Abstract
Ca2+ modulates the visual response in both vertebrates and invertebrates. In Drosophila photoreceptors, an increase of cytoplasmic Ca2+ mimics light adaptation. Little is known regarding the mechanism, however. We explored the role of the sole Drosophila Ca2+/calmodulin-dependent protein kinase II (CaMKII) to mediate light adaptation. CaMKII has been implicated in the phosphorylation of arrestin 2 (Arr2). However, the functional significance of Arr2 phosphorylation remains debatable. We identified retinal CaMKII by anti-CaMKII antibodies and by its Ca2+-dependent autophosphorylation. Moreover, we show that phosphorylation of CaMKII is greatly enhanced by okadaic acid, and indeed, purified PP2A catalyzes the dephosphorylation of CaMKII. Significantly, we demonstrate that anti-CaMKII antibodies co-immunoprecipitate, and CaMKII fusion proteins pull down the catalytic subunit of PP2A from fly extracts, indicating that PP2A interacts with CaMKII to form a protein complex. To investigate the function of CaMKII in photoreceptors, we show that suppression of CaMKII in transgenic flies affects light adaptation and increases prolonged depolarizing afterpotential amplitude, whereas a reduced PP2A activity brings about reduced prolonged depolarizing afterpotential amplitude. Taken together, we conclude that CaMKII is involved in the negative regulation of the visual response affecting light adaptation, possibly by catalyzing phosphorylation of Arr2. Moreover, the CaMKII activity appears tightly regulated by the co-localized PP2A.
Visual transduction is the process that converts the signal of light (photons) into a change of membrane potential in photoreceptors (see Ref. 1 for review). Visual signaling is initiated upon the activation of rhodopsins by light: light switches on rhodopsin to generate metarhodopsin, which activates the heterotrimeric Gq in Drosophila (2). Subsequently, the GTP-bound Gαq subunit activates phospholipase Cβ4 encoded by the norpA (no receptor potential A) gene (3). Phospholipase Cβ4 catalyzes the breakdown of phosphoinositol 4,5-bisphosphate to generate diacylglycerol, which or its metabolite has been implicated in gating the transient receptor potential (TRP)2 and TRP-like channels (4, 5). TRP is the major Ca2+ channel that mediates the light-dependent depolarization response leading to an increase of cytosolic Ca2+ in photoreceptors. The rise of intracellular Ca2+ modulates several aspects of the visual response including activation, deactivation, and light adaptation (6). For example, Ca2+ together with diacylglycerol activates a classical protein kinase C, eye-PKC, which is critical for the negative regulation of visual signaling by modulating deactivation and light adaptation (7–11).
Light adaptation is the process by which photoreceptors adjust the visual sensitivity in response to ambient background light by down-regulating rhodopsin-mediated signaling. Light adaptation can be arbitrarily subdivided into long term and short term adaptation and may involve multiple regulations to reduce the efficiency of rhodopsin, G protein, or cation channels. For example, translocation of both Gq (12, 13) and TRP-like channels (14, 15) out of the visual organelle may contribute to long term adaptation in Drosophila. In contrast, short term adaptation may be orchestrated by modulating the activity of signaling proteins by protein kinases. Hardie and co-workers (16) demonstrated that an increase of cytoplasmic [Ca2+] mimicked light adaptation, leading to inhibition of the light-induced current. These authors also showed that light adaptation is independent of eye-PKC. Thus the effect of cytoplasmic Ca2+ to control light adaptation is likely mediated via calmodulin and CaMKII. The contribution of CaMKII to light adaptation has not been explored.
CaMKII is a multimeric Ca2+/calmodulin-dependent protein kinase that modulates diverse signaling processes (17). Drosophila contains one CaMKII gene (18) that gives rise to at least four protein isoforms (19). These CaMKII isoforms share over 85% sequence identities with the α isoform of vertebrate CaMKII. For insights into the in vivo physiological role of CaMKII, Griffith et al. (20) generated transgenic flies (ala) expressing an inhibitory domain of the rat CaMKII under the control of a heat shock promoter, hsp70. They demonstrated that, upon heat shock treatment, the overexpression of the inhibitory peptide resulted in a suppression of the endogenous CaMKII activity in the transgenic flies (20). It has been shown that inhibition of CaMKII affects learning and memory (20) and neuronal functions (21–24). In photoreceptors, CaMKII has been implicated in the phosphorylation of the major visual arrestin, Arr2 (25, 26). However, how phosphorylation of Arr2 by CaMKII modifies the visual signaling remains to be elucidated.
Here we report the biochemical and electrophysiological analyses of CaMKII in Drosophila retina. We demonstrate that suppression of CaMKII in ala1 transgenic flies leads to a phenotype indicative of defective light adaptation. The ala1 flies also display greater visual response, suggesting a defect in Arr2. These results support the notion that CaMKII plays a role in the negative regulation of the visual response. Our biochemical analyses demonstrate that dephosphorylation of CaMKII is mediated by protein phosphatase 2A (PP2A). Importantly, we show that PP2A interacts with CaMKII, indicating that CaMKII forms a stable protein complex with PP2A to ensure a tight regulation of the kinase activity. Thus a partial loss of function in PP2A would elevate the CaMKII activity. Indeed, we show that mts heterozygotes display reduced prolonged depolarizing potential (PDA) amplitude. This PDA phenotype strongly suggests that Arr2 becomes more effective to terminate the visual signaling in mts flies. Together, our findings indicate that the ability of Arr2 to terminate metarhodopsin is increased upon phosphorylation by CaMKII, and the retinal CaMKII activity is regulated by PP2A.
EXPERIMENTAL PROCEDURES
Recombinant DNA and Molecular Biology—cDNA fragments coding for two Arr2 peptides (Arr2-Ser366, SNAMKKMKSIEQH, and Arr2-Ala366, SNAMKKMKAIEQH) and autocamtide (KKALRRQETVDAL) (27) were subcloned into pGEX-4T-1 (GE Healthcare) for expression as a terminal peptide of glutathione S-transferase (GST). Briefly, for each peptide, a set of oligonucleotides flanking with sequences ready for ligating into vectors with EcoRI (5′) and XhoI (3′) sites were annealed, and ligated with predigested pGEX-4T-1. The recombinant DNA constructs were confirmed by automatic DNA sequencing. The oligonucleotides used were listed below. AA TTC TCG AAT GCC ATG AAG AAA ATG AAG TCC ATC GAG CAG CAC TAA C (Arr2-Ser366-5′), TC GAG TTA GTG CTG CTC GAT GGA CTT CAT TTT CTT CAT GGC ATT CGA G (Arr2-Ser366-3′), AA TTC TCG AAT GCC ATG AAG AAA ATG AAG GCC ATC GAG CAG CAC TAA C (Arr2-Ala366-5′), TC GAG TTA GTG CTG CTC GAT GGC CTT CAT TTT CTT CAT GGC ATT CGA G (Arr2-Ala366-3′), AA TTC AAG AAA GCC CTC CGC AGG CAG GAG ACC GTC GAT GCC TTG TAG C (autocamtide-5′), and AG CTA CAA GGC ATC GAC GGT CTC CTG CCT GCG GAG GGC TTT CTT G (autocamtide-3′). Recombinant pGEX-4T-1 constructs containing Arr2 or CaMKII were obtained by inserting the cDNA sequence generated by PCR, into the EcoRI (5′) and XhoI (3′) sites.
In Vitro Phosphorylation—In vitro kinase assays were carried out according to Alloway and Dolph (28). Briefly, 15 heads (or 30 retinas) from 1–3-day-old dark-reared flies were dissected and homogenized in binding buffer (20 mm Tris, pH 7.5, 150 mm KCl, 5 mm dithiothreitol, plus 1 mm phenylmethylsulfonyl fluoride, 5 ng/ml leupeptin, 5 ng/ml pepstatin, and 5 ng/ml aprotinin). Homogenates (1 μl) were added into the kinase reaction buffer (6 μl) containing 16 mm Tris (pH 7.5), 5 mm MgCl2, 5 mm β-mercaptoethanol, 3 μCi of [γ-32P]ATP (PerkinElmer Life Sciences) with either Ca2+ (0.5 mm) or EGTA (2 mm). Phosphorylation reactions were carried out at 30 °C for 20 min and analyzed by SDS/PAGE. Following electrophoresis, the gel was stained with Coomassie Blue, dried, and exposed to x-ray films or subjected to phosphorimaging analysis (GE Healthcare). Alternatively, the kinase reaction was performed in the presence of cold ATP (200 μm), and the level of phosphorylated Thr287 or Thr306 was determined by Western blotting using phospho-specific antibodies.
Expression of CaMKII1–275 for in Vitro Kinase Assays—cDNA encoding the kinase domain (1–275 amino acids) of Drosophila CaMKII with added EcoRI and XhoI restriction sites was obtained by PCR and subcloned into pGEX-4T-1 for expression as a GST fusion protein. For the kinase assay, affinity-purified CaMKII1–275 fusion protein was mixed with immobilized substrates in the presence of the kinase reaction buffer at 30 °C for 20 min. The incorporation of radioactive phosphate into substrates was analyzed by SDS/PAGE followed by autoradiography or phosphorimaging.
In Vitro Dephosphorylation Assay—Radiolabeled phosphorylated substrates were incubated with purified PP2A (Millipore, Billerica, MA) containing the A/C dimer in the phosphatase buffer (50 mm HEPES, pH 7.0, 0.1 mm EDTA, 5 mm dithiothreitol, 0.025% Tween 20) at 30 °C for 20 min. Phosphorylated proteins were analyzed by SDS/PAGE, followed by autoradiography. Protein bands corresponding to phosphorylated protein were excised and quantitated by liquid scintillation counter.
Preparation of Fly Head Extracts and Western Blotting—Proteins in fly heads were extracted with the extraction buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Triton X-100, and a mixture of protease inhibitors) as previously described (8). Protein content of the extract was determined by BCA (Thermo Scientific, Rockford, IL). For quantitative Western blotting, fluorophore-conjugated secondary antibodies (Alexa Fluor® 680 goat anti-mouse IgG; Invitrogen) were used, and the signals were visualized and quantified by the Odyssey infrared imaging system (LI-COR, Lincoln, NE). Anti-Arr2 antibodies were a gift from Dr. Rangananthan (University of Texas Southwestern Medical Center) (29). Monoclonal anti-Rh1 rhodopsin antibody was from Development Studies Hybridomo Bank (University of Iowa). Monoclonal anti-PP2Acα, which recognizes the catalytic subunit of Drosophila PP2A (MTS) was obtained from BD Biosciences (San Jose, CA). Polyclonal antibodies for phosphorylated CaMKII at Thr306 were obtained from PhosphoSolutions (Aurora, CO), and the antibodies recognizing phosphorylated CaMKII at Thr287 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Pulldown Assays Using GST Fusion Proteins—GST fusion proteins expressed in bacteria were extracted with lysis buffer (50 mm Tris, pH 7.5, 50 mm NaCl, 5 mm MgCl2, 1 mm 1,4-dithiothreitol, plus a mixture of protease inhibitors). Bacterial lysates were incubated with 25 μl of glutathione-Sepharose beads (Thermo Scientific) to recover GST fusion proteins according to the protocol supplied by the manufacturer. Approximately 10 μg of immobilized GST fusion proteins (or GST alone) were incubated at 4 °C for 1 h with wild-type fly head extracts containing 1% Triton X-100 prepared as described above. Following incubation, the pulled down mixture containing GST fusion proteins and interacting proteins was briefly washed three times with lysis buffer to remove nonspecific binding proteins and analyzed by SDS/PAGE. GST fusion proteins and co-purified associating proteins were detected by Coomassie staining or Western blotting (30).
Calmodulin-Agarose Binding—Fly extracts prepared with the extraction buffer containing 1% Triton X-100 were incubated with calmodulin-agarose (Sigma) in the presence of Ca2+ or EGTA, as recommended by the supplier. Following three washes, proteins that bind to the beads were eluted and analyzed by Western blotting.
Generation of Anti-CaMKII Antibodies—Polyclonal antibodies were obtained by immunizing rabbits with the GST fusion protein containing CaMKII382–490 (382–490 amino acids, CG18069-PA) expressed in bacteria. The rabbit sera were collected after the second injection and tested by Western blotting. The antibodies used for both Western blotting and immunoprecipitations were obtained after the sixth injection of the antigen.
Immunoprecipitation and Immunocomplex Kinase Assay—Fly head extracts (300 μg of total protein) were incubated with anti-CaMKII antibodies, and the immunocomplexes were recovered by protein A-agarose beads (Thermo Scientific). Following three washes with extraction buffer, the immunocomplexes were analyzed by SDS/PAGE or proceeded with an in vitro kinase reaction by incubating with 50 μl of kinase reaction buffer containing Ca2+ at 30 °C for 20 min. The kinase reaction was terminated upon the addition of the sample buffer and analyzed by SDS/PAGE followed by phosphorimaging analysis or autoradiography.
Fractionation of Cytosol and Membranes—Fly heads were collected and homogenized in binding buffer. The membrane was separated from the cytosol following centrifugation at 14,000 × g (48). Cytosol and membrane (from approximately four fly heads equivalents, ∼25 and 16 μg of total protein, respectively) were analyzed by Western blotting.
Electroretinogram (ERG) Recordings—ERG was performed on 3–5-day-old flies as previously described (31). A 300-Watt Halogen lamp (OSRAM) was used for light stimuli. The unattenuated intensity at the level of the fly was 810 μW/cm2. Both recording and ground electrodes were filled with Hoyle's solution. Orange and blue stimuli were generated by the use of Corning CS2-73 and 5433 filters, respectively. At each stimulus, the flies were first dark-adapted for 3 min and then given a blue light stimulus of various durations. The PDA amplitude was measured 70 s post-stimulation. Then a pulse of the orange light of the same duration as the preceding blue light was applied, and the peak amplitude was recorded. To ensure photoconversion of metarhodopsin back to rhodopsin, an orange light stimulus was given prior to the blue light stimulation. All of the recordings were made at 25 °C. The signals were sampled at 2 kHz with an analog-to-digital converter (Digidata 1200A), and the data were acquired and analyzed in a computer with Axoscope (Molecular Devices, Sunnyvale, CA).
Fly Stocks—Fly stocks were maintained at 25 °C in a 12-h dark/12-h light cycle. The mtsXE2258 was obtained from Bloomington Stock Center, and the ras transgene was removed (11). The ala1 transgenic line, in which the transgene was integrated in the X-chromosome, was provided by Dr. L. Griffith (Brandeis University). The ala1 fly was crossed into the genetic background of cn, bw to generate white-eyed ala1; cn, bw lines.
RESULTS
Identification of CaMKII in Retinal Extracts—CaMKII is critically involved in the Ca2+-dependent regulation of signal transduction in the nervous system. In particular, CaMKII mediates activity-dependent alteration of neuronal functions by modulating the function of receptors and ion channels. Drosophila has one CaMKII gene (18) whose gene product has been implicated in the light-dependent phosphorylation of Arr2 in photoreceptors (25, 26). However, little is known regarding the biochemistry of CaMKII in the eye and how it may contribute to fine-tuning of the visual response.
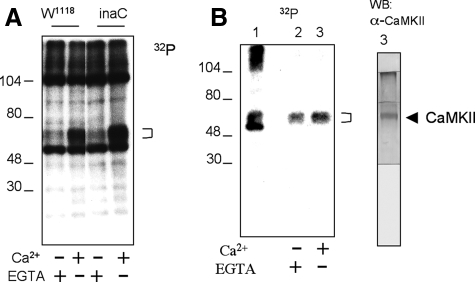
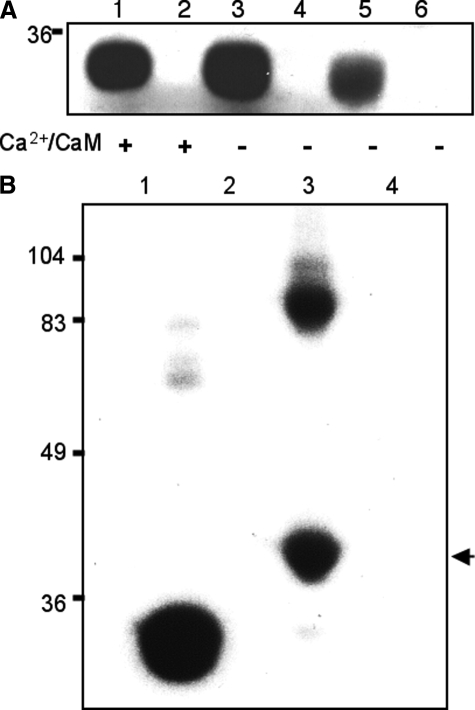
To identify CaMKII in the retina, we first looked for proteins that undergo Ca2+-dependent phosphorylation. Experimentally, retinal homogenates from wild-type flies (W1118) were used for in vitro phosphorylation assays in the presence of radioactive ATP (28). Phosphorylated proteins were analyzed by SDS/PAGE and revealed by autoradiography. As shown in Fig. 1A, several retinal proteins were phosphorylated; however, only a broad band that migrates near 55–65 kDa (bracket) consistently displayed enhanced phosphorylation in the presence of Ca2+ (Fig. 1A). This Ca2+-dependent phosphorylation was also observed in inaCP209 homogenates, indicating that phosphorylation is not regulated by eye-PKC (10). To investigate whether the 55–65-kDa protein that showed Ca2+-dependent phosphorylation is CaMKII, we first tested its enrichment by calmodulin-agarose. We performed calmodulin pulldown assays and show that calmodulin-agarose isolated phosphorylated 55–65-kDa protein (Fig. 1B, left panel, second and third lanes), supporting that this diffuse protein band may be CaMKII. Importantly, polyclonal antibodies raised against Drosophila CaMKII (Fig. 2A) recognized a band in the Western blot, which co-migrated with the putative phosphorylated CaMKII isolated by calmodulin-agarose (Fig. 1B, right panel). Together, we demonstrate the presence of CaMKII in the fly retina.
FIGURE 1.
Identification of CaMKII in retinal extracts. A, in vitro kinase assays. Retinal homogenates from wild type (W1118) or inaC (inaCP209) were used for in vitro kinase assays in the presence of Ca2+ or EGTA, as indicated below. Phosphorylation of retinal proteins was analyzed by SDS/PAGE and detected by autoradiography. A broad band of ∼55–65 kDa (bracket) shows Ca2+-dependent phosphorylation in both wild-type and inaC homogenates. B, isolation of phosphorylated CaMKII by calmodulin-agarose. Retinal homogenates were extracted with extraction buffer containing 1% Triton X-100, and the extract was incubated with calmodulin-agarose. Shown is an autoradiogram depicting total phosphorylated proteins (left panel, lane 1) and those recovered upon binding to calmodulin-agarose in the absence (lane 2) or presence of Ca2+ (lane 3). Phosphorylated proteins were revealed by autoradiography (left panel) following Western blotting (WB) with anti-CaMKII antibodies (right panel). Protein standards are indicated on the left (in kDa).
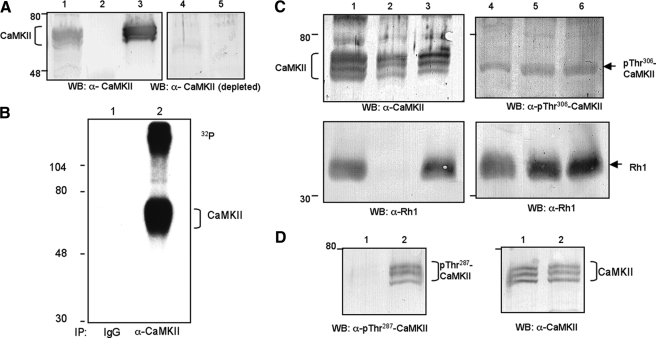
FIGURE 2.
Characterization of anti-CaMKII antibodies. A, Western blot (WB) analysis. Anti-CaMKII recognized multiple polypeptide species in fly head extracts (lane 1), which were enriched by calmodulin-agarose (lane 3), but not by protein A-agarose, a negative control (lane 2). The specificity of antibodies was established as pretreatment with excess antigen greatly diminished the signals in the Western blot (lanes 4 and 5). Lane 4 contains CaMKII enriched by calmodulin-agarose, and lane 5 contains fly head extracts equivalent to the amount loaded in lane 1. B, anti-CaMKII immunoprecipitated CaMKII from fly head extracts. CaMKII was detected by autoradiography following phosphorylation by in vitro kinase assays (lane 2). In contrast, preimmune IgG did not isolate CaMKII (lane 1). The phosphorylated protein of high molecular mass (>104 kDa) may represent undissociated antibody-CaMKII complex. C, subcellular fractionation of CaMKII and phosphorylation of CaMKII at Thr306. CaMKII is present in both cytosol (left panel, lane 2) and membrane (lane 3) following fractionation (see “Experimental Procedures”). In contrast, Rh1 rhodopsin is only detected in the membrane (bottom panels). Lane 1 contains total fly head extracts. The level of phosphorylated CaMKII at Thr306 following in vitro kinase assay with Ca2+ (right panels, lane 6) or EGTA (lane 5) was similar. Lane 4 contains total fly head extracts. D, phosphorylation of CaMKII at Thr287 is greatly increased by Ca2+. Shown is a Western blot probing with antibodies specific for either phosphorylated Thr287 (left panels) or total CaMKII (right panels). The in vitro kinase reactions were performed in the presence of EGTA (lane 1) or Ca2+ (lane 2). Protein size standards are indicated on the left.
Anti-CaMKII Polyclonal Antibodies Immunoprecipitate CaMKII and Recognize CaMKII by Western Blotting—As mentioned, we obtained antibodies against Drosophila CaMKII. Specifically, we immunized rabbits with a fusion protein containing a portion of the association domain, CaMKII382–490 (isoform A, accession: NP_726633; see Fig. 4B). This region of CaMKII is more divergent than the corresponding domain of the vertebrate CaMKII but is present in all predicted Drosophila isoforms. The predicted CaMKII isoforms consist of 490, 509, 516, and 530 amino acids in length with variations in the regulatory domain (32). The regulatory domain of CaMKII encompassing an autoinhibitory and a calmodulin-binding site is important for modulation of the kinase activity.
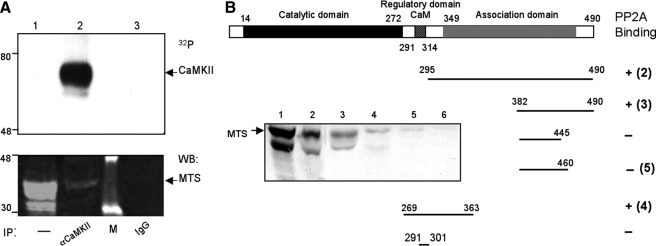
FIGURE 4.
Interactions between PP2A and CaMKII. A, co-purification of PP2A by anti-CaMKII antibodies. Shown is an immunoprecipitation (IP) assay using either anti-CaMKII antibodies (lane 2) or preimmune IgG (lane 3). The presence of CaMKII was revealed by autoradiography following in vitro kinase assay (top panel). The co-precipitation of MTS was detected by Western blotting (bottom panel). Lane 1 contains an aliquot of fly head extracts. Protein standards are indicated on the left. B, PP2A interacts with CaMKII by pulldown assays. Shown is a summary of the pulldown results. Similar amounts of GST fusion proteins containing regulatory or association domain of CaMKII (isoform A) were examined for potential association with PP2A in the fly extracts. The enriched PP2A was revealed by the presence of MTS by Western blotting (inset). Fusion proteins used for pulldown assays were CaMKII295–490 (lane 2), CaMKII382–490 (lane 3), CaMKII269–363 (lane 4), CaMKII382–460 (lane 5), and GST alone (lane 6). Lane 1 contains fly extracts.
As shown in Fig. 2A, Western blotting using anti-CaMKII antibodies revealed multiple protein species of CaMKII near 55–65 kDa from the fly head extracts (lane 1), which can be enriched by calmodulin-agarose (lane 3) but not by protein A-agarose (lane 2). The specificity of our anti-CaMKII antibodies was supported by immunodepletion studies (Fig. 2A, lanes 4 and 5); CaMKII signals in the Western blot were greatly diminished when antiserum was immunodepleted upon incubating with immobilized CaMKII382–490, but not with GST (not shown).
The observed multiple species of CaMKII could be due to different protein isoforms, as well as phosphorylation states. Consistently, a monoclonal antibody specific for Drosophila CaMKII obtained by Takamatsu et al. (33) also recognized multiple bands in fly head extracts.
The polyclonal anti-CaMKII antibodies were also evaluated by immunoprecipitation assays. To reveal immunoprecipitated CaMKII, which co-migrates with the heavy chain of the IgG in SDS/PAGE, we carried out in vitro kinase assays following immunoprecipitation to radiolabeled CaMKII because CaMKII is capable of autophosphorylation in the presence of Ca2+ (Fig. 2B, lane 2). We demonstrate that anti-CaMKII antibodies, but not the preimmune IgG, isolated CaMKII (Fig. 2B). Taken together, we identified CaMKII by both immunological and biochemical methodologies. Like its vertebrate CaMKII, Drosophila CaMKII undergoes Ca2+-dependent autophosphorylation.
CaMKII Is Detected in the Cytosol and Membrane—To investigate whether CaMKII is localized in the cytosol or associated with the membrane, we performed subcellular fractionation. Fly heads were homogenized without detergents, and cytosol was separated from the membrane by centrifugation and analyzed by Western blotting. As shown in Fig. 2C (top left panel), we found that ∼60% of total CaMKII (lane 3) is present in the membrane fraction with the remaining 40% in the cytosol (lane 2). It is interesting to note the differential distribution of the CaMKII isoforms. As a control, Rh1 rhodopsin is detected predominantly in the membrane (bottom left panel, lane 3).
Phosphorylation at Thr287 of CaMKII Is Elevated By Ca2+—It has been well established that CaMKII undergoes autophosphorylation at two residues in its regulatory domain, which critically modulates the kinase activity. The Ca2+-dependent phosphorylation at Thr287 of Drosophila CaMKII is important for its activation, because phosphorylation drastically increases its affinity for calmodulin leading to the Ca2+/calmodulin independent kinase activity (34). In contrast, phosphorylation at Thr306 occurs following phosphorylation at Thr287 and results in a reduction of calmodulin binding and inactivation of the kinase (34).
To investigate whether phosphorylation of CaMKII at Thr306 is increased in the in vitro kinase assays, we estimated the relative level of phosphorylated CaMKII using polyclonal antibodies that recognize Thr(P)306 (35). By Western blotting, anti-Thr(P)306 antibodies detect a protein band (Fig. 2C, top right panel, lane 4) that appears to correspond to the fast moving CaMKII species recognized by our anti-CaMKII antibodies (Fig. 2C, top left panel, lane 1). However, the level of phosphorylated Thr306 was not significantly increased by Ca2+ (Fig. 2C, top right panel, lanes 5 and 6). In contrast, the level of phosphorylated CaMKII containing Thr287 was greatly increased when in vitro kinase assays were performed in the presence of Ca2+ (Fig. 2D). Taken together, we conclude that the observed Ca2+-dependent autophosphorylation of Drosophila CaMKII occurs mostly at Thr287.
Autophosphorylation of CaMKII Is Increased by Okadaic Acid—We noted that relative levels of phosphorylated CaMKII revealed by the in vitro phosphorylation assays sometimes did not show a linear relationship with the incubation time (Fig. 3A), suggesting that phosphorylation states of CaMKII may be regulated by tightly associated protein phosphatases. To uncover the critical protein phosphatase that regulates dephosphorylation of CaMKII, we explored the effect of protein phosphatase inhibitors. We show that okadaic acid (10 nm) led to an increase in phosphorylation of CaMKII (Fig. 3, A and B). By Western blotting employing antibodies specific for phosphorylated CaMKII at Thr287, we show that Thr287 phosphorylation is drastically increased in the presence of okadaic acid (Fig. 3B). In particular, the potentiation is much greater during shorter incubation times (e.g. 1 min). We estimated the IC50 of okadaic acid that elevates phosphorylation of CaMKII to be ∼1 nm, suggesting that dephosphorylation of CaMKII is controlled by members of the PP2A family (36). Together, we conclude that dephosphorylation of CaMKII appears to be regulated by closely localized PP2A.
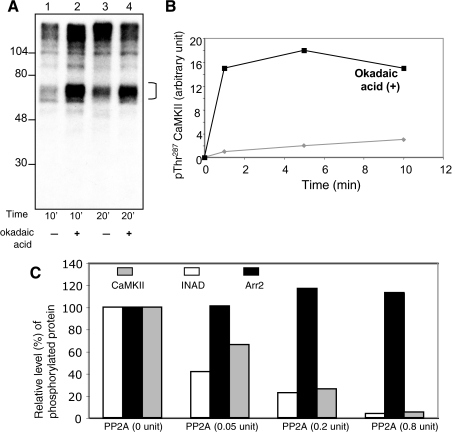
FIGURE 3.
Role of PP2A to catalyze the dephosphorylation of CaMKII and Arr2. A, potentiation of CaMKII autophosphorylation by okadaic acid. Shown is the autoradiography of an in vitro phosphorylation of fly head homogenates in the presence of Ca2+ for either 10 (lanes 1 and 2) or 20 min (lanes 3 and 4), as indicated. The effect of okadaic acid (10 nm) to potentiate autophosphorylation of CaMKII (bracket) was investigated (lanes 2 and 4). B, time-dependent potentiation of autophosphorylation of CaMKII by okadaic acid. The levels of phosphorylated CaMKII with (filled square) or without okadaic acid were determined by Western blotting using Thr(P)287-specific antibodies. Okadaic increased autophosphorylation of CaMKII at Thr287 by 15-fold following 1 min of incubation. C, PP2A dephosphorylates CaMKII, but not Arr2. Purified PP2A was incubated with phosphorylated Arr2305–400 or CaMKII for 20 min, and the remaining phosphorylated proteins were quantitated following SDS/PAGE and shown in a histogram. Phosphorylated INAD containing the first and second PDZ domains was used as a positive control for PP2A.
Purified PP2A Dephosphorylates CaMKII in Vitro—To investigate whether PP2A is directly involved in the dephosphorylation of CaMKII, we performed phosphatase assays using purified PP2A. Experimentally, PP2A was incubated with phosphorylated CaMKII, which was generated by the immunocomplex kinase assays (Fig. 2B). The level of radiolabeled phosphorylated CaMKII following incubation with PP2A was quantitated by scintillation counter. Indeed, we demonstrate that PP2A exhibits a concentration-dependent activity to dephosphorylate CaMKII (Fig. 3C), similar to INAD, a known substrate of PP2A (11).
Interactions between PP2A and CaMKII Revealed by Immunoprecipitation and Pulldown Assays—To investigate whether CaMKII and PP2A form a stable protein complex in photoreceptors, we first examined whether PP2A could be co-immunoprecipitated by anti-CaMKII antibodies. As shown before (Fig. 2B), anti-CaMKII antisera specifically purified CaMKII, which can be detected by autoradiography following autophosphorylation (Fig. 4A, top panel, lane 2). Remarkably, the catalytic subunit of PP2A (MTS) was co-purified in the immunoprecipitates by anti-CaMKII, but not by the preimmune IgG (Fig. 4A, bottom panel). This result is consistent with our observation that okadaic acid significantly enhanced autophosphorylation of immunoprecipitated CaMKII (not shown) and strongly indicates that CaMKII interacts with PP2A to form a protein complex.
To further investigate the formation of the PP2A-CaMKII complex, we performed pulldown assays. Experimentally, immobilized GST fusion proteins containing CaMKII were used to isolate endogenous PP2A from fly extracts, and PP2A was detected by Western blotting for the presence of the catalytic subunit (MTS). The results are summarized in Fig. 4B. We demonstrate that MTS can be co-purified with CaMKII269–363 and CaMKII382–490 (in isoform A). Collectively, these results strongly indicate that CaMKII directly associates with PP2A to form a PP2A-CaMKII protein complex.
Drosophila CaMKII Phosphorylates Arr2 Fusion Proteins Containing Ser366—As indicated before, CaMKII has been implicated in the phosphorylation of Arr2. In addition, Ser366 of Arr2 is the major in vivo phosphorylation site as revealed by mass spectrometric analysis of the endogenous Arr2 (37). To identify the critical kinase from fly extracts, Kahn and Matsumoto (26) demonstrated that the desired kinase activity responsible for the phosphorylation of Arr2 was regulated by both Ca2+ and calmodulin (26). Moreover, these authors showed that purified rat brain CaMKII was capable of phosphorylating a polypeptide substrate of Arr2 spanning Ser366 in vitro (25).
To demonstrate that Drosophila CaMKII indeed catalyzes the phosphorylation of Ser366 in Arr2, we obtained recombinant Drosophila CaMKII by expressing in bacteria. We employed a modified Drosophila CaMKII, CaMKII1–275, which contains only the kinase domain (Fig. 4B). It has been shown that truncated CaMKII missing its regulatory and association domains displays autonomous kinase activity independent of Ca2+ (38). Consistently, we show that in the absence of Ca2+ and calmodulin, CaMKII1–275 phosphorylated fusion proteins containing a terminal autocamtide II sequence (KKALRRQETVDAL) (Fig. 5A, lane 3), a commonly used peptide substrate of CaMKII. Moreover, recombinant CaMKII1–275 phosphorylated a short peptide sequence spanning the Arr2 phosphorylation site (SNAMKKMKS366IEQH) (Fig. 5, A, lane 5, and B, lane 1) and a fusion protein containing the last 96 amino acids of Arr2, Arr2305–400 (Fig. 5B, lane 3). As negative controls, both GST alone and the sequence spanning the first PDZ and second domains of INAD (Fig. 5B, lane 4) were not phosphorylated by CaMKII1–275. Importantly, Ala substitution of the Arr2 peptide at Ser366 greatly diminished the CaMKII-dependent phosphorylation (Fig. 5A, lane 6). Together, our results demonstrate that Drosophila CaMKII is directly involved in the phosphorylation of Arr2 at Ser366 in vitro.
FIGURE 5.
Phosphorylation of Arr2 by recombinant Drosophila CaMKII1–275. A, CaMKII1–275 displays autonomous kinase activity. CaMKII1–275 was employed for the phosphorylation of GST fusion containing the autocamtide sequence with (lane 1) or without Ca2+/calmodulin (lane 3). CaMKII1–275 also phosphorylates fusion protein containing a short peptide of Arr2 encompassing Ser366 (lane 5). Substitution of Ser366 with Ala completely eliminates the phosphorylation (lane 6). Lanes 2 and 4 contain GST alone. B, CaMKII1–275 phosphorylates the C terminus of Arr2. GST fusion proteins containing the C terminus of Arr2 spanning residues 305–400 (∼41 kDa in size, arrow) were phosphorylated by recombinant CaMKII1–275 (lane 3). Note that this Arr2 fusion protein forms dimers. For comparison, phosphorylation of Arr2 peptide fusion is included in lane 1. In contrast, GST alone (lane 2) and fusion proteins containing the N terminus of INAD containing the first and second PDZ domains (∼74 kDa in size) were not phosphorylated (lane 4). Protein size markers are indicated on the left.
Dephosphorylation of Arr2 Does Not Depend on PP2A—As shown before, phosphorylation of Arr2 appears regulated by CaMKII, whose activity may be modulated by the co-localized PP2A. However, PP2A may be also critical to promote the dephosphorylation of Arr2. To investigate whether Arr2 dephosphorylation is directly catalyzed by PP2A, we employed purified PP2A for dephosphorylation assays. Experimentally, equal amounts of phosphorylated Arr2305–400 were incubated with various concentrations of PP2A, and the level of phosphorylated Arr2305–400 was determined by scintillation counting. Interestingly, we found that phosphorylated Arr2305–400 remained relatively constant in the presence of PP2A (Fig. 3C). This finding strongly indicates that PP2A is not directly involved in the dephosphorylation of Arr2 in vitro.
Characterization of ala1 Flies to Reveal the in Vivo Functions of CaMKII in the Visual Signaling—Arr2 is critical for deactivation of the visual response by modulating the interaction between metarhodopsin and the downstream Gq. To investigate the in vivo role of Arr2 phosphorylation by CaMKII in photoreceptors, we characterized ala1 transgenic flies in which CaMKII activity is reduced because of overexpression of the inhibitory peptide (20). The ala1 flies have been well characterized and shown to affect several neuronal processes including learning and memory (20) and synaptic transmission (21–24).
We performed ERG, the extracellular recordings of the compound eye, to analyze visual electrophysiology. First, we examined how CaMKII modulates the ability of Arr2 to inactivate metarhodopsin by investigating the generation of PDA (Fig. 6). Briefly, PDA is the persistent depolarization initiated upon activation of rhodopsin by intense light and is contributed by the continued activity of unquenched metarhodopsin to activate the visual signaling when its inactivation by Arr2 becomes limiting (39). Arrestin inactivates metarhodopsin by stoichiometrically blocking its interaction with the downstream G protein (40, 41). In Drosophila, Arr2 and the major rhodopsin Rh1 are ∼1:4 in molar ratio (39). Therefore when over 30% of Rh1 rhodopsin become activated following intense light stimulation, ∼25% of metarhodopsin will be inactivated by Arr2, and the remaining 5% stay active to trigger PDA. PDA can be more effectively induced by the blue light, which specifically activates Rh1 rhodopsin.
FIGURE 6.
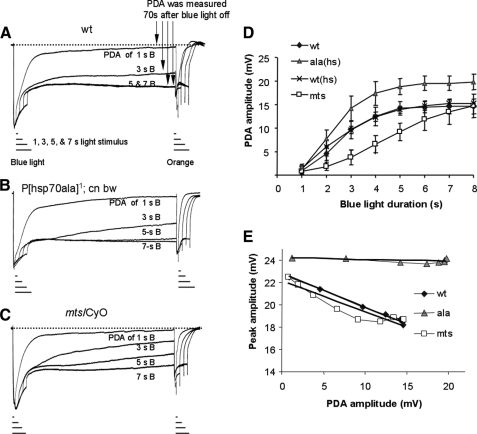
Electrophysiological characterization of ala and mts flies affecting CaMKII and PP2A by ERG. Shown are superimposed ERG recordings of wild type (wt, A), ala1 transgenic flies (with heat shock) (B), and mtsXE2258 heterozygotes affecting PP2A (mts/CyO) (C), in response to a pulse of light of various durations (1, 3, 5, and 7 s). Blue light (λ480) was employed for the initial stimulation, and orange light (λ580) was used following PDA. D, the peak amplitude following the induction of PDA (PDA amplitude) was measured 70 s post-termination of the blue light. The PDA amplitude elicited by different duration of blue light pulse was compared among three different fly strains. Heat shock alone did not modify PDA generation in wild-type flies, whereas heat shock treated ala1 displays greater PDA amplitude. E, relationship between PDA amplitude and the subsequent response to the orange light. The peak amplitude by the orange light following different level of PDA is plotted against PDA amplitude. In wild type and mts/CyO, a greater PDA amplitude results in a decreased peak response.
Experimentally, we stimulated dark-adapted ala1 and wild-type flies with intense blue light of varying durations (1–7 s) and measured PDA 70 s post-stimulation (Fig. 6, A and B). The relationship between PDA amplitude and light duration was examined quantitatively and is shown in Fig. 6D. We show that, compared with wild-type flies, ala1 displays greater PDA amplitude (Fig. 6D). The increased PDA amplitude may be due to either more active metarhodopsin or less efficient Arr2 to shut down metarhodopsin mediated response. The latter is more likely, because phosphorylation of Arr2 is mediated by CaMKII that is suppressed in ala1 flies. Moreover, the impaired activity of Arr2 is not due to a reduction of the Arr2 content in ala1 flies (not shown). Taken together, we conclude that a decreased phosphorylation of Arr2 because of inhibition of endogenous CaMKII reduces the ability of Arr2 to uncouple the metarhodopsin-mediated visual response.
To investigate whether suppression of CaMKII also affects the sensitivity of photoreceptors, we compared the visual response following the generation of PDA. It is important to note that PDA can be readily terminated by a pulse of the orange light (39), which photoconverts metarhodopsin back to rhodopsin, as well as activates rhodopsin (Fig. 6A). We measured the peak amplitude elicited by the orange light stimulation following different levels of PDA and investigated the relationship between peak amplitude (by the orange light) and the prior PDA amplitude (Fig. 6E). In wild-type flies, we show that peak amplitude declines as PDA amplitude increases, a phenomenon indicative of light adaptation, because previous visual exposure down-regulates the response to subsequent light stimulation. Remarkably, in ala1 flies the peak amplitude remains constant, despite the increase of PDA amplitude (Fig. 6E), indicating a lack of light adaptation. Based on the findings, it appears that inhibition of CaMKII leads to less light-adapted visual response. This defect could be contributed by a lack of CaMKII phosphorylation in Arr2 or other substrates. Taken together, our results support the notion that CaMKII is involved in the negative regulation of the visual response, because suppression of CaMKII leads to elevated PDA amplitude and a more constant peak response. We conclude that phosphorylation by CaMKII generates more active Arr2 to inactivate metarhodopsin, which may contribute to light adaptation.
Electrophysiological Analysis of mts for Insights into Regulation of CaMKII by PP2A in Vivo—Our ERG characterization of ala1 flies strongly suggests that inhibition of CaMKII greatly diminishes the ability of Arr2 to inactivate metarhodopsin mediated depolarization response. To gain further insights into the role of CaMKII to modulate Arr2, and how CaMKII is regulated by PP2A in vivo, we examined mtsXE2258 (mts) mutants affecting the catalytic subunit of PP2A (11, 42). We showed earlier that PP2A catalyzes dephosphorylation of CaMKII and is co-localized with CaMKII to regulate autophosphorylation and hence the kinase activity. Therefore the CaMKII activity is expected to increase in a partial loss of function of PP2A mutant. We previously analyzed mts heterozygotes and reported that a reduction of PP2A can compensate for a partial loss of function in eye-PKC to restore fast deactivation of the visual response (11).
Using similar stimulation paradigms as described earlier, we demonstrate that in mts mutant PDA is less easily evoked, because generation of PDA required a longer blue light pulse (Fig. 6C). Therefore, when similar light stimulation was given, the mts mutant displayed reduced PDA amplitude, when compared with wild-type flies (Fig. 6D). The decreased PDA amplitude may be due to decreased output of metarhodopsin and/or increased activity of Arr2 in mts. Although dephosphorylation of Arr2 is not directly regulated by PP2A (Fig. 3C), the latter hypothesis is still more likely because PP2A may affect phosphorylation of Arr2 by modulating CaMKII. It is likely that the CaMKII activity that is elevated in mts flies results in a greater level of Arr2 phosphorylation. This conclusion is in good agreement with our electrophysiological analysis of ala1 flies, which strongly suggests that a reduced Arr2 phosphorylation caused by reduced CaMKII activity impairs the ability of Arr2 to uncouple metarhodopsin. Thus phosphorylated Arr2 appears more effective to terminate metarhodopsin-mediated response. However, it is important to note that the maximum PDA amplitude in mts flies remains similar to that of wild type (16.3 + 3.1 mV, wild type; 14.5 + 3.6 mV, mts) (Fig. 6D), possibly because the levels of Arr2 and rhodopsin are not significantly affected (not shown).
We also analyzed the sensitivity of photoreceptors following PDA by measuring the response to the orange light. We show that mts photoreceptors appear capable of light adaptation similar to wild type, because the peak amplitude declines when PDA amplitude increases (Fig. 6E). This result suggests that an increase of the CaMKII activity caused by a reduction of PP2A does not significantly modify light adaptation. Taken together, our findings support the notion that PP2A is critically involved in modulating the function of CaMKII in vivo, and a reduction of PP2A brings about a more active CaMKII to generate more effective Arr2 for switching off metarhodopsin. Based on our electrophysiological characterization of mts and ala1 flies, we conclude that phosphorylation of Arr2 greatly increases its activity to preclude the interaction between metarhodopsin and G protein, and the CaMKII-dependent phosphorylation of Arr2 may contribute to short term light adaptation.
DISCUSSION
Visual and nonvisual arrestins are multifunctional adaptor proteins that orchestrate as well as regulate several facets of G protein-coupled receptors signaling including receptor trafficking, signaling switching, and receptor desensitization (43, 44). Arrestin is one of the major players involved in the down-regulation of G protein-mediated processes by stoichiometrically interacting with activated receptor to prevent its association with the downstream G protein (40, 41). Moreover, arrestin, by recruiting c-Src, promotes the activation of the mitogen-activated protein kinase (MAPK) pathway (44). Despite playing an important role in G protein-coupled receptor-mediated signaling, how the activity of arrestin is regulated to coordinate various signaling events of G protein-coupled receptors awaits to be explored.
Drosophila photoreceptors express two distinct arrestin homologues, Arr1 and Arr2, both of which are tightly involved in the termination of metarhodopsin (39). Arr1 also participates in the light-dependent endocytosis of rhodopsin (45) and therefore is likely to regulate visual sensitivity. Interestingly, both Arr1 and Arr2 become phosphorylated in a light-dependent manner (28, 46–48), and phosphorylation of Arr2, the major arrestin in photoreceptors, is mediated by CaMKII (25, 26, 37). To date, the functional contribution of light-dependent phosphorylation of Arr2 remains unsettled. One hypothesis proposed that phosphorylation of Arr2 promotes the release of Arr2 from the membrane following its dissociation from rhodopsin (28). The other hypothesis suggested that phosphorylation of Arr2 initiates the interaction with metarhodopsin leading to inactivation of the visual response (26, 48).
In an attempt to elucidate the regulation of Arr2 by CaMKII, we identified and characterized CaMKII in the retina. Drosophila has only one CaMKII gene, which is expressed throughout the development. A loss of function in CaMKII affects the viability of the fly. For insights into the function of CaMKII in photoreceptors, we characterized ala1 transgenic flies in which the endogenous CaMKII activity is reduced by overexpression of an inhibitory peptide. We demonstrate that suppression of CaMKII in vivo leads to increased PDA amplitudes, most likely by decreasing the effectiveness of Arr2. Moreover, a reduced CaMKII activity also results in constant peak amplitudes, indicating that ala photoreceptors display abnormal light adaptation. Our findings support the notion that CaMKII is involved in light adaptation, because inhibition of CaMKII brings about greater visual response. Moreover, CaMKII catalyzes Arr2 phosphorylation, which creates more efficient Arr2 for uncoupling metarhodopsin-mediated signaling.
Our electrophysiological analyses support a new hypothesis that implicates Arr2 phosphorylation in the negative modulation of the visual response. Moreover, our results shed light onto the nature of the negative regulation. In essence, light stimulation leads to the opening of the TRP and TRP-like channels, resulting in an increase of cytoplasmic Ca2+ that activates CaMKII. Activation of CaMKII generates more phosphorylated Arr2 that somehow is more efficient to uncouple metarhodopsin-mediated response. Therefore the level of phosphorylated Arr2 is regulated by cytoplasmic Ca2+, which is increased upon light stimulation. Prolonged light exposure is expected to elevate the level of phosphorylated Arr2 to orchestrate timely inactivation of metarhodopsin.
The light-dependent negative modulation mediated by Ca2+ and CaMKII may underlie the short term light adaptation. Thus a reduction of the CaMKII activity would result in less light-adapted photoreceptors and, consequently, increased peak amplitude. In contrast, increased CaMKII function would result in more light-adapted photoreceptors and, consequently, a reduced visual response. However, the mts flies in which the CaMKII activity may be elevated because of a reduced PP2A activity display light adaptation almost like wild-type flies. This lack of light adaptation defects in mts may be due to the fact that the CaMKII activity is maximally active in wild-type photoreceptors, and a further increase of the CaMKII activity would not modify light adaptation in mts. The molecular basis by which CaMKII modulates light adaptation awaits further investigation. It is very likely that CaMKII, by phosphorylating Arr2, orchestrates the short term light adaptation. Alternatively, additional yet-to-be identified substrates of CaMKII may be also involved.
In summary, by a series of biochemical analyses, we demonstrate that retinal CaMKII undergoes Ca2y+-dependent phosphorylation at Thr287, and autophosphorylation of CaMKII is greatly enhanced by okadaic acid, an inhibitor of protein phosphatase 1/2A. Moreover, we show that PP2A catalyzes the dephosphorylation of CaMKII in vitro. Significantly, CaMKII interacts with PP2A as uncovered by immunoprecipitation and pulldown assays, indicating that PP2A and CaMKII form a stable protein complex. By ERG, we demonstrate that suppression of CaMKII in vivo leads to increased PDA amplitude, most likely by affecting phosphorylation and activity of Arr2. The reduced CaMKII activity also renders photoreceptors to become less light-adapted. To gain insights into the in vivo regulation of CaMKII by PP2A, we characterized the mts mutants in which the PP2A activity is reduced (11, 42). Significantly, the mts mutant displays reduced PDA amplitude, suggesting that increased activity of Arr2 inactivates metarhodopsin, which may be contributed by elevated CaMKII activity. Based on these findings, we conclude that the activity of Arr2 appears indirectly controlled by PP2A, which directly modulates CaMKII. Collectively, our results lend a support for a critical role of CaMKII in mediating the Ca2+-dependent negative regulation of the visual response by phosphorylating Arr2 and possibly other substrates. CaMKII may form a stable protein complex with PP2A that allows timely fine-tuning of the CaMKII activity in photoreceptors. A tight control of CaMKII is vital to achieve temporal regulation of the CaMKII activity for coordinating the negative regulation of the visual response.
Acknowledgments
We thank Dr. Leslie Griffith (Brandeis University) for ala1 transgenic lines and Dr. Rama Rangananthan (UT Southwestern) for anti-Arr2 antibodies. We thank Dr. Jamie McConnell for reading the manuscript and Qin-Xia Chen for excellent technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants EY09743 (to B.-H. S.) and EY00033 (to W. L. P.).
Footnotes
The abbreviations used are: TRP, transient receptor potential; Arr2, arrestin 2; CaMKII, Ca2+/calmodulin-dependent protein kinase II; ERG, electroretinogram; GST, glutathione S-transferase; MTS, microtubule star; PDA, prolonged depolarizing afterpotential; PKC, protein kinase C; PP2A, protein phosphatase 2A.
References
- 1.Wang, T., and Montell, C. (2007) Pfluegers Arch. Eur. J. Physiol. 454, 821–847 [DOI] [PubMed] [Google Scholar]
- 2.Scott, K., Becker, A., Sun, Y., Hardy, R., and Zuker, C. (1995) Neuron 15, 919–927 [DOI] [PubMed] [Google Scholar]
- 3.Bloomquist, B. T., Shortridge, R. D., Schneuwly, S., Perdew, M., Montell, C., Steller, H., Rubin, G., and Pak, W. L. (1988) Cell 54, 723–733 [DOI] [PubMed] [Google Scholar]
- 4.Raghu, P., Usher, K., Jonas, S., Chyb, S., Polyanovsky, A., and Hardie, R. C. (2000) Neuron 26, 169–179 [DOI] [PubMed] [Google Scholar]
- 5.Leung, H. T., Tseng-Crank, J., Kim, E., Mahapatra, C., Shino, S., Zhou, Y., An, L., Doerge, R. W., and Pak, W. L. (2008) Neuron 58, 884–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Tousa, J. E. (2002) Adv. Exp. Med. Biol. 514, 493–505 [PubMed] [Google Scholar]
- 7.Adamski, F. M., Zhu, M. Y., Bahiraei, F., and Shieh, B. H. (1998) J. Biol. Chem. 273, 17713–17719 [DOI] [PubMed] [Google Scholar]
- 8.Popescu, D. C., Ham, A. J., and Shieh, B. H. (2006) J. Neurosci. 26, 8570–8577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie, R. C., Peretz, A., Suss-Toby, E., Rom-Glas, A., Bishop, S. A., Selinger, Z., and Minke, B. (1993) Nature 363, 634–637 [DOI] [PubMed] [Google Scholar]
- 10.Smith, D. P., Ranganathan, R., Hardy, R. W., Marx, J., Tsuchida, T., and Zuker, C. S. (1991) Science 254, 1478–1484 [DOI] [PubMed] [Google Scholar]
- 11.Wang, N., Leung, H. T., Pak, W. L., Carl, Y. T., Wadzinski, B. E., and Shieh, B. H. (2008) J. Neurosci. 28, 1444–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frechter, S., Elia, N., Tzarfaty, V., Selinger, Z., and Minke, B. (2007) J. Neurosci. 27, 5571–5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cronin, M. A., Diao, F., and Tsunoda, S. (2004) J. Cell Sci. 117, 4797–4806 [DOI] [PubMed] [Google Scholar]
- 14.Cronin, M. A., Lieu, M. H., and Tsunoda, S. (2006) J. Cell Sci. 119, 2935–2944 [DOI] [PubMed] [Google Scholar]
- 15.Meyer, N. E., Joel-Almagor, T., Frechter, S., Minke, B., and Huber, A. (2006) J. Cell Sci. 119, 2592–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, Y., Oberwinkler, J., Postma, M., and Hardie, R. C. (2005) Curr. Biol. 15, 1228–1234 [DOI] [PubMed] [Google Scholar]
- 17.Hudmon, A., and Schulman, H. (2002) Annu. Rev. Biochem. 71, 473–510 [DOI] [PubMed] [Google Scholar]
- 18.Cho, K. O., Wall, J. B., Pugh, P. C., Ito, M., Mueller, S. A., and Kennedy, M. B. (1991) Neuron 7, 439–450 [DOI] [PubMed] [Google Scholar]
- 19.Ohsako, S., Nishida, Y., Ryo, H., and Yamauchi, T. (1993) J. Biol. Chem. 268, 2052–2062 [PubMed] [Google Scholar]
- 20.Griffith, L. C., Verselis, L. M., Aitken, K. M., Kyriacou, C. P., Danho, W., and Greenspan, R. J. (1993) Neuron 10, 501–509 [DOI] [PubMed] [Google Scholar]
- 21.Koh, Y. H., Popova, E., Thomas, U., Griffith, L. C., and Budnik, V. (1999) Cell 98, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morimoto-Tanifuji, T., Kazama, H., and Nose, A. (2004) Neuroscience 128, 797–806 [DOI] [PubMed] [Google Scholar]
- 23.Haghighi, A. P., McCabe, B. D., Fetter, R. D., Palmer, J. E., Hom, S., and Goodman, C. S. (2003) Neuron 39, 255–267 [DOI] [PubMed] [Google Scholar]
- 24.Shakiryanova, D., Klose, M. K., Zhou, Y., Gu, T., Deitcher, D. L., Atwood, H. L., Hewes, R. S., and Levitan, E. S. (2007) J. Neurosci. 27, 7799–7806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn, E. S., Kinumi, T., Tobin, S. L., and Matsumoto, H. (1998) Comp Biochem. Physiol B Biochem. Mol Biol 119, 739–746 [DOI] [PubMed] [Google Scholar]
- 26.Kahn, E. S., and Matsumoto, H. (1997) J. Neurochem. 68, 169–175 [DOI] [PubMed] [Google Scholar]
- 27.Hanson, P. I., Kapiloff, M. S., Lou, L. L., Rosenfeld, M. G., and Schulman, H. (1989) Neuron 3, 59–70 [DOI] [PubMed] [Google Scholar]
- 28.Alloway, P. G., and Dolph, P. J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96, 6072–6077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra, P., Socolich, M., Wall, M. A., Graves, J., Wang, Z., and Ranganathan, R. (2007) Cell 131, 80–92 [DOI] [PubMed] [Google Scholar]
- 30.Peng, L., Popescu, D. C., Wang, N., and Shieh, B. H. (2008) J. Neurochem. 104, 1526–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larrivee, D. C., Conrad, S. K., Stephenson, R. S., and Pak, W. L. (1981) J. Gen. Physiol. 78, 521–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GuptaRoy, B., Marwaha, N., Pla, M., Wang, Z., Nelson, H. B., Beckingham, K., and Griffith, L. C. (2000) Brain Res. Mol. Brain Res. 80, 26–34 [DOI] [PubMed] [Google Scholar]
- 33.Takamatsu, Y., Kishimoto, Y., and Ohsako, S. (2003) Brain Res. 974, 99–116 [DOI] [PubMed] [Google Scholar]
- 34.Wang, Z., Palmer, G., and Griffith, L. C. (1998) J. Neurochem. 71, 378–387 [DOI] [PubMed] [Google Scholar]
- 35.Lu, C. S., Hodge, J. J., Mehren, J., Sun, X. X., and Griffith, L. C. (2003) Neuron 40, 1185–1197 [DOI] [PubMed] [Google Scholar]
- 36.Janssens, V., and Goris, J. (2001) Biochem. J. 353, 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto, H., Kurien, B. T., Takagi, Y., Kahn, E. S., Kinumi, T., Komori, N., Yamada, T., Hayashi, F., Isono, K., Pak, W. L., Jackson, K. J., and Tobin, S. L. (1994) Neuron 12, 997–1010 [DOI] [PubMed] [Google Scholar]
- 38.Shoju, H., Sueyoshi, N., Ishida, A., and Kameshita, I. (2005) J. Biochem. (Tokyo) 138, 605–611 [DOI] [PubMed] [Google Scholar]
- 39.Dolph, P. J., Ranganathan, R., Colley, N. J., Hardy, R. W., Socolich, M., and Zuker, C. S. (1993) Science 260, 1910–1916 [DOI] [PubMed] [Google Scholar]
- 40.Kuhn, H., and Wilden, U. (1987) J. Recept. Res. 7, 283–298 [DOI] [PubMed] [Google Scholar]
- 41.Gurevich, V. V., and Gurevich, E. V. (2004) Trends Pharmacol. Sci. 25, 105–111 [DOI] [PubMed] [Google Scholar]
- 42.Wassarman, D. A., Solomon, N. M., Chang, H. C., Karim, F. D., Therrien, M., and Rubin, G. M. (1996) Genes Dev. 10, 272–278 [DOI] [PubMed] [Google Scholar]
- 43.Lefkowitz, R. J., and Shenoy, S. K. (2005) Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 44.Premont, R. T., and Gainetdinov, R. R. (2007) Annu. Rev. Physiol. 69, 511–534 [DOI] [PubMed] [Google Scholar]
- 45.Satoh, A. K., and Ready, D. F. (2005) Curr. Biol. 15, 1722–1733 [DOI] [PubMed] [Google Scholar]
- 46.Yamada, T., Takeuchi, Y., Komori, N., Kobayashi, H., Sakai, Y., Hotta, Y., and Matsumoto, H. (1990) Science 248, 483–486 [DOI] [PubMed] [Google Scholar]
- 47.LeVine, H., 3rd, Smith, D. P., Whitney, M., Malicki, D. M., Dolph, P. J., Smith, G. F., Burkhart, W., and Zuker, C. S. (1990) Mech. Dev. 33, 19–25 [DOI] [PubMed] [Google Scholar]
- 48.Byk, T., Bar-Yaacov, M., Doza, Y. N., Minke, B., and Selinger, Z. (1993) Proc. Natl. Acad. Sci. U. S. A. 90, 1907–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]