypt region of the small bowel (Fig. 4A). Within the crypts there was a significant decline in both E-cadherin and β-catenin expression with TPN. This loss of β-catenin expression was particularly prominent within EC nuclei (Fig. 4B); and this decline in nuclear β-catenin is consistent with an ubiquination of β-catenin.
Figure 4.
A, representative immunofluoresence staining for E-cadherin and β-catenin in the crypt region. B, a detailed imaging of β-catenin in the crypt cells of the small intestine is shown. Note that compared to controls (a and c), TPN administration (b and d) led to a decline in β-catenin expression in crypt cells (shown by white arrows), and a marked loss of cytosolic and nuclear β-catenin expression was detected in the TPN mice.
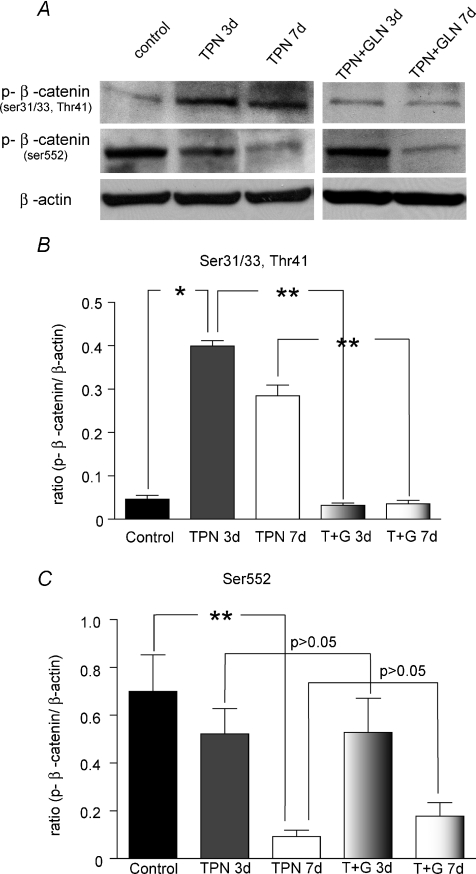
Phosphorylated β-catenin(Ser552) was down-regulated, and phosphorylated β-catenin(Ser31/33,Thr41) was up-regulated with TPN
Site specific phosphorylation of β-catenin is the major mechanism by which this factor is directed to either ubiquination or promotes nuclear transcription of tcf-4. β-Catenin phosphorylated at serine 31 and 33, as well as threonine 41 results in the rapid binding of the Axin/APC complex and ubiquination (Daugherty & Gottardi, 2007), whereas, β-catenin phosphorylated at serine 552 promotes nuclear transcription (Taurin et al. 2006). EC β-catenin phosphorylation was measured with immunoblots. Compared with controls, TPN administration led to an increased phosphorylation of β-catenin on sites Ser33/37/Thr41 (Fig. 5A). This represented a greater than 2-fold increase in protein expression at 7 days (0.66 ± 0.12 versus 0.26 ± 0.07, TPN versus Control, P < 0.01) (Fig. 5B). Additionally, a nearly 10-fold down-regulation of phosphorylation of β-catenin at Ser552 was noted after 7 days of TPN (0.09 ± 0.05 versus 0.70 ± 0.31, TPN versus Control, P < 0.05) (Fig. 5C). Note that we were able to detect these low levels of protein expression by using higher amounts of protein for these immunoblots (100 μg versus 40 μg in the results shown in Fig. 1).
Figure 5. TPN administration altered β-catenin phosphorylation at different sites in the molecule.
A, representative bands for phosphorylated β-catenin expression in ECs. B, results of Western immunoblotting are expressed as the ratio of the protein expression to β-actin protein phosphorylated at Ser31/33,Thr41 relative to β-actin expression. C, results of Western immunoblotting are expressed as the ratio of the protein expression to β-actin protein phosphorylated at Ser552 relative to β-actin expression. Note that phosphorylated β-catenin at Ser31/33,Thr41 increased with TPN at 3 and 7 days, and that glutamine administration prevented this change. Conversely, β-catenin phosphorylated at Ser552 was down-regulated with TPN, and this decline reached significance at 7 days. At both days 3 and 7 of TPN with glutamine administration no significant change in p-β-catenin expression at Ser552 was noted. Note, because of the markedly diminished amount of β-catenin expression in TPN mice, for these experiments 100 μg of protein was used (versus 40 μg in previous studies). Abbreviations: TPN + G, TPN supplemented with glutamine. n= 4–6 per group; *P < 0.01, **P < 0.001.
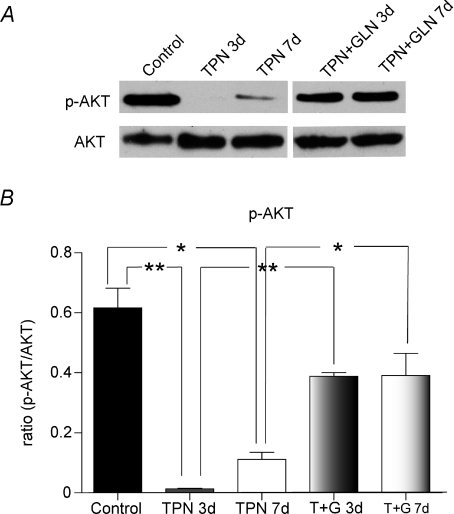
Phosphorylated Akt and phosphorylated GSK3β were down-regulated with TPN administration
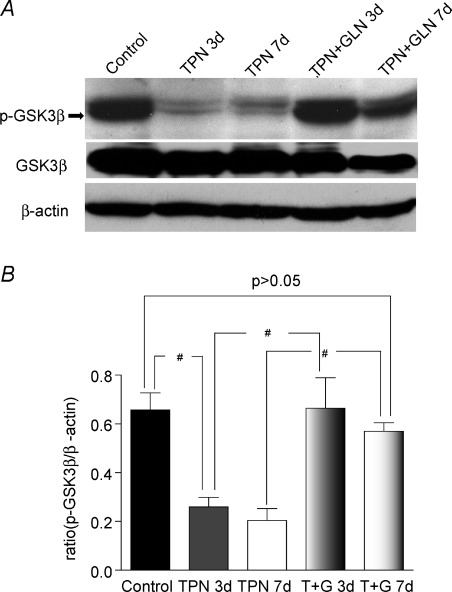
The upstream factors which are known to modulate the phosphorylation of β-catenin were next examined. This included phosphorylated Akt (p-Akt) and p-GSK3β. Almost no p-Akt was detected after 3 days of TPN administration, and this remained significantly lower than enterally fed controls (a 6-fold decline in the protein expression) at 7 days (0.11 ± 0.04 versus 0.62 ± 0.16, P < 0.01) (Fig. 6A and B). For p-GSK3β a 3-fold decline (Fig. 7) was detected in the TPN administration group both at 3 days (0.66 ± 0.12 versus 0.26 ± 0.07, control versus TPN, P < 0.01) and at 7 days (0.66 ± 0.12 versus 0.20 ± 0.09, control versus TPN, P < 0.01).
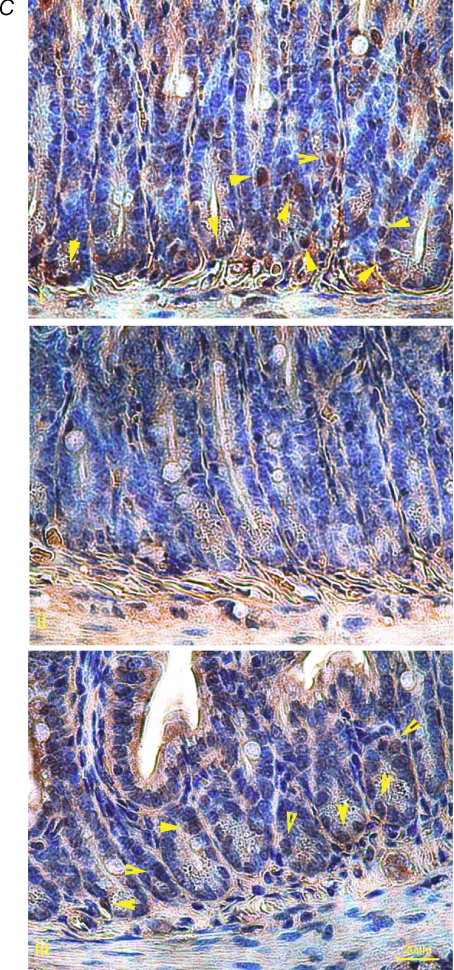
Figure 6. Phosphorylated-Akt (p-AKT) activity decreased with TPN administration.
A, shown are representative bands for p-Akt and total Akt expression for TPN and control groups. B, the ratio of p-AKT to total AKT decreased with TPN administration, at 3 and 7 days. Glutamine administration partially reversed this decline. C, p-AKT immunohistochemical expression showed similar results. In control mice, large numbers of positive staining cells (shown by yellow arrows) were found at the base of the crypt (a upper panel). After 3 days of TPN administration (b middle panel), p-AKT positive cells were markedly decreased in numbers. The lower panel (c) shows p-AKT expression in the TPN + glutamine group. Note that the loss of p-AKT expression was partly prevented with the addition of glutamine. n= 4–6 per group; *P < 0.01 **P < 0.001.
Figure 7. Expression of p-GSK3β was down-regulated with TPN administration.
A, representative bands of p-GSK3β and total GSK3β are shown for each study group, as well as β-actin for normalization. Note that GSK3β is shown as the lower of the two bands; and GSK3α is the upper band. B, results of Western immunoblotting as expressed as the ratio of the protein expression of p-GSK3β to total GSK3β. Note the decline in p-GSK3β with TPN administration, and this down-regulation was reversed with glutamine. #P < 0.05, n= 4–6 per group.
In order to detect the location of these changes in phosphorylation along the crypt–villus axis, p-Akt immunohistochemistry was performed. This showed that in the control group p-Akt positive cells were almost exclusively located at the base of the crypt; however, there were virtually no p-Akt positive cells found after 3 days of TPN (Fig. 6C).
Down-stream tcf/lef transcription factors
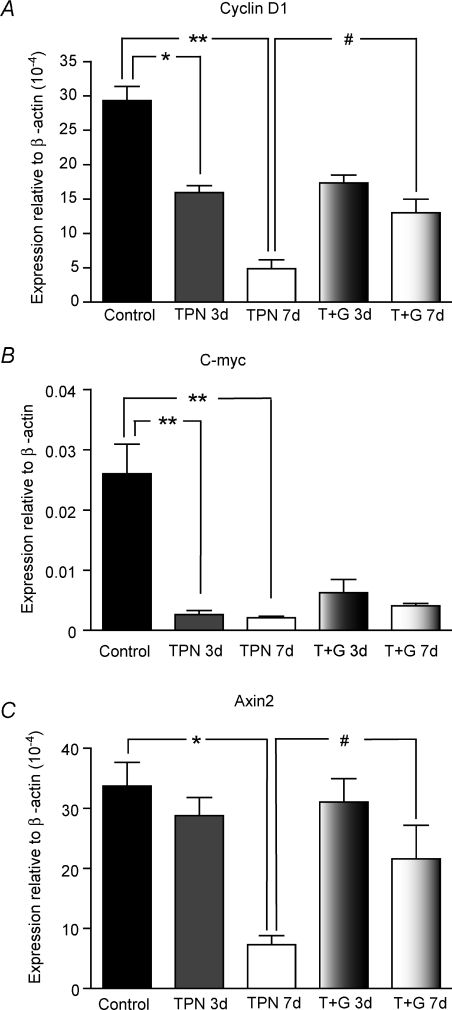
As a marked loss of β-catenin (nuclear and cytoplasmic) was detected in TPN mice, it was next determined if this resulted in a loss of tcf-4 transcriptional activation. c-myc, cyclin D1 and Axin2 are three major regulators of the progression of cells into the proliferative stage of the cell cycle (Sherr, 1996; Shtutman et al. 1999; He et al. 2007); and these factors also represent classic down-stream activation factors following β-catenin activation of tcf-4 transcription. Compared with controls after 7 days of TPN, there was a nearly 10-fold decrease in mRNA expression (0.026 ± 0.009 versus 0.002 ± 0.004, P < 0.01) for c-myc, a 7-fold decrease for cyclin D1 (29.3 ± 5.58 versus 4.86 ± 2.55, P < 0.01) and a 5-fold for Axin2 (33.7 ± 6.82 versus 7.30 ± 3.05, P < 0.01) (Fig. 8). For c-myc and cyclin D1, these declines were noted as earlier as 3 days of TPN (Fig. 8).
Figure 8. TPN administration led to a decreased expression of several down-stream tcf-4 transcription genes.
Cyclin D1 (A), c-myc (B) and Axin2 (C) expression significantly decreased after 7 days of TPN. For c-myc and cyclin D1, this decline was noted as early as 3 days after TPN administration. Glutamine supplemented TPN prevented the observed decline for cyclin D1 and Axin2 expression. *P < 0.01, **P < 0.001, #P < 0.05; n= 4–6 per group.
Intestinal epithelial cell proliferation decreased with TPN
As the decreased expression of cyclin D1, c-myc and Axin2 are representative of critical tcf transcription factors for cell proliferation, we next examined changes in EC growth parameters in TPN mice. Villus length and crypt depth in the TPN groups were significantly reduced as compared to the controls (P < 0.01). Histological examination demonstrated a 22% decline in jejunal villus height (308.4 ± 65.2 versus 397.0 ± 49.5 μm), and a 37% decline in crypt depth (76.1 ± 7.4 versus 121.4 ± 27.2 μm) in TPN mice compared with controls (Table 2).
Table 2.
Morphologic changes in the jejunal crypts and villi
| Groups | Villus length (μm) | Crypt depth (μm) |
|---|---|---|
| Control | 397.4 ± 49.5 | 121.4 ± 27.2 |
| TPN | 308.4 ± 65.2* | 76.1 ± 7.4* |
*Compared with control group: P < 0.01.
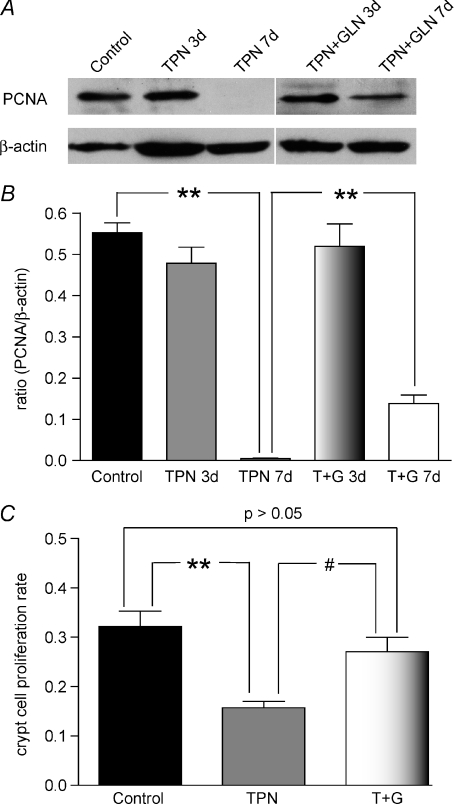
PCNA is synthesized in the S phase of the cell cycle and was used as a marker for proliferating cells. Western blot results showed a significantly decreased expression of PCNA with TPN administration at 7 days (Fig. 9A and B).
Figure 9. Proliferating cell nuclear antigen (PCNA) expression.
A, representative bands of PCNA and β-actin, which was used for normalization. B, results of Western immunoblotting as expressed as the ratio of the PCNA to β-actin. A slight decline was noted at 3 days, and a this decline became significant after 7 days of TPN administration. Glutamine supplemented TPN prevented this decline at 3 days, and partially prevented the decline at day 7. C, crypt cell proliferation is shown as detected by the counting of BrdU positive staining. TPN again led to a significant decline in proliferation compared to control mice; and glutamine supplemented TPN (T + G) prevented this decline in proliferation rates. Results for BrdU staining are shown for day 7. #P < 0.05, **P < 0.001; n= 4–6 per group.
Intestinal EC proliferation rates were also determined by BrdU staining, and were found to be significantly decreased (P < 0.01) compared to controls (13.5 ± 2.4%versus 24.4 ± 4.2%, TPN and controls, respectively; Fig. 9C).
Glutamine administration increased EC proliferation and β-catenin expression
To further examine what factors might contribute to the TPN-associated loss of EC proliferation and the decline in E-cadherin and catenin expression, it was next investigated if a potentially important nutritional factor might be missing from the standard parenteral nutrition solution. It has been previously documented that glutamine, an amino acid not routinely added to TPN solutions in a clinical setting, may be a critical factor for adequate cell growth and survival (Yang et al. 2000; Zarzaur et al. 2002b; Evans et al. 2005; Larson et al. 2007). Further, supplementation of glutamine to intestinal EC has been shown to be effective in restoring tight junctions after acetaldehyde induced injury, and the mechanism appears to be via restoration of the distribution of E-cadherin, catenins and other junctional molecules(Basuroy et al. 2005). We therefore utilized a separate group of TPN mice, which were supplemented with glutamine. We hypothesized that the addition of this amino acid would reverse our observed changes in p-Akt activity, and subsequently improve EC proliferation.
TPN supplemented with glutamine prevented the observed TPN-associated decline in PCNA expression, and resulted in an increase in intestinal EC proliferation rates with TPN (Fig. 9). To investigate the mechanisms driving this increase in EC proliferation, we then looked at the Akt/GSK3 cascade. Glutamine supplementation led to an associated increase in p-Akt expression (Fig. 6), and this increase was noted as early as 3 days after the initiation of TPN, a time at which there was almost no detectable p-Akt in the un-supplemented TPN (standard) group. Immunohistochemical staining of p-Akt (Fig. 6) demonstrated a strong presence of p-Akt with glutamine administration in the crypt region.
Additionally, the significant decline in p-GSK3β observed with TPN administration was completely prevented with glutamine supplementation (Fig. 7). To examine the effect of this sustained p-GSK3β and p-Akt expression, we next examined the expression of p-β-catenin. As noted previously, a significant increase in the p-β-catenin on Ser31/31,Thr41 was found with TPN administration. With glutamine supplementation this increase was partially prevented at both 3 and 7 days after the initiation of TPN (Fig. 5). Glutamine administration, however, did not affect the loss of p-β-catenin expression at Ser552 in the TPN group (Fig. 5). Based on these findings, we next examined the effect of glutamine expression on the presence and stability of unphosphorylated β-catenin. Interestingly, TPN supplemented with glutamine resulted in a partial recovery of β-catenin expression, and this recovery was seen at both days 3 and 7 after TPN administration. However, glutamine had no effect on the decline in E-cadherin expression at either time point (Fig. 1).
This partial prevention of β-catenin loss led to a partial prevention in the decreased expression of the tcf-4 transcription factors (c-myc, Axin2 and cyclin D1) at both day 3 and to a lesser degree at day 7 after TPN administration (Fig. 8). This suggests that the partial prevention in the decline of tcf-4 transcription was a mechanism responsible for sustaining EC proliferation compared to the standard TPN group.
Discussion
Our study demonstrated that TPN in a mouse model led to several significant changes in jejunal epithelial cells, including a loss of E-cadherin and β-catenin, and this was associated with a dramatic loss of EC proliferation. β-Catenin is a key cytoplasmic protein that functions in two different cellular processes: intercellular adhesion and Wnt-mediated transcriptional activation (Daugherty & Gottardi, 2007). The vast majority of β-catenin resides in the cytoskeletal fraction, and binds to E-cadherin and α-catenin. This trimolecular complex of the adherens junctional proteins, β-catenin and α-catenin (Gates & Peifer, 2005) allows for it to maintain its association with the actin cytoskeleton and contribute to cell–cell adhesion(Rimm et al. 1995). The other essential role for β-catenin is to help mediate EC proliferation via transcription of T-cell factor-4 (tcf-4) (Tutter et al. 2001; Batlle et al. 2002; Gregorieff et al. 2005). Each of these actions derive from a common pool of β-catenin (Gottardi Cara & Gumbiner, 2001). Although the loss of β-catenin–E-cadherin association in our TPN model could lead to an increase in β-catenin-mediated tcf-4 signalling, in our TPN model we observed the converse. Regulation of β-catenin has been well-demonstrated to be modulated by its degradation via its association with Axin1, CK1, GSK-3β and APC (abbreviated as the Axin1/APC in this paper) complex. The binding to this Axin1/APC complex results in the prompt ubiquination of β-catenin, and relies on β-catenin being phosphorylated at Ser31/33,Thr41 (Liu et al. 2002; Gottardi & Gumbiner, 2004; Daugherty & Gottardi, 2007), whereas the activation of Wnt signalling leads to the intracellular accumulation of β-catenin, which subsequently translocates to the nucleus to activate the expression of target genes, leading to cell proliferation (Akiyama, 2000; Kikuchi, 2000; Peifer & Polakis, 2000; van Noort & Clevers, 2002; Kikuchi et al. 2006). In our study, TPN administration led to a loss of p-β-catenin at Ser552, a key site of phosphorylation which promotes tcf-4 transcription (Taurin et al. 2006; He et al. 2007), and an increase in p-β-catenin at Ser31/33,Thr41. This corresponded to our observation of decreased expression of tcf-4 transcription factors Axin2, cyclin D1 and c-myc, and a loss of EC proliferation, all of which are critical for cell proliferation (Sherr, 1996; Miller et al. 1999; Shtutman et al. 1999; Takayama et al. 2006). These findings closely fit with the observation of a marked decline in EC proliferation with TPN administration (Yang et al. 2003a, 2004). The observed loss of α-catenin in our study may also have significant implications, as it has been shown that α-catenin can also play a significant role in modulating EC proliferative signalling (Scott & Yap, 2006).
Akt and GSK3β play an important role in the modulation of this Axin1/APC complex. In non-proliferative states, the activity GSK3β is inhibited by the factor existing in a phosphorylated state (Daugherty & Gottardi, 2007). This phosphorylation is modulated by PI3 kinase and Akt signalling (Sheng et al. 2003). In our current study, p-Akt was significantly down-regulated with TPN administration. Such a loss of p-Akt closely corroborates the findings of Sheng et al. where the administration of a diet consisting of glucose, electrolytes and water resulted in a loss of p-Akt expression, and a return of p-Akt expression rapidly occurred with the administration of a solid diet (Sheng et al. 2003). Our observed loss of p-Akt could well be the mechanism contributing to the observed down-regulation in p-GSK3β and the increase in p-β-catenin at Ser31/33,Thr41, as well as the up-regulation of p-β-catenin at Ser552. These actions would clearly account for a subsequent ubiquination of β-catenin. Alternatively, Akt can activate β-catenin–tcf-4/lef-1 transcriptional activity by a direct phosphorylation of β-catenin and this action enhances nuclear accumulation of β-catenin (Fang et al. 2007). In this study, we used PCNA and incorporation of BrdU as markers of EC proliferation. The results showed the expression of PCNA was lost after 7 days of TPN administration, and was consistent with the marked decline in BrdU uptake at this same time point.
A critical question which will need to be investigated with future experiments will be whether this cascade was initiated by the loss of E-cadherin expression, or if the administration of TPN led to changes in the Akt/GSK3 cascade, both of which could lead to a loss of β-catenin and could also affect E-cadherin expression. Our findings of a partial reversal of many of these changes with the addition of glutamine to the TPN solution were quite intriguing. Glutamine is an amino acid which has been demonstrated to affect cellular proliferation in vitro and in vivo (Gu et al. 2001), and glutamine is an important factor for intestinal epithelial (Ziegler et al. 2000; Evans et al. 2005) and lymphoid functionality (DeWitt et al. 1999; Zarzaur et al. 2002a). This increase in EC proliferation was also seen in this present study with glutamine. One of the mechanisms by which glutamine mediates such actions is its ability to alter Akt expression (Larson et al. 2007). Glutamine also prevented the loss of p-Akt expression with TPN administration. In a recent study, glutamine removal for 24 h in rat intestinal epithelial (RIE)-1 cells resulted in an increase in p-Akt expression, and the addition of glutamine resulted in a marked loss of p-Akt (Larson et al. 2007). Although different from our own findings, the authors attributed this decreased expression of p-Akt as a protective effect against the development of EC apoptosis. It also may well be that this finding is a relatively specific finding to this cell line. Although changes in protein synthesis with TPN may have led to some of these changes, this appears less likely. Both groups received similar amounts of nitrogen and calories (Sun et al. 2006), and the fact that protein immunoblots were controlled by the amount of β-actin also makes this less likely.
TPN supplemented with glutamine led to an up-regulation of β-catenin expression. As well, glutamine prevented the increased expression of p-β-catenin at Ser31/33/Thr41; however, it was also intriguing that glutamine did not change the phosphorylation state of β-catenin at Ser552. This was somewhat surprising in that glutamine has been shown to promote other signalling pathways such as activation of extracellular signal-related kinase (ERK) or protein kinase D (PKD), both of which have been shown to be up-regulated in vitro in EC exposed to glutamine (Larson et al. 2007). Because Wnt signalling can affect the phosphorylation status of β-catenin, in the future it will be interesting to investigate the influence of glutamine on Wnt expression (van Noort et al. 2002).
Glutamine also did not have a significant effect on E-cadherin expression. Glutamine has been previously shown to protect acetaldehyde disrupted adherens junctions – most likely by promoting EGF activity (Seth et al. 2004). Additional future work examining the effect of TPN on EGF signalling will also be needed. Further, the PI3K/p-Akt system has been closely associated with sustaining the adherens junction (Laprise et al. 2002). Thus, it is possible that other factors, which have been shown to alter junctional protein expression and prevent normal EC proliferation, such as an up-regulation of pro-inflammatory cytokines, including interferon γ and tumour necrosis factor α in our TPN model, may play a more dominant role, despite the addition of glutamine (Bruewer et al. 2003; Ivanov et al. 2004; Clayburgh et al. 2005; Sipos et al. 2005).
In conclusion, our study reported the loss of β-catenin and E-cadherin in the small bowel of mice given TPN. TPN also led to a decline in p-Akt and p-GSK3β; each of these actions potentially accounted for a differential phosphorylation of β-catenin (increased β-Ser31/33,Thr41, and decreased Ser552), with resultant ubiquination. This suggests a potential mechanism for the loss of cytosolic β-catenin as observed in this study. The implications of this loss are seen by a loss of tcf-4 transcriptional activation, and decreased EC proliferation. The prevention of several of these changes with glutamine supports a potential clinical application of this amino acid. This potential pathway may well explain many of the findings of mucosal atrophy associated with TPN, and may suggest modalities to avoid TPN-associated intestine atrophy.
Acknowledgments
This work was supported by NIH grant 5R01 AI044076-10 (to D.H.T.). The work was also supported with the help of the National Cancer Institute through the University of Michigan's Cancer Center Support Grant (5 P03 CA46592).
References
- Akiyama T. Wnt/β-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–282. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Basuroy S, Sheth P, Mansbach C, Rao R. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367–G375. doi: 10.1152/ajpgi.00464.2004. [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson J, Beghtel H, Van Den Born M, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase–dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antiga L, Dhawan A, Davenport M, Mieli-Vergani G, Bjarnason I. Intestinal absorption and permeability in paediatric short-bowel syndrome: a pilot study. J Pediatr Gastroenterol Nutr. 1999;29:588–593. doi: 10.1097/00005176-199911000-00021. [DOI] [PubMed] [Google Scholar]
- Daugherty R, Gottardi C. Phospho-regulation of β-catenin adhesion and signaling functions. Physiology. 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt RC, Wu Y, Renegar KB, Kudsk KA. Glutamine-enriched total parenteral nutrition preserves respiratory immunity and improves survival to a Pseudomonas pneumonia. J Surg Res. 1999;84:13–18. doi: 10.1006/jsre.1999.5592. [DOI] [PubMed] [Google Scholar]
- Evans M, Jones D, Ziegler T. Glutamine inhibits cytokine-induced apoptosis in human colonic epithelial cells via the pyrimidine pathway. Am J Physiol Gastrointest Liver Physiol. 2005;289:G388–G396. doi: 10.1152/ajpgi.00072.2005. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills G, Kobayashi R, Hunter T, Lu Z. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foligne B, Aissaoui S, Senegas-Balas FCC, Bernard P, Antoine J, Balas D. Changes in cell proliferation and differentiation of adult rat small intestine epithelium after adrenalectomy: kinetic, biochemical, and morphological studies. Dig Dis Sci. 2001;46:1236–1246. doi: 10.1023/a:1010611228730. [DOI] [PubMed] [Google Scholar]
- Frost P, Bihari D. The route of nutritional support in the critically ill: physiological and economical considerations. Nutrition. 1997;13:58S–63S. doi: 10.1016/s0899-9007(97)00207-4. [DOI] [PubMed] [Google Scholar]
- Fukatsu K, Kudsk KA, Zarzaur BL, Wu Y, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15:318–322. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- Gates J, Peifer M. Can 1000 reviews be wrong? Actin, a-catenin, and adherens junctions. Cell. 2005;123:769–772. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gottardi Cara J, Gumbiner B. Adhesion signaling: How α-catenin interacts with its partners. Current Biol. 2001;11:792–794. doi: 10.1016/s0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Gottardi C, Gumbiner B. Distinct molecular forms of β-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Grossmann J, Maxson J, Whitacre C, Orosz D, Berger N, Fiocchi C, Levine A. New isolation technique to study apoptosis in human intestinal epithelial cells. Am J Pathol. 1998;153:53–62. doi: 10.1016/S0002-9440(10)65545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wu ZH, Xie JX, Jin DY, Zhuo HC. Effects of growth hormone (rhGH) and glutamine supplemented parenteral nutrition on intestinal adaptation in short bowel rats. Clin Nutrition. 2001;20:159–166. doi: 10.1054/clnu.2000.0379. [DOI] [PubMed] [Google Scholar]
- Hart M, Concordet J, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- Haxhija E, Yang H, Spencer A, Koga H, Sun X, Teitelbaum D. Modulation of mouse intestinal epithelial cell turnover in the absence of angiotensin coverting enzyme. Am J Physiol Gastrointestinal Liver Physiol. 2008;295:G88–G98. doi: 10.1152/ajpgi.00589.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Yin T, Grindley J, Tian Q, Sato T, Tao W, Dirisina R, Porter-Westpfahl K, Hembree M, Johnson T, Wiedemann L, Barrett T, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Weis W. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell. 2001;10:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- Illig KA, Ryan CK, Hardy DJ, Rhodes J, Locke W, Sax HC. Total parenteral nutrition-induced changes in gut mucosal function: atrophy alone is not the issue. Surgery. 1992;112:631–637. [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to a catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A. Regulation of β-catenin signaling in the Wnt pathway. Biochem Biophys Res Commun. 2000;268:243–248. doi: 10.1006/bbrc.1999.1860. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein–protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- Kiristioglu I, Antony P, Fan Y, Forbush B, Mosley RL, Yang H, Teitelbaum DH. Total parenteral nutrition-associated changes in mouse intestinal intraepithelial lymphocytes. Digestive Dis Sci. 2002;47:1147–1157. doi: 10.1023/a:1015066813675. [DOI] [PubMed] [Google Scholar]
- Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res. 1998;79:91–96. doi: 10.1006/jsre.1998.5408. [DOI] [PubMed] [Google Scholar]
- Laprise P, Chailler P, Houde M, Beaulieu J, Boucher M, Rivard N. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem. 2002;277:8226–8234. doi: 10.1074/jbc.M110235200. [DOI] [PubMed] [Google Scholar]
- Larson S, Li J, Chung D, Evers B. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1262–G1271. doi: 10.1152/ajpgi.00254.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg G, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Miller J, Hocking A, Brown J, Moon R. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Moore F, Feliciano D, Andrassy R, McArdle A, Booth F, Morgenstein-Wagner T, Kellum J, Welling R, Moore E. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F, Moore E, Jones T. TEN vs. TPN following major abdominal trauma: reduced septic morbidity. J Trauma. 1989;29:916–923. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis – a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Peterson CA, Ney DM, Hinton PS, Carey HV. Beneficial effects of insulin-like growth factor I on epithelial structure and function in parenterally fed rat jejunum. Gastroenterology. 1996;111:1501–1508. doi: 10.1016/s0016-5085(96)70011-2. [DOI] [PubMed] [Google Scholar]
- Rimm D, Koslov E, Kebriaei P, Cianci C, Morrow J. α1 (E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz B, Wang F, Shen L, Clayburgh D, Su L, Wang Y, Fu Y, Turner J. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Yap A. Cinderella no longer: a-catenin steps out of cadherin's shadow. J Cell Sci. 2006;119:4599–4605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- Seth A, Basuroy S, Sheth P, Rao R. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G510–G517. doi: 10.1152/ajpgi.00058.2004. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Townsend CJ, Evers B. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos F, Molnar B, Zagoni T, Berczi L, Tulassay Z. Growth in epithelial cell proliferation and apoptosis correlates specifically to the inflammation activity of inflammatory bowel diseases: ulcerative colitis shows specific p53- and EGFR expression alterations. Dis Colon Rectum. 2005;48:775–786. doi: 10.1007/s10350-004-0831-5. [DOI] [PubMed] [Google Scholar]
- Sun X, Spencer AU, Yang H, Haxhija EQ, Teitelbaum DH. Impact of caloric intake on parenteral nutrition-associated intestinal morphology and mucosal barrier function. JPEN J Parenter Enteral Nutr. 2006;30:474–479. doi: 10.1177/0148607106030006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Seth A, Rao R. Role of phospholipase Cγ-induced activation of protein kinase Cɛ (PKCɛ) and PKCβI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem. 2007;283:3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- Takayama S, Rogatsky I, Schwarcz L, Darimont B. The glucocorticoid receptor represses cyclin D1 by targeting the Tcf-β-catenin complex. J Biol Chem. 2006;281:17856–17863. doi: 10.1074/jbc.M602290200. [DOI] [PubMed] [Google Scholar]
- Tang Y, Swietlicki EA, Jiang S, Buhman KK, Davidson NO, Burkly LC, Levin MS, Rubin DC. Increased apoptosis and accelerated epithelial migration following inhibition of hedgehog signaling in adaptive small bowel postresection. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1280–G1288. doi: 10.1152/ajpgi.00426.2005. [DOI] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, Browning D, Dulin N. Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- Tutter A, Fryer C, Jones K. Chromatin-specific regulation of LEF-1-β-catenin transcription activation and inhibition in vitro. Genes Dev. 2001;15:3342–3354. doi: 10.1101/gad.946501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort M, Clevers H. TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev Biol. 2002;244:1–8. doi: 10.1006/dbio.2001.0566. [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, Van Der Zee R, Destree O, Cleevers H. Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Wildhaber BE, Lynn KN, Yang H, Teitelbaum DH. Total parenteral nutrition-induced apoptosis in mouse intestinal epithelium: regulation by the Bcl-2 protein family. Pediatr Surg Int. 2002;18:570–575. doi: 10.1007/s00383-002-0869-1. [DOI] [PubMed] [Google Scholar]
- Wildhaber BE, Yang H, Haxhija EQ, Spencer AU, Teitelbaum DH. Intestinal intraepithelial lymphocyte derived angiotensin converting enzyme modulates epithelial cell apoptosis. Apoptosis. 2005;10:1305–1315. doi: 10.1007/s10495-005-2138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte γδ-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-γ evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003a;284:G629–G637. doi: 10.1152/ajpgi.00290.2002. [DOI] [PubMed] [Google Scholar]
- Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med. 2003b;31:1118–1125. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- Yang H, Kiristioglu I, Fan Y, Forbush B, Bishop DK, Antony PA, Zhou H, Teitelbaum DH. Interferon-γ expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002;236:226–234. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Soderholm JD, Larsson J, Permert J, Lindgren J, Wiren M. Bidirectional supply of glutamine maintains enterocyte ATP content in the in vitro using chamber model. Int J Colorectal Dis. 2000;15:291–296. doi: 10.1007/s003840000258. [DOI] [PubMed] [Google Scholar]
- Yang H, Wildhaber BE, Teitelbaum DH. Keratinocyte growth factor improves epithelial function after massive small bowel resection. J Parenter Enteral Nutr. 2003c;27:198–206. doi: 10.1177/0148607103027003198. [DOI] [PubMed] [Google Scholar]
- Zarzaur B, Ikeda S, Johnson C, Le T, Sacks G, Kudsk K. Mucosal immunity preservation with bombesin or glutamine is not dependent on mucosal addressin cell adhesion molecule-1 expression. JPEN J Parenter Enteral Nutr. 2002a;25:265–270. doi: 10.1177/0148607102026005265. [DOI] [PubMed] [Google Scholar]
- Zarzaur BL, Ikeda S, Johnson CD, Le T, Sacks G, Kudsk KA. Mucosal immunity preservation with bombesin or glutamine is not dependent on mucosal addressin cell adhesion molecule-1 expression. JPEN J Parenter Enteral Nutr. 2002b;26:265–270. doi: 10.1177/0148607102026005265. discussion 270. [DOI] [PubMed] [Google Scholar]
- Ziegler TR, Bazargan N, Leader LM, Martindale RG. Glutamine and the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2000;3:355–362. doi: 10.1097/00075197-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Luo M, Estývariz C, Moore IDA, Sitaraman S, Hao L, Bazargan N, Klapproth J, Tian J, Galloway J, Leader L, Jones D, Gewirtz A. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integ Comp Physiol. 2008;294:R402–R410. doi: 10.1152/ajpregu.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]