Abstract
Acetylation of p53 at carboxyl-terminal lysine residues enhances its transcriptional activity associated with cell cycle arrest and apoptosis. Here we demonstrate that p53 acetylation at Lys-320/Lys-373/Lys-382 is also required for its transcription-independent functions in BAX activation, reactive oxygen species production, and apoptosis in response to the histone deacetylase inhibitors (HDACi) suberoylanilide hydroxamic acid and LAQ824. Knock-out of p53 markedly reduced HDACi-induced apoptosis. Unexpectedly, expression of transactivation-deficient p53 variants sensitized p53-null cells to HDACi-mediated BAX-dependent apoptosis, whereas knockdown of endogenous mutant p53 in cancer cells reduced HDACi-mediated cytotoxicity. Evaluation of the mechanisms controlling this response led to the discovery of a novel interaction between p53 and Ku70. The association between these two proteins was acetylation-independent, but acetylation of p53 could prevent and disrupt the Ku70-BAX complex and enhance apoptosis. These results suggest a new mechanism of acetylated p53 transcription-independent regulation of apoptosis.
The tumor suppressor p53 is a highly regulated transcription factor that has a fundamental role in the prevention of tumorigenesis and response to chemotherapy, making it one of the most comprehensively studied molecules in cancer research. Normal cells exhibit low levels of p53 in resting conditions to maintain cellular homeostasis and prevent aberrant apoptosis. Cellular stress leads to rapid increases in p53 protein levels as a result of combined increases in transcription, translation, and post-translational modifications that repress degradation and enhance its activity. The characterization of p53 function is often accompanied by specific post-translational modifications such as acetylation, methylation, ubiquitination, neddylation, and sumoylation (1). However, even with all that is known about p53, it remains a prominent research topic with hopes of exploiting its proapoptotic functions.
Histone acetyltransferases such as p300/CBP or PCAF and histone deacetylases (HDACs)3 such as HDAC1, -2, -3, or SIRT1 regulate the acetylation of p53 at specific lysines (1). The amount and specificity of p53 acetylation are regulated by diverse mechanisms in response to various cellular stresses (2, 3). Acetylation of p53 enhances its DNA binding activity, stability, and transcriptional activity (3, 4). HDAC inhibitors (HDACi) can also induce hyperacetylation of p53 and trigger transactivation-dependent apoptosis (5, 6). Activated p53 triggers cell cycle arrest and apoptosis by inducing transcription of numerous cell cycle regulators and proapoptotic genes, such as p21, BAX, DR5, and PUMA, and transcriptional repression of the antiapoptotic proteins BCL-2 and MCL-1 (7–9).
In addition to its function as a transcription factor, p53 also promotes cell death through transcription-independent mechanisms as it can cause apoptosis in the presence of transcription and translation inhibitors (10). It has been shown that p53 induces transcription-independent apoptosis through increasing Fas cell surface translocation and caspase-8 activation (11, 12). However, the best characterized transcription-independent proapoptotic function of p53 involves its translocation to the mitochondria in stressed cells where it induces mitochondrial outer membrane permeabilization through direct physical interactions with BCL-2 family members, promoting the activation of BAX (13–15). Mutants of p53 lacking transcriptional activity are fully capable of inducing apoptosis (14, 16, 17). However, unlike their wild type counterparts, some naturally occurring DNA-binding domain mutants of p53 lack the capacity to bind BCL-XL and promote cytochrome c release from isolated mitochondria (18, 19). This leaves open the possibility that mutant p53 may promote apoptosis in a transcription-independent manner other than its role previously described at the mitochondria.
Histone acetyltransferases and HDACs regulate cellular transcriptional machinery by controlling the levels of acetylation of the core histones as well as non-histone proteins, including transcription factors such as p53 (20). HDACi are considered to be promising chemotherapeutic agents because of their ability to induce differentiation, cell cycle arrest, and apoptosis in a variety of cancer cells through altered expression of various cell cycle and apoptosis proteins (20, 21). HDACi-mediated hyperacetylation of core histones causes transcriptional activation of genes that aid in their proapoptotic and cell cycle arrest capabilities (22). Reactive oxygen species (ROS) generation also contributes to the apoptotic response incurred by HDACi (23). An emerging role of HDACi is their ability to modulate the antiapoptotic functions of Ku70. As a crucial component of the nonhomologous end joining DNA repair machinery, HDACi-enhanced acetylation of Ku70 prevents its binding to DNA and sensitizes cancer cells to DNA-damaging agents (24). In addition to its role in DNA damage repair, Ku70 forms an inhibitory complex with BAX that impairs apoptosis initiation. This complex is disrupted by acetylation of Ku70 mediated by CBP/p300, PCAF, SirT1, and HDACi treatment (25–27). The genome-wide effect of HDACi and their varied non-histone targets have made the elucidation of their mechanism of action challenging. As our understanding increases of how this class of anti-neoplastic drugs works, so does its potential for beneficial use in the clinic.
SAHA and LAQ824 are potent inhibitors of class I and II HDACs and are currently in use in clinic settings or under development as chemotherapeutic agents, respectively. HDACi increase the acetylation of Lys-320, Lys-373, and Lys-382 on p53 (6). In this study, we provide evidence that HDACi induce apoptosis through a p53 acetylation-dependent but transactivation-independent mechanism. Expression of transactivation-deficient p53 mutants in p53-null HCT116, H1299, K562, and MEF cells enhances apoptosis initiated by HDACi. Conversely, knockdown of endogenous mutant p53 in HT-29 and SW480 cells abrogates SAHA- or LAQ824-induced apoptosis. Purification of p53-binding proteins and mass spectrometry analysis revealed a novel interaction between p53 and Ku70. This interaction is independent of p53 acetylation. However, p53 acetylation at its carboxyl terminus is required for p53 to prevent and/or displace BAX from its inhibitory interaction with Ku70, thus allowing this key proapoptotic member of the BCL-2 family to target mitochondria, generate ROS, and initiate apoptosis in response to HDACi. Furthermore, knockdown of Ku70 promotes apoptosis in p53-null but not p53 mutant cells treated with HDACi. These results highlight a novel mechanism by which acetylated p53 restrains the BAX/Ku70 interaction to potentiate HDACi-induced apoptosis.
EXPERIMENTAL PROCEDURES
Reagents—LAQ824 and SAHA were kindly provided by Novartis Pharmaceuticals Inc. (East Hanover, NJ) and Merck, respectively. Anti-Ku70 monoclonal and polyclonal antibodies and anti-Myc polyclonal antibodies were purchased from Santa Cruz Biotechnology. Anti-p53 (CM1) polyclonal and monoclonal (DO-1) antibodies, anti-BAX polyclonal and monoclonal antibodies, anti-actin, tubulin, and Myc monoclonal antibodies were described previously (28, 29). Anti-Myc or FLAG-agarose beads were purchased from Sigma. Anti-histone H3 and anti-acetylated p53 (Lys-382) antibodies were purchased from Cell Signaling. Anti-HSP60 and anti-Lamin B antibodies were purchased from BD Biosciences and Calbiochem, respectively.
Cell Culture and Transfection—HCT116 cells were grown in McCoy's 5A medium; K562, SW480, and HT-29 cells were grown in RPMI; and H1299 and MEFs were grown in Dulbecco's modified Eagle's medium. Medium was supplemented with 10% fetal bovine serum and 1× penicillin/streptomycin. p53–/– and p53R172H/R172H MEFs were described previously (30). Cell transfection using Lipofectamine 2000 (Invitrogen) was described previously (28). The plasmids expressing wild type, L22Q/W23S, R175H, R273H, or D281G mutant p53 were described previously (28, 31). The p53L22Q/W23S-3KR mutant was prepared by two-step PCR and subcloned into pcDNA3-Myc vector. To establish stable transfectants, plasmids were co-transfected with pBabe-Puro or pEF6-Myc-His (Invitrogen) empty vector, and selection was performed with puromycin (Puro) or blasticidin S HCl (Bsd). The p53R175H lysine mutants were prepared by site-directed mutagenesis (Stratagene) using pcDNA3-p53 as the template, cloned into the retroviral pKI vector between the BglII/XhoI restriction sites, and transfected into 293 Ampho cells to produce recombinant retroviruses. The lentiviral pLK0.1 empty, scrambled, Ku70 shRNA (TRCN0000039611), and p53 shRNA (TRCN0000010814) constructs were purchased from Sigma. The shRNA lentivirus was produced in 293FT cells using the ViraPower kit from Invitrogen as per the manufacturer's recommendations.
Caspase Assay, ROS Measurement, and Annexin V Staining—Caspase-3 activity was measured using the fluorometric caspase-3 activity assay kit according to the manufacturer's instructions (Sigma). ROS level was determined as described previously (32). Briefly, cells treated or untreated with HDACi for 12–16 h were incubated in PBS containing 10 μm dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes) for 10 min at room temperature and lysed in ROS lysis buffer. H2DCFDA oxidation into 2′,7′-dichlorofluorescein was measured using a spectrofluorometer (excitation 485 nm and emission 535 nm). Data are normalized by protein concentration and expressed as relative change from untreated cells. For flow cytometry measurement of ROS, cells were treated or untreated with 200 nm LAQ824 for 14 h and then trypsinized, washed with PBS once, and incubated in PBS containing 10 μm H2DCFDA for 20 min at room temperature. The cells were analyzed by flow cytometry, and the results were analyzed by FACSDiva software (BD Biosciences). The results were shown as a change of mean area of fluorescence signals (measured by the software). Annexin V-fluorescein isothiocyanate/propidium iodide or annexin V-APC/7AAD staining was completed as per the manufacturer's recommendations (BD Biosciences) and analyzed by FACSCalibur and FlowJo softwares.
Tandem Affinity Purification of p53L22Q/W23S-binding Protein—The p53L22Q/W23S cDNA was subcloned into the EcoRI and XhoI sites of pcDNA3-TAP vector (33). The amino-terminal TAP-tagged p53L22Q/W23S was stably expressed in p53–/– HCT116 cells and treated with 200 nm LAQ824 for 20 h. The cells were lysed in TAP lysis buffer (0.5% Triton X-100, 0.5 mm dithiothreitol, protease inhibitor mixture, phosphatase inhibitor mixture, 100 nm LAQ824) and sonicated. After centrifugation, the cell lysate was subjected to purification/mass spectrometry analysis as described previously (33).
Protein Purification—Human Ku70 cDNA was subcloned into pGEX-4T-1 vector using BamHI and XhoI restriction enzyme cloning sites. The pGEX-4T-1-Ku70 or empty vector was transformed into the BL21 strain of Escherichia coli and grown overnight at 37 °C in LB. One ml of this turbid culture was used to inoculate 200 ml of LB, which was then grown to an A600 between 0.6 and 0.8. At this time, isopropyl 1-thio-β-d-galactopyranoside was added to a final concentration of 0.5 mm and then incubated for 6 h at room temperature with shaking. E. coli was isolated by centrifugation at 25,000 × g for 10 min and resuspended in 2 ml of PBS supplemented with protease inhibitors (Sigma). The resulting E. coli suspension was sonicated, and Triton X-100 was added to a final concentration of 1% and incubated for 30 min at room temperature with rocking. Cell debris was removed by centrifugation at 20,000 × g for 20 min at 4 °C. The supernatant was collected and incubated with glutathione-Sepharose 4B (Amersham Biosciences) for 30 min at room temperature with rocking. After washing three times with 1 ml of PBS and one time with 1 ml of 1% CHAPS lysis buffer, the GST protein was eluted with 10 mm reduced glutathione in 50 mm Tris-HCl (pH 8.0) and dialyzed against PBS (pH 7.4).
Recombinant full-length BAX protein was purified using the IMPACT system (New England Biolabs) as described previously (34). The pYTB1-BAX construct, which expresses a BAX and intein tag fusion protein, was transformed into E. coli BL21. Recombinant proteins were isolated by chitin affinity chromatography according to the manufacturer's protocol. The BAX protein was cleaved off from the intein tag by dithiothreitol and dialyzed in 10 mm HEPES (pH 7.4), 100 mm NaCl, 0.2 mm EDTA.
Purification of nonacetylated and acetylated p53 proteins was performed as described previously (35). Briefly, Sf21 armyworm cells were co-infected with HA-p53 and either empty or p300-His6 baculoviruses. Forty eight hours after infection, nuclear lysates were prepared, and total HA-p53 was purified with 12CA5 monoclonal antibody conjugated to protein A-Sepharose (Pierce) and washed three times in 10 volumes of Buffer D (20 mm HEPES (pH 7.9), 20% glycerol, and 0.2 mm EDTA) containing 0.5 m KCl. A final fourth wash used Buffer D containing 0.1 m KCl. HA-p53 was then eluted with synthetic 12CA5 peptide. HA-p53 isolated from HA-p53/p300-His6 co-infected lysate was subsequently depleted of nonacetylated p53 using Pab421-protein A beads that specifically recognize nonacetylated p53.
In Vitro GST-Ku70 Pulldown Assay—Glutathione-Sepharose 4B-conjugated GST or GST-Ku70 was incubated with 500 ng of recombinant BAX (34) in the presence or absence of 200 ng of purified nonacetylated or acetylated p53 (35) in 300 μl of 1% CHAPS buffer with rocking at 4 °C for 2 h. After centrifugation at 1,000 × g for 1 min, supernatant was removed, and beads were washed three times with 1 ml of ice-cold 1% CHAPS buffer and subjected to SDS-PAGE/immunoblot analysis.
In Vitro Disruption of Endogenous BAX-Ku70 Complex—BAX was immunoprecipitated from HCT116 p53–/– cells using polyclonal BAX antibody immobilized on protein A-agarose beads. Immune complexes were washed three times with 1 ml of ice-cold 1% CHAPS lysis buffer and then resuspended in 20 μl of 1% CHAPS lysis buffer. Approximately 200 ng of purified nonacetylated or acetylated p53 was then added and incubated with shaking at room temperature for 10 min. The immune complexes were then spun down, and the supernatant was collected to detect unbound proteins. The beads were again washed three times with 1 ml of ice-cold 1% CHAPS lysis buffer and then resuspended in Laemmli buffer. The supernatant and immune complexes were analyzed for released and bound proteins, respectively.
Subcellular Fractionation—To isolate the mitochondria-enriched heavy membrane (HM) fraction, cells were homogenized in isotonic mitochondrial buffer (210 mm sucrose, 70 mm mannitol, 10 mm HEPES (pH 7.4), 1 mm EDTA) containing protease inhibitor mixture and centrifuged at 1,000 × g for 10 min to discard nuclei and unbroken cells. The resulting supernatant was centrifuged at 10,000 × g for 15 min to pellet the heavy membrane fraction, and the supernatant was centrifuged further at 100,000 × g for 30 min to obtain cytosolic fraction, which was transferred to new tubes, and the NaCl and CHAPS concentrations were adjusted to 150 mm and 1%, respectively. To isolate the nuclear fraction, cells were resuspended in 5 volumes of Buffer A (10 mm Tris-HCl (pH 8.0), 10 mm NaCl, 1 mm EDTA, 0.5 mm dithiothreitol, and protease inhibitor mixture). After incubation on ice for 15 min, Triton X-100 was added to a final concentration of 0.2%. The lysates were then vortexed for 5 s followed by centrifugation at 10,000 × g for 10 min. The crude nuclear pellets were washed twice with Buffer A, resuspended in Buffer B (10 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1 mm EDTA, 0.5 mm dithiothreitol, 0.5% Triton X-100, and protease inhibitor mixture), and sonicated. The sonicated lysates were then centrifuged at 15,000 × g for 10 min, and the supernatant was used as nuclear fraction. For Ku70/BAX/p53 co-immunoprecipitation experiments, the nuclear and heavy membrane fractions were lysed in 1% CHAPS and sonicated.
Immunoprecipitation—Immunoprecipitation of active BAX by anti-BAX 6A7 antibody and co-immunoprecipitation of Ku70 and BAX were carried out as described previously (28, 36). For co-immunoprecipitation of Ku70 and p53, cells were treated or untreated with HDACi for the indicated times and lysed in 1% CHAPS lysis buffer. After sonication and centrifugation, the lysates were immunoprecipitated with Myc monoclonal, Ku70 monoclonal, or p53 (CM1) polyclonal antibodies followed by immunoblot analysis with the indicated antibodies. For Fig. 7A, 500 μg of proteins were incubated overnight with 4 μl of anti-BAX, 1 μg of anti-Myc (Sigma), or 1 μg of anti-Ku70 (Santa Cruz Biotechnology) polyclonal antibodies. Samples were further incubated with 15 μl of protein A-agarose at 4 °C for 2 h, washed three times, and analyzed by immunoblot.
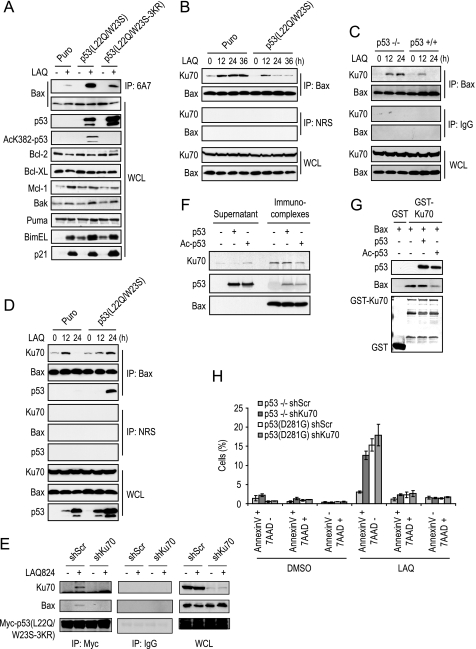
FIGURE 7.
Cellular localization of Ku70/BAX/p53 interactions. A, HCT116 p53–/– (Puro), Myc-p53L22Q/W23S, or Myc-p53L22Q/W23S-3KR cells were treated with DMSO or 200 nm LAQ for 24 h and subjected to cell fractionation and immunoprecipitation (IP) with the indicated antibodies. HM, heavy membrane. B, BAX 6A7 IP from mitochondrial fractions of HCT116 p53–/–, p53L22Q/W23S, and p53L22Q/W23S-3KR cells treated with DMSO, 5 μm SAHA, or 200 nm LAQ for 18 h. C, a proposed model illustrating that acetylated p53 can cause the dissociation of BAX from its inhibitory interaction with Ku70.
RESULTS
Transactivation Activity of p53 Is Dispensable for Apoptosis Induced by SAHA or LAQ824—To investigate the transcription-independent function of p53 in HDACi-mediated cell death, we utilized the previously established p53–/– HCT116 cell line expressing the p53L22Q/W23S transactivation-deficient mutant that retains the proapoptotic function of p53 in which two key amino acids (Leu-22 and Trp-23) in the transactivation domain were replaced with Gln and Ser, respectively (28). The inability of this mutant to transcriptionally activate p53 target genes was confirmed by using p53-responsive luciferase reporter plasmids containing the p21 or Mdm2 promoter (supplemental Fig. S1). When compared with p53+/+ HCT116 cells, p53–/– HCT116 cells displayed a drastically reduced cell death (supplemental Fig. S2A) and caspase-3 activity (Fig. 1A) following SAHA or LAQ824 treatment. Surprisingly, transfection of not only wild type p53 but also the p53L22Q/W23S mutant with puromycin (Puro) selection marker completely resensitized p53–/– HCT116 cells to SAHA and LAQ824 (Fig. 1A and supplemental Fig. S2A), suggesting that HDACi require the presence of p53 but not its transcriptional activity to induce apoptosis. Similar results were obtained in three independent transfection clones (Bsd1–6, Bsd6-3, and Bsd7-1) harboring different levels of p53L22Q/W23S with blasticidin (Bsd) selection marker (Fig. 1A and supplemental Fig. S2A).
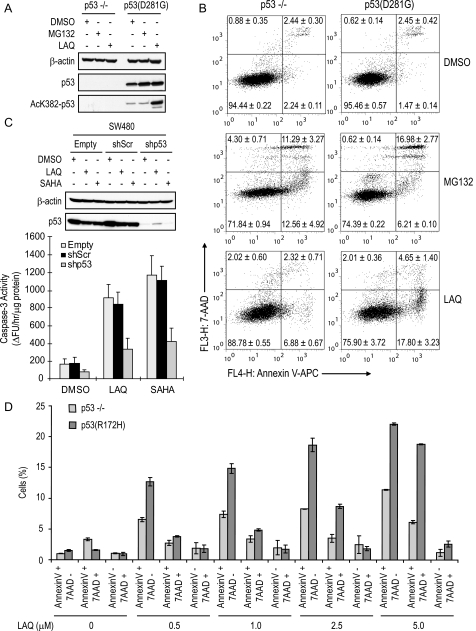
FIGURE 1.
Restoration of transactivation-deficient p53 mutants sensitizes p53–/– cell lines to HDACi-induced apoptosis. A, wild type HCT116 (p53+/+) and HCT116 p53–/– cells stably transfected with empty vector (Puro or Bsd), wild type p53 (WT), or p53L22Q/W23S were treated with either 5 μm SAHA or 200 nm LAQ for 28 h and subjected to caspase-3 assay. B and C, HCT116 p53–/– cells were stably transfected with empty, Myc-p53L22Q/W23S, p53R175H, p53R273H, or p53D281G, treated with DMSO, 5 μm SAHA, or 200 nm LAQ for 24 h, and subjected to anti-BAX 6A7 immunoprecipitation and caspase-3 assay. D and E, K562 and H1299 cells were stably transfected with p53R175H and p53D281G, respectively, treated with DMSO or LAQ (100 nm for K562 and 200 nm for H1299) for 24 h, and subjected to anti-BAX 6A7 immunoprecipitation and caspase-3 assay.
Mutations in p53 have been documented in more than half of all human cancers, of which most arise in the DNA-binding domain. Because there are no known naturally occurring mutations at both Leu-22 and Trp-23 residues of p53 and the transcriptional activity of p53L22Q/W23S is not equal to other common mutations (37), the ability of tumor-associated p53 mutants R175H, R273H, and D281G (31) to promote apoptosis in response to HDACi treatment in cancer cells was assessed. Consistently, all of these transactivation-deficient p53 mutants potentiated HDACi-induced BAX conformational change (Fig. 1B), which was determined by immunoprecipitation with anti-BAX 6A7 monoclonal antibody that only recognizes the active conformer of BAX (38), and caspase-3 activation (Fig. 1C) in p53–/– HCT116 cells, with p53L22Q/W23S remaining the most potent inducer of apoptosis. Additionally, K562 and H1299 cells (both p53-null) stably transfected with p53 constructs containing R175H or D281G mutations, respectively, showed a marked increase in apoptotic response to LAQ824 treatment, as demonstrated by the ability to activate BAX (Fig. 1D) and caspase-3 (Fig. 1E).
Notably, after exposure to SAHA or LAQ824, the exogenously expressed p53 proteins were robustly increased (Fig. 1, B and D, and supplemental Fig. S2B). Proteasome-directed degradation of p53 is tightly regulated through specific ubiquitin ligases such as Mdm2 (39). The increase in p53 protein levels by HDACi coincides with acetylation of p53 (Fig. 1, B and D) that contributes to the inhibition of Mdm2-mediated p53 ubiquitination (40). In addition, microarray analysis indicated that the mRNA levels of exogenous p53 were increased 2–5-fold by HDACi treatment (data not shown). We speculate that the exogenously integrated p53 genes are normally silenced by HDACs and reactivated by HDACi. This may explain at least in part why the up-regulation of endogenous p53 by HDACi was less than that of exogenous p53 (supplemental Fig. S2B). To evaluate the consequences of p53 stabilization after HDAC inhibition, H1299 p53–/– and p53D281G cells were treated with DMSO (vehicle), MG132 (proteasome inhibitor), or LAQ824. Inhibition of the proteasome has been shown to induce p53 accumulation without specific modifications and activation (2). Both MG132 and LAQ824 promoted a similar amount of p53 stabilization, but only LAQ824 treatment resulted in an increase in p53 acetylation (Fig. 2A). Although MG132 caused a similar loss of viability in both p53–/– and p53D281G cell lines, LAQ824 specifically promoted apoptosis in p53D281G-expressing cells (Fig. 2B). Therefore, the presence of mutant p53 does not predispose cells to apoptosis under certain stresses, but HDACi preferentially promote apoptosis in p53-expressing cells. This unique ability of HDACi correlates with the acetylation of p53 but not just its accumulation.
FIGURE 2.
Acetylation rather than stabilization of p53 correlates with apoptotic responses. A and B, H1299 p53–/– and p53D281G cells were treated with DMSO, 200 nm LAQ, or 1 μm MG132 for 24 h and subjected to Western blot analysis and annexin V-APC/7AAD staining. C, SW480 cells were stably transfected with empty, scrambled (shScr), or p53 targeting (shp53) lentiviral shRNA constructs, treated with 200 nm LAQ for 36 h, and subjected to immunoblot and caspase-3 activity assays. D, MEFs derived from p53–/– or p53R172H/R172H knock-in mice were treated with the indicated concentration of LAQ for 24 h and subjected to annexin V-APC/7AAD staining. Experiments are presented as the average ± S.D., n = 3.
To further evaluate the potential of mutant p53 to initiate apoptosis in response to HDACi, two colon carcinoma cell lines, SW480 and HT-29 harboring R273H/P309S and R273H mutations in p53, respectively, were infected with lentiviral constructs expressing p53 shRNA (shp53), scrambled shRNA (shScr), or empty vector. Infection with the shp53 lentivirus effectively knocked down p53 expression in both cell lines (Fig. 2C and supplemental Fig. S3A). Loss of mutant p53 conferred significant resistance to HDACi-induced caspase-3 activation (Fig. 2C and supplemental Fig. S3A) and cell death (supplemental Fig. S3, B and C). These results were also confirmed by annexin V-APC and 7-AAD staining (supplemental Fig. S3D). Furthermore, MEFs derived from p53R172H/R172H (corresponding to human R175H mutation) knock-in mice demonstrated an enhanced apoptotic response over control p53–/– cells when treated with LAQ824 (Fig. 2D). These results highlight the functional significance of endogenous p53 status in determination of apoptotic index in response to HDACi regardless of its transactivational ability.
Carboxyl-terminal Acetylation Is Essential for Mutant p53-mediated Apoptosis in Response to SAHA or LAQ824 Treatment—Inhibition of HDACs by SAHA or LAQ824 resulted in a significant increase in the levels of acetylated p53 (Fig. 1, B and D, and Fig. 2A). To investigate the possible involvement of p53 acetylation in its transcription-independent proapoptotic functions, three lysine residues (Lys-320, Lys-373, and Lys-382), which are known to be acetylated by HDACi treatment (6), in human p53R175H protein were mutated to arginine individually and in triplicate (3KR) to mimic the unacetylated state. These p53 constructs were cloned into retroviral vectors and infected into HCT116 p53–/– cells to comparable levels (Fig. 3A). The apoptotic index was then measured by BAX conformational change (Fig. 3A) and caspase-3 activity (Fig. 3B) after LAQ824 treatment. Single mutations at the individual lysine residues yielded marginal decreases in apoptosis. However, mutation of all three lysines nearly completely abolished the proapoptotic activity of p53R175H in response to HDAC inhibition. To further validate the importance of these residues in transactivation-deficient p53, Myc-tagged p53L22Q/W23S or p53L22Q/W23S-3KR (all three lysines, Lys-320/373/382, were mutated to arginine) was stably expressed in HCT116 p53–/– cells (Fig. 3C). Immunoblot analysis with anti-acetylated Lys-382-p53 antibody confirmed the absence of HDACi-induced Lys-382 acetylation in cells expressing the p53L22Q/W23S-3KR mutant (Fig. 3C). Consistently, the ability of p53L22Q/W23S to induce caspase-3 activation and apoptotic cell death in response to SAHA or LAQ824 was drastically impaired by the 3KR mutations (Fig. 3, D and E).
FIGURE 3.
Substitution of Lys-320, Lys-373, and Lys-382 to Arg attenuates the proapoptotic activity of mutant p53 in response to HDACi. A and B, HCT116 p53–/– cells infected with retroviral empty (pKI), p53R175H, p53R175H/K320R, p53R175H/K373R, p53R175H/K382R, or p53R175H/3KR constructs were analyzed for BAX conformational change and caspase-3 activity after 24 h of 200 nm LAQ treatment. C–E, HCT116 p53–/– stably transfected with empty (Puro), Myc-p53L22Q/W23S, or Myc-p53L22Q/W23S-3KR were treated with DMSO, 5 μm SAHA, or 200 nm LAQ for 18 h and subjected to immunoblot analysis, caspase-3 assay, and annexin V-isothiocyanate/propidium iodide. WCL, whole cell lysate; IP, immunoprecipitation.
SirT1 is a class III HDAC whose activity also influences p53 acetylation status but is not inhibited by LAQ824 or SAHA. To determine whether SirT1 has a functional role in LAQ824-mediated apoptosis, H1299 p53–/– and p53D281G cells were transfected with empty control or SirT1-expressing plasmids. Overexpression of SirT1 resulted in a minimal reduction in acetylated Lys-382-p53 (supplemental Fig. S4A) and did not impact caspase-3 activation in either cell line treated with LAQ824 (supplemental Fig. S4B). Because SirT1 can be specifically inhibited by the compound EX527 (41), we treated p53–/– and p53D281G H1299 cells with EX527, LAQ824, or the combination of both compounds. Although EX527 alone had no effect on Lys-382 acetylation, when combined with LAQ824 there was a significant increase in p53 acetylation (supplemental Fig. S4C). However, EX527 failed to enhance LAQ824-mediated apoptosis (supplemental Fig. S4D). This could possibly be due to a saturation of the acetylated p53 needed for apoptosis induction after LAQ824 treatment. Overall, it appears that SirT1 does not play a significant role in LAQ824-mediated cytotoxicity.
SAHA- or LAQ824-mediated ROS Production Is Dependent on p53 Acetylation and BAX—ROS production plays a central role in HDACi-mediated cell death (23). In addition, ROS is a mediator of p53-induced apoptosis (42). We therefore examined the correlation between ROS generation and p53 transactivation-independent proapoptotic function after HDACi treatment. To this end, HCT116 p53–/– cells stably expressing p53L22Q/W23S or empty control vector (Puro) were treated with SAHA or LAQ824, and the ROS levels were determined as described previously (32). LAQ824 increased the ROS level in p53L22Q/W23S but not control cells (Fig. 4A). The antioxidant N-acetyl-l-cysteine reversed this ROS generation (Fig. 4A) and decreased cell death (Fig. 4B) induced by SAHA or LAQ824 in p53L22Q/W23S cells. Moreover, substitution of Lys-320/Lys-373/Lys-382 to Arg (3KR) completely abolished the ability of p53L22Q/W23S to induce ROS generation after LAQ824 treatment (Fig. 4, C and D). These results suggest that SAHA and LAQ824 require p53 acetylation but not its transcriptional activity to induce ROS production that is essential for HDACi-mediated cytotoxicity.
FIGURE 4.
HDACi-mediated BAX-dependent ROS accumulation requires p53 acetylation but not transactivation. A, HCT116 p53–/– cells stably expressing control Puro or p53L22Q/W23S were treated with 200 nm LAQ with or without 10 mm N-acetyl-l-cysteine (NAC) for 12 h, and the changes in intracellular ROS level were assessed. B, HCT116 p53L22Q/W23S cells were analyzed for viability by trypan blue exclusion after 48 h of treatment with 5 μm SAHA or 200 nm LAQ with or without 10 mm N-acetyl-l-cysteine. C and D, HCT116 p53L22Q/W23S or p53L22Q/W23S-3KR cells were treated with 200 nm LAQ and assayed for ROS levels by spectrofluorometry and flow cytometry. E, BAX+/– and BAX–/– HCT116 cells were treated with 5 μm SAHA or 200 nm LAQ for the indicated times and subjected to trypan blue viability assay. F and G, ROS levels in BAX+/– and BAX–/– HCT116 cells treated with 5 μm SAHA or 200 nm LAQ for 12 h were determined by spectrofluorometry and flow cytometry.
Mitochondria are the main source of cellular ROS, and BCL-2 family proteins are essential mediators of the amount of ROS production by HDACi (43). To determine the role of BAX in HDACi-induced ROS production and cell death, we took advantage of HCT116 BAX knock-out cells (44). Similarly to p53–/– HCT116 cells, BAX–/– HCT116 cells were also resistant to SAHA- or LAQ824-induced cell death regardless of normal p53 expression (Fig. 4E). Furthermore, the up-regulation of ROS levels induced by SAHA or LAQ824 was not observed in BAX-null HCT116 cells (Fig. 4, F and G). Taken together, these data suggest that p53 exerts its transcription-independent function upstream of BAX signaling to control ROS generation and apoptosis in response to HDACi treatment.
Acetylated p53 Binding to Ku70 Activates BAX by Disrupting the BAX-Ku70 Complex—To gain further insight into the transactivation-independent proapoptotic function of p53 in response to HDACi, we took a proteomics approach combined with the TAP (33) to identify p53L22Q/W23S-binding proteins. The amino-terminal TAP-tagged p53L22Q/W23S fusion protein was stably expressed in HCT116 p53–/– cells, and its ability to enhance HDACi-mediated cytotoxicity was confirmed (supplemental Fig. S5). These cells were then treated with LAQ824 and subjected to TAP purification and SDS-PAGE/mass spectrometry analysis. As a result, we identified Ku70 in the p53L22Q/W23S complex (Fig. 5A). This association was not disrupted by DNase or ethidium bromide, indicating that the p53/Ku70 interaction was not mediated by DNA (data not shown). To determine whether p53 acetylation is required for its binding to Ku70, we performed co-immunoprecipitation analysis in p53–/– HCT116 cells stably transfected with empty control vector (Puro), Myc-p53L22Q/W23S, or Myc-p53L22Q/W23S-3KR constructs (Fig. 5B). A specific interaction between Myc-p53L22Q/W23S and endogenous Ku70 was observed after LAQ824 treatment. However, substitution of 3KR in p53L22Q/W23S did not affect this interaction, suggesting that acetylation at these lysines is dispensable for p53 binding to Ku70. The observed increase in p53/Ku70 interaction after LAQ824 treatment is likely due to the increased protein levels of exogenous p53, because stabilization of p53 with MG132 also led to increased p53/Ku70 interaction (Fig. 5C). Moreover, in vitro GST pulldown assays using GST-Ku70 and purified nonacetylated or acetylated recombinant p53 (35) revealed that the interaction between p53 and Ku70 is direct and acetylation-independent (Fig. 5D). Additionally, endogenous p53 could be co-immunoprecipitated with endogenous Ku70 in both LAQ824-treated and untreated SW480 cells (Fig. 5E), providing further evidence that the p53/Ku70 interaction occurs at physiological protein levels in an acetylation-independent manner.
FIGURE 5.
Identification of Ku70 as a novel p53 interacting protein. A, TAP purification and mass spectrometry analysis of cell lysates prepared from TAP and TAP-p53L22Q/W23S HCT116 cells treated with 200 nm LAQ for 20 h. B, anti-Myc immunoprecipitation (IP) of stable HCT116 p53–/– (Puro), Myc-p53L22Q/W23S, or Myc-p53L22Q/W23S-3KR cells treated or untreated with 200 nm LAQ for 16 h. C, Ku70 immunoprecipitations from H1299 p53–/– and p53D281G cells treated with DMSO, 1 μm MG132, or 200 nm LAQ for 24 h. D, recombinant GST or GST-Ku70 was incubated with purified nonacetylated or acetylated p53 for 2 h, washed, and subjected to Western blot analysis. E, SW480 cells were treated with DMSO or 200 nm LAQ for 24 h and subjected to immunoprecipitations with hemagglutinin (HA) or Ku70 monoclonal antibodies, normal rabbit serum (NRS), or p53 polyclonal antibodies. WCL, whole cell lysate.
The interaction between Ku70 and BAX prevents BAX conformational change and mitochondrial translocation (25, 36). Moreover, it has been shown that Ku70 acetylation reduces the BAX/Ku70 association and plays a role in HDACi-induced cell death (26). Thus, we speculated that the interaction between acetylated p53 and Ku70 may be involved in BAX activation induced by HDACi. After LAQ824 treatment, a drastic conformational activation of BAX measured by exposure of the 6A7 epitope was observed in p53L22Q/W23S cells compared with p53L22Q/W23S-3KR and Puro control cells that coincided with increased p53 acetylation (Fig. 6A). Analysis of BCL-2 family proteins in these cell lines revealed that LAQ824 treatment resulted in an increase in BimEL, a somewhat smaller increase in BAK and MCL-1, and a slight decrease in BCL-2 (Fig. 6A). However, there are no clear differences in these BCL-2 family members between p53L22Q/W23S, p53L22Q/W23S-3KR, and control Puro-expressing cells, suggesting that acetylated p53 has other modes of action besides regulating BCL-2 family protein expression for the induction of apoptosis in response to HDACi.
FIGURE 6.
Acetylation of p53 promotes the disruption of BAX-Ku70 complexes. A, anti-BAX 6A7 IP and immunoblot from HCT116 Puro, p53L22Q/W23S, and p533KR cells treated with DMSO or 200 nm LAQ for 18 h. B, HCT116 Puro or Myc-p53L22Q/W23S cells treated with 200 nm LAQ for the indicated times were subjected to immunoprecipitation (IP) with anti-BAX polyclonal antibody or normal rabbit serum (NRS). C, HCT116 p53–/– and p53+/+ cells were treated with 200 nm of LAQ for the indicated times and subjected to immunoprecipitation with anti-BAX polyclonal antibody or normal rabbit serum. D, HCT116 Myc-p53L22Q/W23S or Myc-p533KR were treated with 200 nm of LAQ for the indicated times and subjected to immunoprecipitation with anti-BAX antibody or normal rabbit serum. E, HCT116 p53–/– cells were transiently co-transfected with Myc-p53L22Q/W23S-3KR and scrambled shRNA (shScr) or Ku70 shRNA (shKu70) expression plasmids for 24 h. The cells were then treated with DMSO or 200 nm LAQ for 24 h, lysed in CHAPS buffer, and subjected to immunoprecipitation with anti-Myc monoclonal antibody, followed by immunoblot analysis with the indicated antibodies. F, HCT116 p53–/– cell lysate was immunoprecipitated with anti-BAX polyclonal antibody, and then immunocomplexes were incubated with purified nonacetylated or acetylated p53 for 30 min, and the supernatant and immunocomplexes were assayed by immunoblot. G, recombinant GST or GST-Ku70 was incubated with purified BAX and either nonacetylated or acetylated p53 for 2 h and then washed and subjected to immunoblot analysis. H, H1299 p53–/– and p53D281G cells were infected with shScr or shKu70 lentivirus, 24 h later treated with DMSO or 200 nm LAQ for 24 h, and analyzed for apoptosis by annexin V-APC/7AAD staining. WCL, whole cell lysate.
Interestingly, the BAX/Ku70 interaction was increased at 12 h following LAQ824 treatment (Fig. 6B). However, although the enhanced BAX-Ku70 complex was maintained up to 36 h in p53–/– (Puro) cells, the BAX/Ku70 association was decreased at 24 h in p53L22Q/W23S cells. Similar results were obtained in p53–/– and p53+/+ HCT116 cells (Fig. 6C). Interestingly, the interaction between Ku70 and BAX was increased in p53L22Q/W23S-3KR cells during LAQ824 treatment, and the p53L22Q/W23S-3KR protein was detected in the BAX-Ku70 complex after 24 h of treatment (Fig. 6D). However, knockdown of Ku70 abolished the ability of p53 to co-immunoprecipitate with BAX (Fig. 6E), suggesting that p53 may not form a stable complex with BAX directly. We do not, however, rule out the possibility that there is a transient direct contact between p53 and BAX in the p53-Ku70-BAX complex. Nevertheless, these findings suggest that HDACi treatment initially promotes an increase in the interaction between BAX and Ku70, but prolonged treatment leads to the disruption of this complex, probably through acetylated p53.
Because p53 interacts with Ku70 independently of its acetylation status, the ability of acetylated p53 to disrupt the BAX/Ku70 interaction was assayed in vitro. The BAX-Ku70 immune complexes were isolated from HCT116 p53–/– cell lysate and incubated with purified nonacetylated or acetylated recombinant p53 (35). Only acetylated p53 was able to release Ku70 from the BAX-Ku70 complex when compared with control vehicle and unacetylated p53 (Fig. 6F). Similar results were also obtained in a GST-Ku70 pulldown assay; acetylated p53 could prevent BAX binding to Ku70, whereas nonacetylated p53 had no effect (Fig. 6G). These results clearly indicate that p53 acetylation is important for BAX release from Ku70.
To elucidate the role of Ku70 in HDACi-induced apoptosis, H1299 p53–/– and p53D281G cells were infected with control shScr or shKu70 lentivirus. Knockdown of Ku70 was assayed by Western blot (supplemental Fig. S6). These cells were then treated with LAQ824 for 24 h and subjected to apoptosis analysis with annexin V-APC/7AAD staining and flow cytometry (Fig. 6H). Knockdown of Ku70 in p53–/– cells promotes an enhanced apoptotic response over shScr control cells. However, knockdown of Ku70 in p53D281G cells did not significantly impact LAQ824-induced apoptosis. This result suggests that the BAX-Ku70 complex is essential to the prevention of HDACi-induced apoptosis in p53–/– cells. However, when p53 is present and able to disrupt the BAX-Ku70 complex, reduction in Ku70 expression is redundant and unnecessary to promote HDACi-induced apoptosis.
To determine the cellular localization of the BAX-Ku70 complex, co-immunoprecipitation experiments were performed using heavy membrane, cytosolic, and nuclear fractions isolated from p53–/– HCT116 cells transfected with control empty vector (Puro) or Myc-tagged p53L22Q/W23S or p53L22Q/W23S-3KR (Fig. 7A). The BAX-Ku70 complex was increased in both cytosolic and nuclear fractions of Puro and p53L22Q/W23S-3KR cells after LAQ824 treatment. Because the complex formation was only assayed at 24 h after treatment, the accumulation of the BAX-Ku70 complex was not observed in p53L22Q/W23S cells as it was at 12 h, as seen in Fig. 6D. Furthermore, the Ku70-p53 complex was most readily detected in the nuclear fraction corresponding to the prominent localization of p53 and Ku70 to this cellular compartment. However, p53 could also be found localized to the cytosolic and heavy membrane fractions and in a complex with Ku70 at reduced levels compared with that in the nuclear fraction after LAQ824 treatment.
To determine whether p53L22Q/W23S affects the intracellular redistribution of BAX after HDACi treatment, we performed subcellular fractionation and immunoblot analysis. Ku70 localization was not affected by p53L22Q/W23S after HDACi treatment, whereas the nuclear BAX was decreased in p53L22Q/W23S cells but not in p53–/– cells (supplemental Fig. S7A). Conversely, the cytosolic BAX was increased in p53L22Q/W23S cells after HDACi treatment. These results support the hypothesis that BAX dissociation from Ku70 and subsequent translocation to the cytoplasm after HDACi treatment is mediated by acetylated p53. Consistent with the retained association of Ku70 with BAX, HDACi-induced BAX conformational activation at the mitochondria was attenuated in p53–/– and p53L22Q/W23S-3KR compared with p53L22Q/W23S cells (Fig. 7B), which correlated with an apparent increase in BAX translocation to the mitochondrial fraction specifically in HDACi-treated p53L22Q/W23S cells (supplemental Fig. S7B).
DISCUSSION
HDACi represent a new class of chemotherapeutic agents that have shown promise in pre-clinical and clinical trials and work by modifying the acetylation status of histone and non-histone proteins. Acetylation is accepted as an important post-translational modification that impacts protein structure and/or function, but relatively few non-histone proteins have been identified as regulated by acetylation. Of those identified are several important apoptotic molecules such as Ku70, p53, retinoblastoma protein, and p73 (25, 45, 46). The findings presented in this study indicate that HDACi can induce apoptosis by a p53 transactivation-independent apoptotic mechanism. Expression of various mutants of p53 in p53–/– backgrounds enhances HDACi-induced apoptosis, and knockdown of endogenous mutant p53 results in an abrogated apoptotic response. p53 binds Ku70 and promotes BAX dissociation from Ku70 in an acetylation-dependent manner. Additionally, HDACi can induce substantial apoptosis in p53–/– but not mutant p53 expressing cells when Ku70 is knocked down. Therefore, we propose a model where the interaction between Ku70 and p53 is acetylation-independent; however, acetylation of p53 is required for BAX dissociation from Ku70, conformational activation, and enhanced apoptosis upon HDACi treatment (Fig. 7C). Given that p53 is frequently mutated in human cancers, our findings that mutant p53 sensitizes cancer cells to HDACi-induced apoptosis via BAX activation further explain why this class of inhibitors displays preferential anti-neoplastic potential.
Transcription-independent p53 Mechanism of HDACi-induced Apoptosis—The transcriptional activity of p53 is able to efficiently enhance apoptosis in response to various cellular stresses. Although our experiments predominantly use transactivational repressed p53 mutants, we sought to confirm that p53L22Q/W23S lacks HDACi-induced transcriptional activity by luciferase reporter gene assays (supplemental Fig. S1). Expression of wild type p53 dramatically enhanced transcription from the p21 and Mdm2 promoters, but p53L22Q/W23S also exhibited a low level of activation compared with p53–/– cells. However, this residual transactivation potential of p53L22Q/W23S likely plays a minor role in HDACi-induced apoptosis for several reasons. First, microarray analysis revealed that there was no specific increase in p53-responsive target gene expression after treatment with HDACi (data not shown). The expression of p21 was also found to be similar between p53–/–, p53L22Q/W23S, and been shown that p21 expression is enhanced by HDACi in a p53-independent manner (47). Although p53L22Q/W23S has been reported to retain the potential for transcriptional activation of BAX and repression of BCL-2 (37), we were unable to detect changes in apoptotic proteins controlled by p53, such as BAX, BCL-2, and PUMA after HDACi treatment in p53L22Q/W23S cell lines (Fig. 6A). Additionally, studies with transfection of prominent naturally occurring p53 mutants into p53-deficient HCT116, K562, and H1299 cells, and knockdown of endogenous mutant p53 in SW480 and HT-29 cells further support our hypothesis that p53 retains a transcription-independent proapoptotic function mediated by acetylation.
p53 Localization and HDACi-induced Apoptosis—Although it is widely accepted that p53 has a transcription-independent mechanism of apoptosis induction, several controversies remain. For instance, it has been shown that p53 translocates to mitochondria where it interacts with anti- or proapoptotic BCL-2 family proteins, including BCL-XL, BCL-2, and BAK to induce BAX and BAK activation, thereby leading to the release of cytochrome c from mitochondria to the cytoplasm (18, 48–50). However, other reports suggest that the level of mitochondrial p53 does not correlate with the level of apoptosis (51, 52). Upon HDACi treatment, relatively low levels of p53L22Q/W23S and p53L22Q/W23S-3KR were detected in the mitochondrial fraction (Fig. 7A). Furthermore, there were no differences between wild type p53, p53L22Q/W23S, and p53L22Q/W23S-3KR for their ability to interact with BCL-XL (supplemental Fig. S7C), even though p53L22Q/W23S-3KR cannot enhance HDACi-induced apoptosis, suggesting that the mitochondrial translocation of mutant p53 may not play a major role in HDACi-mediated apoptosis. However, the small increase in HDACi-induced apoptosis in p53L22Q/W23S-3KR over p53–/– cells may rely on its ability to bind BCL-XL at the mitochondria. Similarly, it is worth noting that p53R273H is highly acetylated in response to HDACi, but its pro-apoptotic activity is less in comparison with p53L22Q/W23S (Fig. 1, B and C). This may be explained at least in part by their abilities to bind anti-apoptotic BCL-2 family proteins. It has been shown that naturally occurring p53 mutants, including p53R273H and p53R175H, are deficient in forming a complex with BCL-XL (18), whereas p53L22Q/W23S retains full ability to interact with BCL-XL (28). However, although HDACi apparently enhanced Ku70 binding to p53, it had no effect on the interaction between BCL-XL and p53 (supplemental Fig. S7D), suggesting that there is no competition between Ku70 and BCL-XL for p53 binding.
As a transcription factor, p53 principally localizes in the nucleus, but certain apoptotic stimuli can promote p53 accumulation in the cytosolic and mitochondrial fractions. Ku70 is an important, mainly nuclear protein involved in nonhomologous end joining DNA repair and V(D)J recombination (53). Therefore, it is not surprising that the nucleus is the compartment that contains the highest concentration of p53 associated with Ku70 (Fig. 7A). Alternatively, BAX has been classified as a predominantly cytosolic protein that loosely associates with the mitochondrial membrane in unstressed cells. Enigmatically, BAX has also been classified as a nuclear protein and under certain stresses interacts with p53 (54, 55). Similarly, our results indicate that a substantial amount of BAX can be found in the nucleus of HCT116 cells (Fig. 7A and supplemental Fig. S7A). It seems that HDACi-induced p53 acetylation disrupts the Ku70-BAX complex, which results in BAX conformational change and translocation from the nucleus to the cytoplasm. However, cytosolic p53 is also equally capable of disrupting BAX from Ku70. Although it is less prominent, nuclear BAX localization may predispose cells to enhanced HDACi-induced apoptosis as Ku70/BAX interactions have an increased probability of dissociation because of p53 enrichment.
p53 Acetylation and BAX/Ku70 Disruption—LAQ824 treatment results in a transient increase in the association between Ku70 and BAX (Fig. 6, B–D). This phenomenon could be due to several reasons. First, this apoptotic stimulus may encourage a cellular response designed to prevent BAX activation by sequestration through Ku70 binding. The increased association could be caused by a variety of different mechanisms, such as post-translational modifications or intracellular distribution. Another intriguing possibility is that the increase in Ku70-BAX complex could serve a dual role as Ku70 promotes the deubiquitination of BAX in addition to its sequestration (56). Thus, this interaction could prime cells for apoptosis by increasing the pool of unmodified BAX able to promote apoptosis after release from Ku70. In either case, prolonged HDACi treatment that results in the disruption of the Ku70-BAX complex can enhance the apoptotic response.
Expression of wild type and mutant p53 capable of being acetylated promotes the dissociation of BAX from Ku70 after a prolonged HDACi treatment (Fig. 6, B–D). Moreover, acetylated but not unacetylated recombinant p53 is able to disrupt the Ku70-BAX complex in vitro (Fig. 6, F and G). However, the nonacetylated p53L22Q/W23S-3KR mutant retains the ability to interact with Ku70 and accumulates in the Ku70-BAX complex during HDACi treatment (Fig. 6D and Fig. 7A). Because acetylation of p53 alters its structure (57), it is plausible that once p53 is acetylated changes in conformation lead to competition for the BAX-binding site on Ku70. Acetylation and altered structure of p53 could also lead to conformational changes in Ku70, which may depend on its own acetylation, and the release of BAX as proposed previously (25). Furthermore, past studies into the acetylation of Ku70 and the regulation of apoptosis were carried out primarily in 293T cells, which would preclude p53 transactivational activity but not necessarily its transcription-independent role in HDACi-induced apoptosis (25, 36). Our results suggest that the cellular status of both p53 and Ku70 determines apoptotic responses and warrants further evaluation as biomarkers for clinical therapeutic response to HDACi such as SAHA and LAQ824 (Fig. 6H). Additional assays designed to determine how acetylated p53 affects the Ku70-BAX complex at the structural level will give additional insights into this novel interplay between proteins, with hopes of developing advanced therapeutic strategies to benefit cancer patients.
Supplementary Material
Acknowledgments
We thank Novartis Pharmaceuticals Inc. (East Hanover, NJ) for LAQ824; Merck (Flemington, NJ) for SAHA; Dr. Bert Vogelstein for HCT116 cell lines; Dr. Shigemi Matsuyama for Ku70 plasmids; Dr. Gen Wang for pcDNA3-TAP vector; and Dr. Guillermina Lozano for p53 knock-in MEFs.
This work was supported, in whole or in part, by National Institutes of Health grants. This work was also supported by grants from American Cancer Society and Flight Attendant Medical Research Institute.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures and Figs. S1–S7.
Footnotes
The abbreviations used are: HDAC, histone deacetylase; HDACi, histone deacetylase inhibitor; SAHA, suberoylanilide hydroxamic acid; ROS, reactive oxygen species; PBS, phosphate-buffered saline; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; TAP, tandem affinity purification; H2DCFDA, 2′,7′-dichlorodihydrofluorescein diacetate; MEF, mouse embryonic fibroblast; GST, glutathione S-transferase; Puro, puromycin; Bsd, blasticidin; shRNA, short hairpin RNA; APC, allophycocyanin; 7AAD, 7-amino-actinomycin D.
References
- 1.Toledo, F., and Wahl, G. M. (2006) Nat. Rev. Cancer 6 909–923 [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi, K., Herrera, J. E., Saito, S., Miki, T., Bustin, M., Vassilev, A., Anderson, C. W., and Appella, E. (1998) Genes Dev. 12 2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo, J., Li, M., Tang, Y., Laszkowska, M., Roeder, R. G., and Gu, W. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu, W., and Roeder, R. G. (1997) Cell 90 595–606 [DOI] [PubMed] [Google Scholar]
- 5.Henderson, C., Mizzau, M., Paroni, G., Maestro, R., Schneider, C., and Brancolini, C. (2003) J. Biol. Chem. 278 12579–12589 [DOI] [PubMed] [Google Scholar]
- 6.Terui, T., Murakami, K., Takimoto, R., Takahashi, M., Takada, K., Murakami, T., Minami, S., Matsunaga, T., Takayama, T., Kato, J., and Niitsu, Y. (2003) Cancer Res. 63 8948–8954 [PubMed] [Google Scholar]
- 7.Fridman, J. S., and Lowe, S. W. (2003) Oncogene 22 9030–9040 [DOI] [PubMed] [Google Scholar]
- 8.Pietrzak, M., and Puzianowska-Kuznicka, M. (2008) Biol. Chem. 389 383–393 [DOI] [PubMed] [Google Scholar]
- 9.Miyashita, T., Harigai, M., Hanada, M., and Reed, J. C. (1994) Cancer Res. 54 3131–3135 [PubMed] [Google Scholar]
- 10.Caelles, C., Heimberg, A., and Karin, M. (1994) Nature 370 220–224 [DOI] [PubMed] [Google Scholar]
- 11.Bennett, M., Macdonald, K., Chan, S. W., Luzio, J. P., Simari, R., and Weissberg, P. (1998) Science 282 290–293 [DOI] [PubMed] [Google Scholar]
- 12.Ding, H. F., Lin, Y. L., McGill, G., Juo, P., Zhu, H., Blenis, J., Yuan, J., and Fisher, D. E. (2000) J. Biol. Chem. 275 38905–38911 [DOI] [PubMed] [Google Scholar]
- 13.Dumont, P., Leu, J. I., Della Pietra, A. C., III, George, D. L., and Murphy, M. (2003) Nat. Genet. 33 357–365 [DOI] [PubMed] [Google Scholar]
- 14.Chipuk, J. E., Maurer, U., Green, D. R., and Schuler, M. (2003) Cancer Cell 4 371–381 [DOI] [PubMed] [Google Scholar]
- 15.Erster, S., Mihara, M., Kim, R. H., Petrenko, O., and Moll, U. M. (2004) Mol. Cell. Biol. 24 6728–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haupt, Y., Rowan, S., Shaulian, E., Vousden, K. H., and Oren, M. (1995) Genes Dev. 9 2170–2183 [DOI] [PubMed] [Google Scholar]
- 17.Bissonnette, N., Wasylyk, B., and Hunting, D. J. (1997) Biochem. Cell Biol. 75 351–358 [PubMed] [Google Scholar]
- 18.Mihara, M., Erster, S., Zaika, A., Petrenko, O., Chittenden, T., Pancoska, P., and Moll, U. M. (2003) Mol. Cell 11 577–590 [DOI] [PubMed] [Google Scholar]
- 19.Tomita, Y., Marchenko, N., Erster, S., Nemajerova, A., Dehner, A., Klein, C., Pan, H., Kessler, H., Pancoska, P., and Moll, U. M. (2006) J. Biol. Chem. 281 8600–8606 [DOI] [PubMed] [Google Scholar]
- 20.Minucci, S., and Pelicci, P. G. (2006) Nat. Rev. Cancer 6 38–51 [DOI] [PubMed] [Google Scholar]
- 21.Bhalla, K. N. (2005) J. Clin. Oncol. 23 3971–3993 [DOI] [PubMed] [Google Scholar]
- 22.Johnstone, R. W. (2002) Nat. Rev. Drug. Discov. 1 287–299 [DOI] [PubMed] [Google Scholar]
- 23.Ruefli, A. A., Ausserlechner, M. J., Bernhard, D., Sutton, V. R., Tainton, K. M., Kofler, R., Smyth, M. J., and Johnstone, R. W. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 10833–10838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen, C. S., Wang, Y. C., Yang, H. C., Huang, P. H., Kulp, S. K., Yang, C. C., Lu, Y. S., Matsuyama, S., Chen, C. Y., and Chen, C. S. (2007) Cancer Res. 67 5318–5327 [DOI] [PubMed] [Google Scholar]
- 25.Cohen, H. Y., Lavu, S., Bitterman, K. J., Hekking, B., Imahiyerobo, T. A., Miller, C., Frye, R., Ploegh, H., Kessler, B. M., and Sinclair, D. A. (2004) Mol. Cell 13 627–638 [DOI] [PubMed] [Google Scholar]
- 26.Subramanian, C., Opipari, A. W., Jr., Bian, X., Castle, V. P., and Kwok, R. P. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 4842–4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen, H. Y., Miller, C., Bitterman, K. J., Wall, N. R., Hekking, B., Kessler, B., Howitz, K. T., Gorospe, M., de Cabo, R., and Sinclair, D. A. (2004) Science 305 390–392 [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi, H., Chen, J., Bhalla, K., and Wang, H. G. (2004) J. Biol. Chem. 279 39431–39437 [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi, H., and Wang, H. G. (2006) Mol. Cell. Biol. 26 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang, G. A., Iwakuma, T., Suh, Y. A., Liu, G., Rao, V. A., Parant, J. M., Valentin-Vega, Y. A., Terzian, T., Caldwell, L. C., Strong, L. C., El-Naggar, A. K., and Lozano, G. (2004) Cell 119 861–872 [DOI] [PubMed] [Google Scholar]
- 31.Hinds, P. W., Finlay, C. A., Quartin, R. S., Baker, S. J., Fearon, E. R., Vogelstein, B., and Levine, A. J. (1990) Cell Growth & Differ. 1 571–580 [PubMed] [Google Scholar]
- 32.Chandel, N. S., Schumacker, P. T., and Arch, R. H. (2001) J. Biol. Chem. 276 42728–42736 [DOI] [PubMed] [Google Scholar]
- 33.Wang, G., Barrett, J. W., Nazarian, S. H., Everett, H., Gao, X., Bleackley, C., Colwill, K., Moran, M. F., and McFadden, G. (2004) J. Virol. 78 7097–7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki, M., Youle, R. J., and Tjandra, N. (2000) Cell 103 645–654 [DOI] [PubMed] [Google Scholar]
- 35.Piluso, L. G., Wei, G., Li, A. G., and Liu, X. (2005) Protein Expression Purif. 40 370–378 [DOI] [PubMed] [Google Scholar]
- 36.Li, Y., Yokota, T., Gama, V., Yoshida, T., Gomez, J. A., Ishikawa, K., Sasaguri, H., Cohen, H. Y., Sinclair, D. A., Mizusawa, H., and Matsuyama, S. (2007) Cell Death Differ. 14 2058–2067 [DOI] [PubMed] [Google Scholar]
- 37.Johnson, T. M., Hammond, E. M., Giaccia, A., and Attardi, L. D. (2005) Nat. Genet. 37 145–152 [DOI] [PubMed] [Google Scholar]
- 38.Hsu, Y. T., and Youle, R. J. (1997) J. Biol. Chem. 272 13829–13834 [DOI] [PubMed] [Google Scholar]
- 39.Honda, R., and Yasuda, H. (1999) EMBO J. 18 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, M., Luo, J., Brooks, C. L., and Gu, W. (2002) J. Biol. Chem. 277 50607–50611 [DOI] [PubMed] [Google Scholar]
- 41.Napper, A. D., Hixon, J., McDonagh, T., Keavey, K., Pons, J. F., Barker, J., Yau, W. T., Amouzegh, P., Flegg, A., Hamelin, E., Thomas, R. J., Kates, M., Jones, S., Navia, M. A., Saunders, J. O., DiStefano, P. S., and Curtis, R. (2005) J. Med. Chem. 48 8045–8054 [DOI] [PubMed] [Google Scholar]
- 42.Polyak, K., Zweler, J. L., Kinler, K. W., and Vogelstein, B. (1997) Nature 389 300–305 [DOI] [PubMed] [Google Scholar]
- 43.Ott, M., Gogvadze, V., Orrenius, S., and Zhivotovsky, B. (2007) Apoptosis 12 913–922 [DOI] [PubMed] [Google Scholar]
- 44.Zhang, L., Yu, J., Park, B. H., Kinzler, K. W., and Vogelstein, B. (2000) Science 290 989–992 [DOI] [PubMed] [Google Scholar]
- 45.Chan, H. M., Krstic-Demonacos, M., Smith, L., Demonacos, C., and La Thangue, N. B. (2001) Nat. Cell Biol. 3 667–674 [DOI] [PubMed] [Google Scholar]
- 46.Costanzo, A., Merlo, P., Pediconi, N., Fulco, M., Sartorelli, V., Cole, P. A., Fontemaggi, G., Fanciulli, M., Schiltz, L., Blandino, G., Balsano, C., and Levrero, M. (2002) Mol. Cell 9 175–186 [DOI] [PubMed] [Google Scholar]
- 47.Huang, L., Sowa, Y., Sakai, T., and Pardee, A. B. (2000) Oncogene 19 5712–5719 [DOI] [PubMed] [Google Scholar]
- 48.Chipuk, J. E., Kuwana, T., Bouchier-Hayes, L., Droin, N. M., Newmeyer, D. D., Schuler, M., and Green, D. R. (2004) Science 303 1010–1014 [DOI] [PubMed] [Google Scholar]
- 49.Leu, J. I., Dumont, P., Hafey, M., Murphy, M. E., and George, D. L. (2004) Nat. Cell Biol. 6 443–450 [DOI] [PubMed] [Google Scholar]
- 50.Deng, X., Gao, F., Flagg, T., Anderson, J., and May, W. S. (2006) Mol. Cell. Biol. 26 4421–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahyar-Roemer, M., Fritzsche, C., Wagner, S., Laue, M., and Roemer, K. (2004) Oncogene 23 6226–6236 [DOI] [PubMed] [Google Scholar]
- 52.Essmann, F., Pohlmann, S., Gillissen, B., Daniel, P. T., Schulze-Osthoff, K., and Janicke, R. U. (2005) J. Biol. Chem. 280 37169–37177 [DOI] [PubMed] [Google Scholar]
- 53.Gu, Y., Jin, S., Gao, Y., Weaver, D. T., and Alt, F. W. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 8076–8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishita, M., Inoue, S., Tsuda, M., Tateda, C., and Miyashita, T. (1998) Exp. Cell Res. 244 357–366 [DOI] [PubMed] [Google Scholar]
- 55.Raffo, A. J., Kim, A. L., and Fine, R. L. (2000) Oncogene 19 6216–6228 [DOI] [PubMed] [Google Scholar]
- 56.Amsel, A. D., Rathaus, M., Kronman, N., and Cohen, H. Y. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 5117–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giordano, A., and Avantaggiati, M. L. (1999) J. Cell. Physiol. 181 218–230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.