Abstract
Hec1 (High Expression in Cancer 1) is an oncogene overly expressed in many human cancers. Small molecule INH (Inhibitor of Nek2/Hec1) targeting the Hec1 and its regulator, Nek2, in the mitotic pathway was identified to inactivate Hec1/Nek2 function mediated by protein degradation that subsequently leads to chromosome mis-segregation and cell death. To further improve the efficacy of INH, a series of INH analogues was designed, synthesized and evaluated. Among these 33 newly-synthesized analogues, three of them, 6, 13 and 21, have 6-8 fold more potent cell killing activity than the previous lead compound INH1. Compounds 6 and 21 were chosen for analyzing the underlying action mechanism. They target directly the Hec1/Nek2 pathway and cause chromosome mis-alignment as well as cell death, a mechanism similar to that of INH1. This initial exploration of structural/functional relationship of INH may advance the progress for developing clinically applicable INH analogue.
Introduction
Breast cancer has been one of the leading causes of death in American females for many years. Although multiple combined modality treatment strategies are available and continue to be improved, seeking for alternative therapeutics with a better therapeutic index remains a top priority of the drug development endeavors. Many of the established cancer therapeutics, represented by the microtubule poison taxane and Vinca alkaloids, inhibit cancer cell growth via direct inhibition of mitosis 1, 2. Mitosis is a highly dynamic and delicate process that requires multiple layers of regulation by a variety of molecules. Several innovative agents targeting the key mitotic regulators are currently in preclinical or clinical development, including those inhibiting the spindle-associated kinesin KSP/Eg5, kinetochore-associated kinesin CENP-E, and the mitotic kinase Aurora 3, 4. However, whether the downstream substrate of mitotic kinases may serve as a potential target remains to be explored.
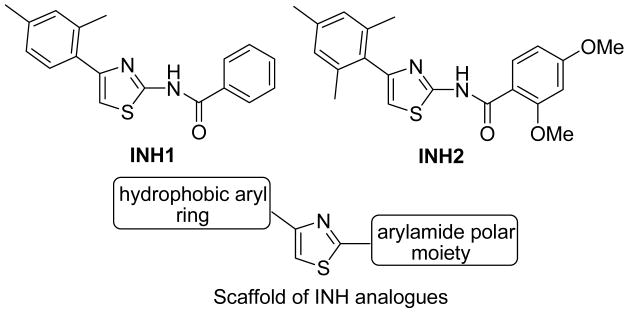
One of the critical mitotic regulators is Hec1a, an oncogene that modulates proper spindle formation between kinetochores and centrosomes. Importantly, overexpression of Hec1 has been documented to associate with poor clinical outcomes of primary breast cancers as well as cases with multiple cancers 5, 6. Inducible overexpression of Hec1 in a transgenic mouse model led to evident tumor formation, primarily lung adenoma and hepatocellular adenoma 7. On the other hand, phosphorylation of Hec1 by Nek2 is known to be critical for its mitotic function and cell survival 8, 9. We have recently identified small molecules INH1 and INH2 that can effectively suppress tumor cell growth in culture and in animals via directly targeting the Hec1/Nek2 mitotic pathway 10. Our leads, INH1 and INH2, sharing an aryl thiazolyl benzamide scaffold, came from screening a library of ∼24,000 synthetic compounds through an inducible reverse yeast two-hybrid system. Although both of them inhibit the proliferation of multiple human breast cancer cell lines in culture, the potency in killing cancer cells and the water solubility remain to be improved for better efficacy. As part of a broad chemical strategy aimed toward the discovery of novel INH analogues, we designed, synthesized and evaluated a series of novel INH analogues. SAR (Structure-activity Relationship) of INH scaffold was also illustrated. INH1 and INH2 both have a core phenyl-thiazolyl-benzamide structures with additional groups on both sides of the thiazolyl rings (Figure 1). The main scaffold of INH was composed of three parts: left part (hydrophobic aryl ring), middle part (thiazolyl ring) and right part (arylamide polar part). The modification of INH analogues was mainly focused on these three parts.
Figure 1.
Structures of INH1 and INH2 and the scaffold of INH analogues
Results and Discussion
Chemistry
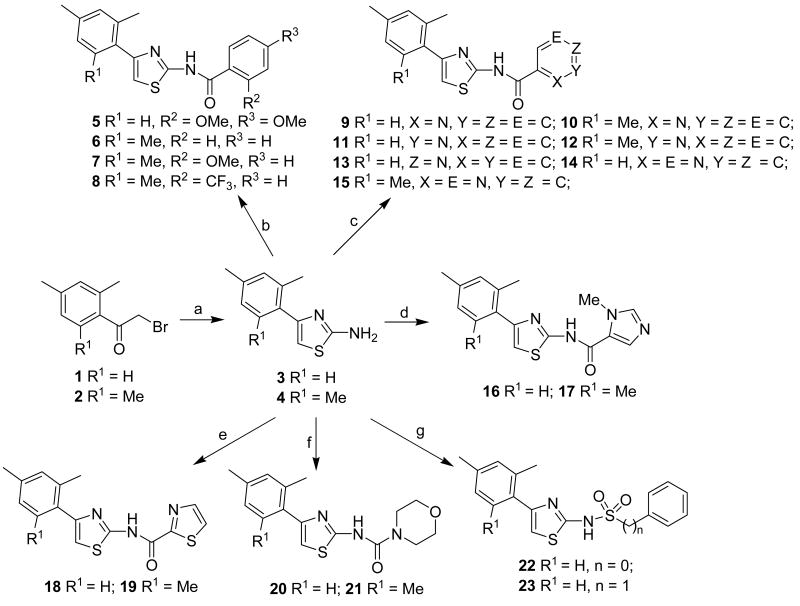
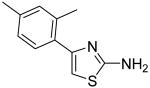
Our synthesis embarked on the preparation of two intermediates 3 and 4 followed by the general synthetic procedure shown in Scheme 1. That is, treatment of bromides 1 and 2 with thiourea in refluxing EtOH afforded 4-(2,4-dimethylphenyl)thiazol-2-ylamine (3) and 4-(2,4,6-trimethylphenyl)thiazol-2-ylamine (4) in 89% and 99% yields, respectively. Acylation of amines 3 and 4 with a series of aryl acyl chloride provided the corresponding INH analogues 5-19 in medium to good yields. In addition, treatment of amines 3 and 4 with 4-morpholinecarbonyl chloride led to the compounds 20 and 21 in 63% and 39% yields, respectively. Sulphonylation of 3 with benzenesulfonyl chloride and benzylsulfonyl chloride furnished the compounds 22 and 23 in 68% and 63% yields, respectively.
Scheme 1.
General Synthetic Procedure for the Preparation of Compounds 5-23a
a Reagents and conditions: (a) Thiourea, EtOH, reflux (89% for 3, 99% for 4); (b) for 5: 2,4-dimethoxy benzoyl chloride, DMAP, CH2Cl2, rt. (67%); for 6: BzCl, Et3N, dioxane, reflux (48%); for 7: 2-methoxyl benzoyl chloride, DMAP, CH2Cl2, 0°C to rt. (77%); for 8: 2-trifluoromethylbenzoyl chloride, DMAP, CH2Cl2, 0°C to rt. (52%); (c) for 9: picolinoyl chloride hydrochloride, DMAP, CH2Cl2, 0°C to rt. (87%); for 10: picolinoyl chloride hydrochloride, DMAP, CH2Cl2, 0°C to rt. (93%); for 11: nicotinoyl chloride hydrochloride, DMAP, CH2Cl2, 0°C to rt. (64%); for 12: nicotinoyl chloride hydrochloride, DMAP, CH2Cl2, 0°C to rt. (73%); for 13: isonicotinoyl chloride hydrochloride, DMAP, CH2Cl2, 0°C to rt. (88%); for 14: pyrazine-2-carbonyl chloride, DMAP, CH2Cl2, 0°C to rt. (77%); for 15: pyrazine-2-carbonyl chloride, DMAP, CH2Cl2, 0°C to rt. (93%); (d) 1-methyl-1H-imidazole-5-carbonyl chloride hydrochloride, DMAP, CH2Cl2, 0°C to rt. (37% for 16, 28% for 17); (e) 1,3-thiazole-2-carbonyl chloride, DMAP, CH2Cl2, 0°C to rt. (76% for 18, 89% for 19); (f) 4-morpholinecarbonyl chloride, DMAP, CH2Cl2, 0°C to rt. (63% for 20, 39% for 21). (g) for 22: PhSO2Cl, DMAP, pyridine, 0°C to rt. (68%); for 23: BnSO2Cl, Et3N, DMAP, THF, 0°C to rt. (63%).
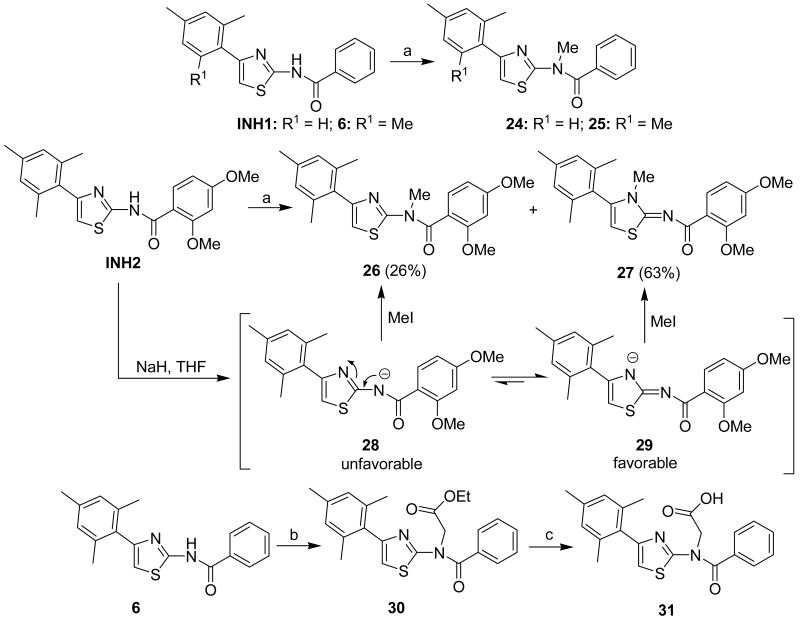
To explore the influence of the hydrogen atom of amide groups on bioactivities, methylated INH analogues 24-26 were prepared (Scheme 2). Treatment of INH1 and compound 6 with NaH / MeI / THF gave the methylated compounds 24 and 25 as the only product in 74% and 89% yields, respectively. Interestingly, subjecting INH2 to the same condition (NaH / MeI / THF) gave two separable methylated compounds 26 and 27 in 26% and 63% yields, respectively. In our opinion, the generation of compound 27 was ascribed to the electron-donating properties of two MeO groups. That is, once INH2 was treated with NaH in THF, the two MeO groups would result in the formation of two intermediates 28 and 29 in tautomeric equilibrium. Intermediate 29 was thought to be preferable to the intermediate 28 because the electronegative center in 29 was more remote from the two electron donating MeO groups than that in the intermediate 28, which was consistent with the higher yield of product 27. Furthermore, a steric contribution to the observed selectivity (higher yield of 27) should also be taken into account as the ortho methoxy group would be occupying the same place as the methyl added to the amide nitrogen. Additionally, amide-protected compound 30 was prepared in 72% yield via treatment of compound 6 with NaH / BrCH2CO2Et / THF. Saponification of the ester 30 with LiOH afforded the acid 31 in 77% yield.
Scheme 2a.
a Reagents and conditions: (a) (i) NaH, THF, 0°C; (ii) MeI, 0°C to rt. (74% for 24, 89% for 25, 26% for 26, 63% for 27); (b) (i) NaH, DMF, 0°C to 90°C; (ii) BrCH2CO2Et, 90°C (72%); (c) LiOH, H2O, MeOH, rt. (77%).
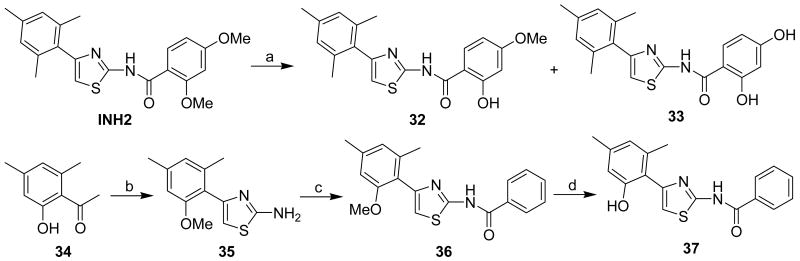
Some phenolic group-containing derivatives were also prepared (Scheme 3). Treatment of INH2 with BBr3 (1.5 eq.) in CH2Cl2 for 2 h smoothly gave the phenol 32 in 72% yield along with diphenol 33 in 3% yield. Further, subjecting INH2 to BBr3 (6.5 eq.) in CH2Cl2 for 3 hr delivered 32 in 20% yield and the diphenol 33 in 64% yield. Synthesis of compound 36 started from 2′-hydroxy-4′,6′-dimethylacetophenone (34), which was converted to the 2-aminothiazoline 35 in three steps including methylation with Me2SO4 / K2CO3, bromination with HBr / BuOOH and final condensation of the resultant α-bromoacetone with thiourea. Benzoylation of amine 35 smoothly gave the compound 36 in 93% yield. Exposure of compound 36 to BBr3 / CH2Cl2 at room temperature provided the phenol analogue 37 in 90% yield.
Scheme 3a.
a Reagents and conditions: (a) BBr3, CH2Cl2, 0°C to rt. (20% for 32, 64% for 33); (b) (i) Me2SO4, K2CO3, acetone, reflux; (ii) HBr, tBuOOH, dioxane, reflux; (iii) thiourea, EtOH, reflux (20% over 3 steps); (c) BzCl, DMAP, CH2Cl2, 0°C to rt. (93%); (d) BBr3, CH2Cl2, 0°C to rt. (90%).
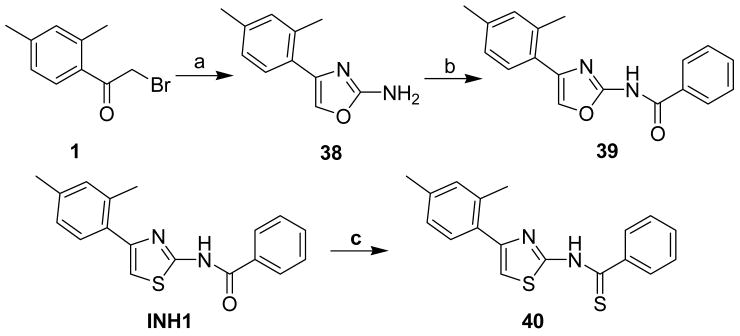
Substitution of an oxazolyl ring for the thiazolinyl ring in INH1 was also accomplished (Scheme 4). Reaction of bromide 1 with urea in refluxing EtOH gave the corresponding 2-aminooxazole 38, which was benzoylated to afford the 2-aminooxazolyl derivative 39. In addition, replacement of amide group in INH1 with thioamide group was performed through treatment with INH1 with Lawesson′s reagent in refluxing toluene and the desired thioamide derivative 40 was obtained in 79% yield.
Scheme 4a.
a Reagents and conditions: (a) urea, EtOH, reflux; (b) BzCl, DMAP, CH2Cl2, 0°C to rt. (7% in 2 steps); (c) Lawesson reagent, toluene, reflux (79%).
SAR analysis of the INH analogues
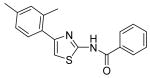
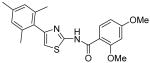
Synthesized analogues 3-27, 30-33, 36-37 and 39-40 were tested for their killing activities on human breast cancer cell lines MDA-MB231 and MDA-MB468, human cervical cancer line HeLa and human erythromyeloblastoid leukemia cell line K562. The results are outlined in Table 1, which are the basis of the current SAR analysis. Upon removal of the benzoyl groups from INH1 and INH2, the resultant thiazolyl amine 3 and 4 did not exhibit any anti-proliferative activity even at > 40 μM, indicating that benzoyl moiety is indispensable. Remarkably, the hybrid compound 5 containing the left moiety of INH1 and the right moiety of INH2 exhibited very low anti-proliferation activities against all the tested cell lines. To our delight, the other hybrid compound 6, containing the right moiety of INH2 and the left moiety of INH1, exhibited significantly improved anti-proliferation activities compared with our leads INH1 and 2. Additionally, deletion of the para-methoxyl group in INH2 resulted in the dramatic loss of bioactivity (7) and replacement of the methoxyl group in 7 with trifluoromethyl group would efficiently improve the bioactivity by approximately 1.5-2 fold (8). Based on the above results and in light of the well-known strong electron-withdrawing properties of trifluoromethyl group that renders neighboring group polar, we proposed that 2,4,6-trimethyl phenyl is preferred to 2,4-dimethyl phenyl, probably due to less rotational flexibility, and that introduction of a more polar group on the right moiety of scaffold seems to improve the bioactivity.
Table 1.
Activity of INH1, INH2 and their analogues
| Compound | Structure | IC50 (μM) | |||
|---|---|---|---|---|---|
| MB231 | MB468 | HeL a | K562 | ||
| INH1 |
|
8.6 | 10.5 | 8.8 | 11.7 |
| INH2 |
|
9.7 | 11.2 | 13.6 | 9.5 |
| 3 |
|
>40 | >40 | >40 | >40 |
| 4 |

|
>40 | >40 | >40 | >40 |
| 5 |
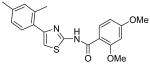
|
23.4 | >40 | >40 | >40 |
| 6 |
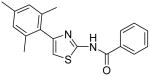
|
1.7 | 2.1 | 2.4 | 2.5 |
| 7 |
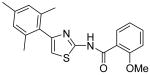
|
>40 | >40 | >40 | >40 |
| 8 |

|
5.9 | 4.1 | 10.6 | 7.5 |
| 9 |
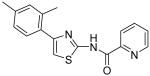
|
7.5 | 6.2 | 14.5 | 13.3 |
| 10 |
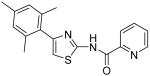
|
4.1 | 2.3 | 5.2 | 4.9 |
| 11 |
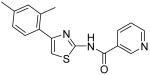
|
4.1 | 5.5 | 5.8 | 8.2 |
| 12 |
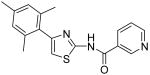
|
3.2 | 3.0 | 3.6 | 3.9 |
| 13 |

|
1.1 | 3.4 | 1.6 | 1.2 |
| 14 |
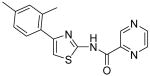
|
>25 | >25 | >25 | >25 |
| 15 |

|
3.2 | 4.7 | 5.7 | 5.9 |
| 16 |
|
>25 | >25 | >25 | >25 |
| 17 |
|
6.8 | 10.7 | 11.2 | 10.1 |
| 18 |
|
2.9 | 6.2 | 7.4 | 10.1 |
| 19 |
|
4.7 | 11.8 | 11.2 | 14.5 |
| 20 |
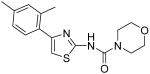
|
5.8 | 4.0 | 4.7 | 4.2 |
| 21 |
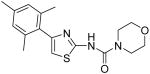
|
1.6 | 4.2 | 2.5 | 3.8 |
| 22 |
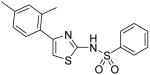
|
>40 | >40 | >40 | >40 |
| 23 |
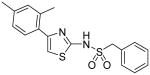
|
>40 | >40 | >40 | >40 |
| 24 |
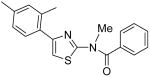
|
13.5 | 16.0 | 20.2 | 14.8 |
| 25 |
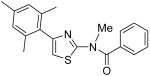
|
11.6 | 10.8 | 14.1 | 9.2 |
| 26 |
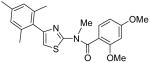
|
10.6 | 18.7 | >40 | 15.5 |
| 27 |
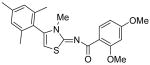
|
22.1 | 21.0 | 22.0 | 23.7 |
| 30 |
|
15.8 | 20.2 | 22.2 | 20.2 |
| 31 |
|
>40 | >40 | >40 | >40 |
| 32 |
|
11.8 | 14.4 | 14.4 | 14.8 |
| 33 |
|
22.5 | 21.3 | >40 | >40 |
| 36 |
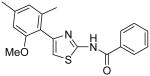
|
7.2 | 10.7 | 10.6 | 7.3 |
| 37 |
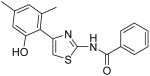
|
11.8 | >40 | 13.1 | 11.9 |
| 39 |
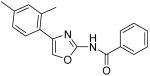
|
14.4 | 16.0 | 18.6 | 19.2 |
| 40 |
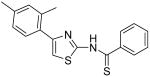
|
21.1 | 19.6 | 18.6 | 22.9 |
A series of analogues 9-21 containing different polar groups on the right part of scaffold was prepared and the biological activities were evaluated. As expected, most analogues exhibited better cell killing activity than the original leads. For most cases, 2,4,6-trimethyl phenyl group was more active than 2,4-dimethyl phenyl group. Apparently, isonicotinoyl group (13) was preferred to pyridine-2-carboxyl group (9, 10) and nicotinoyl group (11, 12). Substitution of pyrazinyl group for isonicotinoyl group led to a considerable reduction in bioactivity (13vs14) although compound 15 still exhibited good bioactivity. The bioactivity variation between 16 and 17, which contain 1-methyl-1H-imidazolyl groups, further consolidated our conclusion that 2,4,6-trimethyl phenyl group was preferred to 2,4-dimethyl phenyl group. Introduction of 1,3-thiazolyl groups did not change the bioactivities significantly (18, 19). Notably, the replacement of phenyl group in lead INH1 with morpholinyl group efficiently improved the activity. Among the analogues made, 6, 13 and 21 displayed the strongest activity, the IC50 values of which were improved by 6-8 fold in comparison to INH1. The para position of the hetero atom (N, O) on the six-membered ring of compounds 13 and 21 clearly provides advantages over the lead INH1 and the pyridine-2-carboxyl derivatives 9-10 (with hetero atom N at ortho-position), suggesting possible existence of an extended hydrophilic groove or pocket in the target binding site.
Replacement of amide moiety in INH1 with sulfonyl amide reduced the activity significantly (22-23, IC50 > 40 μM). Upon methylation of amide groups of INH1 or 2 and compound 6, the resultant derivatives 24-26 showed weakened bioactivities against all the tested cell lines, indicating that methylation of the amide group negatively affects compound activity. Although compound 30 derived from ethoxycarbonylmethylating compound 6 still displayed weak activities, its saponified product 31 is almost inactive (IC50 > 40 μM). Demethylated derivative 33 of INH2 showed very weak activity, but monophenol compound 32 still exhibited slightly weak bioactivity compared with INH2, which indicated that phenol group on the para-position was not preferred. Interestingly, compounds 36-37, derived from substitution of methoxyl group and hydroxyl group for C6 methyl group in 6, were also found to be active although bioactivity decreased significantly. Additionally, we also found that conversion of thiazolyl ring in INH1 to oxazolyl ring (39) or transformation of amide group in INH1 to thioamide group (40) also made the bioactivity decrease, which demonstrated that thiazolyl ring and amide group were important for the cytotoxicity.
Target validation of INH analogues
Next, target validation was performed by using an affinity pulldown assay described previously 10. Because compounds 6, 13 and 21 showed most improved cell killing activities with similar IC50 values in the SAR analysis, they were chosen for target validation by using compound 22 as a negative control. More specifically, 6 was used as a positive compound and 22 as a negative control in the affinity pulldown assay. In principle, compounds 6 and 22 were individually conjugated to an affi-gel matrix through a multistep synthetic route (Supplemental information) (Figure 2A) 10. HeLa cell extract was prepared and subjected to affinity pulldown experiments with control or compound-conjugated matrices. Shown in Figure 2B, 6-conjugated matrix, but not 22-conjugated or unconjugated matrix, selectively co-precipitated with cellular Hec1. In contrast, none of them co-precipitated with cellular Nek2. The results suggest that compound 6 binds to cellular Hec1, similar to the lead, INH1.
Figure 2.
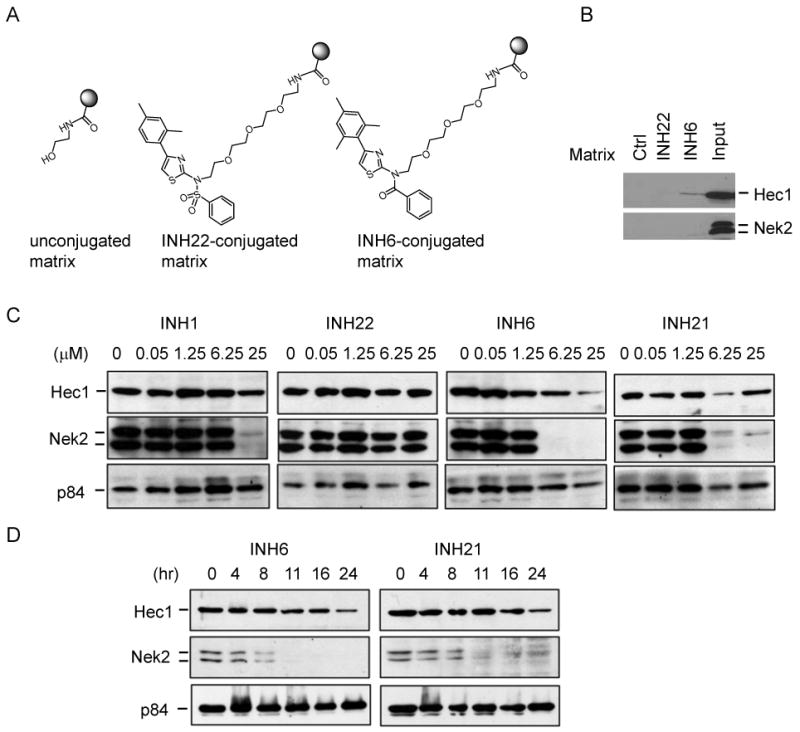
Target validation. (A) Illustrations of compound conjugated affi-gel matrices. (B) HeLa cell extract was used for affinity binding by control matrix, INH22, or INH6 conjugated matrix. Resultant precipitates after sufficient washing were subjected to SDS-PAGE analysis and Western blot to detect Hec1 and Nek2. Whole cell extract was used as input in duplicates. (C) dosage-dependent kinetic study of compound treatment. HeLa cells were treated with 6.25 μM INH6 or INH21, and harvested at various time points for detecting Hec1 and Nek2 by Western blot. P84 is a nuclear matrix protein used a loading control. (D) time-dependent kinetic study with varying dosages of the indicated compounds. Western blot was similarly performed as in (C).
A hallmark mechanism of action by INH1 is to trigger dramatic reduction of Nek2 protein level 10. To determine whether INH analogues retain such bioactivity in cells, both time and dosage-dependent kinetic studies were carried out in HeLa cells. The protein levels of Hec1 and Nek2 were examined by Western blot. In a doses-dependent study, Nek2 was found to be undetectable on treating cells with INH1 (25 μM), or lower dosages of 6 or 21 (both at 6.25 μM), but not by the negative compound 22 (up to 25 μM) (Figure 2C). For the time-dependent study, cells were treated with 6.25 μM of compound 6 or 21. Notably, the Nek2 level was reduced by ∼50% at 8-11 h after treatment with 6 or 21, which was comparable to INH1 treatment at 25 μM with a similar period of time (Figure 2D). In contrast, 22 showed no effect on Nek2 level at any time point tested. In addition, 6 and 21 seemed to trigger a slight Hec1 decrease over time, unlike the treatment with INH1, though the significance of partial Hec1 reduction is not clear at present. These results indicate that 6 and 21 are more efficient in targeting the Hec1/Nek2 complex for degradation.
Compound 6 and 21 triggered mitotic abnormalities
The Hec1/Nek2 complex functions primarily during G2 and M phases. Perturbation of the Hec1 or Nek2 function by antagonists (RNAi or antibody) leads to mitotic abnormalities represented by spindle configuration changes and chromosome misalignment, which contribute to mitotic catastrophe 11-18. Treating cells with INH1 similarly led to mitotic abnormalities, albeit to a lesser extent 10. To test whether compound 6 and 21 can elicit similar mitotic phenotypes via inhibiting Hec1/Nek2, cells were treated with 6, 21 or the negative compound 22. In comparison to the mock or 22-treated cells, 6 or 21 treated cells exhibited increased mitotic population with multipolar spindle configurations, presumably because of defective Hec1/Nek2 functionality (Figure 3A). Consistently, an increased rate of chromosome misalignment was detected upon treatment with 6 or 21 of HeLa cells expressing the chromosome marker protein H2B-GFP (Figure 3B). At the dosage of 6.25 μM, 6 and 21 elicited mitotic abnormalities to a similar extent as INH1 treatment at 25 μM, further confirming the improved efficacy of compound 6 and 21.
Figure 3.
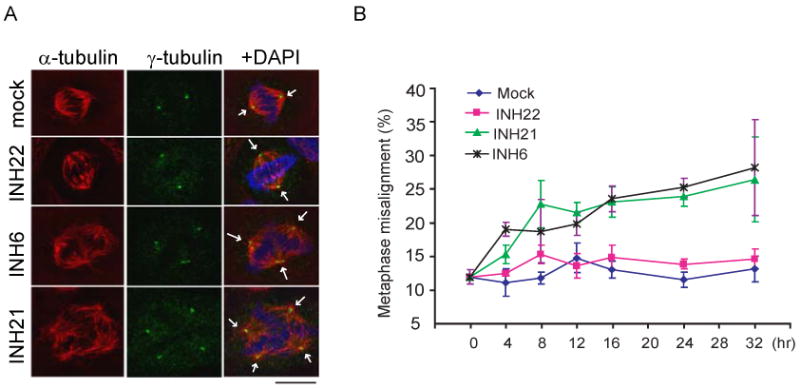
Mitotic abnormality induced by compound 6 and 21, but not 22 and the mock (buffer control). (A) Immunofluorescent staining of HeLa cells treated with mock, compound 22, 6 or 21 (each at 3.75 μM) for 24 hrs. Left column showed merged images of α-tubulin, γ-tubulin and the DAPI dye (DNA). Arrows indicated spindle poles. Note the multipolar spindle configurations in 6 and 21 treated cells. Scale bar, 10 μM. (B) incidence of metaphase chromosome alignment in cells treated individual compounds (2.5 μM each). Triplicate sets of compound-treated HeLa cells expressing the chromosome marker H2B-GFP were measured to obtain the averages and deviations.
Treatment of cells with compound 6 and 21 induced apoptosis
Mitotic abnormalities often lead to mitotic arrest, often followed by mitotic slippage and consequent mitotic catastrophe (i.e., cell death due to abnormal mitosis). Lead compound INH1 is known to trigger mitotic catastrophe. To examine whether compound 6 and 21 may trigger mitotic catastrophe, a time lapse experiment was performed to monitor the cell morphological changes and potential apoptosis. As expected, 6 or 21 treated cells showed progressive morphological changes characteristic of dying cells (e.g., membrane bubbling), which is further confirmed by cell cycle profiling with FACS analysis (Figure 4A and 4B). For instance, approximately 20% of compound 6 or 22 treated cells were apoptotic 72 hrs after treatment. The above results are consistent with the mitosis-targeting activity of compound 6 and 21 with improved efficacy.
Figure 4.
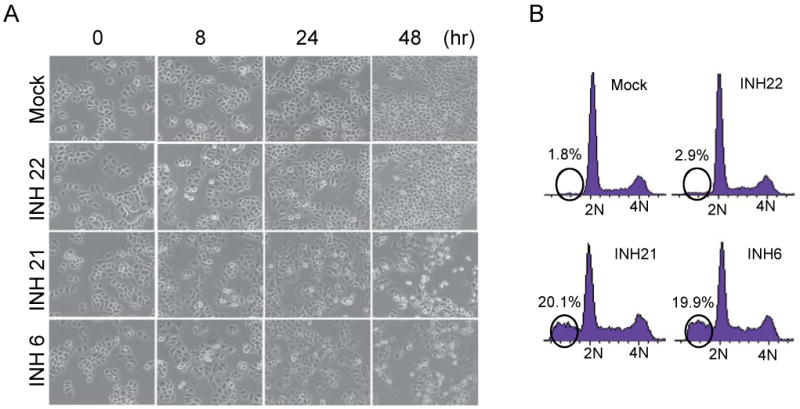
Apoptosis of cells treated with compound 6 and 21, but not 22 and the mock (buffer control). (A) Phase contrast images of cells at various time points after treatment with mock or individual compounds (each at 2.5 μM). (B) Percentages of metaphase population with chromosome alignment in cells treated with individual compounds (2.5 μM each). Triplicate sets of compound-treated HeLa cells expressing the chromosome marker H2B-GFP were measured to obtain the averages and deviations.
Conclusion
This study has identified several INH analogues that show a 6-8 folds increase in cellular killing activity by targeting Hec1/Nek2 pathway. The SAR study identified a few key structural features that may serve as a guideline for further improvement of the current compounds using medicinal chemical approaches. In essence, the presence of a hydrophobic ring with less rotational freedom, such as 2,4,6-trimethyl phenyl, is preferred at the left part of the scaffold (Figure 1). Introducing hetero atoms (N, O) or a polar group to the para positions of the six-membered ring on the right side of the scaffold seems to dramatically improve both solubility and bioactivity. Finally, the integrity of the thiazolyl ring and the amide bond in the middle of the scaffold is important for maintaining compound activity.
Two of the improved versions, compound 6 and 21, are capable of targeting the cellular Hec1/Nek2 complex, triggering mitotic abnormalities and cell death at a much lower dosage than the lead INH1. The structural features identified in this study may serve as a foundation for designing, synthesis and evaluation of the next generation of INH derivatives with further refinement for potential clinical applications.
Experiment Section
Cell lines and antibodies
Human breast cancer cell lines MDA-MB231 and MDA-MB468, and the cervical adenocarcinoma line HeLa were maintained in DMEM medium (Invitrogen) supplemented with 10% FBS. Antibody sources were: mouse anti-Hec1 clone 9G3 and mouse anti-p84, Rabbit anti-Nek2 polyclonal antibodies (Santa Cruz Biotech.), mouse anti-alpha-tubulin and rabbit anti-gamma tubulin (Sigma Aldrich), and secondary antibodies conjugated with Alexa dyes (Invitrogen).
Cell killing assay
Standard XTT assays with a four-day drug treatment procedure were performed to measure the dose-dependent cytotoxicity of INH analogs in cultured cells. Triplicate sets were measured and compiled for final data presentation. The assay was performed by using a commercial kit (Roche Scientific) following the instructions. In principle, cells were plated on 96-well dishes one day before the drug treatment, followed by drug treatment on day 2 and XTT assay on day 5 after drug addition. The absorption at 595 nm was measured with a plate reader and converted to cell survival percentages in comparison to mock treated groups.
Microscopy and FACS analysis
Immunostaining, image processing, and FACS assays were done as detailed previously 19.
Chemistry
All reagents were used as received from commercial sources, unless specified otherwise, or prepared as described in the literature. Reactions requiring anhydrous conditions were performed in vacuum heat-dried glassware under nitrogen atmosphere. Reaction mixtures were stirred magnetically. DMF, dichloromethane and pyridine were distilled from CaH2. 1H NMR spectra were recorded at either 400 MHz or 500 MHz. 13C NMR spectra were recorded at either 125 MHz or 100 MHz. 19F NMR spectra were recorded at 376 MHz with FCCl3 as external standard and low field is positive. Chemical shifts (δ) are reported in ppm, and coupling constants (J) are in Hz. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet.
4-(2,4-Dimethyl-phenyl)thiazol-2-ylamine (3)
The mixture of compound 1 (1.28 g, 5.63 mmol) and thiourea (450 mg, 5.91 mmol) in anhydrous EtOH (20 mL) was heated to reflux for 30min. After that, the solvent was removed in vacuo and the saturated aqueous NaHCO3 was added to make the mixture basic (pH =8-9). Then, the mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were dried with anhydrous Na2SO4. After removal of all the solvent, the residue was purified by silica gel chromatography (Hexane / EtOAc = 2 : 1) to afford product 3 (1.03 g, 89%) as a solid. mp 84.5-85.5 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 8.0 Hz, 1H), 7.06-7.03 (m, 2H), 6.42 (s, 1H), 5.29 (br, 2H), 2.42 (s, 3H), 2.35 (s, 3H); MS (ESI) m/z 205 (M + H+).
N-[4-(2,4-Dimethylphenyl)thiazol-2-yl]-2,4-dimethoxybenzamide (5)
The solution of compound 3 (82 mg, 0.40 mmol), DMAP (55 mg, 0.44 mmol) and 2, 4-dimethoxy benzoyl chloride (83 mg, 0.42 mmol) in CH2Cl2 (3 mL) was stirred for 1h. After that, the mixture was directly subjected to purification by silica gel chromatography (hexane / EtOH = 3 : 1) to give compound 5 (98 mg, 67%) as a solid. mp 159-160 °C; 1H NMR (400 MHz, CDCl3) δ 10.99 (s, 1H), 8.30 (d, J = 8.8 Hz, 1H), 7.49 (d, J = 7.6 Hz, 1H), 7.09-7.06 (m, 2H), 6.88 (s, 1H), 6.69 (dd, J = 8.8, 2.4 Hz, 1H), 6.56 (s, J = 2.4 Hz, 1H), 4.07 (s, 3H), 3.90 (s, 3H), 2.43 (s, 3H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 164.9, 162.7, 159.4, 157.3, 150.4, 137.9, 136.1, 134.7, 132.3, 131.7, 129.8, 126.8, 112.4, 110.6, 106.2, 98.9, 56.5, 55.9, 21.3, 21.2; MS (ESI) m/z 369 (M + H+), 391 (M + Na+); HRMS Calcd for C20H20N2O3SNa (M + Na+), 391.1092 Found: 391.1095.
N-[4-(2,4,6-Trimethylphenyl)thiazol-2-yl]benzamide (6)
The solution of compound 4 (105 mg, 0.48 mmol), Et3N (0.30 mL, 2.15 mmol) and BzCl (75 mg, 0.53 mmol) in dioxane (5 mL) was heated to reflux for 2.5 h. After that, the mixture was cooled to room temperature and all the solvent was removed in vacuo to afford a residue, which was extracted with EtOAc (3 × 30 mL). The combined organic phases were dried with anhydrous Na2SO4. Removal of all the solvent in vacuo resulted in a residue, which was purified with silica gel chromatography (Hexane / EtOAc = 15 : 1) to give compound 6 (48 mg, 31%) as a white solid. mp 192-193 °C; 1H NMR (400 MHz, CDCl3) δ 11.63 (s, 1H), 7.52-7.73 (m, 2H), 7.51 (tt, J = 7.6, 1.2 Hz, 1H), 7.41-7.37 (m, 2H), 6.74 (s, 1H), 6.62 (s, 2H), 2.18 (s, 3H), 1.93 (s, 6H); 13C NMR (100.5 MHz, CDCl3) δ 165.3, 159.1, 148.8, 137.7, 137.0, 132.6, 132.0, 131.5, 128.6, 128.4, 127.7, 111.6, 21.2, 20.5; MS (ESI) m/z 323 (M + H+), 345 (M + Na+); 645 (2M + H+), 667 (M + 2Na+); HRMS Calcd for C19H19N2OS (M + H+), 323.1218 Found: 323.1212.
2-Methoxy-N-[4-(2,4,6-trimethylphenyl)thiazol-2-yl]benzamide (7)
The solution of amine 4 (120 mg, 0.55 mmol) and DMAP (110 mg, 0.90 mmol) in CH2Cl2 (2 mL) was added a solution of 2-methoxyl benzoyl chloride (120 mg, 0.70 mmol) at 0 °C. The resultant mixture was warmed to room temperature and stirred for 20min. After that, H2O (20 mL) was added to quench the reaction and the mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were dried with anhydrous Na2SO4. Removal of all the solvent in vacuo resulted in a residue, which was purified with silica gel chromatography (Hexane / EtOAc = 4 : 1) to give compound 7 (150 mg, 77%) as a white solid. mp 218.5-219.5 °C; 1H NMR (400 MHz, CDCl3) δ 11.11 (s, 1H), 8.34 (dd, J = 7.6, 1.2 Hz, 1H), 7.55 (td, J = 7.6, 1.6 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 7.05 (d, J = 8.4 Hz, 1H), 6.92 (s, 2H), 6.75 (s, 1H), 4.05 (s, 3H), 2.31 (s, 3H), 2.12 (s, 6H); 13C NMR (100.5 MHz, CDCl3) δ 162.5, 157.7, 157.3, 149.1, 137.7, 137.3, 134.3, 132.7, 132.3, 128.2, 121.7, 119.2, 111.5, 111.1, 56.2, 21.1, 20.3; MS (ESI) m/z 353 (M + H+), 375 (M + Na+); 705 (2M + H+), 727 (M + 2Na+); HRMS Calcd for C20H20N2O2SNa (M + Na+), 375.1143 Found: 375.1139.
Pyridine-2-carboxylic acid [4-(2,4-dimethylphenyl)thiazol-2-yl]amide (9)
To a 0 °C solution of amine 3 (90 mg, 0.44 mmol) in CH2Cl2 (4 mL) was added DMAP (120 mg, 0.98 mmol) followed by picolinoyl chloride hydrochloride (80 mg, 0.45 mmol). Then, the mixture was stirred at room temperature for 4h. After that, the whole mixture was subjected to purification by silica gel chromatography to give compound 9 (118 mg, 87%) as a solid. mp 212-213 °C; 1H NMR (400 MHz, CDCl3) δ 11.22 (br, 1H), 8.65 (d, J = 4.0 Hz, 1H), 8.31 (d, J = 8.0 Hz, 1H), 7.93 (t, J = 7.6 Hz, 1H), 7.53 (d, J = 7.2 Hz, 2H), 7.10-7.07 (m, 2H), 6.97 (s, 1H), 2.46 (s, 3H), 2.37 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 162.1, 156.3, 150.8, 148.7, 148.0, 137.9, 136.0, 131.9, 131.8, 129.7, 127.4, 126.8, 122.9, 110.8, 21.3, 21.3; MS (ESI) m/z 310 (M + H+), 332 (M + Na+); HRMS Calcd for C17H15N3OSNa (M + Na+), 332.0833 Found: 332.0825.
N-[4-(2,4-Dimethylphenyl)thiazol-2-yl]nicotinamide (11)
To a 0 °C solution of amine 3 (97 mg, 0.47 mmol) in CH2Cl2 (2 mL) was added DMAP (120 mg, 0.98 mmol) followed by nicotinoyl chloride hydrochloride (108 mg, 0.60 mmol). Then, the mixture was stirred at room temperature for 20min. After that, the whole mixture was subjected to purification by silica gel chromatography to give compound 11 (93 mg, 64%) as a solid. mp 210-211 °C; 1H NMR (500 MHz, CDCl3) δ 12.55 (br, 1H), 8.64 (s, 1H), 8.61 (s, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.20-7.15 (m, 2H), 6.92 (s, 1H), 6.82-6.79 (m, 2H), 2.23 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 164.3, 159.3, 152.9, 148.9, 138.2, 135.7, 135.2, 131.6, 130.9, 129.6, 128.2, 126.7, 123.3, 111.0, 21.2, 20.7; MS (ESI) m/z 310 (M + H+), 332 (M + Na+); HRMS Calcd for C17H15N3OSNa (M + Na+), 332.0833 Found: 332.0835.
N-[4-(2,4-Dimethylphenyl)thiazol-2-yl]isonicotinamide (13)
To a 0 °C solution of amine 3 (75 mg, 0.37 mmol) in CH2Cl2 (2 mL) was added DMAP (90 mg, 0.74 mmol) followed by isonicotinoyl chloride hydrochloride (85 mg, 0.48 mmol). Then, the mixture was stirred at room temperature for 20min. After that, the whole mixture was subjected to purification by silica gel chromatography to give compound 13 (101 mg, 88%) as a solid. mp 148-149 °C; 1H NMR (500 MHz, CDCl3) δ 12.96 (br, 1H), 8.53 (d, J = 4.5 Hz, 2H), 7.38 (d, J = 4.5 Hz, 2H), 7.08 (d, J = 8.0 Hz, 1H), 6.94 (s, 1H), 6.79-6.76 (m, 2H), 2.21 (s, 6H); 13C NMR (100.5 MHz, CDCl3) δ 164.4, 159.2, 150.3, 150.1, 139.0, 138.2, 135.5, 131.7, 130.8, 129.5, 126.8, 121.1, 111.2, 21.1, 20.6; MS (ESI) m/z 310 (M + H+), 332 (M + Na+); HRMS Calcd for C17H15N3OSNa (M + Na+), 332.0833 Found: 332.0826.
Pyrazine-2-carboxylic acid [4-(2,4-dimethylphenyl)thiazol-2-yl]amide (14)
To a 0 °C solution of amine 3 (103 mg, 0.51 mmol) in CH2Cl2 (2 mL) was added DMAP (75 mg, 0.61 mmol) followed by pyrazine-2-carbonyl chloride (87 mg, 0.61 mmol). Then, the mixture was stirred at room temperature for 30min. After that, the whole mixture was subjected to purification by silica gel chromatography to give compound 14 (122 mg, 77%) as a solid. mp 168.5-169.5 °C; 1H NMR (500 MHz, CDCl3) δ 11.05 (br, 1H), 9.51 (s, 1H), 8.85 (d, J = 2.0 Hz, 1H), 8.61 (t, J = 1.5 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.07-7.05 (m, 2H), 6.99 (s, 1H), 2.44 (s, 3H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 160.9, 155.9, 151.0, 148.5, 145.0, 143.2, 142.9, 138.1, 136.0, 131.8, 131.7, 129.7, 126.8, 111.2, 21.3, 21.2; MS (ESI) m/z 311 (M + H+), 333 (M + Na+); HRMS Calcd for C16H14N4OSNa (M + Na+), 333.0786 Found: 333.0781.
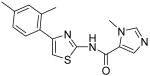
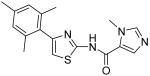
3-Methyl-3H-imidazole-4-carboxylic acid [4-(2,4-dimethylphenyl)thiazol-2-yl]amide (16)
To a 0 °C solution of amine 3 (85 mg, 0.42 mmol) in CH2Cl2 (2 mL) was added DMAP (118 mg, 0.97 mmol) followed by 1-methyl-1H-imidazole-5-carbonyl chloride hydrochloride (180 mg, 0.99 mmol). Then, the mixture was stirred at room temperature for 2h. After that, the whole mixture was subjected to purification by silica gel chromatography to give compound 16 (49 mg, 37%) as a solid. mp 250 °C (decomposed); 1H NMR (500 MHz, DMSO-D6) δ 12.55 (br, 1H), 8.11 (s, 1H), 7.92 (s, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.22 (s, 1H), 7.10 (s, 1H), 7.06 (d, J = 7.5 Hz, 1H), 3.91 (s, 3H), 2.41 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, DMSO-D6) δ 157.9, 156.9, 149.1, 143.6, 136.9, 135.1, 131.7, 131.4, 129.4, 126.4, 124.0, 110.5, 34.0, 21.0, 20.7; MS (ESI) m/z 313 (M + H+), 335 (M + Na+); HRMS Calcd for C16H17N4OS (M + H+), 313.1123 Found: 313.1131.
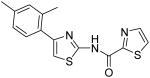
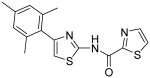
Thiazole-2-carboxylic acid [4-(2,4-dimethylphenyl)thiazol-2-yl]amide (18)
To a 0 °C solution of amine 3 (86 mg, 0.42 mmol) in CH2Cl2 (2 mL) was added DMAP (62 mg, 0.51 mmol) followed by 1, 3-thiazole-2-carbonyl chloride (75 mg, 0.51 mmol). Then, the mixture was stirred at room temperature for 15min. After that, the whole mixture was subjected to purification by silica gel chromatography to give compound 18 (100 mg, 76%) as a solid. mp 146.5-147.5 °C; 1H NMR (500 MHz, CDCl3) δ 10.60 (br, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.71 (d, J = 3.0 Hz, 1H), 7.48 (d, J = 7.5 Hz, 1H), 7.08-7.06 (m, 2H), 6.98 (s, 1H), 2.44 (s, 3H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 161.1, 157.1, 155.6, 151.0, 144.4, 138.0, 136.0, 131.8, 131.7, 129.7, 126.8, 126.3, 111.2, 21.3, 21.2; MS (ESI) m/z 316 (M + H+), 338 (M + Na+); HRMS Calcd for C15H13N3OS2Na (M + Na+), 338.0398 Found: 338.0391.
Morpholine-4-carboxylic acid [4-(2,4-dimethylphenyl)thiazol-2-yl]amide (20)
To a 0 °C solution of amine 3 (94 mg, 0.46 mmol) in CH2Cl2 (2 mL) was added DMAP (281 mg, 2.30 mmol) followed by 4-morpholinecarbonyl chloride (117 mg, 0.78 mmol). Then, the mixture was stirred at room temperature for 3 days. After that, the whole mixture was subjected to purification by silica gel chromatography to give compound 20 (92 mg, 63%) as a solid. mp 168-169 °C; 1H NMR (500 MHz, CDCl3) δ 10.41 (br, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.07 (s, 1H), 7.04 (d, J = 7.5 Hz, 1H), 6.80 (s, 1H), 3.43 (t, J = 4.5 Hz, 4H), 3.21 (t, J = 4.0 Hz, 4H), 2.34 (s, 3H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 161.4, 154.2, 149.1, 138.1, 136.0, 132.0, 131.7, 129.6, 126.9, 110.1, 66.4, 44.1, 21.3, 20.9; MS (ESI) m/z 318 (M + H+), 340 (M + Na+); HRMS Calcd for C16H19N3O2SNa (M + Na+), 340.1096 Found: 340.1092.
Morpholine-4-carboxylic acid [4-(2,4,6-trimethylphenyl)thiazol-2-yl]amide (21)
To a solution of amine 4 (112 mg, 0.51 mmol) in anhydrous CH2Cl2 (4 mL) was added DMAP (69 mg, 0.56 mmol). The resultant solution was cooled to 0 °C and then, a solution of 4-morpholinecarbonyl chloride (80 mg, 0.54 mmol) in anhydrous CH2Cl2 (1 mL) was added dropwise. The mixture was warmed up to room temperature and stirred for 1h. After that, DMAP (150 mg, 1.23 mmol) and 4-morpholinecarbonyl chloride (0.67 mmol) were added. The whole mixture was further stirred for 7h. H2O (20 mL) was added to quench the reaction and the mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic phases were washed with brine and dried with anhydrous Na2SO4. After removal of all the solvent, the residue was purified by silica gel chromatography (Hexan / EtOAc = 3 / 1) to give compound 21 (66 mg, 39%) as a white solid. mp 183-184 °C; 1H NMR (400 MHz, CDCl3) δ 10.62 (s, 1H), 6.91 (s, 2H), 6.60 (s, 1H), 3.53 (s, 4H), 3.21 (s, 4H), 2.32 (s, 3H), 2.01 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 162.41, 154.59, 146.50, 138.08, 137.39, 131.47, 128.60, 110.77, 66.54, 44.00, 21.24, 20.37; MS (ESI) m/z 332 (M + H+), 354 (M + Na+), 662 (2M + H+), 684 (M + 2Na+); HRMS Calcd for C17H22N3O2S (M + H+), 332.1433 Found: 332.1429.
N-[4-(2,4-Dimethylphenyl)thiazol-2-yl]benzenesulfonamide (22)
To a 0 °C solution of amine 3 (99 mg, 0.485 mmol) in pyridine (3 mL) was added DMAP (20 mg, 0.16 mmol) followed by PhSO2Cl (95 mg, 0.53 mmol). Then, the mixture was stirred at room temperature overnight. After that, the mixture was poured into a solution of aqueous 1N HCl (50 mL). The mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were dried with anhydrous Na2SO4. Removal of all the solvent in vacuo resulted in a residue, which was purified with silica gel chromatography (Hexane / EtOAc = 2 : 1) to give compound 22 (113 mg, 68%) as a solid. mp 188-189 °C; 1H NMR (400 MHz, CDCl3) δ 9.93 (br, 1H), 7.93 (d, J = 7.6 Hz, 2H), 7.55-7.43 (m, 3H), 7.17 (d, J = 7.6 Hz, 1H), 7.09-7.04 (m, 2H), 6.26 (s, 1H), 2.34, 2.33 (2s, 6H); 13C NMR (100.5 MHz, CDCl3) δ 168.9, 142.1, 140.3, 136.7, 136.3, 132.4, 132.2, 129.2, 128.9, 127.4, 126.7, 125.9, 103.9, 21.4, 20.6; MS (ESI) m/z 345 (M + H+), 367 (M + Na+); HRMS Calcd for C17H16N2O2S2Na (M + Na+), 367.0551 Found: 367.0551.
N-[4-(2,4-Dimethylphenyl)thiazol-2-yl]-C-phenyl-methanesulfonamide (23)
To a 0 °C solution of compound 3 (116 mg, 0.57 mmol) in THF (5 mL) was added Et3N (0.23 mL, 1.64 mmol) and DMAP (10 mg, 0.08 mmol). Then, BnSO2Cl (130 mg, 0.68 mmol) was added in one portion. The resultant mixture was warmed up to room temperature and stirred for 1.5h. The reaction was quenched with H2O (5ml) and H2O (30 mL) was added. The mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic phases were dried with anhydrous Na2SO4. After removal of all the solvent, the residue was purified by silica gel chromatography (Hexane / EtOAc = 5 : 1 to 2 : 1) to afford product 23 (129 mg, 63%) as a solid. mp 162-163 °C; 1H NMR (400 MHz, CDCl3) δ 10.20 (br, 1H), 7.42-7.40 (m, 2H), 7.30-7.24 (m, 3H), 7.05-7.01 (m, 3H), 6.08 (s, 1H), 4.29 (s, 2H), 2.34 (s, 3H), 2.22 (s, 3H); 13C NMR (100.5 MHz, CDCl3) δ 170.6, 140.1, 136.6, 136.3, 132.0, 131.4, 129.9, 129.1, 128.6, 128.5, 127.3, 126.1, 103.3, 60.2, 21.4, 20.5; MS (ESI) m/z 359 (M + H+), 381 (M + Na+); HRMS Calcd for C18H19N2O2S (M + H+), 359.0888 Found: 359.0880.
N-[4-(2,4-Dimethylphenyl)thiazol-2-yl]-N-methylbenzamide (24)
To a cooled mixture of NaH (20 mg, 60% in oil, 0.5 mmol) in THF (5 mL) was added a solution of compound INH1 (120 mg, 0.39 mmol) in THF (3 ml) dropwise. The mixture was warmed up to room temperature and stirred for 20 min. After that, the mixture was cooled to 0 °C again and MeI (32 μL, 0.5 mmol) was added dropwise. Then, the mixture was warmed up to room temperature and stirred for 2h. Then, H2O (2 mL) was added to quench the reaction and the mixture was diluted with H2O (30 mL). The mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic phases were dried with anhydrous Na2SO4. After removal of all the solvent, the residue was purified by silica gel chromatography (Hexane / EtOAc = 4 : 1) to afford compound 24 (93 mg, 74%) as a solid. mp 146-147 °C; 1H NMR (400 MHz, CDCl3) δ 8.40 (m, 2H), 7.46 (m, 3H), 7.15 (m, 3H), 6.49 (s, 1H), 3.56 (s, 3H), 2.41 (s, 3H), 2.16 (s, 3H); 13C NMR (100.5 MHz, CDCl3) δ 174.40, 168.66, 140.44, 138.69, 137.94, 137.30, 131.61, 131.52, 130.77, 129.43, 128.25, 127.47, 127.22, 106.75, 34.14, 21.53, 19.77; MS (ESI) m/z 323 (M + H+); HRMS Calcd for C19H18N2OSNa (M + H+), 345.1038 Found: 345.1042.
2,4-Dimethoxy-N-methyl-N-[4-(2,4,6-trimethylphenyl)thiazol-2-yl]benzamide (26) and 2,4-dimethoxy-N-[3-methyl-4-(2,4,6-trimethylphenyl)-3H-thiazol-(2Z)-ylidene]benzamide (27)
To a cooled mixture of NaH (100 mg, 60% in oil, 2.5 mmol) in THF (5 mL) was added a solution of compound INH2 (140 mg, 0.37 mmol) in THF (5 mL) dropwise. The mixture was warmed up to room temperature and stirred for 30 min. After that, the mixture was cooled to 0 °C again and MeI (1.0 mL, 16.1 mmol) was added dropwise. Then, the mixture was warmed up to room temperature and stirred for 2h. Then, H2O (2 mL) was added to quenched the reaction and the mixture was diluted with H2O (30 mL). The mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were dried with anhydrous Na2SO4. After removal of all the solvent, the residue was purified by silica gel chromatography (Hexane / EtOAc = 4 : 1 to 2 : 1) to afford compound 26 (less polar, 38 mg, 26%) as a solid and compound 27 (more polar, 93 mg, 63%) as a solid. Compound 26: mp 156-157C; 1H NMR (400 MHz, CDCl3) δ 7.35 (d. J = 8.4 Hz, 1H), 6.94 (s, 2H), 6.79 (s, 1H), 6.59 (dd, J = 10.4, 2.0 Hz, 1H), 6.52 (d, J = 2.0 Hz, 1H), 3.87 (s, 3H), 3.86 (s, 3H), 3.55 (s, 3H), 2.32 (s, 3H), 2.15 (s, 6H); 1C NMR (125MHz, CDCl3) δ 168.9, 162.9, 157.4, 148.5, 137.8, 137.6, 133.0, 131.1, 130.1, 128.5, 117.6, 112.6, 105.2, 98.8, 55.8, 55.7, 37.1, 21.3, 20.7; MS (ESI) m/z 397 (M + H+), 419 (M + Na+); HRMS Calcd for C22H24N2O3SNa (M + Na+), 419.1405 Found: 419.1390. Compound 27: mp 161-162 °C; 8.28 (d, J = 9.6 Hz, 1H), 6.99 (s, 2H), 6.54 (s, 1H), 6.53 (d, J = 6.4 Hz, 1H), 6.39 (s, 1H), 3.95 (s, 3H), 3.87 (s, 3H), 3.44 (s, 3H), 2.35 (s, 3H), 2.07 (s, 6H); 1C NMR (125MHz, CDCl3) δ 173.9, 168.1, 139.9, 138.5, 136.8, 134.0, 128.7, 127.3, 119.8, 106.2, 104.3, 99.1, 56.2, 55.6, 33.4, 21.4, 20.1; MS (ESI) m/z 397 (M + H+), 419 (M + Na+); HRMS Calcd for C22H25N2O3S (M + H+), 397.1586 Found: 397.1584.
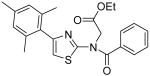
{Benzoyl-[4-(2,4,6-trimethylphenyl)thiazol-2-yl]-amino}acetic acid ethyl ester (30)
NaH (38 mg, 60 in oil, 0.95 mmol) was cooled to 0 °C and a solution of compound 6 (167 mg, 0.52 mmol) in DMF (6 mL) was added. The mixture was carefully heated to 90 °C and stirred for 1h. Then, the mixture was cooled to 0 °C and BrCH2CO2Et (0.60 mL, 5.4 mmol) was added dropwise. After that, the mixture was heated to 90 °C and stirred overnight. H2O (10 mL) was added to quench the reaction and the mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic phases were dried with anhydrous Na2SO4. Removal of all the solvent in vacuo resulted in a residue, which was purified with silica gel chromatography (Hexane / EtOAc = 5 : 1) to give compound 30 as a white solid (152 mg, 72%). mp 160-161 °C; 1H NMR (500 MHz, CDCl3) δ 8.32-8.30 (m, 2H), 7.49 (tt, J = 7.0, 1.5 Hz, 1H), 7.45-7.42 (m, 2H), 6.99 (s, 2H), 6.47 (s, 1H), 4.50 (s, 2H), 4.16 (q, J = 7.0 Hz, 2H), 2.36 (s, 3H), 2.13 (s, 6H), 1.20 (t, J = 7.0 Hz, 3H); 13C NMR (125.5 MHz, CDCl3) δ 174.1, 168.6, 167.3, 140.4, 139.0, 136.9, 136.5, 131.8, 129.5, 129.0, 128.2, 126.0, 106.5, 61.9, 47.9, 21.4, 20.1, 14.3; MS (ESI) m/z 409 (M + H+), 431 (M + Na+); HRMS Calcd for C23H25N2O3S (M + H+), 409.1586 Found: 409.1594.
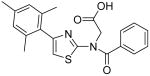
{Benzoyl-[4-(2,4,6-trimethylphenyl)thiazol-2-yl]amino}acetic acid (31)
Compound 30 (75 mg, 0.18 mmol) was dissolved in CH3CN (6 mL). Then, MeOH (3 mL), H2O (1.5 mL) and LiOH·H2O (30 mg, 0.71 mmol) were added subsequently. The resultant mixture was stirred at room temperature for 4h. H2O (20 mL) was added to quench the reaction and HCl (1M, 3 mL) was added. The resultant mixture was extracted with EtOAc (3 × 20 mL). The combined organic phases were dried with anhydrous Na2SO4. Removal of all the solvent in vacuo resulted in a residue, which was purified with silica gel chromatography (Hexane / EtOAc = 2 : 1 to 1 : 2) to give compound 31 (53 mg, 77%) as a white solid. mp 198-199 °C; 1H NMR (500 MHz, CDCl3) δ 10.40 (br, 1H), 8.30 (d, J = 7.5 Hz, 2H), 7.49-7.39 (m, 3H), 7.00 (s, 2H), 6.48 (s, 1H), 4.59 (2H), 2.38 (s, 3H), 2.10 (S, 6H); 13C NMR (100.5 MHz, CDCl3) δ 174.1, 171.1, 168.8, 140.4, 138.8, 136.6, 136.2, 131.8, 129.4, 128.9, 128.2, 125.5, 106.9, 47.7, 21.3, 19.9; MS (ESI) m/z 381 (M + H+), 403 (M + Na+); 761 (2M + H+), 783 (M + 2Na+); HRMS Calcd for C21H20N2O3SNa (M + Na+), 403.1092 Found: 403.1093.
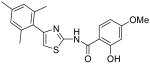
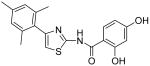
2-Hydroxy-4-methoxy-N-[4-(2,4,6-trimethylphenyl)thiazol-2-yl]benzamide (32) and 2,4-dihydroxy-N-[4-(2,4,6-trimethylphenyl)thiazol-2-yl]benzamide (33)
To a 0°C solution of INH2 (148 mg, 0.39 mmol) in CH2Cl2 (2.5 mL) was added BBr3 (2.5 mL, 1M in CH2Cl2, 2.5 mmol) dropwise. The mixture was warmed up to room temperature and stirred for 3h. Then, MeOH (1 mL) was added and all the solvent was removed in vacuo. The procedure of addition of MeOH (1 mL) was repeated four times. The residue was purified by silica gel chromatography (hexane / EtOAc = 4 : 1) to give compound 32 (less polar, 28 mg, 20%) as a solid and compound 33 (more polar, 88mg, 64%) as a solid. Compound 32: mp 206-207 °C; 1H NMR (500 MHz, CDCl3) δ 11.83 (s, 2H), 7.47 (s, 1H), 6.75 (s, 1H), 6.68 (s, 2H), 6.33 (dd, J = 2.0, 2.0 Hz, 1H), 6.22 (s, 1H), 3.78 (s, 3H), 2.22 (s, 3H), 1.98 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 167.15, 165.02, 162.51, 159.27, 147.98, 138.19, 137.04, 130.88, 129.57, 128.34, 111.62, 107.47, 106.91, 101.54, 55.61, 21.16, 20.51; MS (ESI) m/z 369 (M + H+); HRMS Calcd for C20H20N2O3SNa (M + Na+), 391.1092 Found: 325.1082. Compound 33: mp 236.5-237.5 °C; 1H NMR (400 MHz, DMSO-D6) δ 11.83 (s, 2H), 10.31 (s, 1H), 7.93 (d, J = 6.4 Hz, 1H), 6.98 (s, 1H), 6.91 (s, 2H), 6.42 (d, J = 6.4 Hz, 2H), 2.25 (s, 3H), 2.05 (s, 6H); 13C NMR (100.5 MHz, DMSO-D6) δ 164.33, 163.08, 159.88, 156.88, 148.24, 136.62, 132.48, 131.85, 127.89, 111.16, 108.28, 107.38, 102.74, 20.69, 20.04; MS (ESI) m/z 355 (M + H+); HRMS Calcd for C19H18N2O3SNa (M + Na+), 377.0936 Found: 377.0932.
4-(2-Methoxy-4,6-dimethylphenyl)thiazol-2-ylamine (35)
To a solution of compound 34 (500 mg, 3.05 mmol) in acetone (30 mL) was added K2CO3 (7.6 g, 55 mmol) and Me2SO4 (4.5 mL, 47.5 mmol). The resultant mixture was heated to reflux for 4h. Then, the mixture was cooled to room temperature and NaOH (2N, 100 mL) was added. The whole mixture was extracted with CH2Cl2 (3 × 60 mL) and the combined organic phases were dried over anhydrous Na2SO4. Removal of all the solvent in vacuo gave a residue, which was dissolved in dioxane (8 mL). To the resultant solution, HBr (0.6 ml, 5.3 mmol) and tBuOOH (0.6 mL, 5.0-6.0M in hexane, ∼3.4 mmol) were added subsequently. The mixture was refluxed overnight. After cooled to room temperature, all the solvent was removed in vacuo to give a residue. The residue was dissolved in H2O (20 mL) and the whole mixture was extracted with CH2Cl2 (3 × 60 mL). The combined organic layers were dried over anhydrous Na2SO4. All the solvent was removed in vacuo and the resultant residue was purified by silica gel chromatography (hexane / EtOAc = 100 : 1) to give a compound (169 mg) as a oil. This oil (169 mg) was dissolved in EtOH (4 mL) and thiourea (59 mg, 0.78 mmol) was added. The resultant mixture was heated to reflux for 1h. After cooled to room temperature, all the solvent was removed in vacuo to provide a residue. To this residue, saturated aqueous NaHCO3 (15 mL) was added and the mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic layers were dried over anhydrous Na2SO4. All the solvent was removed in vacuo and the resultant residue was purified by silica gel chromatography (hexane / EtOAc = 2 : 1 to 1 : 1) to give compound 35 (140 mg, 20%) as a solid. 1H NMR (400 MHz, CDCl3) δ 6.68 (t, J = 0.8 Hz, 1H), 6.60 (s, 1H), 6.35 (s, 1H), 5.05 (s, 2H), 3.74 (s, 3H), 2.34 (s, 3H), 2.17 (s, 3H); 13C NMR (100.5 MHz, CDCl3) δ 166.65, 158.02, 147.15, 139.11, 138.93, 123.36, 121.95, 109.47, 107.12, 56.07, 21.84, 20.32; MS (ESI) m/z 235.17 (M + H+); HRMS Calcd for C12H15N2OS (M + H+), 235.0905 Found: 235.0907.
N-[4-(2,4-Dimethylphenyl)oxazol-2-yl]benzamide (39)
The mixture of compound 1 (1.0 g, 4.41 mmol) and urea (290 mg, 4.79 mmol) in anhydrous EtOH (17 mL) was heated to reflux for 4.0 h. After that, the solvent was removed in vacuo and the aqueous saturated NaHCO3 was added to make the mixture base (PH =8-9). Then, the mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were dried with anhydrous Na2SO4. After removal of all the solvent, the residue was purified by silica gel chromatography (Hexane / EtOAc = 3 : 1) to afford the desired amine (100 mg) as a solid. A cooled solution of the above solid (100 mg) and DMAP (129 mg, 1.06 mmol) in CH2Cl2 (3 mL) was added a solution of BzCl (100 mg, 0.71 mmol) in CH2Cl2 (1 mL) dropwise. The mixture was warmed up to room temperature and stirred for 40min. After that, the mixture was poured into H2O (20 mL) and the resultant mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic phases were dried with anhydrous Na2SO4. After removal of all the solvent, the residue was purified by silica gel chromatography (Hexane / EtOAc = 8 : 1) to give the compound 39 (92 mg) as a solid. mp 137-138 °C; 1H NMR (400 MHz, CDCl3) δ 10.77 (br, 1H), 7.88 (s, 2H), 7.55 (s, 1H), 7.46 (s, 2H), 7.28 (s, 2H), 7.06-7.10 (m, 2H), 2.39 (s, 3H), 2.33 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 138.6, 135.7, 132.8, 131.9, 128.8, 128.5, 128.2, 127.1, 21.5, 21.3; MS (ESI) m/z 292 (M + H+); HRMS Calcd for C18H17N2O2 (M + H+), 293.1290 Found: 293.1298.
N-[4-(2,4-Dimethylphenyl)thiazol-2-yl]thiobenzamide (40)
The mixture of INH1 (152 mg, 0.49 mmol) and Lawesson reagent (210 mg, 0.49 mmol) in toluene (10 mL) was heated to reflux for 2h. After that, the mixture was cooled to room temperature and all the solvent was removed in vacuo. The resultant residue was purified by silica gel chromatography (Hexane / EtOAc = 20 : 1) to give compound 40 (126 mg, 79%) as a solid. mp 161.5-162.5 °C; 1H NMR (400 MHz, CDCl3) δ 12.37 (br, 1H), 7.68 (d, J = 7.6 Hz, 2H), 7.44 (t, J = 7.2 Hz, 1H), 7.30 (t, J = 7.6 Hz, 2H), 7.20 (d, J = 8.0 Hz, 1H), 6.97-6.93 (m, 2H), 6.88 (s, 1H), 2.34 (s, 3H), 2.29 (s, 3H); 13C NMR (100.5 MHz, CDCl3) δ 195.0, 160.5, 149.6, 141.0, 138.1, 135.7, 131.8, 131.8, 130.9, 129.6, 128.6, 127.3, 126.8, 122.2, 109.7, 21.3, 21.0; MS (ESI) m/z 325 (M + H+), 347 (M + Na+); HRMS Calcd for C18H16N2S2Na (M + Na+), 347.0653 Found: 347.0644.
Supplementary Material
Acknowledgments
This work is supported by NIH grant to W.-H. Lee (CA107568), postdoctoral-multidisciplinary fellowship to X.-L. Qiu (W81XWH-06-1-0418) from USA Department of Defense Congressionally Directed Medical Research Programs (DOD CDMRP), postdoctoral fellowship to G. Wu (PDF0600907) from Susan Komen Breast Cancer Foundation, postdoctoral fellowship to J. Zhu from the California Breast Cancer Research Program and postdoctoral fellowship to L. Zhou (W81XWH-04-1-0470) from USA DOD CDMRP.
Footnotes
Abbreviations: Hec1, high expression in cancer 1; Nek2, NIMA (never in mitosis gene a)-related kinase 2; INH, Inhibitor of Nek2/Hec1; RNAi, RNA interference; SAR, structure-activity relationship; DAPI, 4′,6-diamidino-2-phenylindole; DMAP, 4,4-dimethylaminopyridine.
Supporting Information Available: (A) Analytic data of compounds 4, 8, 10, 12, 15, 17, 19, 25, 36-37. (B) Detailed procedure for preparation of compound-conjugated matrix. Both of these materials are available free of charge via http://pubs.acs.org.
References
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Trudeau ME. Docetaxel: a review of its pharmacology and clinical activity. Can J Oncol. 1996;6:443–457. [PubMed] [Google Scholar]
- 3.Taylor S, Peters JM. Polo and Aurora kinases: lessons derived from chemical biology. Curr Opin Cell Biol. 2008;20:77–84. doi: 10.1016/j.ceb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN, Jr, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14:1639–1648. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 5.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 6.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–1521. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Rodriguez E, Sotillo R, Schvartzman JM, Benezra R. Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc Natl Acad Sci U S A. 2008;105:16719–16724. doi: 10.1073/pnas.0803504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH. Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem. 2002;277:49408–49416. doi: 10.1074/jbc.M207069200. [DOI] [PubMed] [Google Scholar]
- 9.Du J, Cai X, Yao J, Ding X, Wu Q, Pei S, Jiang K, Zhang Y, Wang W, Shi Y, Lai Y, Shen J, Teng M, Huang H, Fei Q, Reddy ES, Zhu J, Jin C, Yao X. The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability. Oncogene. 2008;27:4107–4114. doi: 10.1038/onc.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R, Lau J, Chen PL, Lee WH. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res. 2008;68:8393–8399. doi: 10.1158/0008-5472.CAN-08-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 12.DeLuca JG, Moree B, Hickey JM, Kilmartin JV, Salmon ED. hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J Cell Biol. 2002;159:549–555. doi: 10.1083/jcb.200208159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deluca JG, Dong Y, Hergert P, Strauss J, Hickey JM, Salmon ED, McEwen BF. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell. 2005;16:519–531. doi: 10.1091/mbc.E04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YT, Chen Y, Wu G, Lee WH. Hec1 sequentially recruits Zwint-1 and ZW10 to kinetochores for faithful chromosome segregation and spindle checkpoint control. Oncogene. 2006;25:6901–6914. doi: 10.1038/sj.onc.1209687. [DOI] [PubMed] [Google Scholar]
- 15.Fry AM, Descombes P, Twomey C, Bacchieri R, Nigg EA. The NIMA-related kinase X-Nek2B is required for efficient assembly of the zygotic centrosome in Xenopus laevis. J Cell Sci. 2000;113(Pt 11):1973–1984. doi: 10.1242/jcs.113.11.1973. [DOI] [PubMed] [Google Scholar]
- 16.Sonn S, Khang I, Kim K, Rhee K. Suppression of Nek2A in mouse early embryos confirms its requirement for chromosome segregation. J Cell Sci. 2004;117:5557–5566. doi: 10.1242/jcs.01476. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher L, Cerniglia GJ, Yen TJ, Muschel RJ. Live cell imaging reveals distinct roles in cell cycle regulation for Nek2A and Nek2B. Biochim Biophys Acta. 2005;1744:89–92. doi: 10.1016/j.bbamcr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Prigent C, Glover DM, Giet R. Drosophila Nek2 protein kinase knockdown leads to centrosome maturation defects while overexpression causes centrosome fragmentation and cytokinesis failure. Exp Cell Res. 2005;303:1–13. doi: 10.1016/j.yexcr.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Wu G, Lin YT, Wei R, Chen Y, Shan Z, Lee WH. Hice1, a novel microtubule-associated protein required for maintenance of spindle integrity and chromosomal stability in human cells. Mol Cell Biol. 2008;28:3652–3662. doi: 10.1128/MCB.01923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.