Abstract
Fetal growth is decreased at high altitude (> 2700 m). We hypothesized that variation in fetal O2 delivery might account for both the altitude effect and the relative preservation of fetal growth in multigenerational natives to high altitude. Participants were 168 women of European or Andean ancestry living at 3600 m or 400 m. Ancestry was genetically confirmed. Umbilical vein blood flow was measured using ultrasound and Doppler. Cord blood samples permitted calculation of fetal O2 delivery and consumption. Andean fetuses had greater blood flow and oxygen delivery than Europeans and weighed more at birth, regardless of altitude (+208 g, P < 0.0001). Fetal blood flow was decreased at 3600 m (P < 0.0001); the decrement was similar in both ancestry groups. Altitude-associated decrease in birth weight was greater in Europeans (−417 g) than Andeans (−228 g, P < 0.005). Birth weight at 3600 m was > 200 g lower for Europeans at any given level of blood flow or O2 delivery. Fetal haemoglobin concentration was increased,  decreased, and the fetal
decreased, and the fetal  /
/ curve was left-shifted at 3600 m. Fetuses receiving less O2 extracted more (r2= 0.35, P < 0.0001). These adaptations resulted in similar fetal O2 delivery and consumption across all four groups. Increased umbilical venous O2 delivery correlated with increased fetal O2 consumption per kg weight (r2= 0.50, P < 0.0001). Blood flow (r2= 0.16, P < 0.001) and O2 delivery (r2= 0.17, P < 0.001) correlated with birth weight at 3600 m, but not at 400 m (r2= 0.04, and 0.03, respectively). We concluded that the most pronounced difference at high altitude is reduced fetal blood flow, but fetal haematological adaptation and fetal capacity to increase O2 extraction indicates that deficit in fetal oxygen delivery is unlikely to be causally associated with the altitude- and ancestry-related differences in fetal growth.
curve was left-shifted at 3600 m. Fetuses receiving less O2 extracted more (r2= 0.35, P < 0.0001). These adaptations resulted in similar fetal O2 delivery and consumption across all four groups. Increased umbilical venous O2 delivery correlated with increased fetal O2 consumption per kg weight (r2= 0.50, P < 0.0001). Blood flow (r2= 0.16, P < 0.001) and O2 delivery (r2= 0.17, P < 0.001) correlated with birth weight at 3600 m, but not at 400 m (r2= 0.04, and 0.03, respectively). We concluded that the most pronounced difference at high altitude is reduced fetal blood flow, but fetal haematological adaptation and fetal capacity to increase O2 extraction indicates that deficit in fetal oxygen delivery is unlikely to be causally associated with the altitude- and ancestry-related differences in fetal growth.
Among the most pervasive effects of altitude (> 2700 m) on mammalian reproductive outcome is reduced fetal growth (Lichty et al. 1957; Yip, 1987; Han, 1993). While all data support that lower ambient  is the ultimate cause of reduced fetal growth, the proximate mechanisms remain unknown. We have shown that Andean women have larger infants, and greater uterine blood flow and oxygen delivery to the uterus regardless of altitude. However, maternal oxygen transport was not related to birth weight; the altitude-associated reduction in these parameters was equivalent in Andean versus recent European migrants, and when normalized to the weight of the uterine contents uteroplacental oxygen delivery was similar (Zamudio et al. 2007a). Since ultimately it is fetal blood flow that determines fetal oxygen and nutrient supply, we focused this study on fetal umbilical blood flow, oxygen delivery and oxygen consumption.
is the ultimate cause of reduced fetal growth, the proximate mechanisms remain unknown. We have shown that Andean women have larger infants, and greater uterine blood flow and oxygen delivery to the uterus regardless of altitude. However, maternal oxygen transport was not related to birth weight; the altitude-associated reduction in these parameters was equivalent in Andean versus recent European migrants, and when normalized to the weight of the uterine contents uteroplacental oxygen delivery was similar (Zamudio et al. 2007a). Since ultimately it is fetal blood flow that determines fetal oxygen and nutrient supply, we focused this study on fetal umbilical blood flow, oxygen delivery and oxygen consumption.
Aside from the factors mediating environmental impacts, the mechanisms contributing to population differences in fetal growth have not been elucidated. For example, infants born to Han (Chinese) women are smaller and lighter than infants born to persons of European ancestry (Yip et al. 1991). Likewise, infants of Native American Aymara or Quechua ancestry in South America are larger and heavier than infants born to the admixed Hispanic population, regardless of altitude and despite lower socioeconomic status (Haas et al. 1980; Giussani et al. 2001; Zamudio et al. 2007a). Native Americans have greater birth weights across multiple environments (Munroe et al. 1984; Thomson, 1990), as do Tibetans (Zamudio et al. 1993; Tripathy & Gupta, 2005). These populations share an ancestral origin in Northern Mongolia (Torroni et al. 1994; Merriwether et al. 1996). This suggests that both Andean and Tibetan protection from altitude-associated reduction in growth may be due to enhanced fetal growth in general, due to shared population origins, rather than a specific genetic adaptation to the altitude environment. The natural experiment thus afforded by coresident Andean and European populations at high and low altitude in Bolivia permits the testing of hypotheses regarding the influence of altitude and of ancestry on fetal growth and the delineation of mechanisms contributing to the relative preservation of fetal growth among Andeans at high altitude.
To our knowledge, measures of human fetal O2 delivery and consumption have not been previously reported, nor have human fetal blood gases been determined to see whether the high-altitude fetus adapts, or suffers from reduced O2 tension, as in fetal growth restriction at sea level (Pardi et al. 1993; Richardson & Bocking, 1998). We tested the hypothesis that a decrease in fetal blood flow, O2 delivery and/or O2 consumption at high altitude contributes to reduced fetal growth. We further hypothesized that Andean fetuses would have greater blood flows/O2 delivery or consumption and that this would contribute to their growth advantage at both low and high altitude.
Methods
Research design, subjects and sites
The study is a cross-sectional comparison of Andean pregnancies at high altitude (3600 m) versus genetically similar women who migrated to low altitude (400 m) and of European (largely Hispanic) pregnancies at low altitude compared with genetically similar women who migrated to high altitude. All participants gave written, informed consent to Bolivian National Bioethics Committee and USA IRB approved protocols. Inclusion criteria were good health, enumeration of three generations of ancestors’ names, birthplaces and ethnicity, conception, gestation and delivery at the altitude of study, and election of non-urgent caesarean delivery. The reasons for caesarean delivery were prior caesarean, fetal malpresentation (e.g. breech) or personal preference. Women were excluded for drug, alcohol or tobacco use, or complications of pregnancy. Sites were the Instituto Boliviano de Biología de Altura, Universidad de San Andreas Mayor and the Hospital Materno-Infantil in La Paz, Bolivia (3600 m, 494 mmHg), Centro Nacional de Enfermedades Tropicales, the Clinica Sirani, and the Hospital Hernandez Vera in Santa Cruz, Bolivia (400 m, 760 mmHg). Women of Asian or African ancestry or with partners whose ancestry was incongruent with their own were excluded in order to minimize genetic heterogeneity in the studies of the ancestry-informative markers discussed below.
The method for ancestry assignment was previously reported (Zamudio et al. 2007a). Briefly, women who identified themselves and three generations of their ancestors as born and raised at > 3000 m, and as of Aymara or Quechua ethnicity were provisionally assigned to the Andean group. Women who excluded Aymara or Quechua from their ancestry, and who could enumerate three generations of sea level ancestors were provisionally assigned to the European group. Group assignments were finalized using 133 ancestry-informative markers (AIMs), single nucleotide polymorphisms located across the genome in non-coding regions and known to vary between continents (Shriver et al. 2004, 2005). As the fetus represents the genetic contribution of both mother and father to the pregnancy of interest, fetal DNA was isolated from core villous placental tissue samples using the Qiagen Midi DNA isolation kit (Qiagen, Chatsworth, CA, USA) according to the manufacturer's instructions. The AIM analyses showed significant Native American admixture in the sea level ancestry group, as is true of all Hispanic/Latino populations in the Americas studied to date (Merriwether, 1997; Bonilla et al. 2004, 2005). However of the > 50 tribes known in Bolivia, only Aymara and Quechua are of high-altitude origin, as supported in analyses of ancient DNA showing continuity between the pre-Colombian and current Andean population inhabiting the highlands (Martinez-Laso et al. 2006; Shinoda et al. 2006). The sea-level ancestry group is hereafter termed European both for convenience and because the AIM data indicated that geographical ancestry primarily derived from the European continent.
We report here on maternal and neonatal characteristics and fetal blood flow from 168 pregnancies. We report data on fetal O2 extraction and consumption from a subset of 142 of these pregnancies. The inclusion criteria for the subset of births for which O2 consumption was calculated was that labour had not commenced prior to delivery, oxygen was not administered to the mother prior to clamping of the umbilical cord, umbilical arterial pH differed from venous by > 0.02, and that arterial  was > 3.8 mmHg higher than venous
was > 3.8 mmHg higher than venous  (Lackman et al. 2001). This ensured that cord blood samples were not inadvertently collected from the same vessel, or from mixed venous and arterial cord blood.
(Lackman et al. 2001). This ensured that cord blood samples were not inadvertently collected from the same vessel, or from mixed venous and arterial cord blood.
Protocol
Prior to delivery (4 ± 1 day, mean ±s.d.), a medical/obstetric history, ancestry and health screen, and arterialized blood gases were obtained in the mothers. Maternal data on haematology and blood gases are reported in Table 2, but since the methods and interpretation have been previously published and discussed, they are not considered further here except where pertinent to the interpretation of fetal data. The umbilical cord was doubly clamped at caesarean delivery prior to the infant's first breath and the placenta and cord were delivered intact for cord blood sampling. Placental weight was measured after trimming the cord and membranes. Ponderal index, which estimates neonatal body mass, was calculated as 100 × birth weight (g)/birth length (cm)3. All subjects in this study were delivered under spinal or epidural anaesthesia. The four subjects in whom these techniques failed were delivered under general anaesthesia and were excluded from analysis as supplemental oxygen was administered to the mother.
Table 2.
Fetal haematology and blood gases
| 400 m European | 3600 m European | 400 m Andean | 3600 m Andean | P-values | |
|---|---|---|---|---|---|
| n= 44 (range) | n= 37 (range) | n= 44 (range) | n= 43 (range) | ||
| Haemoglobin (g dl−1) | 14.7 ± 0.2 | 17.1 ± 0.3 | 14.3 ± 0.2 | 16.8 ± 0.2 | P < 0.0001 altitude |
| (12.2–17.1) | (12.6–20.2) | (11.2–16.9) | (13.3–19.2) | P= 0.10 ancestry | |
| Hematocrit (%) | 44 ± 1 | 50 ± 1 | 44 ± 1 ± 0.6 | 49 ± 1 | P < 0.0001 altitude |
| (36–55) | (38–70) | (34–55) | (39–56) | ||
| Transferrin (mg/ml) | 3.0 ± 0.1 | 3.1 ± 0.2 | 3.4 ± 0.2 | 3.2 ± 0.1 | NS |
| (1.5–3.9) | (1.8–4.9) | (1.5–4.8) | (1.3–4.8) | ||
| Erythropoietin (IU ml−1) | 23.5 ± 2.4 | 41.6 ± 4.5 | 24.6 ± 2.4 | 30.7 ± 2.2 | P < 0.0001 altitude |
| 5.8–74.5 | 15.6–102.6 | 11.5–63.9 | (8.1–54.4) | P < 0.05 interaction | |
| Umbilical vein | n= 41 | n= 30 | n= 37 | n= 41 | |
 (mmHg) (mmHg) |
29.8 ± 1.0 | 28.2 ± 1.2 | 31.4 ± 1.0 | 27.0 ± 0.9 | P < 0.005 altitude |
| (20–45) | (15–41) | (18–44) | (15–38) | ||
 (%) (%) |
52 ± 2 | 53 ± 3 | 55 ± 2 | 57 ± 3 | NS ancestry |
| (26–78) | (22–75) | (26–76) | (18–78) | ||
 (mmHg) (mmHg) |
46.6 ± 1.0 | 42.1 ± 1.0 | 44.1 ± 0.9 | 39.2 ± 0.7 | P < 0.0001 altitude |
| (35.6–61.3) | (23.0–52.0) | (32.0–57.2) | (31.6–51.0) | P= 0.005 ancestry | |
| pH | 7.34 ± 0.01 | 7.34 ± 0.01 | 7.35 ± 0.01 | 7.35 ± 0.01 | P= 0.08 ancestry |
| (7.28–7.40) | (7.27–7.40) | (7.31–7.38) | (7.21–7.40) | ||
| Bicarbonate (mmol l−1) | 23 ± 0.4 | 21 ± 0.2 | 23 ± 0.4 | 21 ± 0.2 | P < 0.0001 altitude |
| (17–28) | (18–23) | (18–27) | (17–27) | ||
| Base excess (mmol l−1) | –1.5 ± 0.3 | – 3.2 ± 0.2 | –1.8 ± 0.3 | – 4.4 ± 0.2 | P < 0.0001 altitude |
| (–6.0–3.2) | –5.3 to – 0.4) | (–6.0–1.9) | (–7.3 to –1.7) | P < 0.01 ancestry | |
| Calculated O2 content (ml l−1) | 103 ± 4 | 118 ± 6 | 104 ± 4 | 129 ± 6 | P < 0.0001 altitude |
| (53–141) | (30–175) | (56–160) | (45–187) | ||
| Umbilical artery | n= 38 | n= 29 | n= 37 | n= 38 | |
 (mmHg) (mmHg) |
16.6 ± 0.8 | 15.4 ± 1.0 | 18.0 ± 0.9 | 16.9 ± 0.7 | P= 0.09 ancestry |
| (7.9–32.0) | (5.3–28.2) | (9.3–38.3) | (8.5–28.7) | ||
 (%) (%) |
20 ± 2 | 16 ± 2 | 22 ± 2 | 25 ± 2 | P < 0.005 ancestry |
| (7.9–32) | (5.3–26.1) | (7.8–32.0) | 9.3–28.3) | P= 0.06 interaction | |
 (mmHg) (mmHg) |
56.0 ± 1.1 | 51.1 ± 0.9 | 55.1 ± 1.1 | 47.6 ± 0.9 | P < 0.0001 altitude |
| (38.9–76.0) | (44.0–79.7) | (40.5–84.8) | (40.0–61.5) | P < 0.05 ancestry | |
| pH | 7.28 ± 0.01 | 7.29 ± 0.01 | 7.30 ± 0.01 | 7.30 ± 0.01 | P < 0.05 ancestry |
| (7.21–7.35) | (7.24–7.39) | (7.22–7.36) | (7.16–7.34) | ||
| Bicarbonate (mmol l−1) | 24.3 ± 0.6 | 22.1 ± 0.6 | 24.9 ± 0.5 | 22.6 ± 0.3 | P < 0.0001 altitude |
| (16.3–29.7) | (18.9–26.8) | (18.6–29.7) | (18.9–26.8) | ||
| Calculated O2 content (ml l−1) | 39 ± 3 | 36 ± 4 | 43 ± 3 | 52 ± 4 | P < 0.005 ancestry |
| (10–85) | (5–97) | (16–110) | (12–137) | P < 0.05 interaction | |
| Maternal ‘arterialized’ | n= 44 | n= 37 | n= 44 | n= 43 | |
| Haemoglobin (gm dl−1) | 12.0 ± 0.2 | 15.3 ± 0.2 | 11.1 ± 0.2 | 14.6 ± 0.2 | P < 0.0001 altitude |
| (9.1–14.3) | (12.6–17.8) | (8.5–13.8) | (11.3–18.0) | P < 0.001 ancestry | |
| Haematocrit (%) | 36.7 ± 0.4 | 42.0 ± 0.6 | 34.4 ± 0.6 | 40.7 ± 0.6 | P < 0.0001 altitude |
| (31.0–42.0) | (32.5–48.0) | (28.0–44.0) | (34.1–49.0) | P < 0.005 ancestry | |
 (%) (%) |
97.6 ± 0.1 | 91.4 ± 0.4 | 97.8 ± 0.1 | 91.1 ± 0.3 | P < 0.0001 altitude |
| (92.2–99.3) | (80.2–94.1) | (94.0–99.3) | (80.3–93.8) | ||
 (mmHg) (mmHg) |
90.8 ± 1.7 | 54.6 ± 1.2 | 93.7 ± 1.3 | 53.2 ± 1.7 | P < 0.0001 altitude |
| (86.2–110.4) | (41.0–70.5) | (85.6–115.0) | (41.5–71.8) | ||
 (mmHg) (mmHg) |
35.2 ± 0.4 | 28.0 ± 0.6 | 35.7 ± 0.4 | 26.5 ± 0.4 | P < 0.0001 altitude |
| (29.6–40.8) | (23.0–37.0) | (29.7–42.1) | (19.8–34.2) | P < 0.05 interaction | |
| pH | 7.42 ± 0.004 | 7.44 ± 0.004 | 7.43 ± 0.004 | 7.44 ± 0.003 | P < 0.001 altitude |
| (7.37–7.38) | (7.38–7.48) | (7.39–7.48) | (7.39–7.48) | P < 0.05 ancestry | |
| Bicarbonate | 22.3 ± 0.3 | 19.0 ± 0.4 | 23.2 ± 0.3 | 17.8 ± 0.3 | P < 0.0001 altitude |
| (18.3–27.1) | (15.7–23.4) | (18.5–28.0) | (14.8–22.7) | P < 0.005 interaction | |
| Calculated arterial O2 content (ml l−1) | 157 ± 2 | 187 ± 2 | 146 ± 3 | 178 ± 3 | P < 0.0001 altitude |
| (118–188) | (158–219) | (108–179) | (136–219) | P < 0.001 ancestry |
Data are means ±s.e.m.
Ultrasound
The ultrasound methods have been described in detail in prior publications (Palmer et al. 1992; Zamudio et al. 2007a). Our protocol was modified to include measurement of the pulsatility index (PI) and peak systolic velocity (PSV) and end-diastolic velocity (EDV) of the fetal middle cerebral artery and the umbilical artery at the placental and fetal insertion sites, as changes in vascular resistance in these vessels are well-known correlates of fetal growth restriction (Giles et al. 1985; Todros et al. 1996; Galan et al. 2002). We additionally measured the umbilical vein diameter and blood mean flow velocity to calculate volumetric blood flow to the fetus (Sutton, 1990; Barbera et al. 1999). Estimates of volumetric blood flow are known to vary depending on where the umbilical cord is insonated and we therefore measured umbilical vein parameters as close as possible to the cord insertion at the fetal abdomen (Skulstad et al. 2002). Studies were conducted at both altitudes between 15.00 h and 19.00 h, with no food eaten for at least 2 h. Studies were conducted with the patient supine (with a hip wedge to prevent compression of the vena cava) or in the lateral position. Postural changes (supine versus lateral) do not affect uterine or umbilical blood flows (Jaffa et al. 2001). An ATL model 3000 (3600 m) or 5000 (400 m) was used for all studies. Umbilical venous internal diameters were measured in duplicate (without colour), in triplicate if values differed by ≥ 0.03 cm. At the same location, the Doppler time-averaged mean flow velocity (MFV) was obtained, with 30 s of the non-pulsatile flow averaged for mean flow velocity, the average speed of the blood travelling through the vein in one cardiac cycle (MFV, cm s−1). In the middle cerebral and umbilical arteries, pulsatility index was calculated as the peak systolic – end diastolic flow velocity/mean (Thompson et al. 1986). Elevated values in the umbilical artery are an indicator of increased impedance to blood flow and impaired placental development (Kreczy et al. 1995), while decreased pulsatility in the middle cerebral artery is indicative of brain sparing in the hypoxic fetus (Galan et al. 2002). Volumetric flow (ml min−1) was calculated as the cross-sectional area of the umbilical vein × the mean flow velocity of blood travelling through the vein (MFV) × 60. Studies were videotaped and a subset of 10 per group obtained on the ATL 5000 were re-examined (blinded to identity) on an ATL 3000 to derive measures of variation between sites. The coefficient of variation (CV) for umbilical venous diameter was 3 ± 1%. Variability in duplicate measures of blood mean flow velocity in the umbilical vein was 5 ± 2%. These CVs did not differ between altitudes.
O2 delivery and consumption
Cord venous and arterial blood was collected from the doubly clamped cord into heparinized blood gas syringes, immediately sealed and measured within 1 h of collection. Blood gases were measured using a Radiometer ABL 5000 (Copenhagen, Denmark) at high altitude and an Eschweiler ECOSYS II (Kiel, Germany) at low altitude; the machines were calibrated prior to every study using the manufacturers’ standard calibration solutions. Analyses were repeated where duplicate values for pH varied by > 0.02 or  by > 1 mmHg. The CV of duplicate measures did not exceed 2% for any blood gas or haematological parameters. Haemoglobin was measured using a Radiometer OSM 3 (Copenhagen, Denmark) calibrated daily using the manufacturer's standards, or by the cyanomethaemoglobin technique. Note that where fetal haemoglobin is referred to, it is the haemoglobin concentration measured by the OSM or cyanomethemoglobin technique. We cannot distinguish between fetal haemoglobin (Hgb F) and adult haemoglobin (Hgb A). O2 delivery (ml min−1) was calculated as oxygen content ((fetal haemoglobin (g l−1) × 1.34) ×
by > 1 mmHg. The CV of duplicate measures did not exceed 2% for any blood gas or haematological parameters. Haemoglobin was measured using a Radiometer OSM 3 (Copenhagen, Denmark) calibrated daily using the manufacturer's standards, or by the cyanomethaemoglobin technique. Note that where fetal haemoglobin is referred to, it is the haemoglobin concentration measured by the OSM or cyanomethemoglobin technique. We cannot distinguish between fetal haemoglobin (Hgb F) and adult haemoglobin (Hgb A). O2 delivery (ml min−1) was calculated as oxygen content ((fetal haemoglobin (g l−1) × 1.34) × ) × volumetric blood flow (l min−1). Fetal O2 consumption was calculated as (umbilical venous O2 content – umbilical artery O2 content) × blood flow. Oxygen consumption is expressed as absolute O2 consumption (ml min−1) or normalized for birth weight (ml min−1 kg−1) or for uterine contents (ml (kg fetus and placenta)−1 min−1). Fetal fractional O2 extraction, the percentage of oxygen taken up by the fetus between the umbilical vein and artery, was calculated as (umbilical venous O2 content – umbilical artery O2 content)/(umbilical vein O2 content) × 100. Near term fetal weight estimates by ultrasound are relatively inaccurate, varying by ± 10% of the actual value measured at birth. Because delivery was very close to the time of the research ultrasound, anywhere we refer to fetal weight we are using birth weight as the closest approximation of fetal weight at the time of the research ultrasound.
) × volumetric blood flow (l min−1). Fetal O2 consumption was calculated as (umbilical venous O2 content – umbilical artery O2 content) × blood flow. Oxygen consumption is expressed as absolute O2 consumption (ml min−1) or normalized for birth weight (ml min−1 kg−1) or for uterine contents (ml (kg fetus and placenta)−1 min−1). Fetal fractional O2 extraction, the percentage of oxygen taken up by the fetus between the umbilical vein and artery, was calculated as (umbilical venous O2 content – umbilical artery O2 content)/(umbilical vein O2 content) × 100. Near term fetal weight estimates by ultrasound are relatively inaccurate, varying by ± 10% of the actual value measured at birth. Because delivery was very close to the time of the research ultrasound, anywhere we refer to fetal weight we are using birth weight as the closest approximation of fetal weight at the time of the research ultrasound.
Statistics
All continuous data presented in this report were normally distributed as indicated by the Kolmogarov–Smirnoff test with the exception of fetal erythropoietin concentrations, which were analysed by the Dunn multiple comparisons test. Data were initially analysed using SAS (version 8.0). ANOVA and regression models were used for analyses of birth weight and body size in the infants. Only significant terms were included in the final regression models used to adjust neonatal anthropometrics. Birth weight was thus adjusted for maternal age and parity, infant sex and gestational age. Birth length, abdominal and head circumferences and the ponderal index were adjusted for differences in gestational age and infant sex. A two-way ANOVA with altitude and ancestry as the independent variables was used for the remaining variables and for comparison of the adjusted data on infant size and weight. Interaction between altitude and ancestry was further investigated using Sheffe's test when the interaction term carried a P-value of < 0.05. Chi square tests were used to compare the infant sex ratio between groups. Correlation analyses were used to evaluate relationships between independent and dependent variables (Prism, GraphPad Software Inc., San Diego, CA, USA). The data in figures and tables are presented as means ±s.e.m. unless otherwise indicated and are reported as significant where P < 0.05. P-values ≥ 0.05 and ≤ 0.10 are reported in tables and figures as trends.
For Tables 1–3 if an effect of altitude is noted as significant, it is significant in both ancestry groups. Likewise if an effect of ancestry is noted it is significant for both altitudes. Significant interactions between altitude and ancestry indicate that the response of one ancestry group to altitude differs from the other. In Tables 1–3 the values are given for the entire sample size. Comparison of data from all subjects versus those in whom validated cord blood gases were obtained did not differ in any parameter listed with the exception of gestational age. The research ultrasound and delivery occurred 4 days earlier in European than Andean women in the subset with validated blood gases (P < 0.05). However, our initial analyses showed that gestational age in this narrow range did not influence any of the dependent variables.
Table 1.
Maternal and infant characteristics
| 400 m European n= 44 | 3600 m European n= 37 | 400 m Andean n= 44 | 3600 m Andean n= 43 | P-values | |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Gestational age at ultrasound (weeks) | 37.7 ± 03 | 37.8 ± 0.2 | 38.3 ± 0.2 | 38.0 ± 0.2 | NS |
| Age (years) | 27 ± 1 | 30 ± 1 | 29 ± 1 | 33 ± 1 | P < 0.0001 altitude |
| P < 0.01 ancestry | |||||
| Parity (#) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.2 | P < 0.01 ancestry |
| Height (cm) | 161 ± 1 | 162 ± 1 | 156 ± 1 | 152 ± 1 | P < 0.0001 ancestry |
| Non-pregnant weight (kg) | 62 ± 2 | 61 ± 1 | 59 ± 2 | 58 ± 2 | NS |
| Non-pregnant Body Mass Index (kg m−2) | 24 ± 1 | 24 ± 1 | 24 ± 1 | 25 ± 1 | NS |
| Weight gain with pregnancy (kg) | 11 ± 1 | 13 ± 2 | 12 ± 1 | 14 ± 1 | P < 0.05 altitude |
| Infant characteristics | |||||
| Birth weight (grams (unadjusted values) | 3420 ± 65 | 2993 ± 56 | 3526 ± 44 | 3316 ± 59 | P < 0.0001 altitude |
| P < 0.0001 ancestry | |||||
| P= 0.10 interaction | |||||
| Birth weight (grams, adjusted values) | 3414 ± 33 | 2978 ± 37 | 3567 ± 24 | 3367 ± 36 | P < 0.0001 altitude |
| P < 0.0001 ancestry | |||||
| P < 0.005 interaction | |||||
| Fetal heart rate (BPM) | 140 ± 2 | 142 ± 1 | 143 ± 1 | 142 ± 2 | NS |
| Placental weight (g) | 477 ± 16 | 478 ± 20 | 463 ± 14 | 497 ± 17 | NS |
| Birth/placental weight ratio | 7.4 ± 0.2 | 6.5 ± 0.2 | 7.8 ± 0.2 | 6.9 ± 0.2 | P < 0.0001 altitude |
| P= 0.06 ancestry | |||||
| Ponderal index | 2.69 ± 0.03 | 2.64 ± 0.04 | 2.68 ± 0.04 | 2.70 ± 0.05 | P= 0.08 ancestry |
| Birth length (cm) | 50.1 ± 0.1 | 48.3 ± 0.1 | 50.9 ± 0.2 | 48.8 ± 0.1 | P < 0.0001 altitude |
| P < 0.0001 ancestry | |||||
| Abdominal circumference (cm) | 34.0 ± 0.1 | 32.9 ± 0.1 | 34.5 ± 0.1 | 34.6 ± 0.1 | P < 0.0001 ancestry |
| P < 0.001 interaction | |||||
| Head circumference (cm) | 34.7 ± 0.1 | 34.2 ± 0.1 | 34.9 ± 0.1 | 34.6 ± 0.1 | P < 0.01 altitude |
| P < 0.001 ancestry | |||||
| Clinically assessed gestational age at birth (weeks) | 38.5 ± 0.2 | 38.2 ± 0.2 | 38.8 ± 0.1 | 38.6 ± 0.2 | P= 0.08 ancestry |
| Days (from LMP) | 271 ± 1 | 270 ± 1 | 273 ± 1 | 272 ± 1 | P= 0.09 ancestry |
| Sex ratio M/F | 22/22 | 26/11 | 21/23 | 22/21 | NS |
Birth weights (BW) were adjusted for maternal age (MA) and parity (P) and for the infant's gestational age (GA, days from LMP) and sex (S, 0 male, 1 female), as follows: 400 m European BW =–4366 – 0.7(MA) + 89.3(P) + 29.5(GA) – 97(S); 3600 m European BW =–3559 + 8.3(MA) – 23.2(P) + 24.1(GA) – 251(S); 400 m Andean BW =–1552 – 0.9(MA) + 1.7(P) + 18.9(GA) – 51.0(S); 3600 m Andean BW =–2817 – 0.1(MA) + 90.1(P) + 22.3(GA) – 10 (S)).
Table 3.
Fetal vascular resistance indices and flow velocities
| 400 m European n= 44 | 3600 m European n= 37 | 400 m Andean n= 44 | 3600 m Andean n= 43 | P-values | |
|---|---|---|---|---|---|
| Umb. A PI (placental insertion) | 0.83 ± 0.02 | 0.92 ± 0.04 | 0.84 ± 0.02 | 0.88 ± 0.04 | P < 0.05 altitude |
| Umb. A PSV (cm s−1 placental insertion) | 46 ± 2 | 41 ± 3 | 52 ± 3 | 39 ± 2 | P < 0.0001 altitude |
| Umb. A EDV (cm s−1 placental insertion) | 20 ± 1 | 17 ± 1 | 23 ± 1 | 16 ± 1 | P < 0.0001 altitude |
| Umb. A PI (fetal insertion) | 0.84 ± 0.03 | 0.96 ± 0.04 | 0.84 ± 0.02 | 0.90 ± 0.04 | P < 0.01 altitude |
| Umb. A PSV (cm s−1, fetal insertion) | 53 ± 2 | 45 ± 2 | 54 ± 2 | 49 ± 2 | P < 0.005 altitude |
| Umb. A EDV (cm s−1, fetal insertion) | 23 ± 1 | 18 ± 1 | 23 ± 1 | 20 ± 1 | P < 0.0001 altitude |
| MCA PI | 1.49 ± 0.06 | 1.50 ± 0.06 | 1.48 ± 0.06 | 1.53 ± 0.08 | NS |
| MCA PSV (cm s−1) | 50 ± 2 | 54 ± 2 | 52 ± 2 | 60 ± 2 | P < 0.01 altitude |
| MCA EDV (cm s−1) | 12 ± 1 | 13 ± 1 | 13 ± 1 | 15 ± 1 | NS |
Abbreviations: Umb. A-Umbilical Artery, PI-Pulsatility Index, PSV-Peak Systolic Velocity, EDV-End Diastolic Velocity, MCA-Middle Cerebral Artery.
Results
Ancestry
The AIM analysis showed that in the 142 studies in which all data were obtained Andean infants were 78 ± 1% Native American and 15 ± 2% European. The European sample was of 60 ± 2% European and 34 ± 2% Native American (non-Andean) ancestry. Low levels of sub-Saharan African (2–6%) and/or East Asian admixture (3–5%) were detected in all four groups. There was no difference between altitudes in the measures of geographical ancestry, i.e. Andean women at low versus high altitude did not differ in their proportional admixture, nor did women of European ancestry between the low and high altitude sites.
Altitude effects
Altitude reduced birth weight (Table 1) whether considered as raw values or adjusted values. Women delivering at high altitude were older, and weight gain with pregnancy was greater (Table 1).
Fetal haemoglobin concentration, haematocrit and erythropoietin levels increased in response to altitude whilst fetal transferrin concentrations were similar (Table 2). At 3600 m  and bicarbonate levels were lower in both the umbilical vein and the umbilical artery and base excess was increased in the umbilical vein. Despite lower
and bicarbonate levels were lower in both the umbilical vein and the umbilical artery and base excess was increased in the umbilical vein. Despite lower  (−3.0 mmHg, P < 0.005), umbilical venous O2 content was increased at 3600 m (+24 ml l−1, P < 0.0001), due to the higher fetal haemoglobin concentrations (Table 2).
(−3.0 mmHg, P < 0.005), umbilical venous O2 content was increased at 3600 m (+24 ml l−1, P < 0.0001), due to the higher fetal haemoglobin concentrations (Table 2).
Umbilical arterial resistance to blood flow was increased in the high altitude pregnancies at both the placental and the abdominal junctures (Table 3). The middle cerebral artery pulsatility index was similar at 400 and 3600 m. Peak systolic and end diastolic blood flow velocities were diminished in the umbilical artery at high altitude, as was the peak systolic velocity of the middle cerebral artery (Table 3).
The diameter of the umbilical vein was reduced at high altitude (Fig. 1A), with no significant difference in mean flow velocities (Fig. 1B). As a result volumetric blood flow to the fetus was lower at high altitude (Fig. 1C). When normalized to fetal weight, blood flow was still decreased at high altitude (95 ± 4 ml min−1 kg−1 at 400 m versus 74 ± 3 ml min−1 kg−1 at 3600 m in Europeans; 107 ± 4 ml min−1 kg−1 at 400 m versus 82 ± 3 ml min−1 kg−1 at 3600 m in Andeans (P < 0.0001). The altitude-associated reduction was similar in both ancestry groups (−22% European, −23% Andean). Including placental weight as part of the uterine contents perfused by the fetal circulation did not change the altitude-associated reduction in blood flow because placental weights were similar in all four groups (Table 1).
Figure 1. Umbilical vein diameter, flow velocity and volumetric blood flows in low- and high-altitude fetuses of European versus Andean ancestry.
A, the diameter of the umbilical vein was smaller in both ancestry groups at high altitude (*P < 0.001). The magnitude of the reduction was similar between ancestry groups (–12% in Europeans, –13% in Andean, P= 0.87). Umbilical venous diameter was smaller in women of European ancestry, regardless of altitude (†P < 0.0001). B, there were no differences in the mean flow velocity of blood travelling through the umbilical vein within each altitude. However, there was a trend towards reduced mean flow velocity at high altitude among the European women only (P= 0.06). C, altitude decreased volumetric blood flow (*P < 0.0001) to a similar degree in both ancestry groups (–32% European, –27% Andean, P= 0.61). Volumetric blood flow through the umbilical vein was lower in European than in Andean women regardless of altitude (†P < 0.0001).
The fetal umbilical venous  /
/ curve was left-shifted at 3600 m, such that the blood of high-altitude fetuses was 50% saturated with oxygen at a
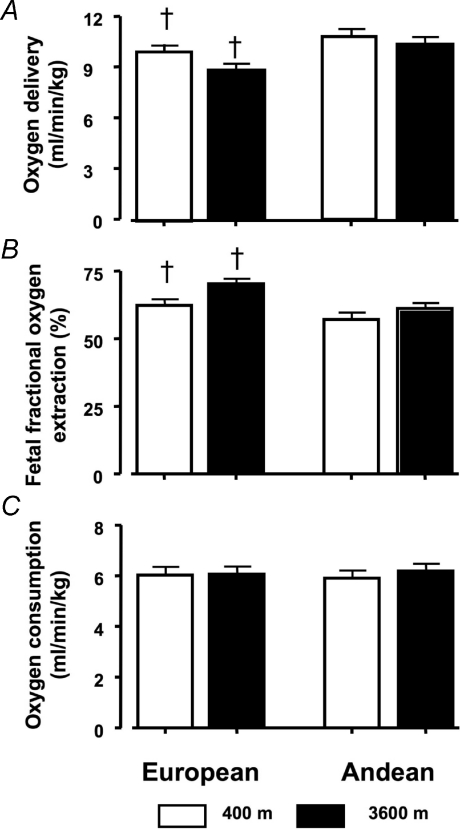
curve was left-shifted at 3600 m, such that the blood of high-altitude fetuses was 50% saturated with oxygen at a  ∼3–5 mmHg lower than their low-altitude counterparts (Fig. 2A). As a result of lower flow, umbilical venous oxygen delivery was lower at 3600 m (European 35 ± 3 versus 27 ± 2 ml min−1; Andean 45 ± 4 versus 39 ± 3 ml min−1 at low and high altitude, respectively, P < 0.01). This is true, however, only when the data are considered as absolute values. When normalized to fetal weight (ml min−1 kg−1), fetal oxygen delivery was the same at low versus high altitude within each ancestry group (9.6 ± 0.5 at 400 m and 9.0 ± 0.5 at 3600 m, Europeans (P= 0.88; 10.7 ± 0.5 at 400 m and 10.3 ± 0.5 at 3600 m in Andeans P= 0.97). High-altitude fetuses extracted 13% (European) and 7% (Andean) more of the oxygen delivered by the umbilical vein (Fig. 3B), but these differences were not significant. Fetal oxygen consumption per kg was the same in all four groups (Fig. 3C).
∼3–5 mmHg lower than their low-altitude counterparts (Fig. 2A). As a result of lower flow, umbilical venous oxygen delivery was lower at 3600 m (European 35 ± 3 versus 27 ± 2 ml min−1; Andean 45 ± 4 versus 39 ± 3 ml min−1 at low and high altitude, respectively, P < 0.01). This is true, however, only when the data are considered as absolute values. When normalized to fetal weight (ml min−1 kg−1), fetal oxygen delivery was the same at low versus high altitude within each ancestry group (9.6 ± 0.5 at 400 m and 9.0 ± 0.5 at 3600 m, Europeans (P= 0.88; 10.7 ± 0.5 at 400 m and 10.3 ± 0.5 at 3600 m in Andeans P= 0.97). High-altitude fetuses extracted 13% (European) and 7% (Andean) more of the oxygen delivered by the umbilical vein (Fig. 3B), but these differences were not significant. Fetal oxygen consumption per kg was the same in all four groups (Fig. 3C).
Figure 2. Fetal blood gas relationships.
A, the correlation between the umbilical venous  and
and  determined by the blood gas analyses was plotted for each of the four groups using a third order polynomial (best fit regression). The low altitude curves overlap for Europeans (□) and Andeans (○). High altitude left-shifts the
determined by the blood gas analyses was plotted for each of the four groups using a third order polynomial (best fit regression). The low altitude curves overlap for Europeans (□) and Andeans (○). High altitude left-shifts the  /
/ curve (P < 0.05), with Andean fetuses (•, continuous line) showing a progressively greater leftward shift with increasing
curve (P < 0.05), with Andean fetuses (•, continuous line) showing a progressively greater leftward shift with increasing  when compared with the Europeans (▪, dashed–dotted line). This results in differences at which the blood is 50% saturated (indicated by the line at 50% on the y axis). The P50 for
when compared with the Europeans (▪, dashed–dotted line). This results in differences at which the blood is 50% saturated (indicated by the line at 50% on the y axis). The P50 for  is equivalent at 400 m (28.4 versus 28.5 for European (dotted line) and Andean (dashed line), respectively). In contrast the blood is half-saturated with oxygen at 25.8 mmHg at 3600 m in Europeans, and 23.4 mmHg at 3600 m in Andean, P < 0.05). The Andean fetuses at 3600 m thus maintain saturation at higher levels for a given
is equivalent at 400 m (28.4 versus 28.5 for European (dotted line) and Andean (dashed line), respectively). In contrast the blood is half-saturated with oxygen at 25.8 mmHg at 3600 m in Europeans, and 23.4 mmHg at 3600 m in Andean, P < 0.05). The Andean fetuses at 3600 m thus maintain saturation at higher levels for a given  when compared with Europeans. (400 m European y=–92.8 + 8.64x– (–0.16x2) + 0.001x3, r2= 0.94, P < 0.0001; 400 m Andean y=–16.4 + 1.09x+ 0.81x2– 0.001x3, r2= 0.97, P < 0.0001; 3600 m European y=–122.4 + 13.33x–0.32x2+ 0.003x3, r2= 0.90, P < 0.0001; 3600 m Andean y=–7.0 – 0.98x+ 0.24x2– 0.004x3, r2= 0.97, P < 0.0001.) B, the maternal curves relating
when compared with Europeans. (400 m European y=–92.8 + 8.64x– (–0.16x2) + 0.001x3, r2= 0.94, P < 0.0001; 400 m Andean y=–16.4 + 1.09x+ 0.81x2– 0.001x3, r2= 0.97, P < 0.0001; 3600 m European y=–122.4 + 13.33x–0.32x2+ 0.003x3, r2= 0.90, P < 0.0001; 3600 m Andean y=–7.0 – 0.98x+ 0.24x2– 0.004x3, r2= 0.97, P < 0.0001.) B, the maternal curves relating  to
to  are also left-shifted at high altitude, but without any apparent difference due to ethnicity at low or high altitude. The line symbols are the same as in A. (400 m European y=–9.6 + 2.94x– 0.81x2– 0.001x3, r2= 0.97, P < 0.0001; 3600 m European y=–122.4 + 13.13x– 0.32x2+ 0.003x3, r2= 0.91, P < 0.0001; 400 m Andean y=–15.7 + 1.02x+ 0.08x2– 0.001x3, r2= 0.97, P < 0.0001; 3600 m Andean y=–1.9 – 1.59x+ 0.27x2– 0.005x3, r2= 0.98, P < 0.0001.) C shows the correlation between maternal
are also left-shifted at high altitude, but without any apparent difference due to ethnicity at low or high altitude. The line symbols are the same as in A. (400 m European y=–9.6 + 2.94x– 0.81x2– 0.001x3, r2= 0.97, P < 0.0001; 3600 m European y=–122.4 + 13.13x– 0.32x2+ 0.003x3, r2= 0.91, P < 0.0001; 400 m Andean y=–15.7 + 1.02x+ 0.08x2– 0.001x3, r2= 0.97, P < 0.0001; 3600 m Andean y=–1.9 – 1.59x+ 0.27x2– 0.005x3, r2= 0.98, P < 0.0001.) C shows the correlation between maternal  and fetal
and fetal  at the two altitude combined. For any given value of maternal
at the two altitude combined. For any given value of maternal  , the Andean fetus has a lower
, the Andean fetus has a lower  than the European fetus. (y= 34.1 + 0.33x, r2= 0.06, P < 0.05 European; y= 30.4 + 0.46x, r2= 0.12, P < 0.005.)
than the European fetus. (y= 34.1 + 0.33x, r2= 0.06, P < 0.05 European; y= 30.4 + 0.46x, r2= 0.12, P < 0.005.)
Figure 3. Oxygen delivery, fetal fractional O2 extraction and consumption.
A, umbilical oxygen delivery did not differ between altitudes within each ancestry group (P= 0.77 European, P= 0.99 Andean), but was lower in European than Andean women regardless of altitude (P < 0.001). B, fetal oxygen extraction was similar at low and high altitude in Andean pregnancies (P= 0.96) and in European pregnancies (P= 0.13). However, probably because European women have lower oxygen delivery to begin with, percentage fetal O2 extraction from the umbilical vein to the umbilical artery was greater in European than in Andean women, regardless of altitude (†P < 0.01). The range for fetal O2 extraction was 35–93% at low altitude and 20–93% at high altitude. C, oxygen consumption per kg fetal weight was similar in all four groups.
In summary, altitude increased resistance to umbilical arterial blood flow, and decreased umbilical venous blood flow, absolute oxygen delivery and fetal growth. Fetal haemotological adjustments compensated for lower blood flow, such that when normalized to fetal weight or uterine contents, O2 delivery and consumption were preserved at high altitude.
Ancestry effects
European women were taller, younger in age and of lower parity than Andean women (Table 1), but similar in prepregnant body mass index and in weight gain with pregnancy. Birth weight was lower in European neonates, regardless of altitude, whether considered as the raw values or adjusted for differences in maternal age, parity, infant sex and gestational age (Table 1). European infants were smaller than Andean infants, having smaller adjusted birth length, abdominal circumference and head circumference than their Andean counterparts. Based on the ponderal index, there was a trend towards greater body mass among Andean infants (Table 1).
There was a trend towards higher haemoglobin concentrations in European than Andean neonates (Table 2). Umbilical venous and arterial carbon dioxide tensions were greater and venous base excess lower in European than Andean neonates (Table 2).  of the umbilical arterial blood tended to be lower in the European infants across both altitudes (Table 2).
of the umbilical arterial blood tended to be lower in the European infants across both altitudes (Table 2).
Umbilical vein diameter was smaller in European than Andean fetuses, regardless of altitude (Fig. 1A, P < 0.0001), while mean flow velocity was similar (Fig. 1B). Therefore umbilical blood flow was lower in European women (Fig. 1C, P < 0.0001) at both 400 m and 3600 m. This was also true when normalized to fetal weight (European 95 ± 4 ml min−1 kg−1versus Andean 107 ± 4 ml min−1 kg−1 at 400 and European 74 ± 3 versus Andean 82 ± 3 ml min−1 kg−1 at 3600 m P < 0.0001). Absolute fetal O2 delivery was also lower in the European versus Andean pregnancies, regardless of altitude (−15%, 33 ± 2 versus 38 ± 2 ml min−1 at 400 m; −26%, 27 ± 2 versus 34 ± 2 at 3600 m, P < 0.005 Fig. 3A), but not when normalized to fetal weight (9.6 versus 10.7 ml O2 min−1 kg−1 in Europeans versus Andeans at 400 m, P= 0.54, and 9.0 versus 10.5 ml O2 min−1 kg−1 in Europeans versus Andeans at 3600 m P= 0. 30).
In summary European ancestry is associated with smaller babies and reduced umbilical venous blood flow, but not oxygen delivery, at both low and high altitude. The altitude-associated decrement in blood flow and oxygen delivery is similar between ancestry groups.
Interaction between ethnicity and altitude
The altitude-associated reduction in fetal growth is greater in European than Andean pregnancies (−417 g versus−228 g, P < 0.005 Table 1). Andean infants preserve low altitude values for abdominal circumference, head circumference and ponderal index, while the European neonates showed a reduction in these parameters.
Of the haematological parameters presented in Table 2, interaction was present in the erythropoietin concentrations in the fetal cord blood, which increased to a greater extent in Europeans at 3600 m than in Andeans. The O2 content of the umbilical artery was decreased in Europeans at high altitude, consistent with a trend towards lower 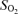 and with the greater fetal venous to arterial oxygen extraction observed in European pregnancies (Fig. 3B).
and with the greater fetal venous to arterial oxygen extraction observed in European pregnancies (Fig. 3B).
Greater umbilical venous O2 delivery among Andeans at 3600 m was due not only to preservation of mean flow velocity (Fig. 1B) but possibly also to a greater leftward shift in the fetal  /
/ curve (Fig. 2A). This permitted Andean versus European fetuses to maintain a higher
curve (Fig. 2A). This permitted Andean versus European fetuses to maintain a higher  for a given
for a given  at 3600 m (P50 (the oxygen tension at which the fetal blood is half-saturated with oxygen) = 23.8 versus 25.7, P < 0.01).
at 3600 m (P50 (the oxygen tension at which the fetal blood is half-saturated with oxygen) = 23.8 versus 25.7, P < 0.01).
Maternal contributions to fetal oxygenation
We examined the extent to which our previously published data on maternal oxygen transport might have correlated with those present in the fetus. There was no relationship between maternal  , pH, or uterine blood flow and the same parameters in the umbilical vein. Only in relationship to the entire sample did maternal uterine oxygen delivery correlate with fetal oxygen delivery (y= 26.4 + 0.08x, r2= 0.07, P < 0.005), but this explained only 7% of the variation in fetal oxygen delivery.
, pH, or uterine blood flow and the same parameters in the umbilical vein. Only in relationship to the entire sample did maternal uterine oxygen delivery correlate with fetal oxygen delivery (y= 26.4 + 0.08x, r2= 0.07, P < 0.005), but this explained only 7% of the variation in fetal oxygen delivery.  is lower in Andean than European women at high altitude (Table 2), and we therefore tested whether the maternal
is lower in Andean than European women at high altitude (Table 2), and we therefore tested whether the maternal  /
/ curve was left-shifted at high altitude and might contribute to the altered fetal curves (Fig. 2B). The maternal curve was left-shifted at high versus low altitude, but did not differ in the Andean versus European women. Across the entire sample maternal and fetal
curve was left-shifted at high altitude and might contribute to the altered fetal curves (Fig. 2B). The maternal curve was left-shifted at high versus low altitude, but did not differ in the Andean versus European women. Across the entire sample maternal and fetal  were positively associated (r2= 0.13, P < 0.0001), but the correlation was greater in Andean (r2= 0.17, P < 0.0001) than European women (r2= 0.08, P < 0.05), and anchored at a lower intercept such that for a given maternal
were positively associated (r2= 0.13, P < 0.0001), but the correlation was greater in Andean (r2= 0.17, P < 0.0001) than European women (r2= 0.08, P < 0.05), and anchored at a lower intercept such that for a given maternal  , fetal
, fetal  is ∼3 mmHg lower in the Andean than European fetus (y= 33.2 + 0.343x European, y= 27.9 + 0.431x Andean) (Fig. 2C).
is ∼3 mmHg lower in the Andean than European fetus (y= 33.2 + 0.343x European, y= 27.9 + 0.431x Andean) (Fig. 2C).
Factors influencing fetal oxygen delivery and consumption
The altitude and ancestry associated differences in umbilical blood flow were largely mediated by diameter, even though in a low resistance vessel such as the umbilical vein it is possible that diameter adjusts to accommodate flow velocity rather than the reverse (Fig. 4A). We found a strong negative correlation between blood flow velocity and diameter, regardless of altitude or ancestry and with a similar slope present in all four groups (Fig. 4A and centre left inset). Haemoglobin and haematocrit were not related to umbilical vein diameter, nor were any of the blood gases.
Figure 4. Correlations among fetal variables.
A, the mean flow velocity of the blood travelling through the umbilical vein is inversely related to the diameter of the vein velocity (r2= 0.45, P < 0.0001 all subjects). (400 m Europeans (dotted line) y= 18.6 – 9.69x, r2= 0.26, P < 0.0005; 3600 m Europeans (dashed–dotted line) y= 18.4 – 12.91x, r2= 0.30, P < 0.0005; 400 m Andean (dashed line) y= 18.41 – 9.58x, r2= 0.28, P < 0.0002; 3600 m Andean (continuous line) y= 18.26 – 10.96x, r2= 0.22, P < 0.005.) The slope of the relationship is similar between altitudes (centre inset left, P= 0.40) and did not differ between ancestry groups (P= 0.56). B, fetal fractional O2 extraction is increased when umbilical venous O2 delivery is reduced (r2= 0.35, P < 0.0001, all subjects). Fetuses receiving less O2 through the umbilical vein increase their umbilical venous to arterial oxygen extraction. (400 m Europeans (dotted line) y= 83.3–0.62x, r2= 0.21, P < 0.005; 3600 m Europeans (dashed–dotted line) y= 93.22 – 0.84x, r2= 0.33, P < 0.005; 400 m Andean (dashed line) y= 78.31 – 0.50x, r2= 0.20, P < 0.01; 3600 m Andean (continuous line) y= 80.0 – 0.62x, r2= 0.28, P < 0.0005). The slope of the relationship is similar between altitudes (centre inset right, P= 0.81) and did not differ between ancestry groups (P= 0.61).
Umbilical venous oxygen delivery was negatively correlated with fetal oxygen extraction. Fetuses receiving less oxygen through the umbilical vein extracted a greater proportion of the oxygen from the venous to arterial circulation (Fig. 4B, centre right inset). In turn, those who extracted more oxygen had lower umbilical artery  (Fig. 5B, centre inset right) and
(Fig. 5B, centre inset right) and  (r2= 0.74), with no differences in slope between the groups (P < 0.0001, data not shown). Fetuses receiving more oxygen consumed more relative to their weight (Fig. 5A, centre inset left). These data highlight the adaptive range of fetuses in compensating for variation in oxygen delivery to preserve normal consumption (Fig. 3C).
(r2= 0.74), with no differences in slope between the groups (P < 0.0001, data not shown). Fetuses receiving more oxygen consumed more relative to their weight (Fig. 5A, centre inset left). These data highlight the adaptive range of fetuses in compensating for variation in oxygen delivery to preserve normal consumption (Fig. 3C).
Figure 5. Fetal adaptive range. Geometric and line symbols are the same as in prior figures.
A, fetal oxygen consumption per kg fetal weight increases as oxygen delivery is increased (r2= 0.50, P < 0.0001, all subjects). Fetuses receiving more O2 through the umbilical vein have greater O2 consumption relative to their weight (400 m Europeans y= 6.3 + 0.41x, r2= 0.491, P < 0.0001; 3600 m Europeans y= 6.1 + 0.46x, r2= 0.45, P < 0.0001; 400 m Andean y= 6.2 + 0.42x, r2= 0.56, P < 0.0001; 3600 m Andean y= 7.9 + 0.33x, r2= 0.35, P < 0.0001.) The slope of the relationship is similar between altitudes (centre inset left, P= 0.59) and did not differ between ancestry groups (P= 0.50). B, fetal oxygen extraction is correlated with umbilical arterial  , such that those fetuses with the greatest O2 extraction have the lowest arterial
, such that those fetuses with the greatest O2 extraction have the lowest arterial  (r2= 0.64, P < 0.0001 all subjects). (400 m Europeans y= 34.6 – 0.29x, r2= 0.71, P < 0.0001; 3600 m Europeans y= 42.3 – 0.38x, r2= 0.69, P < 0.0001; 400 m Andean y= 29.6 + 0.20x, r2= 0.31, P < 0.0005; 3600 m Andean y= 32.0 – 0.26x, r2= 0.75, P < 0.0001.) The slope of the relationship is virtually identical between altitudes (centre inset right, P= 0.98), and did not differ between ancestry groups (P= 0.26).
(r2= 0.64, P < 0.0001 all subjects). (400 m Europeans y= 34.6 – 0.29x, r2= 0.71, P < 0.0001; 3600 m Europeans y= 42.3 – 0.38x, r2= 0.69, P < 0.0001; 400 m Andean y= 29.6 + 0.20x, r2= 0.31, P < 0.0005; 3600 m Andean y= 32.0 – 0.26x, r2= 0.75, P < 0.0001.) The slope of the relationship is virtually identical between altitudes (centre inset right, P= 0.98), and did not differ between ancestry groups (P= 0.26).
Finally, we asked whether blood flow or oxygen delivery is related to fetal size. Umbilical venous blood flow is positively associated with birth weight across all four groups (r2= 0.17, P < 0.0001), but this is largely due to the greater correlation between blood flow and birth weight at high altitude (Fig. 6A and inset, r2= 0.16, P < 0.001). When we examined O2 delivery, a similar relationship emerged, but accounted for only 12% of the variation in birth weight across all four groups (Fig. 5B, r2= 0.12, P < 0.005). Within each sample significance was not attained, and again, the relationship was stronger at high than low altitude (r2= 0.17, P < 0.001, Fig. 5B and inset). Birth weight thus seemed to be more dependent on blood flow and O2 delivery at high altitude than at low altitude.
Figure 6. Blood flow, oxygen delivery and birth weight.
Geometric and line symbols are the same as in prior figures. A, across the entire sample, variation in blood flow through the umbilical vein (the total fetal nutrient supply) accounted for 17% of the variation in birth weight (P < 0.0001), but did not attain significance within each of the 4 groups (400 m Europeans y= 3049 + 1.15x, r2= 0.06, P= 0.11; 3600 m Europeans y= 2624 + 1.68x, r2= 0.09, P= 0.08; 400 m Andean y= 3443 + 0.221x, r2= 0.00, P= 0.64; 3600 m Andean y= 2867 + 1.65x r2= 0.08, P= 0.08). However when considered by altitude alone, birth weight is more closely correlated with blood flow at high altitude (inset left, 3600 m y= 3576 + 2.32x, r2= 0.16, P < 0.001; 400 m y= 3201 + 0.78x, r2= 0.04, P= 0.05), while the slopes between ancestry groups did not differ (P= 0.11). Nonetheless, for any given level of blood flow the European babies weigh ∼230 g less than Andean infants at high altitude. B, across the entire sample, variation in umbilical oxygen delivery accounted for 12% of the variation in birth weight (P < 0.0001) but this is due largely to the high altitude subjects (inset, right). Among all high altitude subjects 17% of the variation in birth weight was accounted for by oxygen delivery (y= 2724 + 13.72x, r2= 0.17, P < 0.001), while at low altitude only 3% of the variation was explained by oxygen delivery (y= 3271 + 6.17x, P= 0.12). The slopes did not differ between ancestry groups (0.56). The data again indicate that for any given oxygen delivery, the European neonate at high altitude is smaller.
Discussion
Fetal oxygen delivery and consumption at 3600 m are preserved at sea level values, despite pronounced reduction in fetal blood flow. The hypothesis that reduced fetal oxygen delivery may contribute to diminished fetal growth at high altitude is not supported. The hypothesis that Andean fetuses would have greater blood flow and oxygen delivery, irrespective of altitude, is supported. For any given level of oxygen delivery, Andean fetuses were larger. The magnitude of the ancestry effect is +208 g. Oxygen extraction increases linearly across the entire sample depending on the amount of O2 delivered, highlighting the general ability of the fetus to compensate for variation in oxygen delivery. The similarity of the slope of this relationship in all four groups supports that the altitude and ancestry associated differences in birth weight are not due to differences in O2 availability. Oxygen delivery is in excess of fetal consumption. This excess combined with the similarity in oxygen delivery and consumption support that oxygen supply is sufficient to meet fetal needs. European infants were > 200 g lighter than Andean infants for any given level of blood flow or oxygen delivery at high altitude. In contrast, the relationship at low altitude was similar. These data argue against oxygen deficit as causally associated with diminished fetal growth at high altitude. Reduced fetal blood flow may be of relevance, as blood flow was diminished at 3600 m both absolutely and relative to fetal weight.
Study limitations
There are several limitations in this study including the potential effects of anaesthesia, the inherent variability in biological measures such as blood gases and blood flow and the inability to gather certain data to fully characterize the fetal  /
/ dissociation curve. All patients were delivered under regional blocks (spinal or epidural). Maternal hypotension or respiratory suppression secondary to anaesthesia may have altered fetal blood gases. However our APGAR scores, pH values and the prior literature argue against anaesthetic regimes as significantly influencing our results. There are no differences in fetal
dissociation curve. All patients were delivered under regional blocks (spinal or epidural). Maternal hypotension or respiratory suppression secondary to anaesthesia may have altered fetal blood gases. However our APGAR scores, pH values and the prior literature argue against anaesthetic regimes as significantly influencing our results. There are no differences in fetal  and
and  with spinal versus epidural anaesthesia (Roberts et al. 1995; Tonni et al. 2007). Breathing room air at caesarean delivery does increase the incidence of fetal acidaemia (reviewed by Backe & Lyons, 2002). However in clinically relevant measures such as the rate of depressed infants (APGAR < 7, pH > 7.0), asphyxiated infants (APGAR < 7, pH < 7.0) and those who require mechanical ventilation, regional blocks do not differ from each other (Tonni et al. 2007). None of our infants met the clinical criteria for asphyxiated or depressed. Umbilical arterial blood gas analysis is the most widely accepted standard for assessing fetal oxygenation and acid–base status. Our arterial blood gases and pH were well within the range reported for normally grown infants at sea level (Lackman et al. 2001).
with spinal versus epidural anaesthesia (Roberts et al. 1995; Tonni et al. 2007). Breathing room air at caesarean delivery does increase the incidence of fetal acidaemia (reviewed by Backe & Lyons, 2002). However in clinically relevant measures such as the rate of depressed infants (APGAR < 7, pH > 7.0), asphyxiated infants (APGAR < 7, pH < 7.0) and those who require mechanical ventilation, regional blocks do not differ from each other (Tonni et al. 2007). None of our infants met the clinical criteria for asphyxiated or depressed. Umbilical arterial blood gas analysis is the most widely accepted standard for assessing fetal oxygenation and acid–base status. Our arterial blood gases and pH were well within the range reported for normally grown infants at sea level (Lackman et al. 2001).
Measures of blood flow by Doppler and ultrasound are variable; the techniques used here required an hour or more per patient in order to minimize variation. Our reproducibility and variability measurements are similar to those reported by other investigators engaged in similar ultrasound techniques for research on fetal blood flow (see Methods). These data therefore represent the best estimates of variable biological parameters. It is reassuring that our values agree with recent data collected in a similar fashion and published in abstract form by Cetin and colleagues (Taricco et al. 2008) and with other studies of umbilical volumetric blood flow in the near term human fetus. Values ranging from 78 to 121 ml min−1 kg−1 are reported in normal third trimester pregnancies (Barbera et al. 1999; Ferrazzi et al. 2000; Rigano et al. 2001; Boito et al. 2002b; Di Naro et al. 2002). Our value of 90 ml min−1 kg−1 for the entire sample is between the 50th and 75th percentile for week 38 of pregnancy whilst the range of values we observed (35–171 ml min−1 kg−1) encompasses the 5th–97th percentiles reported in a large series of serially studied pregnancies at sea level (Acharya et al. 2005).
The fetal oxyhaemoglobin dissociation is left shifted, and approximates that of the adult at a pH of 7.6, which yields a P50 of ∼20 mmHg (Severinghaus, 1979), 6 mmHg lower than in the adult. The left shift facilitates the transfer of oxygen from the mother to the fetoplacental circulation. This is largely due to the presence of high levels of fetal haemoglobin (HbF), a high O2 affinity variant comprising 75–95% of fetal blood at birth (Richardson et al. 2004). Moreover, as the haemoglobin binds the greater levels of carbon dioxide present in the fetus, oxygen affinity is decreased favouring oxygen diffusion to the fetal tissues. In the intervillous circulation, the opposite effect, offloading of carbon dioxide from the fetal to the maternal circulation, promotes oxygen binding (the double Bohr effect), which can actually double the oxygen tension gradient (Metcalfe, 1963). We show in our raw data plotting  against
against  that this shift is greater in the umbilical vein of Andeans than Europeans at 3600 m, and greater in both groups than at low altitude. However, determination of the true oxygen dissociation curve would require titration to fully establish the sigmoidal response curve characteristic of oxygen dissociation in both fetal and adult circulations. The limited range of
that this shift is greater in the umbilical vein of Andeans than Europeans at 3600 m, and greater in both groups than at low altitude. However, determination of the true oxygen dissociation curve would require titration to fully establish the sigmoidal response curve characteristic of oxygen dissociation in both fetal and adult circulations. The limited range of  values in our fetal blood required the use of a three-parameter equation as a best fit, whilst in the adult a seven-parameter equation is used for the full curve (Kelman, 1966). This introduces error and limits the interpretation of the data. Previous studies have shown that a greater proportion of HbF is retained in high-altitude neonates (Ballew & Haas, 1986), which would be consistent with a greater left-shift, but in the absence of direct measurement, this remains speculative.
values in our fetal blood required the use of a three-parameter equation as a best fit, whilst in the adult a seven-parameter equation is used for the full curve (Kelman, 1966). This introduces error and limits the interpretation of the data. Previous studies have shown that a greater proportion of HbF is retained in high-altitude neonates (Ballew & Haas, 1986), which would be consistent with a greater left-shift, but in the absence of direct measurement, this remains speculative.
Blood flows and vascular resistance
The high altitude infants show asymmetric growth restriction, but no evidence for brain-sparing flow redistribution as is seen in the chronically hypoxic sheep model (Kamitomo et al. 1993). The middle cerebral artery pulsatility index was unaffected by altitude in both our study and the only other human study, at an even greater elevation of 4300 m (Krampl et al. 2001). On the other hand the higher umbilical artery resistance we observed at 3600 m is associated with reduced placental return in sea level growth restricted pregnancies (Kiserud et al. 2006). Greater umbilical artery resistance to flow may thus contribute to the diminished umbilical venous blood flow observed in sea level growth restriction (Boito et al. 2002a) and in these normal high altitude pregnancies. Our data are consistent with those obtained in humans at 4300 m: there is an elevated pulsatility index in the umbilical artery and lower peak systolic and end-diastolic flow velocities in multiple fetal vessels at 4300 m altitude (Krampl et al. 2001). These differences were attributed to elevated fetal haematocrit but this study and prior studies indicate that blood viscosity does not impact on the pulsatility index (Fairlie et al. 1991), nor does the umbilical or middle cerebral artery appear sensitive to acute changes in oxygenation (Simchen et al. 2005).
We speculate that these differences are due to fetal vasoconstriction, lower fetal blood volume and hence global reductions in blood flow. Fetal heart rate and blood pressure are primary regulators of umbilical blood flow (Itskovitz et al. 1983a), and could explain the differences we report between ancestry and altitude groups. However we found no differences in fetal heart rate between our four groups and pressure could not be measured. In chronic severe hypoxia there is a sustained elevation in fetoplacental vascular resistance and vasoconstrictor reactivity (Jakoubek et al. 2008) that elevates blood pressure (Alonso et al. 1989). This would be expected to diminish fetal blood volume and hence placental return, consistent with our findings in the high-altitude human fetus.
Oxygen delivery and consumption
Altitude impacted both ancestry groups equally, reducing blood flow, but not oxygen delivery or consumption when normalized for fetal mass or uterine contents. The mean difference in low- versus high-altitude fetal O2 delivery per kg fetal weight was 0.84 ml min−1 (kg fetal weight)−1 in Europeans and 0.096 in Andeans. Sample sizes > 350 for Europeans and well in excess of 5000 for Andeans would be needed for these differences to be significant. We are therefore confident in concluding that differences in fetal O2 delivery do not directly mediate the ancestry and altitude-related growth differences reported here.
We found that human fetuses increase their oxygen extraction when oxygen delivery is diminished, a phenomenon well-described in sheep (Bocking et al. 1992). We found a positive correlation between fetal oxygen delivery and consumption, replicating experimental animal studies (reviewed in Carter, 1989). Decreased oxygen consumption by the fetus occurs only after an ∼50% fall in blood flow (Itskovitz et al. 1983b). The difference between our study and the sheep and rat studies in which these data were derived is that we find that fetal growth is depressed while in the presence of sufficient oxygen and normal oxygen consumption. This contrasts with sheep in which fetal compensation for chronic high-altitude exposure can result in normally grown fetuses. Finally, our data are consistent with experimental animal studies showing that oxygen delivery is in excess of fetal demand (reviewed in Carter, 1989): near-term fetal O2 delivery was 34 ± 1 ml min−1, ∼5-fold greater than near-term fetal O2 consumption. Expressed relative to fetal weight, O2 delivery was 10.2 ± 0.3 ml min−1 (kg fetal weight)−1, 41% greater than consumption. The 90th percentile for fetal oxygen extraction was 78% at low altitude and 81% at high altitude; we therefore conclude that there is some reserve available to the majority of the fetuses, and that this reserve may be greater in Andean than European fetuses given their higher oxygen delivery and lower extraction.
Reduced fetal growth
The altered growth trajectory in high altitude fetuses appears at ∼23 weeks gestational age, just as the placenta finishes it's period of most rapid growth and the fetus begins its most rapid growth (Krampl et al. 2000). It is possible that the placenta, whose size is maintained or even increased at high altitude (Zamudio, 2003), consumes key resources to support its own growth at the expense of the fetus. It is also possible that placental function is modified by the chronically lower  ; we have shown that the term high-altitude placenta is responding to hypoxia with increased HIF-1α and HIF-dependent protein production (Zamudio et al. 2007b). If true, this human model of slowed fetal growth differs from experimental animal models of growth restriction. All, to our knowledge, are associated with reduced placental as well as fetal growth (Wallace et al. 2005), and at least some of the alterations in placental function, e.g. glucose transport, are due to smaller placental size (Wallace et al. 2003), and not to altered transporter densities as we have reported for the high altitude placenta (Zamudio et al. 2006).
; we have shown that the term high-altitude placenta is responding to hypoxia with increased HIF-1α and HIF-dependent protein production (Zamudio et al. 2007b). If true, this human model of slowed fetal growth differs from experimental animal models of growth restriction. All, to our knowledge, are associated with reduced placental as well as fetal growth (Wallace et al. 2005), and at least some of the alterations in placental function, e.g. glucose transport, are due to smaller placental size (Wallace et al. 2003), and not to altered transporter densities as we have reported for the high altitude placenta (Zamudio et al. 2006).
Normal near-term umbilical venous  at sea level ranges ∼24–30 mmHg with 2 standard deviations equivalent to ∼±12 mmHg (Haruta et al. 1986; Soothill et al. 1986, 1987; Pardi et al. 1987; Fujikura & Yoshida, 1996; Lackman et al. 2001). We found a modest, but significant, 3 mmHg reduction in umbilical venous
at sea level ranges ∼24–30 mmHg with 2 standard deviations equivalent to ∼±12 mmHg (Haruta et al. 1986; Soothill et al. 1986, 1987; Pardi et al. 1987; Fujikura & Yoshida, 1996; Lackman et al. 2001). We found a modest, but significant, 3 mmHg reduction in umbilical venous  at 3600 m, but the value is within 1 s.d. of the range of normal values reported in the largest study to date in human pregnancy (Lackman et al. 2001). This appears to be of physiological significance, as our erythropoietin data suggest the high altitude fetuses were sensing and responding to hypoxia, a response that was more marked in the European than Andean fetuses. Fetal hypoxia is also consistent with our prior data showing that placental hypoxia is present at high altitude (Soleymanlou et al. 2005; Zamudio et al. 2007b). It is possible that even very modest decrement in fetal
at 3600 m, but the value is within 1 s.d. of the range of normal values reported in the largest study to date in human pregnancy (Lackman et al. 2001). This appears to be of physiological significance, as our erythropoietin data suggest the high altitude fetuses were sensing and responding to hypoxia, a response that was more marked in the European than Andean fetuses. Fetal hypoxia is also consistent with our prior data showing that placental hypoxia is present at high altitude (Soleymanlou et al. 2005; Zamudio et al. 2007b). It is possible that even very modest decrement in fetal  , and not oxygen delivery or consumption, is a key issue in altitude-associated reduction in fetal growth. Umbilical venous
, and not oxygen delivery or consumption, is a key issue in altitude-associated reduction in fetal growth. Umbilical venous  differences of as little as 2–3 mmHg are associated with extremes of the growth spectrum at sea level (Lackman et al. 2001). A recent study of chick embryos found that 3600 m altitude decreased embryonic
differences of as little as 2–3 mmHg are associated with extremes of the growth spectrum at sea level (Lackman et al. 2001). A recent study of chick embryos found that 3600 m altitude decreased embryonic  by ∼30% and fetal growth by > 40%, and was entirely reversed with supplemental oxygen to raise
by ∼30% and fetal growth by > 40%, and was entirely reversed with supplemental oxygen to raise  (Giussani et al. 2007). In the reverse experiment high-altitude eggs incubated at low altitude had higher
(Giussani et al. 2007). In the reverse experiment high-altitude eggs incubated at low altitude had higher  and enhanced fetal growth, perhaps due to a thinner eggshell or chorioallantoic membrane that permitted increased diffusion, or perhaps, as the authors suggest, due to epigenetic modification of haematological responses. The latter is unlikely in humans, while the former is similar to what is observed in the human placenta. Altitude reduces the distance for diffusion across the syncytiotrophoblast, although there is not sufficient evidence to determine whether this varies by ancestry (Zamudio, 2003). Arguing against
and enhanced fetal growth, perhaps due to a thinner eggshell or chorioallantoic membrane that permitted increased diffusion, or perhaps, as the authors suggest, due to epigenetic modification of haematological responses. The latter is unlikely in humans, while the former is similar to what is observed in the human placenta. Altitude reduces the distance for diffusion across the syncytiotrophoblast, although there is not sufficient evidence to determine whether this varies by ancestry (Zamudio, 2003). Arguing against  as a factor directly moderating fetal growth is the lack of any association between
as a factor directly moderating fetal growth is the lack of any association between  and fetal weight in the present study. Moreover, the 10% altitude-associated reduction in fetal
and fetal weight in the present study. Moreover, the 10% altitude-associated reduction in fetal  is present in both Andean and European residents of high altitude and yet Europeans suffer nearly twice the altitude-associated decrement in birth weight.
is present in both Andean and European residents of high altitude and yet Europeans suffer nearly twice the altitude-associated decrement in birth weight.
Altitude effects were similar between ancestry groups. The most pronounced altitude-associated difference was decreased fetal blood flow, despite normal oxygen delivery. That Europeans have lower blood flows than Andeans, even at 400 m, and smaller babies, implies that flow rather than oxygen may be a more important determinant of the fetal growth trajectory in these normal healthy pregnancies. We suspect that flow-limited substrates, e.g. glucose and amino acids, may play an important role in the decreased fetal growth at altitude, as has been suggested in studies of intrauterine growth restriction at sea level (Cetin et al. 1988).
In summary, having Native American origins is associated with greater blood flow, oxygen delivery and fetal growth regardless of altitude. Fetal haemoglobin was increased at altitude, thereby increasing oxygen content. This, perhaps combined with a left-shifted  /
/ curve, and the ability of the fetus to extract more oxygen when delivery is lowered defends fetal oxygen supply and consumption, and hence specific genetic adaptations to high altitude among Andeans are unlikely for any of the parameters we report here. Placental growth is maintained, and this perhaps diverts resources from the fetus. We conclude that fetal oxygen delivery and consumption is preserved at high versus low altitude and does not appear to directly contribute to the reduction in fetal growth.
curve, and the ability of the fetus to extract more oxygen when delivery is lowered defends fetal oxygen supply and consumption, and hence specific genetic adaptations to high altitude among Andeans are unlikely for any of the parameters we report here. Placental growth is maintained, and this perhaps diverts resources from the fetus. We conclude that fetal oxygen delivery and consumption is preserved at high versus low altitude and does not appear to directly contribute to the reduction in fetal growth.
Acknowledgments
This work was funded by the National Science Foundation (USA) BCS 0309142 and the National Institutes of Health (USA) HD42737. The extraordinary generosity of the following physician–colleagues in Bolivia made this work possible: Dra Maria Luz Almendros Pasantino, Dr Gonzalo Azurduy, Dra Diva Bellido, Dr Roberto Bohrt, Dra Patricia Canido, Dr Juan Carlos Carazas, Dra Karina Chávez Vila, Dr Ciro Ciompi, Dra Edith Claros Mercado, Dra Yohelma Eid, Dr Alfredo Graña Aguirre, Dra Rosario Justiniano Velásquez, Dr Marcelo Koziner, Dr Jorge La Fuente Mendez, Dr Helmut Lema, Dr Fernando Meswith, Dr David Molina Mery, Dra Sandra O’Connor, Dra Rosario Palacios Parada, Dra Jesica Pardo Quiroga, Dr Juan Carlos Quinteros La Fuente, Dr Erwin Rendón Vaca, Dr Jose Luís Rivero Fleidler, Dra Patricia Suaréz Peña, Dr Rudy Soria, Dr Jaime Terán, Dra Lilian Toledo, Dr Marco Vargas, Dra Mercedes Villena, Dra Claudia Yepéz and Dra Elizabeth Zelada.
References
- Acharya G, Wilsgaard T, Berntsen GKR, Maltau JM, Kiserud T. Doppler-derived umbilical artery absolute velocities and their relationship to fetoplacental volume blood flow: a longitudinal study. Ultrasound Obstet Gynecol. 2005;25:444–453. doi: 10.1002/uog.1880. [DOI] [PubMed] [Google Scholar]
- Alonso JG, Okai T, Longo LD, Gilbert RD. Cardiac function during long-term hypoxemia in fetal sheep. Am J Physiol Heart Circ Physiol. 1989;257:H581–H589. doi: 10.1152/ajpheart.1989.257.2.H581. [DOI] [PubMed] [Google Scholar]
- Backe SK, Lyons G. Oxygen and elective caesarean section. Br J Anaesth. 2002;88:4–5. doi: 10.1093/bja/88.1.4. [DOI] [PubMed] [Google Scholar]
- Ballew C, Haas JD. Hematologic evidence of fetal hypoxia among newborn infants at high altitude in Bolivia. Am J Obstet Gynecol. 1986;155:166–169. doi: 10.1016/0002-9378(86)90104-3. [DOI] [PubMed] [Google Scholar]
- Barbera A, Galan HL, Ferrazzi E, Rigano S, Jozwik M, Battaglia FC, Pardi G. Relationship of umbilical vein blood flow to growth parameters in the human fetus. Am J Obstet Gynecol. 1999;181:174–179. doi: 10.1016/s0002-9378(99)70456-4. [DOI] [PubMed] [Google Scholar]
- Bocking AD, White SE, Homan J, Richardson BS. Oxygen consumption is maintained in fetal sheep during prolonged hypoxaemia. J Dev Physiol. 1992;17:169–174. [PubMed] [Google Scholar]
- Boito S, Struijk PC, Ursem NT, Stijnen T, Wladimiroff JW. Umbilical venous volume flow in the normally developing and growth-restricted human fetus. Ultrasound Obstet Gynecol. 2002a;19:344–349. doi: 10.1046/j.1469-0705.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- Boito S, Struijk PC, Ursem NTC, Stijnen T, Wladimiroff JW. Umbilical venous volume flow in the normally developing and growth-restricted human fetus. Ultrasound Obstet Gynecol. 2002b;19:344–349. doi: 10.1046/j.1469-0705.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- Bonilla C, Gutierrez G, Parra EJ, Kline C, Shriver MD. Admixture analysis of a rural population of the state of Guerrero, Mexico. Am J Phys Anthropol. 2005;128:861–869. doi: 10.1002/ajpa.20227. [DOI] [PubMed] [Google Scholar]
- Bonilla C, Parra EJ, Pfaff CL, Dios S, Marshall JA, Hamman RF, Ferrell RE, Hoggart CL, McKeigue PM, Shriver MD. Admixture in the Hispanics of the San Luis Valley, Colorado, and its implications for complex trait gene mapping. Ann Hum Genet. 2004;68:139–153. doi: 10.1046/j.1529-8817.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- Carter AM. Factors affecting gas transfer across the placenta and the oxygen supply to the fetus. J Devel Physiol. 1989;12:305–322. [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol. 1988;158:120–126. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- Di Naro E, Raio L, Ghezzi F, Franchi M, Romano F, Addario VD. Longitudinal umbilical vein blood flow changes in normal and growth-retarded fetuses. Acta Obstet Gynecol Scand. 2002;81:527–533. [PubMed] [Google Scholar]
- Fairlie FM, Lang GD, Lowe GG, Walker JJ. Umbilical artery flow velocity waveforms and cord blood viscosity. Am J Perinatol. 1991;8:239–243. doi: 10.1055/s-2007-999387. [DOI] [PubMed] [Google Scholar]
- Ferrazzi E, Rigano S, Bozzo M, Bellotti M, Giovannini N, Galan H, Battaglia FC. Umbilical vein blood flow in growth-restricted fetuses. Ultrasound Obstet Gynecol. 2000;16:432–438. doi: 10.1046/j.1469-0705.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- Fujikura T, Yoshida J. Blood gas analysis of placental and uterine blood during cesarean delivery. Obstet Gynecol. 1996;87:133–136. doi: 10.1016/0029-7844(95)00300-2. [DOI] [PubMed] [Google Scholar]
- Galan HL, Ferrazzi E, Hobbins JC. Intrauterine growth restriction (IUGR): biometric and Doppler assessment. Prenat Diagn. 2002;22:331–337. doi: 10.1002/pd.311. [DOI] [PubMed] [Google Scholar]
- Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985;92:31–38. doi: 10.1111/j.1471-0528.1985.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res. 2001;49:490–494. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Salinas CE, Villena M, Blanco CE. The role of oxygen in prenatal growth: studies in the chick embryo. J Physiol. 2007;585:911–917. doi: 10.1113/jphysiol.2007.141572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J, Frongillo EJ, Stepcik C, Beard J, Hurtado L. Altitude, ethnic and sex difference in birth weight and length in Bolivia. Am J Human Biol. 1980;52:459–477. [PubMed] [Google Scholar]
- Han VK. Pathophysiology, cellular and molecular mechanisms of foetal growth retardation. Equine Vet J. 1993;(Suppl.):12–16. doi: 10.1111/j.2042-3306.1993.tb04802.x. [DOI] [PubMed] [Google Scholar]
- Haruta M, Funato T, Sumida T, Naka Y, Saeki N, Shinkai T. Intervillous blood-gas status, especially oxygenation during cesarean section. Nippon Sanka Fujinka Gakkai Zasshi. 1986;38:909–916. [PubMed] [Google Scholar]
- Itskovitz J, LaGamma EF, Rudolph AM. Heart rate and blood pressure responses to umbilical cord compression in fetal lambs with special reference to the mechanism of variable deceleration. Am J Obstet Gynecol. 1983a;147:451–457. doi: 10.1016/s0002-9378(16)32243-8. [DOI] [PubMed] [Google Scholar]
- Itskovitz J, LaGamma EF, Rudolph AM. The effect of reducing umbilical blood flow on fetal oxygenation. Am J Obstet Gynecol. 1983b;145:813–818. doi: 10.1016/0002-9378(83)90684-1. [DOI] [PubMed] [Google Scholar]
- Jaffa AJ, Gull I, Amster R, Lessing JB, Wolman I. Effect of the supine position on uterine and umbilical blood flow during the third trimester of uncomplicated pregnancies in multiparous patients. Gynecol Obstet Invest. 2001;52:252–256. doi: 10.1159/000052985. [DOI] [PubMed] [Google Scholar]
- Jakoubek V, Bibova J, Herget J, Hampl V. Chronic hypoxia increases fetoplacental vascular resistance and vasoconstrictor reactivity in the rat. Am J Physiol Heart Circ Physiol. 2008;294:H1638–H1644. doi: 10.1152/ajpheart.01120.2007. [DOI] [PubMed] [Google Scholar]
- Kamitomo M, Alonso JG, Okai T, Longo LD, Gilbert RD. Effects of long-term, high-altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol. 1993;169:701–707. doi: 10.1016/0002-9378(93)90646-z. [DOI] [PubMed] [Google Scholar]
- Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol. 1966;21:1375–1376. doi: 10.1152/jappl.1966.21.4.1375. [DOI] [PubMed] [Google Scholar]
- Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol. 2006;28:126–136. doi: 10.1002/uog.2832. [DOI] [PubMed] [Google Scholar]
- Krampl E, Lees C, Bland JM, Espinoza Dorado J, Moscoso G, Campbell S. Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound Obstet Gynecol. 2000;16:9–18. doi: 10.1046/j.1469-0705.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- Krampl E, Lees C, Bland JM, Espinoza Dorado J, Moscoso G, Campbell S. Fetal Doppler velocimetry at high altitude. Ultrasound Obstet Gynecol. 2001;18:329–334. doi: 10.1046/j.0960-7692.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- Kreczy A, Fusi L, Wigglesworth JS. Correlation between umbilical arterial flow and placental morphology. Int J Gynecol Pathol. 1995;14:306–309. doi: 10.1097/00004347-199510000-00004. [DOI] [PubMed] [Google Scholar]
- Lackman F, Capewell V, Gagnon R, Richardson B. Fetal umbilical cord oxygen values and birth to placental weight ratio in relation to size at birth. Am J Obstet Gynecol. 2001;185:674–682. doi: 10.1067/mob.2001.116686. [DOI] [PubMed] [Google Scholar]
- Lichty JL, Ting R, Bruns PD, Dyar E. Studies of babies born at high altitude. I. Relationship of altitude to birth weight. Am J Dis Child. 1957;93:666–669. doi: 10.1001/archpedi.1957.02060040668009. [DOI] [PubMed] [Google Scholar]
- Martinez-Laso J, Siles N, Moscoso J, Zamora J, Serrano-Vela JI, Ji RA-C, Castro MJ, Serrano-Rios M, Arnaiz-Villena A. Origin of Bolivian Quechua Amerindians: their relationship with other American Indians and Asians according to HLA genes. Eur J Med Genet. 2006;49:169–185. doi: 10.1016/j.ejmg.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Merriwether DA, Hall WW, Vahlne A, Ferrell RE. mtDNA variation indicates Mongolia may have been the source for the founding population for the New World. Am J Hum Genet. 1996;59:204–212. [PMC free article] [PubMed] [Google Scholar]
- Merriwether DA, Huston S, Iyengar S, Hamman R, Norris JM, Shetterly SM, Kamboh MI, Ferrell RE. Mitochondrial versus nuclear admixture estimates demonstrate a past history of directional mating. Am J Phys Anthropol. 1997;102:153–159. doi: 10.1002/(SICI)1096-8644(199702)102:2<153::AID-AJPA1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. Placental gas transfer. Anesthesiology. 1963;26:460–464. doi: 10.1097/00000542-196507000-00010. [DOI] [PubMed] [Google Scholar]
- Munroe M, Shah CP, Badgley R, Bain HW. Birth weight, length, head circumference and bilirubin level in Indian newborns in the Sioux Lookout Zone, northwestern Ontario. Can Med Assoc J. 1984;131:453–456. [PMC free article] [PubMed] [Google Scholar]
- Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol. 1992;80:1000–1006. [PubMed] [Google Scholar]
- Pardi G, Buscaglia M, Ferrazzi E, Bozzetti P, Marconi A, Cetin I, Battaglia F, Makowski E. Cord sampling for the evaluation of oxygenation and acid-base balance in growth-retarded human fetuses. Am J Obstet Gynecol. 1987;157:1221–1228. doi: 10.1016/s0002-9378(87)80298-3. [DOI] [PubMed] [Google Scholar]
- Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Farrazzi E, Buscaglia M, Battaglia FC. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993;328:692–696. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol. 1998;119:717–723. doi: 10.1016/s1095-6433(98)01010-1. [DOI] [PubMed] [Google Scholar]
- Richardson DB, Wing S, Lorey F, Hertz-Picciotto I. Adult hemoglobin levels at birth and risk of sudden infant death syndrome. Arch Pediatr Adolesc Med. 2004;158:366–371. doi: 10.1001/archpedi.158.4.366. [DOI] [PubMed] [Google Scholar]
- Rigano S, Bozzo M, Ferrazzi E, Bellotti M, Battaglia FC, Galan HL. Early and persistent reduction in umbilical vein blood flow in the growth-restricted fetus: a longitudinal study. Am J Obstet Gynecol. 2001;185:834–838. doi: 10.1067/mob.2001.117356. [DOI] [PubMed] [Google Scholar]
- Roberts SW, Leveno KJ, Sidawi JE, Lucas MJ, Kelly MA. Fetal acidemia associated with regional anesthesia for elective cesarean delivery. Obstet Gynecol. 1995;85:79–83. doi: 10.1016/0029-7844(94)p4401-9. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Adachi N, Guillen S, Shimada I. Mitochondrial DNA analysis of ancient Peruvian highlanders. Am J Phys Anthropol. 2006;131:98–107. doi: 10.1002/ajpa.20408. [DOI] [PubMed] [Google Scholar]
- Shriver MD, Kennedy GC, Parra EJ, Lawson HA, Sonpar V, Huang J, Akey JM, Jones KW. The genomic distribution of population substructure in four populations using 8,525 autosomal SNPs. Hum Genomics. 2004;1:274–286. doi: 10.1186/1479-7364-1-4-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver MD, Mei R, Parra EJ, Sonpar V, Halder I, Tishkoff SA, Schurr TG, Zhadanov SI, Osipova LP, Brutsaert TD, Friedlaender J, Jorde LB, Watkins WS, Bamshad MJ, Gutierrez G, Loi H, Matsuzaki H, Kittles RA, Argyropoulos G, Fernandez JR, Akey JM, Jones KW. Large-scale SNP analysis reveals clustered and continuous patterns of human genetic variation. Hum Genomics. 2005;2:81–89. doi: 10.1186/1479-7364-2-2-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen MJ, Tesler J, Azami T, Preiss D, Fedorko L, Goldszmidz E, Fisher J, Kingdom J, Slorach C, Hornberger LK. Effects of maternal hyperoxia with and without normocapnia in uteroplacental and fetal Doppler studies. Ultrasound Obstet Gynecol. 2005;26:495–499. doi: 10.1002/uog.1995. [DOI] [PubMed] [Google Scholar]
- Skulstad SM, Kiserud T, Rasmussen S. Degree of fetal umbilical venous constriction at the abdominal wall in a low-risk population at 20–40 weeks of gestation. Prenat Diagn. 2002;22:1022–1027. doi: 10.1002/pd.462. [DOI] [PubMed] [Google Scholar]
- Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soothill PW, Nicolaides KH, Campbell S. Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br Med J (Clin Res Ed) 1987;294:1051–1053. doi: 10.1136/bmj.294.6579.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soothill PW, Nicolaides KH, Rodeck CH, Campbell S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther. 1986;1:168–175. doi: 10.1159/000262264. [DOI] [PubMed] [Google Scholar]
- Sutton MS, Theard MA, Bhatia SJ, Plappert T, Saltzman DH, Doubilet P. Changes in placental blood flow in the normal human fetus with gestational age. Pediatr Res. 1990;28:383–387. doi: 10.1203/00006450-199010000-00016. [DOI] [PubMed] [Google Scholar]
- Taricco E, Radaelli T, Cozzi V, Rossi G, Puglia D, Pardi G, Cetin I. Fetal oxygen uptake in normal and GDM pregnancies. Reprod Sci. 2008;15:69A. [Google Scholar]
- Thompson RS, Trudinger BJ, Cook CM. A comparison of Doppler ultrasound waveform indices in the umbilical artery – I. Indices derived from the maximum velocity waveform. Ultrasound Med Biol. 1986;12:835–844. doi: 10.1016/0301-5629(86)90001-3. [DOI] [PubMed] [Google Scholar]
- Thomson M. Heavy birthweight in Native Indians of British Columbia. Can J Public Health. 1990;81:443–446. [PubMed] [Google Scholar]
- Todros T, Ronco G, Fianchino O, Rosso S, Gabrielli S, Valsecchi L, Spagnolo D, Acanfora L, Biolcati M, Segnan N, Pilu G. Accuracy of the umbilical arteries Doppler flow velocity waveforms in detecting adverse perinatal outcomes in a high-risk population. Acta Obstet Gynecol Scand. 1996;75:113–119. doi: 10.3109/00016349609033301. [DOI] [PubMed] [Google Scholar]
- Tonni G, Ferrari B, De Felice C, Ventura A. Fetal acid-base and neonatal status after general and neuraxial anesthesia for elective cesarean section. International J Gynaecol Obstet. 2007;97:143–146. doi: 10.1016/j.ijgo.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Torroni A, Miller JA, Moore LG, Zamudio S, Zhuang J, Droma T, Wallace DC. Mitochondrial DNA analysis in Tibet: implications for the origin of the Tibetan population and its adaptation to high altitude. Am J Phys Anthropol. 1994;93:189–199. doi: 10.1002/ajpa.1330930204. [DOI] [PubMed] [Google Scholar]
- Tripathy V, Gupta R. Birth weight among Tibetans at different altitudes in India: are Tibetans better protected from IUGR? Am J Hum Biol. 2005;17:442–450. doi: 10.1002/ajhb.20400. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Milne JS, Hay WW., Jr Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2003;547:85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Regnault TR, Limesand SW, Hay WW, Jr, Anthony RV. Investigating the causes of low birth weight in contrasting ovine paradigms. J Physiol. 2005;565:19–26. doi: 10.1113/jphysiol.2004.082032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip R. Altitude and birth weight. J Pediatr. 1987;111:869–876. doi: 10.1016/s0022-3476(87)80209-3. [DOI] [PubMed] [Google Scholar]
- Yip R, Li Z, Chong WH. Race and birth weight: the Chinese example. Pediatrics. 1991;87:688–693. [PubMed] [Google Scholar]
- Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta. 2006;27:49–55. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Droma T, Norkyel KY, Acharya G, Zamudio JA, Niermeyer SN, Moore LG. Protection from intrauterine growth retardation in Tibetans at high altitude. Am J Phys Anthropol. 1993;91:215–224. doi: 10.1002/ajpa.1330910207. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007a;582:883–895. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, Post M, Illsley NP, Caniggia I. Human placental hypoxia-inducible factor-1α expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007b;170:2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]