Abstract
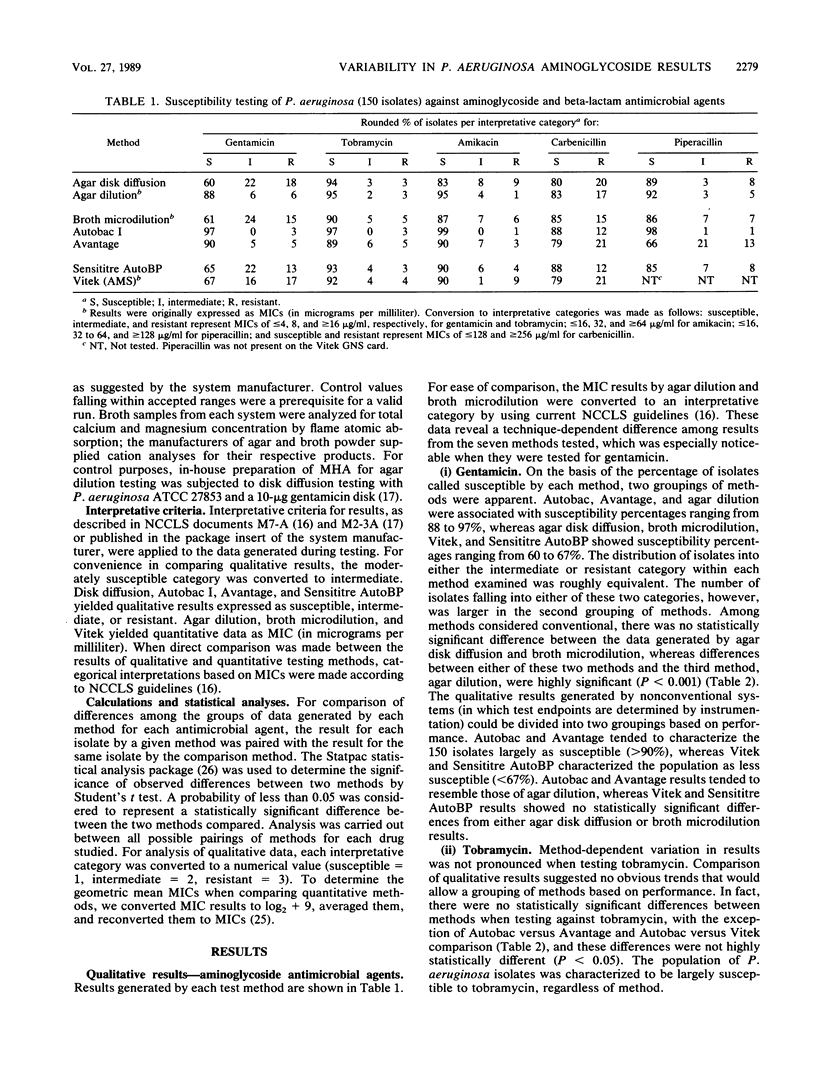
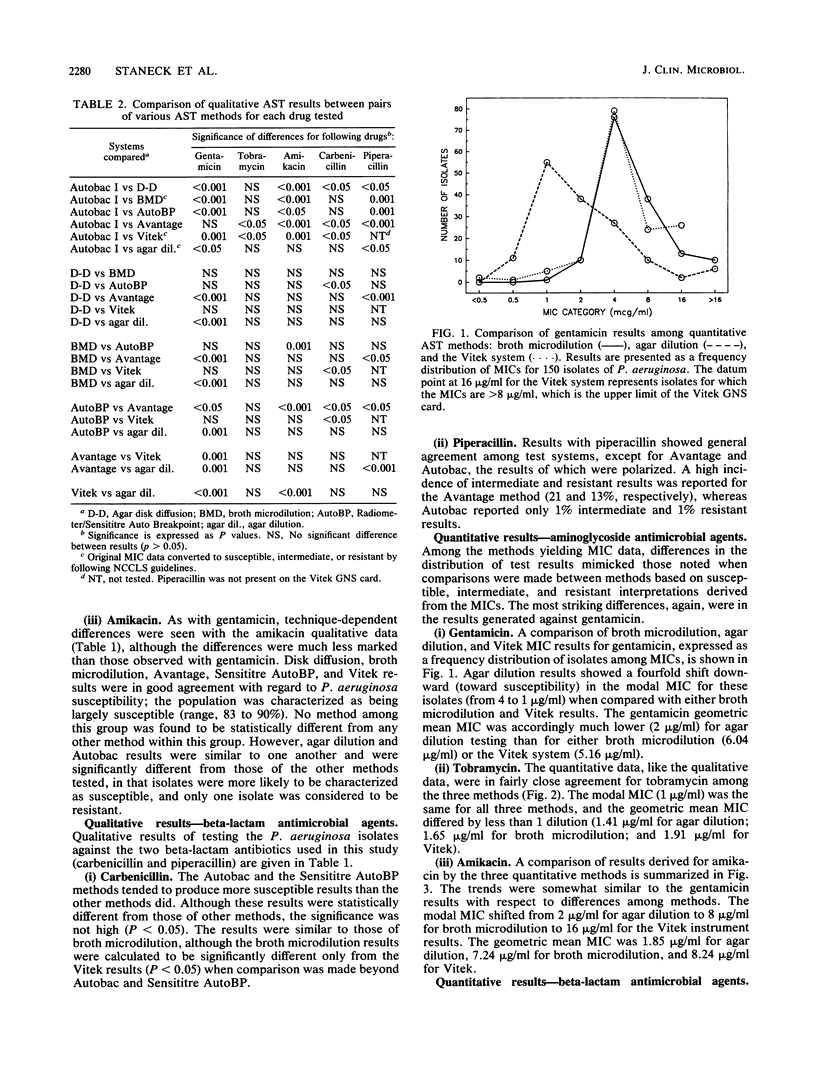
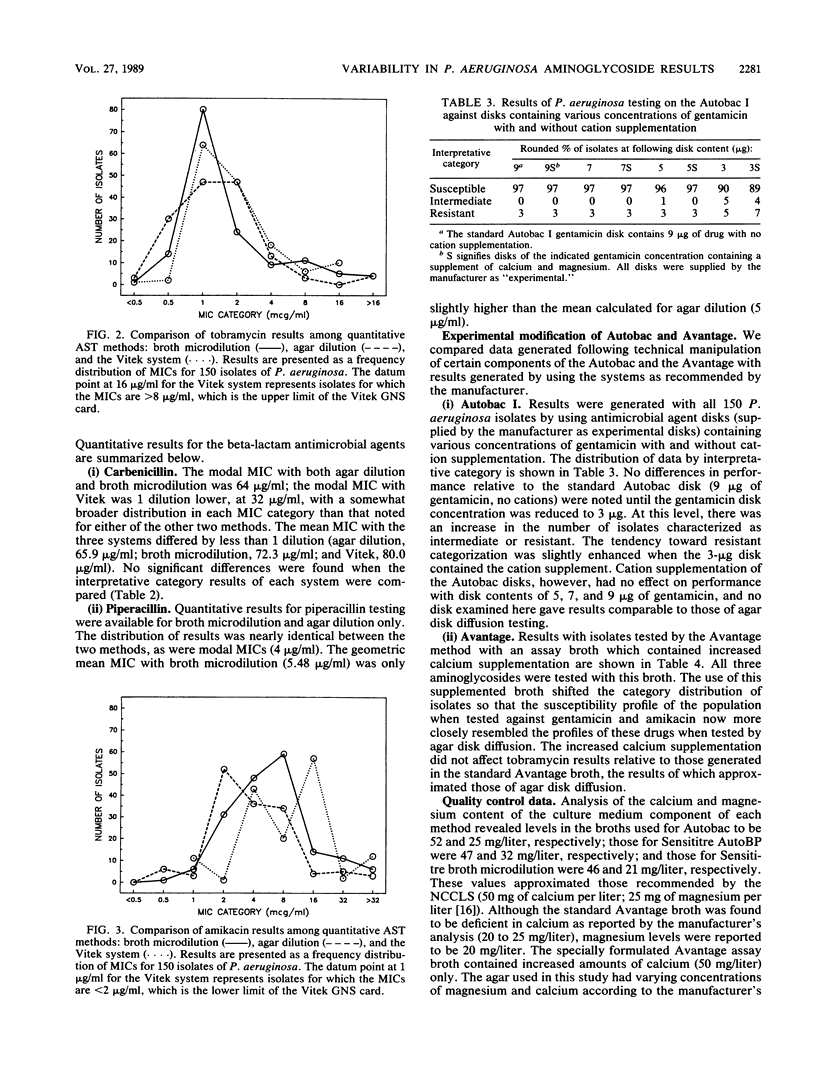
Seven commonly used antimicrobial susceptibility testing methods were used to test the susceptibility of 150 isolates of Pseudomonas aeruginosa against gentamicin, tobramycin, amikacin, carbenicillin, and piperacillin. Results were compared with respect to the susceptibility characteristics of the population of isolates as defined by each method. Conventional methods included agar disk diffusion and agar dilution, carried out in accordance with current recommendations of the National Committee for Clinical Laboratory Standards, as well as broth microdilution testing with cation-supplemented Mueller-Hinton broth (CSMHB). Methods in which instrumentation was used for result determination included the Autobac I, Avantage, Sensititre Autoreader (using a breakpoint panel at 18 h of incubation), and Vitek (AMS-240, using the GNS susceptibility card). When necessary for comparison, MIC data were converted to categorical interpretations (susceptible, intermediate, and resistant). With respect to gentamicin, no significant differences were noted among the results of disk diffusion, broth microdilution, Sensititre Auto breakpoint, or Vitek methods which characterized 60 to 67% of isolates as susceptible, 16 to 22% as intermediate, and 13 to 17% as resistant. In contrast, agar dilution, Autobac, and Avantage, although yielding gentamicin results similar to those of one another, were each significantly different in result reporting from the other four methods above for gentamicin results, and they characterized the Pseudomonas population largely as susceptible (88 to 97%), with 0 to 6% intermediate and only 3% to 6% resistant. More isolates were characterized as being resistant to gentamicin in the Avantage test if an assay broth supplemented with increased amounts of calcium was used. Cation impregnation of Autobac disks did not appreciably change Autobac results. The geometric mean MIC of gentamicin was 4 micrograms/ml lower in the agar dilution method than in the CSMHB microdilution method, despite monitoring of the agar for cation content through performance disk diffusion testing with P. aeruginosa ATCC 27853. Tobramycin activity was greater than gentamicin activity, and susceptibility to tobramycin ranged from 89 to 97%, with few statistically significant differences noted among the seven methods studied. Differences in MIC distribution and geometric mean MIC between agar dilution and CSMHB microdilution testing were minimal and suggested less of a cation influence on tobramycin than gentamicin results. Although amikacin was also more active than gentamicin (83 to 99% of isolates were susceptible), differences in the amikacin results among methods tended to reflect the same trends in reporting as seen with gentamicin testing, with the exception that results of Avantage testing were similar to those of disk diffusion, CSMHB microdilution, Sensititre, and Vitek. A difference in geometric mean MIC of 5 micrograms/ml between CSMHB testing and agar dilution testing suggested the influence of divalent cations on amikacin results. Few highly significant differences were noted among methods when isolates were tested against carbenicillin and piperacillin, except that Avantage piperacillin results (66% susceptible) and Autobac piperacillin results (98% susceptible) were noticeably different from the percent piperacillin susceptibility (range, 85 to 92%) measured by the other methods. Method-dependent variability among aminoglycoside susceptibility results, particularly when testing gentamicin, prevents meaningful comparison of Pseudomonas susceptibility trends among hospitals when different methods are used and promotes confusion and frustration among clinical microbiologists and clinicians owing to the uncertainties of clinical meaning of these data.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Miller G. H., Thornsberry C., Hare R. S., Jones R. N., Lorber R. R., Ferraresi R., Cramer C. Influence of cation supplements on activity of netilmicin against Pseudomonas aeruginosa in vitro and in vivo. Antimicrob Agents Chemother. 1987 Oct;31(10):1514–1518. doi: 10.1128/aac.31.10.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Schoenknecht F. D., Norton R., O'Brien T. F., Matsen J. M., Thornsberry C., Thrupp L. D., Markley E., Gavan T. L. Inter- and intralaboratory variability in antibiotic susceptibility tests with Pseudomonas aeruginosa and Enterobacteriaceae. J Infect Dis. 1976 Oct;134(4):328–335. doi: 10.1093/infdis/134.4.328. [DOI] [PubMed] [Google Scholar]
- Casillas E., Kenny M. A., Minshew B. H., Schoenknecht F. D. Effect of ionized calcium and soluble magnesium on the predictability of the performance of Mueller-Hinton agar susceptibility testing of Pseudomonas aeruginosa with gentamicin. Antimicrob Agents Chemother. 1981 Jun;19(6):987–992. doi: 10.1128/aac.19.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherne J. E., Peterson L. R. Microdilution aminoglycoside susceptibility testing of Pseudomonas aeruginosa and Escherichia coli with a cation-supplemented inoculum. Antimicrob Agents Chemother. 1980 Jul;18(1):210–211. doi: 10.1128/aac.18.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'amato R. F., Thornsberry C., Baker C. N., Kirven L. A. Effect of calcium and magnesium ions on the susceptibility of Pseudomonas species to tetracycline, gentamicin polymyxin B, and carbenicillin. Antimicrob Agents Chemother. 1975 May;7(5):596–600. doi: 10.1128/aac.7.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. D., Iannetta A. Antagonistic effect of calcium in serum on the activity of tobramycin against Pseudomonas. Antimicrob Agents Chemother. 1972 Jun;1(6):466–469. doi: 10.1128/aac.1.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. D., Iannetta A. Relative antagonism in vitro of calcium in serum to the bactericidal activities of gentamicin and tobramycin on Pseudomonas aeruginosa. Chemotherapy. 1973 Oct;19(4):243–253. doi: 10.1159/000221461. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Staneck J. L., Needham C., Tubert T. Sensititre autoreader for same-day breakpoint broth microdilution susceptibility testing of members of the family Enterobacteriaceae. J Clin Microbiol. 1987 Aug;25(8):1481–1485. doi: 10.1128/jcm.25.8.1481-1485.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod L. P., Waterworth P. M. Effect of medium composition on the apparent sensitivity of Pseudomonas aeruginosa to gentamicin. J Clin Pathol. 1969 Sep;22(5):534–538. doi: 10.1136/jcp.22.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N., Kutscher E., Ireland P., Barnett J. A., Sanford J. P. Effect of the concentrations of magnesium and calcium on the in-vitro susceptibility of Pseudomonas aeruginosa to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S37–S45. doi: 10.1093/infdis/124.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- Kenny M. A., Pollock H. M., Minshew B. H., Casillas E., Schoenknecht F. D. Cation components of Mueller-Hinton agar affecting testing of Pseudomonas aeruginosa susceptibility to gentamicin. Antimicrob Agents Chemother. 1980 Jan;17(1):55–62. doi: 10.1128/aac.17.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo J. B., Kiehn T. E., Wong B., Bernard E. M., Armstrong D. Examination of Pseudomonas aeruginosa-gentamicin discrepancies encountered in an Autobac I-disk diffusion comparison. Antimicrob Agents Chemother. 1982 Mar;21(3):412–415. doi: 10.1128/aac.21.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A., O'Brien T. F., Wacker W. E., Yulug N. F. Effect of salt concentration on the apparent in-vitro susceptibility of Pseudomonas and other gram-negative bacilli to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S59–S64. doi: 10.1093/infdis/124.supplement_1.s59. [DOI] [PubMed] [Google Scholar]
- Pollock H. M., Minshew B. H., Kenny M. A., Schoenknecht F. D. Effect of different lots of Mueller-Hinton agar on the interpretation of the gentamicin susceptibility of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Sep;14(3):360–367. doi: 10.1128/aac.14.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Effects of procedural variations on the activity of aminoglycosides in vitro. Am J Clin Pathol. 1975 Mar;63(3):438–445. doi: 10.1093/ajcp/63.3.438. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., Barry A. L., Jones R. N., Baker C. N., Badal R. E., Packer R. R. Antibacterial activity of fortimicin A compared with those of five other aminoglycosides, and factors affecting susceptibility tests. Antimicrob Agents Chemother. 1981 Jan;19(1):122–129. doi: 10.1128/aac.19.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. C., Lieberman L., Gerlach E. H. Microdilution antibiotic susceptibility test: examination of certain variables. Appl Microbiol. 1973 Nov;26(5):658–665. doi: 10.1128/am.26.5.658-665.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Snyder R. J., Kohner P. C., Wiltse C. G., Ilstrup D. M., McCall J. T. Effect of cation content of agar on the activity of gentamicin, tobramycin, and amikacin against Pseudomonas aeruginosa. J Infect Dis. 1978 Feb;137(2):103–111. doi: 10.1093/infdis/137.2.103. [DOI] [PubMed] [Google Scholar]
- Woolfrey B. F., Fox J. M., Quall C. O. Comparison of minimum inhibitory concentration values determined by three antimicrobic dilution methods for Pseudomonas aeruginosa. Am J Clin Pathol. 1981 Jan;75(1):39–44. doi: 10.1093/ajcp/75.1.39. [DOI] [PubMed] [Google Scholar]
- Woolfrey B. F., Lally R. T., Ederer M. N., Quall C. O. Evaluation of the automicrobic system for susceptibility testing of Pseudomonas aeruginosa to gentamicin, tobramycin, and amikacin. J Clin Microbiol. 1984 Apr;19(4):502–505. doi: 10.1128/jcm.19.4.502-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey B. F., Ramadei W. A., Quall C. O. Inability of the standardized disk agar-diffusion test to measure susceptibility of the fluorescent group of pseudomonads to gentamicin. Am J Clin Pathol. 1978 Sep;70(3):337–342. doi: 10.1093/ajcp/70.3.337. [DOI] [PubMed] [Google Scholar]
- Zimelis V. M., Jackson G. G. Activity of aminoglycoside antibiotics aganst Pseudomonas aeruginosa: specificity and site of calcium and magnesium antagonism. J Infect Dis. 1973 Jun;127(6):663–669. doi: 10.1093/infdis/127.6.663. [DOI] [PubMed] [Google Scholar]



