Abstract
In ischemic preconditioning (IPC) brief ischemia/reperfusion renders the heart resistant to infarction from any subsequent ischemic insult. Protection results from binding of surface receptors by ligands released during the preconditioning ischemia. The downstream pathway involves redox signaling as IPC will not protect in the presence of a free radical scavenger. To determine when the redox signaling occurs, five groups of isolated rabbit hearts were studied. All hearts underwent 30 min of coronary branch occlusion and 2 h of reperfusion. IPC groups were subjected to 5 min of regional ischemia followed by 10 min of reperfusion prior to the 30-min coronary occlusion. The Control group had only the 30-min occlusion and 2-h reperfusion. The second group had IPC alone. The third group was also preconditioned, but the free radical scavenger N-2-mercaptopropionyl glycine (MPG, 300 µM) was infused during the 10-min reperfusion and therefore was present in the myocardium in the distribution of the snared coronary artery during the entire reperfusion phase and also during the subsequent 30-min ischemia. In another preconditioned group MPG was added to the perfusate before the preconditioning ischemia and therefore was present in the tissue only during the preconditioning ischemia and then was washed out during reperfusion. In the fifth group MPG was added to the perfusate for only the last 5 min of the preconditioning reperfusion and therefore was present in the tissue during the last minutes of the reperfusion phase and the 30 min of ischemia. Infarct size and risk size were measured by triphenyltetrazolium staining and fluorescent microspheres, resp. IPC reduced infarct size from 31.3±2.7% of the ischemic zone in control hearts to only 8.4±1.9%. MPG completely blocked IPC’s protection in the 3rd group (39.4±2.8%) but did not affect its protection in groups 4 (8.1±1.5%) or 5 (7.8±1.1%). Hence redox signaling occurs during the reperfusion phase of IPC.
Introduction
Exposure of the heart to a brief period of ischemia followed by reperfusion causes it to become very resistant to infarction when exposed to a subsequent ischemic insult. Protection of this ischemic preconditioning (IPC) is triggered by binding of surface receptors by ligands released during the preconditioning ischemia [22, 26]. IPC’s actual protection results from inhibition of mitochondrial permeability transition pores (mPTP) following restoration of blood flow after the lethal ischemic insult [11]. In a non-preconditioned heart production of reactive oxygen species (ROS) and/or calcium entry in the first minutes of reperfusion lead to the opening of mPTP which destroy many of the heart’s mitochondria [23, 25]. In the IPC heart less ROS are produced during reperfusion and mPTP formation is inhibited. Because reactive oxygen species (ROS) are believed to play an important role in the pathogenesis of myocardial ischemia/reperfusion injury [3, 8–10, 23], they were considered to be undesirable. However ROS have recently been shown to be involved in IPC’s cardioprotective signaling [1]. IPC’s trigger phase is dependent on both mitochondrial ATP-sensitive potassium channels (mKATP) [3, 21] and ROS production [21]. mKATP opening of leads to production of ROS by the mitochondria that then trigger protection through redox signaling [15, 19, 20].
It is unknown when ROS signaling actually occurs. We have proposed that the ROS release that triggers IPC occurs during the reperfusion phase of IPC when oxygen tension is high in the myocardium. Release of agonists by ischemic cells and binding of surface receptors would occur in both preconditioned and naïve hearts, but ROS generation and triggering of protection during the brief reperfusion would occur in only IPC hearts. Thus both ischemia and well as reperfusion are needed to put the heart into a protected state. Unfortunately direct measurements of ROS production in ischemic heart cells have not supported our hypothesis. In chick myocytes dichlorofluorescein (DCF), a probe that becomes fluorescent in the presence of H2O2 [12], has indicated that ROS are made during simulated ischemia and production actually stops during reoxygenation [3]. Of course we do not know if the ROS detected by DCF are the same ROS that are responsible for IPC’s redox signaling. We, therefore, designed protocols to determine when redox signaling is required. We confined the free radical scavenger N-2-mercaptopropionyl glycine (MPG) to either the ischemic or the reperfusion phase of IPC and observed markedly different effects.
Methods
Isolated heart model
All animal care adhered to published guidelines [17] and the procedures were approved by the university’s Institutional Animal Care and Use Committee. New Zealand White rabbits were anesthetized with sodium pentobarbital (30 mg/kg) and ventilated with 100% oxygen. Hearts were exposed through a left thoracotomy, and a suture was passed around a branch of the left coronary artery to form a snare. The heart was excised and perfused on a Langendorff apparatus with modified Krebs-Henseleit bicarbonate buffer that contained (in mM) 118.5 NaCl, 24.7 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, and 10.0 glucose. The buffer was gassed with 95% O2/5%CO2. A fluid-filled latex balloon was inserted into the left ventricle to measure pressure. All hearts were allowed to equilibrate for 20 min before the protocol was started.
Protocol for infarct studies
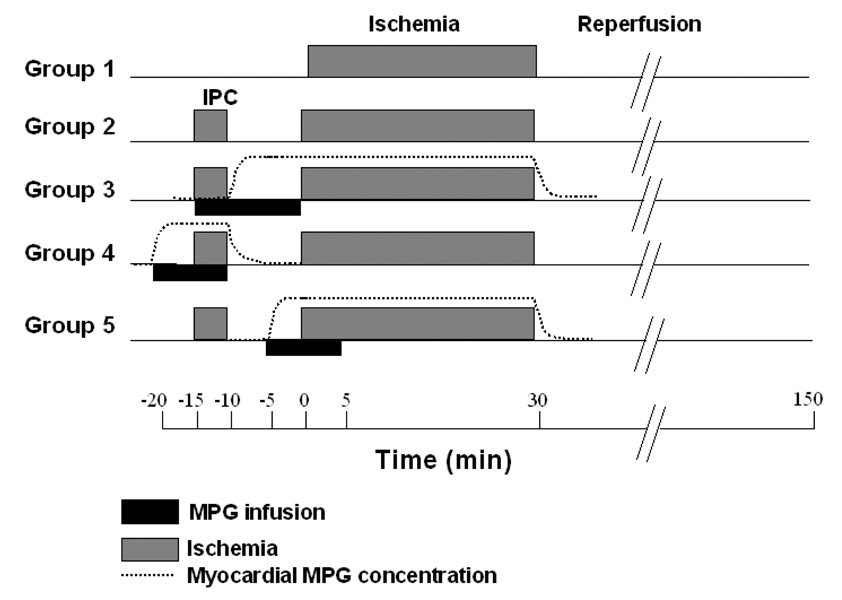
Five groups of isolated rabbit hearts were studied. All groups underwent 30 min of coronary branch occlusion and 2 h of reperfusion. IPC groups were subjected to 5 min of regional ischemia followed by 10 min of reperfusion prior to the 30 min of coronary occlusion. A detailed diagram of the protocols appears in Fig 1. The control group had only the 30-min occlusion and 2-h reperfusion. The second group had only IPC. In the third group also with IPC MPG (300 µM) was added to the perfusate for 15 min starting just after the beginning of the preconditioning ischemia and ending with the onset of the 30-min ischemic period. Because rabbit hearts have very sparse collateralization MPG would not be expected to enter the tissue in the distribution of the snared artery until the onset of IPC’s reperfusion period and would not be washed out until after the 30-min ischemic period. The fourth group was also preconditioned and MPG was infused for 10 min starting 5 min prior to IPC’s ischemia and ending with release of the 5-min coronary occlusion. Therefore, MPG would have been present in the tissue during the preconditioning ischemia and then quickly washed out during IPC’s reperfusion phase. In the fifth group a 10-min infusion of MPG was started in the fifth minute of preconditioning’s reperfusion period. Thus MPG would have entered the tissue during the last 5 min of reperfusion and been present during the subsequent 30-min ischemia. The three groups were necessary because the washin and washout times would have been in the order of several minutes making it impossible to have MPG present in the tissue during only ischemia or during only reperfusion. For example, in group 3 a small amount of MPG may have entered the tissue during the preconditioning ischemia and definitely would have been present during the 30-min ischemic period. Group 5 then tested whether the MPG during the 30-min ischemic period was critical.
Fig. 1.
Infarct protocols. Abbreviations: MPG= N-2-mercaptopropionyl glycine,
Measurement of infarct size
At the end of the experiment, the coronary artery was reoccluded, and 2–9 µm fluorescent microspheres (Microgenics Corp., Freemont, CA) were infused to delineate the ischemic zone (region at risk) as the area of tissue without fluorescence. The heart was cut into 2-mm-thick slices. The slices were incubated in 1% triphenyltetrazolium chloride in sodium phosphate buffer (pH 7.4) at 37°C for 10 min. The slices were then immersed in 10% formalin to preserve the stained (viable) and unstained (necrotic) tissue. The risk zone was identified by illuminating the slices with ultraviolet light which excited the fluorescent microspheres present in the non-ischemic muscle. The areas of infarct and risk zone were determined by planimetry of each slice, and volumes were calculated by multiplying each area by the slice thickness and summing them for each heart. Infarct size was expressed as a percentage of the risk zone.
Data analysis
All data are expressed as mean ± SEM. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed on baseline hemodynamic variables, risk zone, and infarct size. A value of p<0.05 was considered to be significant.
Results
Hemodyamics
There were no differences in baseline hemodynamics among the 5 groups (see Table 1). Coronary occlusion caused an expected decrease in left ventricular developed pressure and coronary flow in all groups. There was partial recovery following reperfusion.
Table 1.
Hemodynamic data
|
Ventricular Pressure and Coronary Flow | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Intervention* |
30 min occlusion |
30 min reperfusion |
|||||
| Group | LVDP mmHg | CF ml/min/g | LVDP mmHg | CF ml/min/g | LVDP mmHg | CF ml/min/g | LVDP mmHg | CF ml/min/g |
| Control (Group 1) | 113 ± 6.2 | 8.0 ± 0.3 | N/A | N/A | 45 ± 5.1† | 6.0 ± 0.3† | 86 ± 5.8† | 7.0 ± 0.5† |
| IPC (Group 2) | 116 ± 3.8 | 9.8 ± 0.5 | 52 ± 4.4† | 5.5 ± 0.4† | 51 ± 7.6† | 5.8 ± 0.5† | 79 ± 3.7† | 8.1 ± 0.5 |
| IPC +MPG (Group 3) | 112 ± 4.5 | 9.0 ± 0.7 | 61 ± 3.7† | 6.0 ± 0.3† | 52 ± 6.7† | 6.1 ± 0.4† | 72 ± 4.3† | 7.0 ± 0.5 |
| IPC +MPG (Group 4) | 110 ± 3.7 | 9.3 ± 0.6 | 109 ± 3.7 | 9.3 ± 0.6 | 47 ± 6.9† | 6.0 ± 0.4† | 77 ± 6.2† | 8.0 ± 0.6 |
| IPC +MPG (Group 5) | 111 ± 4.8 | 10.1 ± 0.6 | 99 ± 5.1 | 9.9 ± 0.8 | 53 ± 9.7† | 6.6 ± 0.4† | 80 ± 3.6† | 9.0 ± 0.6 |
|
Heart Rate (bpm) | ||||||||
|
Group |
Baseline |
Intervention* |
30 min occlusion |
30 min reperfusion |
||||
| Control (Group 1) | 203 ± 4.9 | N/A | 197 ± 5.8 | 200 ± 4.6 | ||||
| IPC (Group 2) | 194 ± 6.9 | 178 ± 9.9 | 177 ± 9.2 | 192 ± 4.9 | ||||
| IPC +MPG (Group 3) | 199 ± 8.8 | 186 ± 11.5 | 216 ± 7.7 | 216 ± 6.2 | ||||
| IPC +MPG (Group 4) | 185 ± 8.5 | 190 ± 6.6 | 176 ± 8.6 | 196 ± 6.5 | ||||
| IPC +MPG (Group 5) | 182 ± 4.5 | 180 ± 4.4 | 174 ± 9.2 | 175 ± 11.4 | ||||
Values are means±SEM. Abbreviations: CF = coronary flow, IPC = ischemic preconditioning, LVDP = left ventricular developed pressure, MPG = N-(2-mercaptopropionyl) glycine.
Hemodynamics measured at beginning of preconditioning ischemia in IPC, at beginning of MPG infusion after beginning of IPC in Group 3, during the fifth min of MPG infusion prior to IPC in Group 4, and at the beginning of the MPG infusion in the sixth minute of reperfusion in group 5.
p<0.05 vs. Baseline
Infarct size
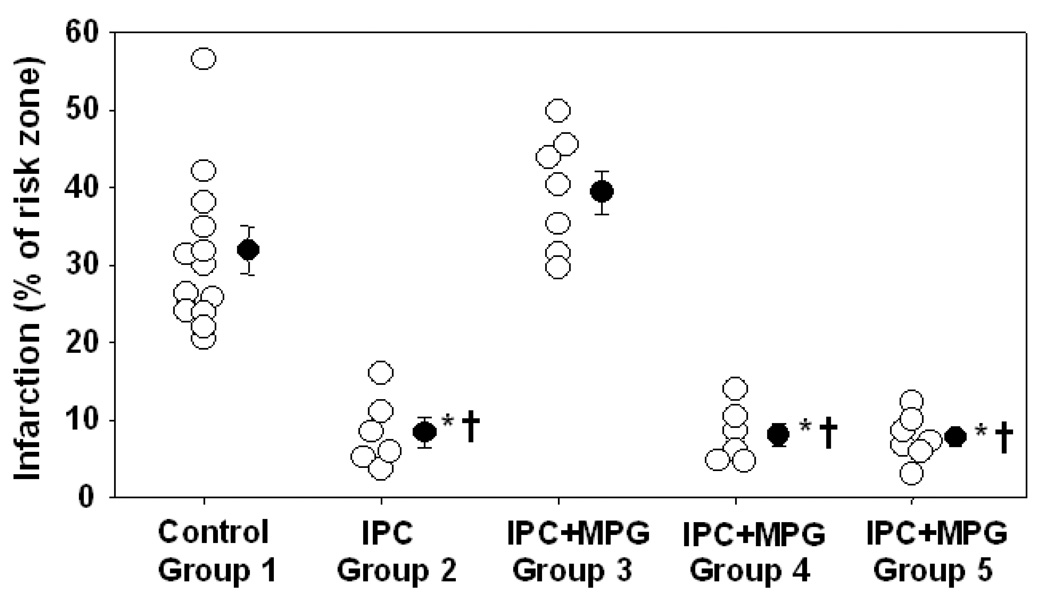
There was no significant difference in body weight or heart weight among the groups (see Table 2). Preconditioning reduced infarct size from 31.3±2.7% of the ischemic zone in control hearts to only 8.4±1.9% (p<0.001) (Fig. 2). MPG completely blocked preconditioning’s protection (p<0.001) in the third group (39.4±2.8%), but did not alter preconditioning’s protection in the fourth (8.1±1.5%) or fifth (7.8±1.1%) groups. Group 3 is the only group that would have had MPG present in the myocardium in the distribution of the snared artery during the early minutes of preconditioning’s reperfusion phase.
Table 2.
Infarct size data
| Group | n | Body Weight (kg) | Heart Weight (g) | Risk Zone Volume (cm3) | Infarct Volume (cm3) | I/R (%) |
|---|---|---|---|---|---|---|
| Control (Group 1) | 13 | 2.2 ± 0.0 | 7.3 ± 0.2 | 1.27 ± 0.07 | 0.39 ± 0.04 | 31.3 ± 2.7 |
| IPC (Group 2) | 6 | 2.1 ± 0.0 | 6.9 ± 0.2 | 1.30 ± 0.08 | 0.10 ± 0.00 | 8.4 ± 1.9*† |
| IPC+MPG (Group 3) | 7 | 2.0 ± 0.0 | 6.8 ± 0.3 | 1.28 ± 0.13 | 0.51 ± 0.07 | 39.4 ± 2.8 |
| IPC+MPG (Group 4) | 6 | 2.1 ± 0.0 | 6.8 ± 0.2 | 1.16 ± 0.06 | 0.10 ± 0.02 | 8.1 ± 1.5*† |
| IPC+MPG (Group 5) | 7 | 2.0 ± 0.0 | 6.5 ± 0.1 | 1.13 ± 0.08 | 0.09 ± 0.01 | 7.8 ± 1.1*† |
Values are means±SEM.
Abbreviations: see Table 1; I/R=infarction as a % of risk zone; n=number of animals
p<0.001 vs. Control,
p<0.001 vs. Group 3
Fig. 2.
Infarct size as a percentage of risk zone for individual hearts and group means with SEM. The protective effect of ischemic preconditioning (IPC) was blocked in group 3 (2-N-mercaptopropionyl glycine infused for 15 min during IPC ischemia and reperfusion). *p<0.001 vs. Control, †p<0.001 vs. group 3
Discussion
Free radicals have been proposed to be an important part of the mechanism of preconditioning [1, 6, 20]. ROS have a physiological role in modulating cell signaling pathways. Despite the fact that ROS act as second messengers in normal cells at low concentrations, at high concentrations ROS are toxic and can induce cell damage. It is well known that a burst of ROS occurs when molecular oxygen is returned to cells that have endured prolonged ischemia [2, 7]. ROS are also generated during ischemia. [3, 13] Residual oxygen during ischemia apparently provides sufficient molecular O2 for generation of superoxide anion (O2−•) by mitochondria. When isolated cardiomyocytes are incubated with DCF, simulated ischemia increases fluorescence in cells with functioning mitochondria [2, 3, 24], and if the hypoxic period is brief reoxygenation actually reduces ROS production. It has been proposed that the ROS oxidizing DCF are the same ones that trigger preconditioning [2]. The present data would argue against that conclusion, however. In our experiments only when MPG was present throughout most of IPC’s reperfusion phase were hearts not protected by IPC (group 3). When MPG was present in the myocardium only during the preconditioning ischemia (group 4), protection of that tissue was not affected. Also loading the tissue with MPG prior to the prolonged ischemic insult also failed to affect protection. Thus the redox signaling that triggers IPC’s protection occurs during early reperfusion rather than during the preceding ischemia. Therefore, any H2O2 produced during ischemia is probably unrelated to the signaling responsible for triggering of preconditioning.
Mitochondria make small quantities of superoxide because of the leak of electrons in their electron transport chain. Dismutation of superoxide anion produces hydrogen peroxide (H2O2) which DCF detects [12]. Although, other potential source of ROS, such as nitric oxide synthase, NADPH oxidases, and xanthine oxidase, contribute to ROS generation, mitochondria are thought to be the source of the ROS that trigger IPC because those ROS are under the control of mKATP [5, 18, 20].
Regardless of the identity of the ROS signal that triggers IPC’s protection, MPG must be capable of scavenging it. MPG is actually a very selective scavenger of hydroxyl radical and peroxynitrite and does not react with H2O2 or superoxide [4, 16]. DCF should be insensitive to a low level hydroxyl radical signal. We found that reduced Mitotracker red which is readily oxidized by ROS to a fluorescent product is a very sensitive reporter for ROS generated by mKATP openers in well-oxygenated isolated cardiomyocytes [18]. We were unable to oxidize reduced Mitotracker red with H2O2 in a test tube indicating that it does not detect this species. In our pilot studies DCF could not detect the mKATP-induced ROS signal while Mitotracker could (unpublished observation). Also MPG completely abolished the Mitotracker signal. [14] These observations suggest that DCF detects a different ROS species than that which is responsible for triggering IPC.
Conclusion
This study clearly indicates that the critical time for the redox signaling that triggers entrance into the preconditioned state is during IPC’s early reperfusion phase rather than during the preconditioning ischemia. These data suggest that the H2O2 seen during simulated ischemia in cells is unrelated to the redox signaling responsible for triggering of preconditioning. Finally they explain why ischemia interrupted by a brief period of reperfusion is required to trigger a protected state.
Acknowledgments
This work was supported in part by grants HL-20468 from the Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Dost was supported by a grant from the Scientific and Technological Research Council of Turkey (TUBITAK).
Reference List
- 1.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 2.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Becker LB, Vanden Hoek TL, Shao Z-H, Li C-Q, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Jeroudi MO, Patel BS, Aruoma OI, Halliwell B, Lai EK, McCay PB. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion: evidence that myocardial "stunning" is a manifestation of reperfusion injury. Circ Res. 1989;65:607–622. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- 5.Costa ADT, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 6.Facundo HT, Carreira RS, de Paula JG, Santos CC, Ferranti R, Laurindo FR, Kowaltowski AJ. Ischemic preconditioning requires increases in reactive oxygen release independent of mitochondrial K+ channel activity. Free Radic Biol Med. 2006;40:469–479. doi: 10.1016/j.freeradbiomed.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Fantinelli JC, Cingolani HE, Mosca SM. Na+/H+ exchanger inhibition at the onset of reperfusion decreases myocardial infarct size: role of reactive oxygen species. Cardiovasc Pathol. 2006;15:179–184. doi: 10.1016/j.carpath.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 9.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 10.Halestrap AP, Kerr PM, Javadov S, Woodfield K-Y. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol. 2004;287:H841–H849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 12.Keston AS, Brandt R. The Fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- 13.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 14.Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, Downey JM, Benoit JN. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol. 2002;97:365–373. doi: 10.1007/s003950200045. [DOI] [PubMed] [Google Scholar]
- 15.Krieg T, Qin Q, Philipp S, Alexeyev MF, Cohen MV, Downey JM. Acetylcholine and bradykinin trigger preconditioning in the heart through a pathway that includes Akt and NOS. Am J Physiol. 2004;287:H2606–H2611. doi: 10.1152/ajpheart.00600.2004. [DOI] [PubMed] [Google Scholar]
- 16.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42:812–825. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Research Council. Guide for the Care and Use of Laboratory Animals. 7th ed. National Academy Press: Washington, D.C; 1996. [Google Scholar]
- 18.Oldenburg O, Critz SD, Cohen MV, Downey JM. Acetylcholine-induced production of reactive oxygen species in adult rabbit ventricular myocytes is dependent on phosphatidylinositol 3- and Src-kinase activation and mitochondrial KATP channel opening. J Mol Cell Cardiol. 2003;35:653–660. doi: 10.1016/s0022-2828(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 19.Oldenburg O, Qin Q, Krieg T, Yang X-M, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol. 2004;286:H468–H476. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 20.Pain T, Yang X-M, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 21.Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, Losano G, Pagliaro P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006;101:180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- 22.Schulz R, Cohen MV, Behrends M, Downey JM, Heusch G. Signal transduction of ischemic preconditioning. Cardiovasc Res. 2001;52:181–198. doi: 10.1016/s0008-6363(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 23.Solaini G, Harris DA. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J. 2005;390:377–394. doi: 10.1042/BJ20042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X-M, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]