Abstract
Risk of developing obesity and diabetes may be influenced by the nutritional environment early in life. We examined the effects of high fibre or protein diets on satiety hormones and genes involved in glucose and lipid metabolism during postnatal development and on adult fat mass. At 21 days of age, Wistar rat pups were weaned onto control (C), high fibre (HF) or high protein (HP) diet. Tissue and blood were collected at 7, 14, 21, 28 and 35 days after birth. A second group of rats consumed the weaning diets until 4 months when they were switched to a high fat–high sugar diet for 6 weeks, after which body and fat mass and plasma glucose were determined. In young rats, HF diet increased plasma glucagon-like peptide (GLP-1) compared to C and HP and decreased leptin compared to C at postnatal days 28 and 35. Hepatic fatty acid synthase mRNA was down-regulated by HF and HP compared to C at days 28 and 35. In brown adipose tissue, HF increased uncoupling protein-3 mRNA whereas HP increased mRNA of the inflammatory cytokine interleukin-6. Body weight, fat mass and glycaemia in adult males and fat mass in females were greater after the high fat challenge in rats that consumed the HP diet from weaning. Increasing fibre or protein in postnatal diets causes rapid change in satiety hormone secretion and genes involved in glucose and lipid metabolism which appear to influence fat mass and glycaemia in adulthood, high protein being associated with increased susceptibility to obesity.
It is well-established that nutrition can have a direct impact on normal physiological function, as well as on pathological conditions such as obesity, cardiovascular disease (CVD), hypertension and diabetes. These diseases are highly prevalent in society today and are interrelated in complex ways. A change in diet can attenuate certain conditions even in a progressed state. There is little scientific unity on the causes of the rising prevalence of obesity or a consensus on effective weight loss/maintenance prescriptions (Eisenstein et al. 2002). Therefore, diet recommendations for the treatment and prevention of obesity based on non-scientific evidence are abundant. In addition, there is a growing body of evidence that suggests risk of developing CVD, obesity and diabetes may be influenced by events that occurred decades prior to disease onset, a concept known as the developmental origins of health and disease (DOHaD) (Waterland & Garza, 1999; Cottrell & Ozanne, 2007).
It has been suggested that prenatal life, childhood and adolescence are critical periods that are characterized by a high degree of plasticity (Silveira et al. 2007). Plasticity during development allows a range of different phenotypes to emerge from a single genotype largely in response to the nature of the environmental cues received during that period (Gluckman & Hanson, 2004). It also appears that the more divergent the environmental cues are between the developmental period and later life, the greater the susceptibility to obesity, leptin and insulin resistance, elevated blood pressure and cardiovascular dysfunction (Hanson & Gluckman, 2008). The detrimental effects of this ‘mismatch’ between the pre- and postnatal environment has largely been examined in developed societies but may also provide insight into the increased risk of chronic diseases seen with nutrition transition in populations experiencing economic improvement or in migrant groups (Hanson & Gluckman, 2008).
Considering that the plastic phase of life continues well past birth (Hanson & Gluckman, 2008), it is likely that nutritional variances outside of the traditionally studied patterns of maternal undernutrition and suckling overnutrition (Taylor & Poston, 2007) contribute to disease susceptibility, perhaps due to mechanisms distinct from the epigenetics that characterize the DOHaD models, although this remains to be tested. There is a paucity of literature examining the influence of weaning diet in rodents and whether or not the composition of that diet influences obesity risk later in life if that animal suddenly encounters a diet (e.g. high fat/sucrose) that differs significantly from that experienced from weaning onwards.
Critical to our understanding of dietary influences on disease susceptibility is the response of key metabolic pathways to that diet pattern. Glucose and lipid metabolism, and the genes controlling these systems, are integral to the utilization and storage of nutrients by the body and are pivotal pathways involved in the pathogenesis of diabetes and obesity. A clear understanding of the developmental changes that occur in these pathways with dietary manipulations is lacking, especially manipulations that reflect the current dietary habits of western cultures.
While the consequences of undernutrition, and specifically low maternal protein intake, have been widely examined in the literature, much less is known regarding overnutrition or the provision of diets with higher levels of certain nutrients (Armitage et al. 2005; McArdle et al. 2006). A diet high in fibre is often recommended for various health benefits, including the prevention and management of diabetes (Marlett et al. 2002; Lairon et al. 2007). We have previously shown that a high fibre diet fed to adult rats increases the secretion of glucagon-like peptide-1 (GLP-1), a potent insulin secretagogue that also slows gastric emptying, inhibits glucagon secretion, enhances β-cell proliferation and regulates food intake (Reimer & McBurney, 1996; Reimer et al. 1997). In addition, mothers advised to eat a high animal protein, low carbohydrate diet during pregnancy had offspring with lower birth weight and increased blood pressure in adulthood (Herrick et al. 2003). Whether or not diets high in fibre or protein introduced at weaning bring about early changes in satiety hormones and genes involved in glucose and lipid metabolism which could then predict weight gain in response to a high energy diet in adulthood remains to be elucidated.
Given that a large segment of the North American population experiences positive energy balance to a greater extent than negative energy balance, and often engage in periods of enhanced intake of select nutrients, a greater understanding of the longitudinal changes of genes related to metabolism under these nutritional environments is needed. Our objective therefore was to examine the early (postnatal days 7–35) changes in satiety hormones and genes related to glucose and lipid metabolism in young rats weaned onto a control, high fibre or high protein diet. A separate group of animals were allowed to continue consuming one of the three diets until 4 months of age when they were switched to a high fat–high sucrose (HF/HS) diet and body fat mass and glycaemia determined.
Methods
Animals and diets
Young animals
The experimental protocol was approved by the University of Calgary Animal Care Committee and conformed to the Guide for the Care and Use of Laboratory Animals. Eighteen virgin female Wistar rats were obtained from Charles River (Montreal, PQ, Canada) and housed in a temperature and humidity controlled room with a 12 h light–12 h dark cycle. After a period of acclimatization, females were mated with Wistar males (Charles River, Montreal, PQ, Canada) in wire-bottomed cages. On the day a copulation plug was found, the females were isolated and given free access to control diet (formulation based on AIN-93G; Reeves et al. 1993). The day following birth, litters were culled to 10 pups (5 males and 5 females where possible). At weaning (day 21), the males and females were separated and six litters were placed on each of three experimental diets: control, high fibre (25% wt/wt) and high protein (40% wt/wt). Composition of the diets is given in Table 1. The high fibre diet used a combination of the prebiotic fibres inulin and oligofructose at a ratio of 1: 1 (by weight). Diets met all nutritional requirements of pregnant, lactating and growing rats. Control and high protein diets were made isocaloric by replacing an equal energy content of cornstarch with casein resulting in an energy density of 3.76 kcal g−1 for both diets. Due to the lower caloric content of the fibre, it is impossible to completely match energy density of the high fibre diet and it was therefore slightly lower at 3.30 kcal g−1. This adjustment in starch content has been used previously (Reimer & Russell, 2008) and minimizes changes in fat or other essential nutrient intake. The formulation of diets allows for a near equal percentage contribution of each ingredient to overall energy with the exception of the casein in the high protein diet and cornstarch manipulation for the fibre content in the high fibre diet. Food and water were provided ad libitum throughout the experiment.
Table 1.
Experimental diet composition
| Composition (g kg−1) | Control* | High protein | High fibre |
|---|---|---|---|
| Cornstarch | 397.5 | 197.5 | 262 |
| Casein | 200 | 400 | 173 |
| Dextrinized cornstarch | 132 | 132 | 114 |
| Sucrose | 100 | 100 | 87 |
| Soybean oil | 70 | 70 | 61 |
| Alphacel | 50 | 50 | 43 |
| AIN-93-MX | 35 | 35 | 30 |
| AIN-93-VX | 10 | 10 | 9 |
| l-Cystine | 3 | 3 | 2.8 |
| Choline bitartrate | 2.5 | 2.5 | 2.2 |
| Inulin/oligofructose† | 0 | 0 | 216 |
Based on AIN-93G purified diet for pregnant, lactating, or growing rats to meet all nutritional requirements. The digestible energy of the control and high protein diets is 3.76 kcal g−1 and the high fibre diet slightly lower at 3.30 kcal g−1 due to the reduced energy content of the inulin and oligofructose.
Inulin supplied as Orafti Raftiline HP and oligofructose as Orafti Raftilose P95 in a 1: 1 blend by weight (Quadra Chemicals Ltd, Burlington, ON, Canada). The energy value of the fibre blend (1.5 kcal g−1) (Roberfroid, 1999) was used to substitute for an equicaloric amount of cornstarch in the high fibre diet.
On days 7, 14, 21, 28 and 35 after birth, two pups (1 male and 1 female) from each litter were killed at approximately the same time each morning. For each diet treatment this represents six different dams to minimize the influence of any one dam on a diet group. On each test day, body weights were measured and rats anaesthetized with isoflurane (inhalation at 1 l min−1 O2 flow 5%). Non-fasted blood was collected by cardiac puncture with the exception of 7-day-old pups when trunk blood was collected following rapid decapitation. Due to the young age of the rats at the time of killing (from 7 to 35 days old), no overnight fast was employed to ensure that all rats were killed in a similar metabolic state representing approximately 2 h into the light cycle. Blood was collected with the addition of EDTA (1 mg ml−1) and aprotinin (5 × 105 KIU l−1). Diprotin A, an inhibitor of dipeptidyl peptidase IV (DPP-IV) was added at 34 μg ml−1 (Calbiochem, La Jolla, CA, USA) to inhibit GLP-1 degradation (Drucker, 2001). Blood was centrifuged at 1600 g for 15 min at 4°C and plasma stored at −80°C until analysis. Following over-anaesthetization and aortic cut, tissues were collected. The small intestine was excised, flushed, measured and weighed, then divided into three segments designated duodenum, jejunum and ileum. A segment of each section was immersed in liquid nitrogen and stored at −80°C for later mRNA analysis. The colon, stomach, liver and brown adipose tissue (BAT) were also excised, measured, weighed and stored at −80°C for mRNA expression analysis.
Long-term feeding study into adulthood
A second set of rats were included to determine if the composition of diet introduced at weaning and then consumed until a switch in adulthood to a high energy diet had any influence on body fat content and glycaemia. As per the young rats described above, pups were weaned onto a control, high fibre or high protein diet at 21 days of age. Rats consumed these diets until 4 months of age when they were all given a high fat–high sucrose (HF/HS) diet ad libitum for 6 weeks. The HF/HS diet provided 40% of energy from fat and 45% from sucrose and was composed of (g per 100 g): cornstarch (5), casein (14), sucrose (51), soybean oil (10), lard (10), Alphacel (5), AIN-93M mineral mix (3.5), AIN-93 vitamin mix (1), l-cystine (0.3), and choline bitartrate (0.25). At the end of this long-term feeding protocol, final body weight was measured and body composition determined under light anaesthetic (isoflurane) using dual energy X-ray absorptiometry (DEXA) with software for small animal analysis (Hologic QDR 4500, Hologic, Inc., Bedford, MA, USA). An oral glucose tolerance test was performed after an overnight fast according to our previous work (Reimer & Russell, 2008).
Plasma analysis
A multiplex hormone assay kit and Luminex instrument were used to measure plasma hormone concentrations in the young rats according to the manufacturer's directions (Rat Endocrine LincoPlex Kit, Millipore, St Charles, MO, USA). Antibody-immobilized beads were included in the kit for insulin, active GLP-1, leptin, total amylin and glucagon. The sensitivity of the multiplex kit is 55.6 pm for insulin and 6.2 pm for all other analytes. Plasma glucose concentrations were measured in duplicate using a glucose trinder assay (Stanbio Laboratory, Boerne, TX, USA).
RNA extraction and real time PCR
Total RNA was extracted from the stomach, small intestine, colon, liver and BAT of young rats using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) (Deng et al. 2005). Reverse transcription was performed with an input of 1 μg of total RNA using the first strand cDNA synthesis kit for RT-PCR (Invitrogen) with oligo d(T)15 as a primer. The resultant cDNA was amplified using primers synthesized by the University of Calgary Core DNA Services (Calgary, AB, Canada) and analysed by real time PCR. Primer sequences are provided in Table 2. The PCR reaction was heated for 1 min 30 s then 40 cycles at 95°C for 30 s, 60°C for 30 s and 72°C for 20 s in a DNA iCycler apparatus (Bio-Rad, Hercules, CA, USA). A melt curve showed the melting point of the PCR product of interest. Actin was verified as a suitable housekeeping gene for the tissues of interest and actin primers included as an internal control in the reactions. The 2−ΔCT method was utilized for the data analysis:
where threshold cycle (CT) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold (Livak & Schmittgen, 2001). The ΔCT is the difference in threshold cycles for the gene of interest and actin.
Table 2.
Primer sequences for real time PCR
| Gene | Forward primer | Reverse primer | PCR size (bp) |
|---|---|---|---|
| PRG | ACCGCCCTGAGATTACTTTTCTG | AGTTCTCTTTCCAGGTTCACCAC | 122 |
| GLUT2 | GTTGCTGGATAAGTTCACCTGGAT | GATTGGACCTGGCCCAATCT | 133 |
| GLUT5 | GGCCTCATCTTCCCATTCATTCAA | GGGTAGCAGGTGGGAGGTCATTA | 223 |
| SGLT1 | CTACATCCAGTCCATCACCCAGTTAC | CCAATCAGGAAGCCGAGAATCAG | 126 |
| Ghrelin | AGAGGCGCCAGCTAACAAGTAA | GCAGGAGAGTGCTGGGAGTT | 112 |
| PYY | AGCGGTATGGGAAAAGAGAAGTC | ACCACTGGTCCACACCTTCTG | 111 |
| Leptin | TCACACACGCAGTCGGTATCC | GTCTCGCAGGTTCTCCAGGTC | 183 |
| Amylin | CTGCCACTGCCCACTGAAAG | CACTTCCGTTTGTCCACCTGAG | 150 |
| FAS | GAGGACTTGGGTGCCGATTAC | GCTGTGGATGATGTTGATGATAGAC | 132 |
| ACC | CCTTCTTCTACTGGCGACTGAG | TAAGCCTTCACTGTGCCTTCC | 148 |
| GLK | CCGAGTGGCTTACAGTTCTG | ACCTGAGTGTTGGAGATGATTC | 140 |
| SREBP1c | TCATCAACAACCAAGACAGTG | AGAGAAGCAGGAGAAGAGAAG | 130 |
| SREBP2 | GTGATTGTCTTGAGCGTCTTC | CGGATAAGCAGGTCTGTAGG | 169 |
| HMGCR | GGGACCAACCTTCTACCTCAG | GACAACTCACCAGCCATCAC | 134 |
| CYP7A1 | GCTCTGGAGGGAGTGCCATTTAC | GCTGTGCGGATATTCAAGGATGC | 118 |
| LCAT | ATGGGTATGTGCGGGATG | CCAAGGCTGTGTCCAATAAG | 157 |
| IL-6 | CTCCGCAAGAGACTTCCAG | GGTCTGTTGTGGGTGGTATC | 120 |
| TNF-α | GTCGTAGCAAACCACCAAG | AGAGAACCTGGGAGTAGATAAG | 145 |
| UCP1 | GAGGTGGTGAAGGTCAGAATGC | GTCGTCCCTTTCCACAGTGTTG | 131 |
PRG, proglucagon; GLUT, glucose transporter; SGLT, sodium-dependent glucose transporter; PYY, peptide YY; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; GLK, glucokinase; SREBP, sterol regulatory element binding protein; HMGCR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; CYP, cholesterol 7 α-hydroxylase; LCAT, lecithin:cholesterol acyltransferase; IL-6, interleukin-6; TNF-α, tumour necrosis factor-α; UCP-1, uncoupling protein-1.
Statistics
All data are presented as means ±s.e.m. A multivariate ANOVA was used to evaluate differences between diet groups with Tukey's post hoc test. If a sex difference was found independent of diet, an independent-samples t test was conducted. When no sex differences were found, males and females were combined. Differences were considered significant when P≤ 0.05. Statistical analyses were performed using SPSS v 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
Consequences of switching from diets introduced at weaning to a high energy diet in adulthood
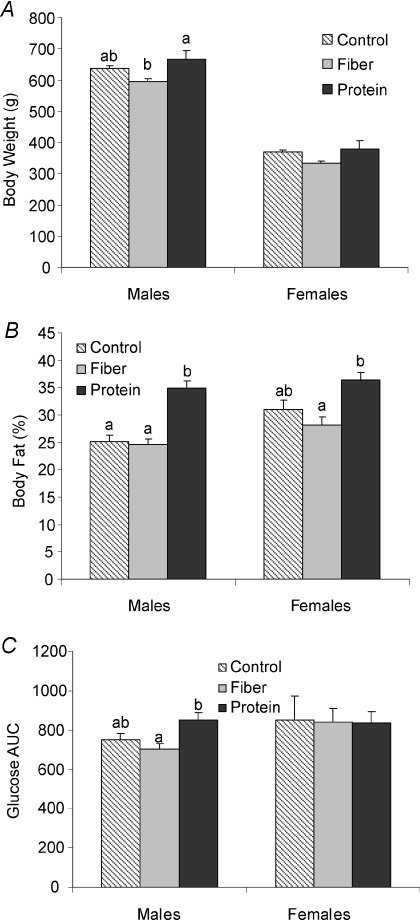
Male rats weaned onto the high protein diet and then switched to a high fat–high sucrose diet for 6 weeks in adulthood had significantly higher body weight compared to those weaned onto the high fibre diet (P= 0.019) (Fig. 1). Although final body weight in females was also lower in the fibre group compared to the protein group this was not significant. When body fat content was expressed as a percentage, the high protein group had markedly higher body fat compared to both the control (P < 0.001) and high fibre (P < 0.001) groups in the males and higher than the fibre group in the females (P= 0.02) (Fig. 1). Control females had a mean body fat percentage that was intermediate between the high protein and high fibre groups. During the OGTT, male rats in the high protein group had significantly greater area under the curve for glucose compared to the high fibre group (Fig. 1). There were no differences in glucose AUC for females.
Figure 1. Body weight, percentage fat mass and glycaemic response in adult rats fed control, high fibre or high protein weaning diets.
Rats were weaned onto a control, high fibre or high protein diet at 21 days of age and then from days 100–128 of age consumed a high fat–high sucrose diet. A, final body weight in males and females; B, final percentage body fat in males and females; C, glucose area under the curve (AUC) during an oral glucose tolerance test. Results are presented as means ±s.e.m. (n= 8–10 (males) and n= 8–10 (females)). Diet treatments with different letters represent a significant difference within males or females (P < 0.05).
Growth parameters of pups
The mean number of pups in a litter at birth was 13.7 ± 0.5 with a mean of 6.3 ± 0.4 males and 7.2 ± 0.4 females per litter. Mean pup weight at birth was 6.4 ± 0.2 g. As expected with growth, body weight and the weight of the liver, stomach, small intestine and colon increased significantly with age from day 7 to day 35 after birth (P < 0.001). At days 28 and 35, there were no diet differences in body or organ weight but body weight was significantly lower in female rats versus males at 35 days (P < 0.001; 133.0 ± 5.7 versus 149.4 ± 6.1 in female and males, respectively). Organ weight, expressed as a proportion of individual total body weight, was calculated and is presented in Table 3. Liver weight, when adjusted for total body weight, was significantly higher in female rats consuming the high protein diet at 35 days compared to control (P= 0.01) and high fibre (P= 0.01). In males, at 28 days, the high protein diet resulted in a proportionately greater liver weight compared to the fibre diet (P= 0.04), and at day 35 both the protein and control diets had greater liver weights than fibre (P= 0.04 and 0.04, respectively) (Table 3). Small intestine weight at 35 days was also proportionately larger in male rats fed high protein compared to control (P= 0.01). Diet differences observed in both sexes were confined to the large intestine with proportionately greater colon weight (P < 0.001) and length (P= 0.03) in both males and females with the high fibre diet compared to control and protein at 28 days and 35 days. Age differences depicted in Table 3 largely reflect increasing organ weight with growth from 7 to 35 days.
Table 3.
Organ weights of rats adjusted for total body weight
| Age (days) | |||||
|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 (on diet) | 35 (on diet) | |
| Total body weight (g) | |||||
| Control | 16.7 ± 0.5a | 37.0 ± 1.3b | 62.0 ± 1.1c | 92.1 ± 2.9d | 137.4 ± 6.9e |
| High fibre | 92.7 ± 3.8d | 145.5 ± 4.5e | |||
| High protein | 90.0 ± 3.3d | 140.7 ± 6.3e | |||
| Liver weight (mg g−1) | |||||
| Control | 32.9 ± 2.0a | 33.6 ± 4.5ab | 39.9 ± 8.5c | 43.2 ± 2.04d | 47.2 ± 4.0d |
| High fibre | 41.3 ± 2.7d | 42.9 ± 3.9d | |||
| High protein | 46.2 ± 1.8 d‡ | 49.9 ± 3.5e‡ | |||
| Stomach weight (mg g−1) | |||||
| Control | 7.8 ± 1.0ab | 7.4 ± 4.0ab | 8.1 ± 5.0ab | 8.7 ± 0.5a | 7.8 ± 0.8a |
| High fibre | 8.5 ± 0.9a | 7.8 ± 1.2a | |||
| High protein | 9.3 ± 0.6a | 7.7 ± 0.7b | |||
| Small intestine length (m g−1) | |||||
| Control | 2.2 ± 0.2a | 1.4 ± 0.2b | 1.1 ± 0.1c | 0.9 ± 0.1d | 0.7 ± 0.1e |
| High fibre | 1.0 ± 0.1d | 0.7 ± 0.1e | |||
| High Protein | 1.0 ± 0.1d | 0.7 ± 0.1e | |||
| Small intestine weight (mg g−1) | |||||
| Control | 34.1 ± 1.5a | 29.6 ± 4.5b | 30.1 ± 7.0bc | 33.1 ± 1.8d | 30.5 ± 2.3e |
| High fibre | 35.4 ± 1.0d | 33.2 ± 2.5e | |||
| High protein | 34.2 ± 1.3d | 34.3 ± 2.7 d‡ | |||
| Colon length (mm g−1) | |||||
| Control | 2.55 ± 0.01a | 1.65 ± 0.01b | 1.26 ± 0.01c | 1.12 ± 0.01c,x | 0.86 ± 0.01d,x |
| High fibre | 1.30 ± 0.01c,y | 1.02 ± 0.01d,y | |||
| High protein | 1.03 ± 0.01c,x | 0.89 ± 0.01d,x | |||
| Colon weight (mg g−1) | |||||
| Control | 3.0 ± 1.0a | 3.8 ± 4.0a | 4.4 ± 1.1ab | 5.2 ± 0.3c,x | 5.1 ± 0.6c,x |
| High fibre | 6.3 ± 0.3c,y | 6.4 ± 0.7c,y | |||
| High protein | 5.5 ± 0.4c,x | 4.9 ± 0.8c,x | |||
Values are means ±s.e.m. with an n= 20 males (M) and 16 females (F) on day 7; n= 16 (M) and 19 (F) on day 14; n= 16 (M) and 17 (F) on day 21; n= 6 (M) and 6 (F) per diet group on days 28 and 35 each. Superscripts ‘a’ to ‘e’ represent significant differences (P < 0.05) between ages within a row. Superscripts ‘x’ and ‘y’ represent a significant difference (P < 0.05) between diet groups for both males and females within an age category. Values with different letters are significantly different from each other. ‡Significant difference in one sex only; in males only on d28 between the high protein diet versus control and fibre for liver weight (P < 0.05); in females only on d35 between the high protein diet compared to control (P= 0.01) and high fibre for liver weight (P= 0.01); and in males only on d35 between high protein versus control (P < 0.05) only for small intestine weight.
Plasma hormones in young rats
Concentrations of plasma GLP-1, leptin, insulin, amylin and glucagon are presented in Table 4. On postnatal days 28 and 35 (i.e. 1 week and 2 weeks, respectively, on experimental diets), high fibre diet caused a significant increase in plasma active GLP-1 concentrations compared to the control and high protein diets (P < 0.001). The high fibre diet also caused a significant decrease in plasma leptin concentrations in rats fed the high fibre diet compared to the control diet on postnatal days 28 and 35 (Table 4). There were no changes to insulin, glucagon or amylin concentrations. Independent of diet, there was a significant age effect on plasma GLP-1 (P < 0.001) wherein plasma concentrations consistently fell between 7 and 35 days. There were no diet differences in plasma glucose on postnatal days 28 and 35.
Table 4.
Plasma hormone concentrations from postnatal days 7–35 in response to weaning diets high in fibre or protein
| Age (days) | |||||
|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 (on diet) | 35 (on diet) | |
| GLP-1 (pm) | |||||
| Control | 139.5 ± 23.0† | 87.1 ± 18.12 | 43.34 ± 4.90 | 24.0 ± 0.74 | 26.6 ± 2.6 |
| High fibre | 37.4 ± 2.19** | 38.9 ± 3.22** | |||
| High protein | 26.8 ± 2.05 | 27.6 ± 1.78 | |||
| Leptin (pm) | |||||
| Control | 292.9 ± 44.0 | 397.5 ± 39.1 | 376.2 ± 45.3 | 346.8 ± 51.3 | 321.8 ± 48.9 |
| High fibre | 208.4 ± 25.0* | 216.7 ± 20.6* | |||
| High protein | 273.2 ± 30.5 | 269.5 ± 32.1 | |||
| Insulin (pm) | |||||
| Control | 232.7 ± 46.2 | 163.9 ± 21.5 | 237.9 ± 73.1 | 175.8 ± 45.6 | 215.5 ± 38.3 |
| High fibre | 123.5 ± 17.5 | 154.4 ± 40.5 | |||
| High protein | 111.4 ± 11.5 | 188.0 ± 23.4 | |||
| Glucagon (pm) | |||||
| Control | 178.6 ± 42.0 | 222.1 ± 43.2 | 234.0 ± 45.5 | 118.7 ± 22.6 | 175.2 ± 36.7 |
| High fibre | 156.6 ± 31.9 | 160.7 ± 17.6 | |||
| High protein | 159.1 ± 40.9 | 192.5 ± 35.9 | |||
| Amylin (pm) | |||||
| Control | 11.8 ± 0.3 | 11.6 ± 0.6 | 12.9 ± 1.4 | 12.5 ± 0.6 | 15.2 ± 1.2 |
| High fibre | 17.3 ± 2.8 | 15.9 ± 3.2 | |||
| High protein | 11.8 ± 0.2 | 12.7 ± 0.7 | |||
Values are means ±s.e.m. with an n= 11 males (M) and 5 females (F) on day 7; n= 8 (M) and 8 (F) on day 14; n= 7 (M) and 9 (F) on day 21; n= 6 (M) and 6 (F) per diet group on days 28 and 35. No sex differences were found for plasma hormones and therefore sexes were combined. *Significant difference between high fibre versus control (P < 0.01) in both sexes at d28 and d35. **Significant difference between high fibre versus control and high protein diets (P < 0.001) in both sexes at d28 and d35. †Significant age difference, independent of diet where day 7 is different from days 21, 28 and 35; and day 14 is different from days 28 and 35 (P < 0.05).
Gene expression in young rats
Intestinal genes
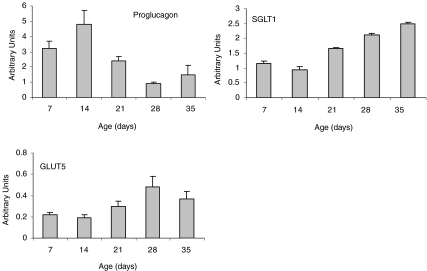
There were no significant sex or diet differences in the expression of the glucose transporters GLUT2, GLUT5 or SGLT-1 in the small intestine of the young rats (data not shown). Likewise, there were no significant sex or diet differences in proglucagon mRNA in the distal small intestine and colon or for ghrelin in the stomach (data not shown). There were, however, significant age effects for three of these genes (P < 0.05) (Fig. 2). While the glucose transporters SGLT-1 and GLUT5 increased with increasing age, proglucagon was significantly lower at day 28 compared to days 7 and 14.
Figure 2. Age effects on intestinal gene expression in control rats.
Results are presented as means ±s.e.m. (n= 12, 12, 10, 12, 12 for days 7, 14, 21, 28 and 35, respectively, for proglucagon and SGLT-1 and n= 12, 12, 6, 10, 10 for GLUT5). Arbitrary units represent the relative mRNA expression of the gene of interest to the housekeeping gene. There was a significant age effect (P < 0.05) for all genes presented. For proglucagon, 28d was different from 7d and 14d (both P= 0.02). For SGLT-1, 35d was different from 7d (P= 0.01), 14d (P= 0.01), and 21 d (P= 0.02). For GLUT5, 35d was different from 14d (P= 0.04).
Liver genes
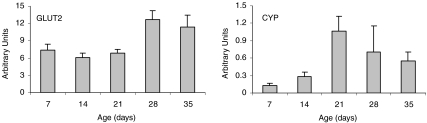
Both the high fibre and high protein diets caused a significant decrease in FAS mRNA on postnatal days 28 and 35 when compared to the control diet (P= 0.023) (Fig. 3). There were, however, no other significant diet differences for GLUT2, acetyl CoA carboxylase (ACC), glucokinase, sterol regulatory-element binding protein (SREBP-1c), SREBP-2, lecithin:cholesterol acyl transferase (LCAT), cholesterol 7 α-hydroxylase (CYP), 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) or leptin mRNA in the liver. Sex differences were found in the expression of certain liver genes independent of diet effects. Females exhibited significantly higher expression of mRNA for GLUT2 (P= 0.029), ACC (P= 0.003), and cholesterol 7 α-hydroxylase (P= 0.031) when compared to males (data not shown). There was a significant age effect for GLUT2 and CYP (P < 0.05) (Fig. 4). A marked increase in both genes occurred around the time of weaning or shortly thereafter.
Figure 3. Expression of fatty acid synthase mRNA in liver of young rats fed high fibre or high protein diets on postnatal days 28 and 35.
Results are presented as mean ±s.e.m. (n= 10–12). Arbitrary units represent the relative mRNA expression of the gene of interest to the housekeeping gene. Diet treatments with different letters represent a significant difference (P < 0.05).
Figure 4. Age effects on expression of GLUT2 and CYP mRNA in liver in control rats.
Results are presented as means ±s.e.m. (n= 12, 7, 12, 12, 11 for days 7 through 35, respectively). Arbitrary units represent the relative mRNA expression of the gene of interest to the housekeeping gene. A significant age effect was seen for both genes (P < 0.05). For GLUT2, 28d was different from 14d and 21d (both P= 0.03). For CYP, 21d was different from 7d (P= 0.01).
Brown adipose tissue genes
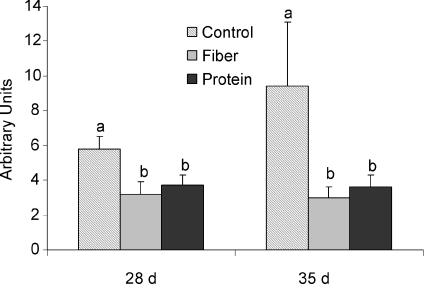
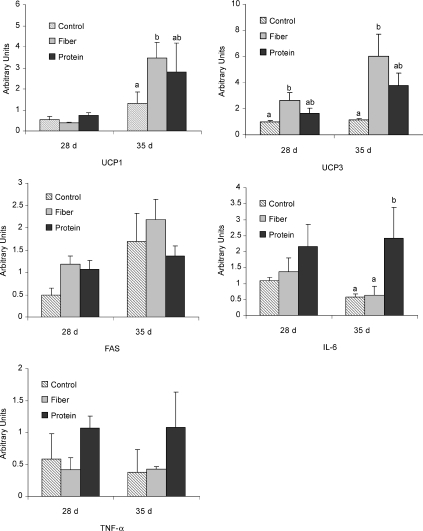
Changes in gene expression in brown adipose tissue (BAT) are shown in Fig. 5. The high fibre diet resulted in a significant increase in uncoupling protein 1 (UCP-1) mRNA in BAT on day 35 (P= 0.02) and a significant increase in UCP-3 mRNA on postnatal days 28 and 35 compared to control (P < 0.05). While FAS mRNA appeared to increase in the high fibre and protein diets compared to control on day 28 this was not significant. Levels of mRNA for the inflammatory cytokine IL-6 were significantly higher with high protein on day 35 (P < 0.05). While tumour necrosis factor-α (TNF-α) mRNA followed this same trend on days 28 and 35, the difference was not significant (P= 0.11). In general, the pattern of gene expression seen in the brown adipose tissue on day 28 was accentuated by day 35.
Figure 5. Expression of UCP-1, UCP-3, FAS, IL-6 and TNF-α mRNA in brown adipose tissue of young rats fed high fibre or high protein diets.
Individual graphs represent UCP-1, UCP-3, FAS, IL-6 and TNF-α mRNA expression on postnatal day 28 and day 35, respectively. Results are presented as means ±s.e.m. (n= 9). Arbitrary units represent the relative mRNA expression of the gene of interest to the housekeeping gene. Diet treatments with different letters represent a significant difference (P < 0.05) within an age category.
Discussion
The present study demonstrates that weaning diets high in fibre or protein result in early changes in the secretion of select satiety hormones and the expression of genes involved in glucose and lipid metabolism that reflect the response to a high energy diet in adulthood. The longitudinal developmental changes of five plasma hormones and 19 genes involved in energy balance were examined at days 7, 14, 21, 28 and 35 after birth. Body weight and fat mass in response to high energy diet challenge were also determined in adulthood in response to the control, high fibre or high protein diet initiated at weaning. This work provides evidence of a long-term effect of weaning diet on fat mass development in adulthood and the first developmental profile of gene expression and plasma hormones involved in the metabolism of energy in response to these weaning diets. Our major findings include: (1) an increase in adult body weight, fat mass and glycaemia in males and fat mass in females in response to high energy diets after a weaning diet high in protein compared to fibre; (2) an increase in plasma GLP-1 and decrease in plasma leptin in young rats weaned onto a high fibre diet; (3) a proportional increase in the size of the colon with a high fibre diet and proportional increase in liver and small intestine size with a high protein diet in young rats; (4) a decrease in hepatic FAS mRNA with the fibre and protein diets; (5) an increase in BAT mRNA expression of the inflammatory cytokine IL-6 with high protein and an increase in UCP-1 and UCP-3 mRNA with the fibre diet; and (6) a profile of the longitudinal developmental changes in expression of genes involved in glucose and lipid metabolism from postnatal days 7–35.
Evidence for the link between early nutritional environments and risk of adult chronic disease is well-established from a range of experimental evidence (Symonds & Gardner, 2006). This work has in part been driven by the need to identify factors contributing to the rapid rise in the incidence of type 2 diabetes and obesity. The risk of developing the metabolic syndrome, a combination of risk factors predisposing individuals to cardiovascular disease and type 2 diabetes, has been shown to be particularly sensitive to nutritional influences early in life, particularly during fetal and early postnatal and infant development (Barker et al. 2002; Gillman, 2005). The majority of animal studies have used two major models to elucidate the dietary origins of health and disease, that of maternal undernutrition to probe prenatal influences, and litter manipulation to examine postnatal under- and overnutrition during the suckling period. Much less work has examined the effects of maternal hypernutrition on the development of obesity in offspring and even less work has probed the effect of increased intake of select nutrients in the early postnatal period (suckling) and as addressed by our study, during weaning to solid food (Taylor & Poston, 2007).
Our data demonstrating a significant increase in body weight and percentage fat mass in male rats (fat mass alone in females) weaned onto a high protein diet and then challenged in adulthood with a high fat and sugar diet suggest that lasting changes may result from altering the composition of the first solid food. Our study is unique in that it extended the typical postnatal period of dietary intervention beyond suckling to span the time from weaning to early adulthood. By examining the changes in satiety hormone secretion and genes involved in metabolism during development (day 7–35) we were able to identify certain early responses to diet that may reflect the ability to respond to high energy diets later in life.
While several of the changes we observed would suggest that dietary fibre may be protective, fewer parameters we examined in young rats can explain the marked predisposition to obesity that the high protein diet brought about in adulthood. Our data showing an increase in the relative mass and length of the colon with fibre on postnatal days 28 and 35 is consistent with our previous work in adult rats with fibre-enriched diets (Reimer & McBurney, 1996; Reimer et al. 1997; Reimer & Russell, 2008). Addition of readily fermentable fibres to a diet is known to cause a significant proliferative effect in the colon and distal small intestine (Jacobs & Lupton, 1984). The effect is likely to reflect the bulking effect in the lower gastrointestinal tract and more importantly the production of short chain fatty acids (SCFA) from microbial fermentation of the fibre (Sakata, 1989; Rombeau & Kripke, 1990). We specifically examined a blend of the prebiotic fibres oligofructose and inulin. These fibres are soluble and non-gel-forming and selectively stimulate growth of lactic acid bacteria in the gut (Delzenne, 2003). Inulin is chiefly extracted from chicory root and has a degree of polymerization (DP) or number of glucose or fructose units of 2 to >60 (Roberfroid, 2001). Partial enzymatic hydrolysis of inulin results in the formation of oligofructose, a shorter chain fibre with a DP < 10. In humans, oligofructose has been shown to enhance satiety (Cani et al. 2006), improve glucose control in hyperglycaemic subjects (Yamashita et al. 1984), raise levels of plasma GLP-1 (Piche et al. 2003) and improve blood lipid profiles (Delzenne & Williams, 2002).
Our data suggest that introduction of a high fibre diet at weaning alters the trajectory of circulating GLP-1 and leptin compared to control. While the increase in GLP-1 concentrations is consistent with findings from numerous studies utilizing various fibre sources in adult rats (Reimer & McBurney, 1996; Reimer et al. 1997; Cani et al. 2004; Cani et al. 2005a,b; Delmee et al. 2006; Reimer & Russell, 2008), our work is the first to demonstrate this change during development from weaning to days 28 and 35 after birth. Our lack of accompanying increase in proglucagon expression, however, differs from work in adult rats (Reimer & McBurney, 1996; Reimer et al. 1997; Cani et al. 2005b) and may reflect a difference in younger versus older rats. While the increased plasma GLP-1 without an up-regulation of proglucagon could occur via increased stability of the mRNA, this remains to be tested.
Furthermore, while evidence for the effect of dietary fibre on plasma leptin is less prevalent and most often associated with weight loss as a result of increased fibre intake (Koh-Banerjee & Rimm, 2003; Artiss et al. 2006), we demonstrate a marked reduction in circulating leptin independent of changes in body weight in young rats fed the high fibre diet compared to control rats at postnatal days 28 and 35. There is evidence for the role of leptin in growth and development and specifically puberty, with obese children exhibiting higher leptin levels, a proven permissive factor in initiating lutenizing hormone pulsatility (Dunger et al. 2006). Much earlier in the developmental timeline, however, there is a suggestion that low levels of leptin reduce neural projections from the arcuate nucleus to other hypothalamic nuclei involved in energy balance (Bouret et al. 2004). Vickers et al. (2005) have shown that leptin injection from postnatal days 3–13 normalizes the negative phenotype associated with maternal undernutrition. Attig et al. (2008) showed that administration of leptin antagonist to neonatal rats induced in adult rats leptin resistance and higher accumulation of fat mass in response to high energy diet. Conversely, others have shown that neonatal rats treated with leptin had higher body weight and leptin resistance in adulthood (de Oliveira Cravo et al. 2002; Toste et al. 2006). In the neonatal rat hypothalamus, leptin appears to promote the development of the appetite stimulatory neuropeptide Y (NPY) and agouti-related protein (AgRP) projections to the arcuate nucleus. Whether or not the lower circulating leptin levels seen with the high fibre weaning diet play a clear role in the lower body and fat mass seen in this group in adulthood compared to the high protein group remains to be elucidated.
In terms of the longitudinal changes in gene expression related to glucose and lipid metabolism, expression of the lipogenic enzyme which catalyses the synthesis of long-chain fatty acids in the cytosol, FAS, was down-regulated in the livers of rats fed the high fibre and the high protein diet compared to controls at postnatal days 28 and 35. Delzenne and Nadine (2001) have shown that the prebiotic fibre oligofructose is able to reduce de novo fatty acid synthesis in the liver through inhibition of lipogenic enzymes, including ACC, FAS, malic enzyme, ATP citrate lyase, and glucose-6-phosphate dehydrogenase, in adult rats. The lower FAS mRNA observed in the young rats consuming the high protein diet is likely to reflect the lower levels of carbohydrate in this diet. It has been previously shown that carbohydrate free and/or high protein diets decrease hepatic FAS mRNA in normal and genetically obese rats (Morris et al. 2003; Pichon et al. 2006; Zhang et al. 2007).
Brown adipose tissue is present in newborn mammals and plays a role in maintenance of body temperature through non-shivering thermogenesis associated with mitochondrial UCP-1. The function of UCP-3, which shares ∼60% homology with UCP-1, is less clear with recent suggestions that it may play a role in mitigating reactive oxygen species formation and altering fatty acid translocation (Bezaire et al. 2007). Consumption of the high fibre diet in our study resulted in an increase in UCP-3 on day 28 and an increase in both UCP-1 and UCP-3 by day 35. Somewhat unexpected was the increase in FAS mRNA in BAT in the high fibre group although this did not reach significance. Although the majority of work performed with oligofructose has shown a decrease in FAS mRNA in the liver (Delzenne & Kok, 1999; Delzenne & Nadine, 2001) and indeed reflects that which we observed in the liver of our fibre-fed rats, the expression of FAS in brown adipose tissue in response to oligofructose has not been examined, particularly when introduced at an early age. BAT is known to be a major site for fatty acid synthesis with fatty acids being the main fuel to drive the thermogenic capacity of the tissue (Rousset et al. 2004). De novo lipid synthesis occurs in BAT and serves as much as a supply of fatty acids to be oxidized in the mitochondria during thermogenesis as a storage depot (Valverde et al. 2005). Given the marked rise in UCP-1 and UCP-3 in our young rats fed the high fibre diet, the concomitant rise in expression of the lipogenic gene, FAS, may reflect the increased demand for thermogenic fuel. While these changes in UCP mRNA may explain in part some of the protective effect of the high fibre weaning diet on adulthood body weight gain they do not provide an explanation for the highly detrimental effects seen with the high protein diet. Future studies should examine in greater detail the response of white adipose tissue to weaning diets high in fibre and protein as well. Furthermore, while the use of real time PCR allowed us to examine transcriptional changes in response to diet manipulation in this study, future use of proteonomics that measures the functional product of gene expression (protein) (de Roos & McArdle, 2008) would be a valuable tool to elucidate the activation or inactivation of proteins in response to our diets.
Several cytokines, including TNF-α and IL-6, play a pivotal role in the low-grade inflammation seen in obesity and the metabolic dysregulation and adipose tissue development accompanying it (Xu et al. 2003). TNF-α acts as a negative regulator of adipogenic and thermogenic differentiation and stimulates insulin resistance in brown adipose tissue (Valverde et al. 1998). In addition, the inflammatory cytokine IL-6 is thought to play a role in the development of coronary heart disease (Yudkin et al. 2000) and obese humans have elevated circulating levels of IL-6 (Kralisch et al. 2007). While evidence for the effects of dietary fibre or protein on inflammation and cytokine production is limited, Esposito et al. (2003) demonstrated that a high fat meal increased IL-18 and adiponectin concentrations from baseline, while a high fibre meal decreased IL-18 from baseline in healthy subjects and patients with type 2 diabetes. Our data suggests that introducing a high protein diet at weaning up-regulates the expression of IL-6 in brown adipose tissue of young rats. This finding appears to be one plausible explanation for the increased body weight and fat mass seen after a high energy diet challenge in adulthood when the weaning diet was high in protein. None of the five plasma hormones we examined were grossly abnormal in the young rats fed the high protein diet; for the most part their concentrations were intermediate between the fibre and control diets but were most different from fibre. This suggests that although detrimental changes can be seen very early on in terms of inflammatory cytokine expression in the BAT, overt metabolic disturbance reflected in insulin, GLP-1 and other circulating hormones may occur past the 35 days of age we examined in this study and may in fact only manifest when the animals are presented with a high fat–high sugar diet. In our study it appears the high energy diet in adulthood unmasked the susceptibility to increased body fat mass in rats weaned onto a high protein diet.
In conclusion, we have shown that weaning diets high in fibre or protein, when consumed into early adulthood, alter the early trajectory of circulating satiety hormones and gene expression of factors known to influence energy homeostasis. We suggest that the current study provides a snap-shot of the developmental changes in hormone and mRNA profiles occurring in young rats that reflect the difference in propensity to increased body fat mass and glycaemia in adulthood. Ultimately it may be possible to use the hormonal and gene expression profiles generated in this work to identify biomarkers that are predictive of future obesity risk in relation to dietary manipulation initiated during growth. The results of this study from adult rats suggest that introduction of a high protein diet at weaning results in marked increases in body fat mass and worsened glycaemic response when rats are switched to a high fat–high sucrose diet in adulthood. Compared to the high protein diet, the high fibre diet introduced at weaning and consumed into adulthood was protective against increased body fat and glycaemic dysregulation even in the face of a high energy diet.
Acknowledgments
This work was supported in part by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research. No conflict of interest is reported.
References
- Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiss JD, Brogan K, Brucal M, Moghaddam M, Jen KL. The effects of a new soluble dietary fiber on weight gain and selected blood parameters in rats. Metabolism. 2006;55:195–202. doi: 10.1016/j.metabol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes. 2008;32:1153–1160. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Seifert EL, Harper M-E. Uncoupling protein-3: clues in an ongoing mitochondrial mystery. FASEB J. 2007;21:312–324. doi: 10.1096/fj.06-6966rev. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1 (7–36) amide on glycemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol. 2005a;185:457–465. doi: 10.1677/joe.1.06100. [DOI] [PubMed] [Google Scholar]
- Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–526. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy humans: a pilot study. Eur J Clin Nutr. 2006;60:567–572. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res. 2005b;13:1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Ozanne SE. Developmental programming of energy balance and the metabolic syndrome. Proc Nutr Soc. 2007;66:198–206. doi: 10.1017/S0029665107005447. [DOI] [PubMed] [Google Scholar]
- Delmee E, Cani PD, Gual G, Knauf C, Burcelin R, Maton N, Delzenne NM. Relation between colonic proglucagon expression and metabolic response to oligofructose in high fat diet-fed mice. Life Sci. 2006;79:1007–1013. doi: 10.1016/j.lfs.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Delzenne NM. Oligosaccharides: state of the art. Proc Nutr Soc. 2003;62:177–182. doi: 10.1079/pns2002225. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Kok NN. Biochemical basis of oligofructose-induced hypolipidemia in animal models. J Nutr. 1999;129:1467S–1470S. doi: 10.1093/jn/129.7.1467S. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Nadine K. Effects of fructans-type of prebiotics on lipid metabolism. Am J Clin Nutr. 2001;73:456S–458S. doi: 10.1093/ajcn/73.2.456s. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Williams CM. Prebiotics and lipid metabolism. Curr Opin Lipidol. 2002;13:61–67. doi: 10.1097/00041433-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Deng MY, Wang H, Ward GB, Beckham TR, McKenna TS. Comparison of six RNA extraction methods for the detection of classical swine fever virus by real-time and conventional reverse transcriptase-PCR. J Vet Diagn Invest. 2005;17:574–578. doi: 10.1177/104063870501700609. [DOI] [PubMed] [Google Scholar]
- de Oliveira Cravo C, Teixeira CV, Passos MC, Dutra SC, de Moura EG, Ramos C. Leptin treatment during the neonatal period is associated with higher food intake and adult body weight in rats. Horm Metab Res. 2002;34:400–405. doi: 10.1055/s-2002-33473. [DOI] [PubMed] [Google Scholar]
- de Roos B, McArdle HJ. Proteonomics as a tool for the modelling of biological processes and biomarker development in nutrition research. Br J Nutr. 2008;99(Suppl. 3):S66–S71. doi: 10.1017/S0007114508006909. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Development of glucagon-like peptide-1-based pharmaceuticals as therapeutic agents for the treatments of diabetes. Curr Pharm Des. 2001;7:1399–1412. doi: 10.2174/1381612013397401. [DOI] [PubMed] [Google Scholar]
- Dunger DB, Ahmed ML, Ong KK. Early and late weight gain and the timing of puberty. Mol Cell Biol. 2006;254–255:140–145. doi: 10.1016/j.mce.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? Nutr Rev. 2002;60:189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Giugliano F, Di Palo C, Ciotola M, Barbieri M, Paolisso G, Giugliano D. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2003;78:1135–1140. doi: 10.1093/ajcn/78.6.1135. [DOI] [PubMed] [Google Scholar]
- Gillman M. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development and patterns of disease. Science. 2004;305:1773–1776. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Herrick K, Phillips DI, Haselden S, Shiell AW, Campbell-Brown M, Godfrey KM. Maternal consumption of a high-meat, low-carbohydrate diet in late pregnancy: relation to adult cortisol concentrations in the offspring. J Clin Endocrinol Metab. 2003;88:3554–3560. doi: 10.1210/jc.2003-030287. [DOI] [PubMed] [Google Scholar]
- Jacobs LR, Lupton JR. Effect of dietary fibers on rat large bowel mucosal growth and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 1984;246:G378–G385. doi: 10.1152/ajpgi.1984.246.4.G378. [DOI] [PubMed] [Google Scholar]
- Koh-Banerjee P, Rimm EB. Whole grain consumption and weight gain: a review of the epidemiological evidence, potential mechanisms and opportunities for future research. Proc Nutr Soc. 2003;62:25–29. doi: 10.1079/PNS2002232. [DOI] [PubMed] [Google Scholar]
- Kralisch S, Sommer G, Deckert CM, Linke A, Bluher M, Stumvoll M, Fasshauer M. Adipokines in diabetes and cardiovascular diseases. Minerva Endocrinol. 2007;32:161–171. [PubMed] [Google Scholar]
- Lairon D, Play B, Jourdheuil-Rahmani D. Digestible and indigestible carbohydrates: interactions with postprandial lipid metabolism. J Nutr Biochem. 2007;18:217–227. doi: 10.1016/j.jnutbio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/s0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats – a review. Placenta. 2006;27(Suppl. A):S56–S60. doi: 10.1016/j.placenta.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Morris KL, Namey TC, Zemel MB. Effects of dietary carbohydrate on the development of obesity in heterozygous Zucker rats. J Nutr Biochem. 2003;14:32–39. doi: 10.1016/s0955-2863(02)00249-8. [DOI] [PubMed] [Google Scholar]
- Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003;124:894–902. doi: 10.1053/gast.2003.50159. [DOI] [PubMed] [Google Scholar]
- Pichon L, Huneau J-F, Fromentin G, Tome D. A high-protein, high-fat, carbohydrate-free diet reduces energy intake, hepatic lipogenesis, and adiposity in rats. J Nutr. 2006;136:1256–1260. doi: 10.1093/jn/136.5.1256. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–3956. doi: 10.1210/endo.137.9.8756571. [DOI] [PubMed] [Google Scholar]
- Reimer RA, Russell JC. Glucose tolerance, lipids and GLP-1 secretion in JCR: LA-cp rats fed a high protein fiber diet. Obesity. 2008;16:40–46. doi: 10.1038/oby.2007.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer RA, Thomson ABR, Rajotte R, Basu TK, Ooraikul B, McBurney MI. A physiological level of rhubarb fiber increases proglucagon gene expression and modulates intestinal glucose uptake in rats. J Nutr. 1997;127:1923–1928. doi: 10.1093/jn/127.10.1923. [DOI] [PubMed] [Google Scholar]
- Roberfroid M. Caloric value of inulin and oligofructose. J Nutr. 1999;129:1436S–1437S. doi: 10.1093/jn/129.7.1436S. [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. Prebiotics: preferential substrates for specific germs? Am J Clin Nutr. 2001;73:406S–409S. doi: 10.1093/ajcn/73.2.406s. [DOI] [PubMed] [Google Scholar]
- Rombeau JL, Kripke SA. Metabolic and intestinal effects of short chain fatty acids. J Parenter Enteral Nutr. 1990;14(Suppl):S181–S185. doi: 10.1177/014860719001400507. [DOI] [PubMed] [Google Scholar]
- Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillard F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl. 1):S130–S135. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation of isolated and denervated jejunal segment of the rat. Scan J Gastroenterol. 1989;24:886–890. doi: 10.3109/00365528909089230. [DOI] [PubMed] [Google Scholar]
- Silveira PP, Portella AK, Goldani MZ, Barbieri MA. Developmental origins of health and disease (DOHaD) J Pediatr (Rio J) 2007;83:494–504. doi: 10.2223/JPED.1728. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Gardner DS. Experimental evidence for early nutritional programming of later health in animals. Curr Opin Clin Nutr Metab Care. 2006;9:278–283. doi: 10.1097/01.mco.0000222112.46042.19. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–298. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr. 2006;95:830–837. doi: 10.1079/bjn20061726. [DOI] [PubMed] [Google Scholar]
- Valverde AM, Benito M, Lorenzo M. The brown adipose cell: a model for understanding the molecular mechanisms of insulin resistance. Acta Physiol Scand. 2005;183:59–73. doi: 10.1111/j.1365-201X.2004.01384.x. [DOI] [PubMed] [Google Scholar]
- Valverde AM, Teruel R, Navarro P, Benito M, Lorenzo M. Tumor necrosis factor-α causes insulin receptor substrate-2 mediated insulin resistance and inhibits insulin-induced adipogenesis in fetal brown adipocytes. Endocrinology. 1998;139:1229–1238. doi: 10.1210/endo.139.3.5854. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am J Clin Nutr. 1999;69:179–197. doi: 10.1093/ajcn/69.2.179. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Itakura M, Kawai K. Effect of fructooligosaccharides on blood glucose and serum lipids in diabetic subjects. Nutr Res. 1984;4:961–966. [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohammed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Hua JZ, Wang SR, Sun CH. Post-weaning isocaloric hyper-soybean oil versus a hyper-carbohydrate diet reduces obesity in adult rats induced by a high-fat diet. Asia Pac J Clin Nutr. 2007;16(Suppl.):368–373. [PubMed] [Google Scholar]