Abstract
Members of the CLC gene family either function as chloride channels or as
anion/proton exchangers. The plant AtClC-a uses the pH gradient across the
vacuolar membrane to accumulate the nutrient
 in this organelle. When AtClC-a was
expressed in Xenopus oocytes, it mediated
in this organelle. When AtClC-a was
expressed in Xenopus oocytes, it mediated
 exchange
and less efficiently mediated Cl–/H+ exchange.
Mutating the “gating glutamate” Glu-203 to alanine resulted in an
uncoupled anion conductance that was larger for Cl– than
exchange
and less efficiently mediated Cl–/H+ exchange.
Mutating the “gating glutamate” Glu-203 to alanine resulted in an
uncoupled anion conductance that was larger for Cl– than
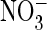 . Replacing the “proton
glutamate” Glu-270 by alanine abolished currents. These could be
restored by the uncoupling E203A mutation. Whereas mammalian endosomal ClC-4
and ClC-5 mediate stoichiometrically coupled
2Cl–/H+ exchange, their
. Replacing the “proton
glutamate” Glu-270 by alanine abolished currents. These could be
restored by the uncoupling E203A mutation. Whereas mammalian endosomal ClC-4
and ClC-5 mediate stoichiometrically coupled
2Cl–/H+ exchange, their
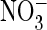 transport is largely uncoupled from
protons. By contrast, the AtClC-a-mediated
transport is largely uncoupled from
protons. By contrast, the AtClC-a-mediated
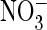 accumulation in plant vacuoles
requires tight
accumulation in plant vacuoles
requires tight
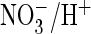 coupling. Comparison of AtClC-a and ClC-5 sequences identified a proline in
AtClC-a that is replaced by serine in all mammalian CLC isoforms. When this
proline was mutated to serine (P160S), Cl–/H+
exchange of AtClC-a proceeded as efficiently as
coupling. Comparison of AtClC-a and ClC-5 sequences identified a proline in
AtClC-a that is replaced by serine in all mammalian CLC isoforms. When this
proline was mutated to serine (P160S), Cl–/H+
exchange of AtClC-a proceeded as efficiently as
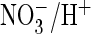 exchange, suggesting a role of this residue in
exchange, suggesting a role of this residue in
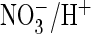 exchange. Indeed, when the corresponding serine of ClC-5 was replaced by
proline, this Cl–/H+ exchanger gained efficient
exchange. Indeed, when the corresponding serine of ClC-5 was replaced by
proline, this Cl–/H+ exchanger gained efficient
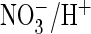 coupling. When inserted into the model Torpedo chloride channel
ClC-0, the equivalent mutation increased nitrate relative to chloride
conductance. Hence, proline in the CLC pore signature sequence is important
for
coupling. When inserted into the model Torpedo chloride channel
ClC-0, the equivalent mutation increased nitrate relative to chloride
conductance. Hence, proline in the CLC pore signature sequence is important
for 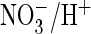 exchange and
exchange and 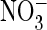 conductance both in
plants and mammals. Gating and proton glutamates play similar roles in
bacterial, plant, and mammalian CLC anion/proton exchangers.
conductance both in
plants and mammals. Gating and proton glutamates play similar roles in
bacterial, plant, and mammalian CLC anion/proton exchangers.
CLC proteins are found in all phyla from bacteria to humans and either
mediate electrogenic anion/proton exchange or function as chloride channels
(1). In mammals, the roles of
plasma membrane CLC Cl– channels include transepithelial
transport
(2–5)
and control of muscle excitability
(6), whereas vesicular CLC
exchangers may facilitate endocytosis
(7) and lysosomal function
(8–10)
by electrically shunting vesicular proton pump currents
(11). In the plant
Arabidopsis thaliana, there are seven CLC isoforms
(AtClC-a–AtClC-g)2
(12–15),
which may mostly reside in intracellular membranes. AtClC-a uses the pH
gradient across the vacuolar membrane to transport the nutrient nitrate into
that organelle (16). This
secondary active transport requires a tightly coupled
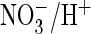 exchange. Astonishingly, however, mammalian ClC-4 and -5 and bacterial EcClC-1
(one of the two CLC isoforms in Escherichia coli) display tightly
coupled Cl–/H+ exchange, but anion flux is largely
uncoupled from H+ when
exchange. Astonishingly, however, mammalian ClC-4 and -5 and bacterial EcClC-1
(one of the two CLC isoforms in Escherichia coli) display tightly
coupled Cl–/H+ exchange, but anion flux is largely
uncoupled from H+ when 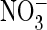 is transported
(17–21).
The lack of appropriate expression systems for plant CLC transporters
(12) has so far impeded
structure-function analysis that may shed light on the ability of AtClC-a to
perform efficient
is transported
(17–21).
The lack of appropriate expression systems for plant CLC transporters
(12) has so far impeded
structure-function analysis that may shed light on the ability of AtClC-a to
perform efficient
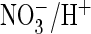 exchange. This dearth of data contrasts with the extensive mutagenesis work
performed with CLC proteins from animals and bacteria.
exchange. This dearth of data contrasts with the extensive mutagenesis work
performed with CLC proteins from animals and bacteria.
The crystal structure of bacterial CLC homologues (22, 23) and the investigation of mutants (17, 19–21, 24–29) have yielded important insights into their structure and function. CLC proteins form dimers with two largely independent permeation pathways (22, 25, 30, 31). Each of the monomers displays two anion binding sites (22). A third binding site is observed when a certain key glutamate residue, which is located halfway in the permeation pathway of almost all CLC proteins, is mutated to alanine (23). Mutating this gating glutamate in CLC Cl– channels strongly affects or even completely suppresses single pore gating (23), whereas CLC exchangers are transformed by such mutations into pure anion conductances that are not coupled to proton transport (17, 19, 20). Another key glutamate, located at the cytoplasmic surface of the CLC monomer, seems to be a hallmark of CLC anion/proton exchangers. Mutating this proton glutamate to nontitratable amino acids uncouples anion transport from protons in the bacterial EcClC-1 protein (27) but seems to abolish transport altogether in mammalian ClC-4 and -5 (21). In those latter proteins, anion transport could be restored by additionally introducing an uncoupling mutation at the gating glutamate (21).
The functional complementation by AtClC-c and -d
(12,
32) of growth phenotypes of a
yeast strain deleted for the single yeast CLC Gef1
(33) suggested that these
plant CLC proteins function in anion transport but could not reveal details of
their biophysical properties. We report here the first functional expression
of a plant CLC in animal cells. Expression of wild-type (WT) and mutant
AtClC-a in Xenopus oocytes indicate a general role of gating and
proton glutamate residues in anion/proton coupling across different isoforms
and species. We identified a proline in the CLC signature sequence of AtClC-a
that plays a crucial role in
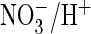 exchange. Mutating it to serine, the residue present in mammalian CLC proteins
at this position, rendered AtClC-a Cl–/H+ exchange
as efficient as
exchange. Mutating it to serine, the residue present in mammalian CLC proteins
at this position, rendered AtClC-a Cl–/H+ exchange
as efficient as
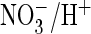 exchange. Conversely, changing the corresponding serine of ClC-5 to proline
converted it into an efficient
exchange. Conversely, changing the corresponding serine of ClC-5 to proline
converted it into an efficient
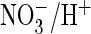 exchanger. When proline replaced the critical serine in Torpedo
ClC-0, the relative
exchanger. When proline replaced the critical serine in Torpedo
ClC-0, the relative 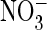 conductance of
this model Cl– channel was drastically increased, and
“fast” protopore gating was slowed.
conductance of
this model Cl– channel was drastically increased, and
“fast” protopore gating was slowed.
EXPERIMENTAL PROCEDURES
Molecular Biology—cDNAs of A. thaliana AtClC-a (12), rat ClC-5 (34), and Torpedo marmorata ClC-0 (35) were cloned into the pTLN (36) expression vector. Mutations were generated by recombinant PCR and confirmed by sequencing. Capped cRNA was transcribed from linearized plasmids using the Ambion mMESSAGE mMACHINE kit (SP6 RNA polymerase for pTLN) according to the manufacturer's instructions.
Expression in Xenopus Oocyte and Two-electrode Voltage-Clamp Studies—Pieces of ovary were obtained by surgery from deeply anesthetized (0.1% tricaine; Sigma) pigmented or albino Xenopus laevis frogs. Oocytes were prepared by manual dissection and collagenase A (Roche Applied Science) digestion. 23 ng (ClC-5), 46–50 ng (AtClC-a), or 1–3 ng (ClC-0) of cRNA were injected into oocytes. Oocytes were kept in ND96 solution (containing 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl .2, 1 mm MgCl2, 5 mm HEPES, pH 7.5) at 17 °C for 1–2 days (ClC-0), 3–4 days (ClC-5), or for 5–6 days (AtClC-a). Two-electrode voltage clamping was performed at room temperature (20–24 °C) using a TEC10 amplifier (npi Electronics, Tamm, Germany) and pClamp9 software (Molecular Devices). The standard bath solution contained 96 mm NaCl, 2 mm K+ gluconate, 5 mm Ca2+ d-gluconate, 1.2 mm MgSO4, 5 mm HEPES, pH 7.5 (or MES for buffering to pH 5.5 or 6.5; Tris for pH 8.5). For some experiments, NaCl was substituted with equal amounts of either NaNO3, NaBr, or NaI. Ag/AgCl electrodes and 3 m KCl agar bridges were used as reference and bath electrodes, respectively.
Measurement of Relative Intracellular pH Changes Using the “Fluorocyte” Device—Proton transport was measured semiquantitatively by monitoring intracellular fluorescence signal changes using the Fluorocyte device (21). Briefly, 23 nl of saturated aqueous solution of the pH indicator BCECF (Molecular Probes) were injected into oocytes 10–30 min before measurements. Oocytes were placed over a hole 0.8 mm in diameter, through which BCECF fluorescence changes were measured in response to pulse trains, which served to reduce the possible activation of endogenous oocyte currents. Starting from a holding voltage of –60 mV, depolarizing pulse trains clamped oocytes to +90 mV for 400 ms and –60 mV for 100 ms, whereas hyperpolarizing pulse trains started from –30 mV and clamped to –160 mV for 400 ms and to –30 mV for 100 ms. BCECF fluorescence was measured with a photodiode and digitally Bessel-filtered at 0.3 Hz. These nonratiometric measurements generally show drifts owed to bleaching or intracellular dye distribution (21) but allow for sensitive measurements of pH changes upon changes in voltage or external ion composition.
RESULTS
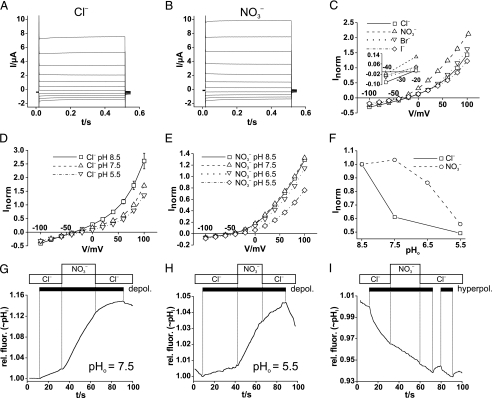
Previous attempts to functionally express plant CLC proteins in animal
cells proved unsuccessful
(12). However, when
Xenopus oocytes were measured 5 or more days after injecting AtClC-a
cRNA, currents well above background levels were observed in two-electrode
voltage-clamp experiments (Fig.
1). These outwardly rectifying currents were roughly 30% larger
when extracellular chloride was replaced by nitrate
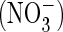 (Fig. 1, A–C),
smaller with extracellular iodide, and nearly unchanged with a replacement by
bromide (Fig. 1C).
Reversal potentials indicated that the apparent anion permeability was larger
for
(Fig. 1, A–C),
smaller with extracellular iodide, and nearly unchanged with a replacement by
bromide (Fig. 1C).
Reversal potentials indicated that the apparent anion permeability was larger
for 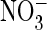 than for the other anions
tested. It should be noted that for CLC exchangers, the observed apparent
permeabilities and conductances represent those of coupled anion/proton
exchange rather than diffusive anion transport. Contrasting with the slow
activation of currents by depolarization observed in plant vacuoles
(16), heterologously expressed
AtClC-a currents almost totally lacked time-dependent relaxations
(Fig. 1, A and
B). Like currents elicited by ClC-4 or -5
(26), AtClC-a currents were
reduced by acidic extracellular pH (pHo)
(Fig. 1, D–F).
With extracellular
than for the other anions
tested. It should be noted that for CLC exchangers, the observed apparent
permeabilities and conductances represent those of coupled anion/proton
exchange rather than diffusive anion transport. Contrasting with the slow
activation of currents by depolarization observed in plant vacuoles
(16), heterologously expressed
AtClC-a currents almost totally lacked time-dependent relaxations
(Fig. 1, A and
B). Like currents elicited by ClC-4 or -5
(26), AtClC-a currents were
reduced by acidic extracellular pH (pHo)
(Fig. 1, D–F).
With extracellular 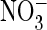 , the
extracellular pH had to be more acidic to obtain the same degree of current
decrease as with Cl– (Fig.
1F).
, the
extracellular pH had to be more acidic to obtain the same degree of current
decrease as with Cl– (Fig.
1F).
FIGURE 1.
Electrophysiological characterization of AtClC-a in Xenopus
oocytes. A and B, voltage-clamp traces of
oocyte-expressed AtClC-a in either Cl–-containing
(A) or 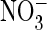 -containing
(B) solutions. From a holding potential of –30 mV, the oocyte
was clamped in 20-mV steps to voltages between –100 and +100 mV for 500
ms. C, steady-state I/V curves of AtClC-a with
different extracellular anions. Currents were normalized for individual
oocytes to a current at +80 mV in Cl– solution (with a mean
value of 3.38 ± 0.40 μA). AtClC-a has a
-containing
(B) solutions. From a holding potential of –30 mV, the oocyte
was clamped in 20-mV steps to voltages between –100 and +100 mV for 500
ms. C, steady-state I/V curves of AtClC-a with
different extracellular anions. Currents were normalized for individual
oocytes to a current at +80 mV in Cl– solution (with a mean
value of 3.38 ± 0.40 μA). AtClC-a has a
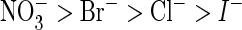 conductance sequence (mean values from 13 oocytes from four batches; error
bars, S.E.). Inset, higher magnification of the
I/V curve to show reversal potentials. D and
E, steady-state I/V curves of AtClC-a at different
values of extracellular pH (pHo) in
Cl–-containing (D) or
conductance sequence (mean values from 13 oocytes from four batches; error
bars, S.E.). Inset, higher magnification of the
I/V curve to show reversal potentials. D and
E, steady-state I/V curves of AtClC-a at different
values of extracellular pH (pHo) in
Cl–-containing (D) or
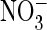 -containing (E) solutions.
The voltage-clamp protocol was performed as in A and B.
Currents were normalized for individual oocytes to the respective current at
+80 mV at pH 7.5 (five oocytes for each data point; error bars,
S.E.). F, currents at +80 mV as a function of pHo
with extracellular Cl– (□) or
-containing (E) solutions.
The voltage-clamp protocol was performed as in A and B.
Currents were normalized for individual oocytes to the respective current at
+80 mV at pH 7.5 (five oocytes for each data point; error bars,
S.E.). F, currents at +80 mV as a function of pHo
with extracellular Cl– (□) or
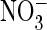 (○). For each curve, currents
were normalized to currents at pHo 8.5.
G–I, AtClC-a mediated, voltage-driven H+ transport
assayed by pH-sensitive BCECF fluorescence using the Fluorocyte system
(21). Proton transport was
stimulated by trains (black bars) of 400-ms long voltage pulses,
either to +90 mV (depolarization) in (G and H) or to
–160 mV (hyperpolarization) in I in the presence of either
extracellular Cl– or
(○). For each curve, currents
were normalized to currents at pHo 8.5.
G–I, AtClC-a mediated, voltage-driven H+ transport
assayed by pH-sensitive BCECF fluorescence using the Fluorocyte system
(21). Proton transport was
stimulated by trains (black bars) of 400-ms long voltage pulses,
either to +90 mV (depolarization) in (G and H) or to
–160 mV (hyperpolarization) in I in the presence of either
extracellular Cl– or
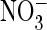 (shown in boxes above).
Depolarizing pulses elicited intracellular alkalinization both with
pHo 7.5 (G) and pHo 5.5, when
proton transport occurs against a gradient (H). Extracellular
(shown in boxes above).
Depolarizing pulses elicited intracellular alkalinization both with
pHo 7.5 (G) and pHo 5.5, when
proton transport occurs against a gradient (H). Extracellular
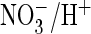 accelerates depolarization (depol.)-driven alkalinization (G
and H) but not hyperpolarization (hyperpol.)-induced
acidification (I, pHo 7.5). Only changes in
relative fluorescence (rel. fluor.) induced by changes in voltage or
external anions are relevant because of the drift inherent to the Fluorocyte
technique (21). Results as in
G–I were obtained in 26, 6, and 7 independent experiments,
respectively.
accelerates depolarization (depol.)-driven alkalinization (G
and H) but not hyperpolarization (hyperpol.)-induced
acidification (I, pHo 7.5). Only changes in
relative fluorescence (rel. fluor.) induced by changes in voltage or
external anions are relevant because of the drift inherent to the Fluorocyte
technique (21). Results as in
G–I were obtained in 26, 6, and 7 independent experiments,
respectively.
We next explored proton transport of AtClC-a by measuring
semiquantitatively the pHi of voltage-clamped oocytes
using the Fluorocyte system
(21). Net ion transport was
elicited by strongly depolarizing oocytes to +90 mV. Because prolonged strong
depolarization can elicit endogenous transport processes in oocytes, trains of
depolarizing pulses were used instead
(20,
21). An inside-positive
voltage should lead to anion influx and proton efflux through electrogenic
anion/proton exchangers. Pulsing AtClC-a-expressing oocytes to positive
voltages indeed induced intracellular alkalinization
(Fig. 1G).
Importantly, alkalinization was also observed when protons were extruded
against their electrochemical potential (at pHo 5.5),
suggesting that their transport is driven by the coupled anion entry
(Fig. 1H). Under
either condition (pHo 7.5 or 5.5), alkalinization occurred
more rapidly with extracellular 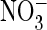 than with Cl–. This shows that AtClC-a more efficiently
mediates
than with Cl–. This shows that AtClC-a more efficiently
mediates 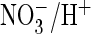 exchange than Cl–/H+ exchange, a finding
compatible with its role in accumulating
exchange than Cl–/H+ exchange, a finding
compatible with its role in accumulating
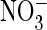 in plant vacuoles
(16,
37). In contrast with ClC-4
and -5 (26), AtClC-a mediates
robust currents also at negative voltages
(Fig. 1, A–E).
Accordingly, and in contrast to ClC-5 (see
Fig. 5H), AtClC-a
mediated proton influx when oocytes were pulsed to negative voltages
(–160 mV) (Fig.
1I). Substituting extracellular Cl– by
in plant vacuoles
(16,
37). In contrast with ClC-4
and -5 (26), AtClC-a mediates
robust currents also at negative voltages
(Fig. 1, A–E).
Accordingly, and in contrast to ClC-5 (see
Fig. 5H), AtClC-a
mediated proton influx when oocytes were pulsed to negative voltages
(–160 mV) (Fig.
1I). Substituting extracellular Cl– by
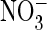 failed to influence the rate of
acidification because proton influx is coupled to an efflux of anions from the
interior of oocytes.
failed to influence the rate of
acidification because proton influx is coupled to an efflux of anions from the
interior of oocytes.
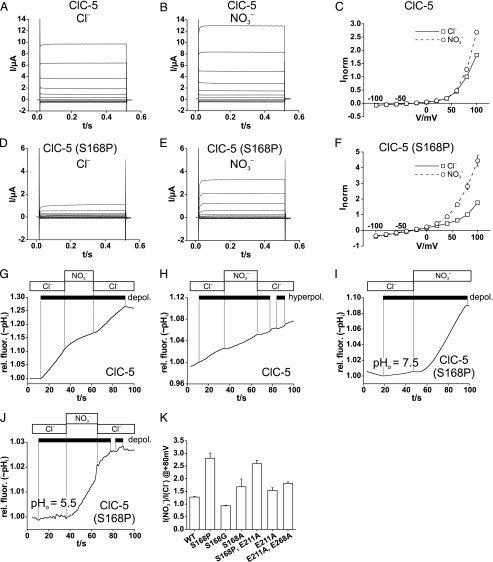
FIGURE 5.
Effect of the S168P mutation on ClC-5 conductance and proton
coupling. A and B, shown are the typical two-electrode
voltage-clamp traces of oocyte-expressed ClC-5 in
Cl–-containing (A) or
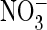 -containing (B) solutions.
C, shown are the steady-state I/V curves from such
experiments (averaged from 19 oocytes from seven batches; normalized for each
oocyte to current in Cl– at +80 mV, with mean current of 3.01
± 0.35 μA). D–F, shown are the current properties of
the S168P mutant measured as in A–C. The data in F
show means from 23 oocytes from five batches, normalized to I in
Cl– at +80 mV, with mean I of 0.79 ± 0.17
μA. Voltage-clamp protocols were performed in A–F as
described in the legend to Fig. 1.
G, shown is an example of less efficient depolarization
(depol.)-induced alkalinization by ClC-5 in the presence of
-containing (B) solutions.
C, shown are the steady-state I/V curves from such
experiments (averaged from 19 oocytes from seven batches; normalized for each
oocyte to current in Cl– at +80 mV, with mean current of 3.01
± 0.35 μA). D–F, shown are the current properties of
the S168P mutant measured as in A–C. The data in F
show means from 23 oocytes from five batches, normalized to I in
Cl– at +80 mV, with mean I of 0.79 ± 0.17
μA. Voltage-clamp protocols were performed in A–F as
described in the legend to Fig. 1.
G, shown is an example of less efficient depolarization
(depol.)-induced alkalinization by ClC-5 in the presence of
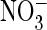 compared with Cl–.
Similar results were obtained in 17 experiments. H, hyperpolarization
(hyperpol.) does not elicit intracellular acidification with ClC-5,
in contrast to AtClC-a (Fig.
1I). Similar results were obtained in 13 experiments.
I and J, shown is the drastically increased
compared with Cl–.
Similar results were obtained in 17 experiments. H, hyperpolarization
(hyperpol.) does not elicit intracellular acidification with ClC-5,
in contrast to AtClC-a (Fig.
1I). Similar results were obtained in 13 experiments.
I and J, shown is the drastically increased
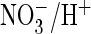 exchange
activity with ClC-5 (S168P), as shown by Fluorocyte with
pHo 7.5 (I) or with pHo 5.5
(transport against gradient) (J), resembling in this respect AtClC-a
(Fig. 1G and
H). Similar results were obtained in 21 (I) and
eight (J) experiments. K, shown is a ratio of currents at
+80 mV measured with extracellular
exchange
activity with ClC-5 (S168P), as shown by Fluorocyte with
pHo 7.5 (I) or with pHo 5.5
(transport against gradient) (J), resembling in this respect AtClC-a
(Fig. 1G and
H). Similar results were obtained in 21 (I) and
eight (J) experiments. K, shown is a ratio of currents at
+80 mV measured with extracellular 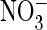 and Cl–, respectively, for ClC-5 and several mutants,
determined as in Fig.
4F. The number of oocytes used for averages are as
follows: ClC-5, 19; ClC-5(S168P), 23; ClC-5(S168G), 17; ClC-5(S168A), 2;
ClC-5(S168P,E211A), 6; ClC-5(E211A), 10; ClC-5(E211A,E268A), 34). Error
bars indicate S.E. rel. fluor., relative fluorescence.
and Cl–, respectively, for ClC-5 and several mutants,
determined as in Fig.
4F. The number of oocytes used for averages are as
follows: ClC-5, 19; ClC-5(S168P), 23; ClC-5(S168G), 17; ClC-5(S168A), 2;
ClC-5(S168P,E211A), 6; ClC-5(E211A), 10; ClC-5(E211A,E268A), 34). Error
bars indicate S.E. rel. fluor., relative fluorescence.
In EcClC-1 and ClC-4 and -5, a certain gating glutamate is important for
coupling chloride fluxes to proton countertransport
(17,
19,
20). In crystals of the
bacterial protein, the negatively charged side chain of this glutamate seems
to block the permeation pathway
(22,
23). Mutating this glutamate
to alanine in ClC-4 and -5 not only leads to flux uncoupling but also
abolishes the strong outward rectification of either transporter
(26). Likewise, currents of
the E203A mutant lost their outward rectification
(Fig. 2). Rather than being
linear and again similar to the analogous mutants of ClC-4 and -5
(19,
20,
26), currents showed slight
outward rectification at positive voltages and slight inward rectification at
negative voltages. Contrasting with the moderate changes in ion selectivity
observed with such mutations in ClC-4 and -5
(26), the E203A mutation
drastically altered the anion selectivity of AtClC-a. Whereas reversal
potentials indicated that the mutant remained more permeable for
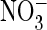 than for Cl–
(Fig. 2C,
inset), its conductance was now reduced rather than increased when
extracellular Cl– was replaced by
than for Cl–
(Fig. 2C,
inset), its conductance was now reduced rather than increased when
extracellular Cl– was replaced by
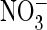 (Fig. 2, A–C;
see Fig. 4F). With
either external anion, currents were insensitive to changes in
pHo (Fig. 2, D
and E), and trains of depolarizing pulses failed to
change pHi (Fig.
2F). Hence, the E203A mutant displayed uncoupled anion
transport and drastically reduced conductance in the presence of
(Fig. 2, A–C;
see Fig. 4F). With
either external anion, currents were insensitive to changes in
pHo (Fig. 2, D
and E), and trains of depolarizing pulses failed to
change pHi (Fig.
2F). Hence, the E203A mutant displayed uncoupled anion
transport and drastically reduced conductance in the presence of
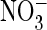 .
.
FIGURE 2.
Impact of gating glutamate mutant E203A on AtClC-a properties.
A and B, voltage-clamp traces of oocytes expressing the
E203A mutant of AtClC-a with extracellular Cl– (A)
or 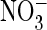 (B) using the pulse
protocol of Fig. 1 (A and
B). C, steady-state I/V curves
of AtClC-a(E203A) with different extracellular anions reveal a changed
conductance sequence of
(B) using the pulse
protocol of Fig. 1 (A and
B). C, steady-state I/V curves
of AtClC-a(E203A) with different extracellular anions reveal a changed
conductance sequence of
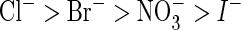 (mean of 19 oocytes; error bars, S.E.). Currents were normalized to
current in Cl– at + 80 mV (with mean current of 3.05 ±
0.22 μA). Inset, higher magnification of the I/V
curve to show reversal potentials. D and E, currents of
AtClC-a(E203A) are insensitive to pHo, both in the
presence of extracellular Cl– (D) or
(mean of 19 oocytes; error bars, S.E.). Currents were normalized to
current in Cl– at + 80 mV (with mean current of 3.05 ±
0.22 μA). Inset, higher magnification of the I/V
curve to show reversal potentials. D and E, currents of
AtClC-a(E203A) are insensitive to pHo, both in the
presence of extracellular Cl– (D) or
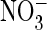 (E). The number of oocytes
is 10 for D and 14 for E (error bars, S.E.).
F, lack of depolarization (depol.)-induced alkalinization
with the E203A mutant. 25 independent experiments gave similar results.
rel. fluor., relative fluorescence.
(E). The number of oocytes
is 10 for D and 14 for E (error bars, S.E.).
F, lack of depolarization (depol.)-induced alkalinization
with the E203A mutant. 25 independent experiments gave similar results.
rel. fluor., relative fluorescence.
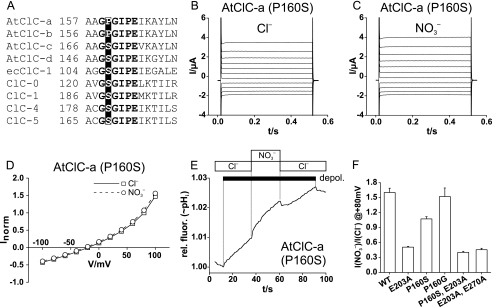
FIGURE 4.
A critical role of proline residue in AtClC-a in determining anion
selectivity. A, shown is the sequence alignment of several CLC
proteins in the region of the GSGIPE signature sequence. E. coli
EcClC-1 as well as all animal CLC proteins have a serine between the two
glycines. It is replaced by proline in plant AtClC-a and -b, but not in
AtClC-c and -d. B and C, shown are typical two-electrode
voltage-clamp traces of the AtClC-a P160S mutant in Xenopus oocytes
with external Cl– (B) or
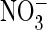 (C) (voltage protocol as
in Fig. 1). D, the
current-voltage relationship of the mutant appears to be identical in
extracellular Cl– and
(C) (voltage protocol as
in Fig. 1). D, the
current-voltage relationship of the mutant appears to be identical in
extracellular Cl– and
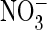 (mean of nine oocytes from six
batches each). Currents were normalized to those in Cl– at
+80 mV (where mean current was 2.33 ± 0.44 μA). E,
depolarization (depol.)-induced H+ transport of AtClC-a
(P160S) is similarly effective with extracellular Cl– or
(mean of nine oocytes from six
batches each). Currents were normalized to those in Cl– at
+80 mV (where mean current was 2.33 ± 0.44 μA). E,
depolarization (depol.)-induced H+ transport of AtClC-a
(P160S) is similarly effective with extracellular Cl– or
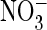 , in stark contrast to the WT
(Fig. 1G,H). Similar
results were obtained in 14 experiments (14 ooyctes from six batches).
F, shown is the ratio of currents at + 80 mV in the presence of
, in stark contrast to the WT
(Fig. 1G,H). Similar
results were obtained in 14 experiments (14 ooyctes from six batches).
F, shown is the ratio of currents at + 80 mV in the presence of
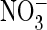 and Cl– for
AtClC-a and several mutants, respectively. The number of oocytes is as
follows: AtClC-a, 25; AtClC-a(E203A), 24; AtClC-a(P160S), 9; AtClC-a(P160G),
5; AtClC-a(P160S,E203A), 4; and AtClC-a(E203A,E270A), 9. rel. fluor.,
relative fluorescence.
and Cl– for
AtClC-a and several mutants, respectively. The number of oocytes is as
follows: AtClC-a, 25; AtClC-a(E203A), 24; AtClC-a(P160S), 9; AtClC-a(P160G),
5; AtClC-a(P160S,E203A), 4; and AtClC-a(E203A,E270A), 9. rel. fluor.,
relative fluorescence.
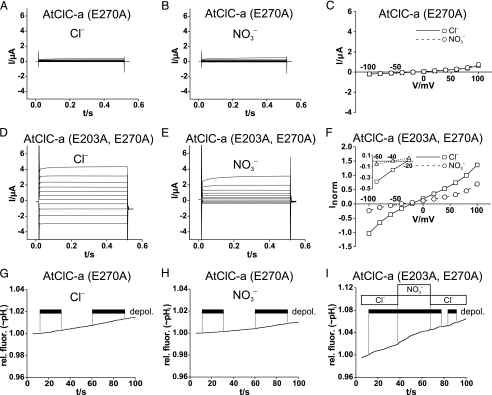
We next studied the AtClC-a mutant E270A, which is equivalent to the proton glutamate mutations of EcClC-1 (27) and ClC-4 and -5 (Fig. 3) (21). Neither currents (Fig. 3, A–C) nor depolarization-induced H+ transport (Fig. 3, G and H) were different from background levels with this mutant. However, when combined with the uncoupling E203A mutation in the gating glutamate, the AtClC-a(E203A,E270A) double mutant gave currents that resembled those of the single E203A mutant (Fig. 3, D–F). Like AtClC-a(E203A), the double mutant failed to transport protons in response to depolarization (Fig. 3I).
FIGURE 3.
Effect of proton glutamate E270A mutation alone and in combination with
the uncoupling E203A mutation on AtClC-a transport. A and
B, voltage-clamp traces of oocyte-expressed AtClC-a(E270A) in either
Cl–-containing (A) or
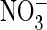 -containing (B) solutions
are not different from those from uninjected control oocytes (not shown).
C, steady-state I/V curves of AtClC-a(E270A) are
not different from controls (10 oocytes per data point). Currents were
normalized to current at +80 mV in Cl–. D and
E, currents of the AtClC-a(E203A,E270A) double mutant are larger with
Cl– (D) than with
-containing (B) solutions
are not different from those from uninjected control oocytes (not shown).
C, steady-state I/V curves of AtClC-a(E270A) are
not different from controls (10 oocytes per data point). Currents were
normalized to current at +80 mV in Cl–. D and
E, currents of the AtClC-a(E203A,E270A) double mutant are larger with
Cl– (D) than with
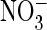 (E). These results also
suggest that the failure to observe AtClC-a(E270A) currents is not due to an
absence from the plasma membrane. F, I/V curves of
currents averaged from nine oocytes from four batches are shown. Currents were
normalized to those in Cl– at 80 mV (with mean current of
1.86 ± 0.47 μA). Inset, a higher magnification of the
I/V curve shows reversal potentials. G and
H, shown is a lack of detectable depolarization
(depol.)-induced alkalinization with AtClC-a(E270A) in the presence
of Cl– (G) or
(E). These results also
suggest that the failure to observe AtClC-a(E270A) currents is not due to an
absence from the plasma membrane. F, I/V curves of
currents averaged from nine oocytes from four batches are shown. Currents were
normalized to those in Cl– at 80 mV (with mean current of
1.86 ± 0.47 μA). Inset, a higher magnification of the
I/V curve shows reversal potentials. G and
H, shown is a lack of detectable depolarization
(depol.)-induced alkalinization with AtClC-a(E270A) in the presence
of Cl– (G) or
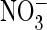 (H). Similar negative
results were obtained with nine oocytes (from four batches) for G and
H. I, shown is a lack of depolarization-induced proton
transport with AtClC-a(E203A,E270A). Similar results were obtained with five
oocytes from three batches. Voltage-clamp protocols are identical to those
described in the legend to Fig.
1. rel. fluor., relative fluorescence.
(H). Similar negative
results were obtained with nine oocytes (from four batches) for G and
H. I, shown is a lack of depolarization-induced proton
transport with AtClC-a(E203A,E270A). Similar results were obtained with five
oocytes from three batches. Voltage-clamp protocols are identical to those
described in the legend to Fig.
1. rel. fluor., relative fluorescence.
Two main biophysical differences between AtClC-a and ClC-4 and -5 are (i)
the much stronger rectification of the mammalian isoforms and (ii) their
partial uncoupling of anion from proton transport in the case of nitrate. We
therefore searched for differences in their primary sequences that may
underlie these differences. A salient feature is the presence of a proline in
a stretch of highly conserved “signature” sequences
(Fig. 4A). In AtClC-a,
this proline replaces a serine that is found at this position in most CLC
proteins. The importance of that serine was recognized early on
(25). Even the conservative
exchange of this serine for threonine (S123T) changed the ion selectivity,
single-channel conductance, and open-channel rectification of the ClC-0
Cl– channel
(25). In the crystal of
EcClC-1, the equivalent serine (Ser-107) participates in the coordination of
Cl– in the central binding site
(22). Mutating this particular
proline in AtClC-a to the “consensus” serine (P160S) resulted in
less rectifying currents (Fig. 4,
B–D), indicating that the difference in
rectification between WT AtClC-a and ClC-5 proteins is not determined by the
residue at this position. Importantly, both the apparent permeability (from
reversal potentials) and conductance no longer differed measurably between
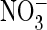 and Cl– solutions
(Fig. 4, B–D).
Furthermore, the mutant performed Cl–/H+- and
and Cl– solutions
(Fig. 4, B–D).
Furthermore, the mutant performed Cl–/H+- and
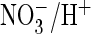 exchange
with similar efficiencies (Fig.
4E). When this proline was mutated to glycine (P160G),
the ratio of currents measured in the presence of extracellular
exchange
with similar efficiencies (Fig.
4E). When this proline was mutated to glycine (P160G),
the ratio of currents measured in the presence of extracellular
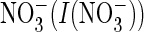 to those measured with extracellular Cl–
(I(Cl–)) resembled the ratio
to those measured with extracellular Cl–
(I(Cl–)) resembled the ratio
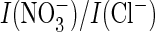 of WT AtClC-a (Fig.
4F), with which it also shares the higher efficiency of
of WT AtClC-a (Fig.
4F), with which it also shares the higher efficiency of
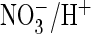 exchange
as compared with Cl–/H+ exchange (data not shown).
When the P160S mutation was combined with the uncoupling mutation in the
gating glutamate, the resulting double mutant AtClC-a(P160S,E203A) displayed a
low
exchange
as compared with Cl–/H+ exchange (data not shown).
When the P160S mutation was combined with the uncoupling mutation in the
gating glutamate, the resulting double mutant AtClC-a(P160S,E203A) displayed a
low
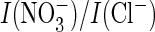 ratio that was similar to the single E203A mutant
(Fig. 4F) and had lost
proton coupling (data not shown).
ratio that was similar to the single E203A mutant
(Fig. 4F) and had lost
proton coupling (data not shown).
The above experiments indicate that the presence of proline in the CLC
signature sequence may be responsible for the efficient
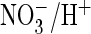 coupling
of AtClC-a. We asked next whether changing the equivalent serine to proline in
ClC-5 would increase its efficiency of
coupling
of AtClC-a. We asked next whether changing the equivalent serine to proline in
ClC-5 would increase its efficiency of
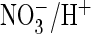 coupling. In the presence of extracellular chloride, currents from the
ClC-5(S168P) mutant (Fig.
5D) were much smaller (∼5–10-fold) than WT
currents (Fig. 5A).
They increased ∼3-fold in the presence of extracellular
coupling. In the presence of extracellular chloride, currents from the
ClC-5(S168P) mutant (Fig.
5D) were much smaller (∼5–10-fold) than WT
currents (Fig. 5A).
They increased ∼3-fold in the presence of extracellular
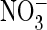 (Fig. 5E), coinciding
with the recent parallel work by Zifarelli and Pusch
(38). Whereas currents of WT
ClC-5 are also larger with
(Fig. 5E), coinciding
with the recent parallel work by Zifarelli and Pusch
(38). Whereas currents of WT
ClC-5 are also larger with 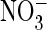 than
with Cl– (Fig. 5,
A–C), the
than
with Cl– (Fig. 5,
A–C), the
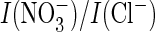 ratio is markedly increased in the mutant
(Fig. 5, D–F and
K). The strong rectification of ClC-5 currents is not
appreciably affected by the S168P mutation
(Fig. 5, A–F),
but currents showed somewhat more pronounced and slower “gating”
relaxations when jumping to positive voltages. In contrast to WT ClC-5
(Fig. 5G), trains of
depolarizing pulses alkalinized the oocyte interior much more rapidly when
extracellular Cl– was replaced by
ratio is markedly increased in the mutant
(Fig. 5, D–F and
K). The strong rectification of ClC-5 currents is not
appreciably affected by the S168P mutation
(Fig. 5, A–F),
but currents showed somewhat more pronounced and slower “gating”
relaxations when jumping to positive voltages. In contrast to WT ClC-5
(Fig. 5G), trains of
depolarizing pulses alkalinized the oocyte interior much more rapidly when
extracellular Cl– was replaced by
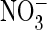 (Fig. 5I).
ClC-5(S168P) transported H+ efficiently against its electrochemical
gradient (with pHo 5.5) using
(Fig. 5I).
ClC-5(S168P) transported H+ efficiently against its electrochemical
gradient (with pHo 5.5) using
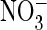 as a driving ion
(Fig. 5J). When
Ser-168 of ClC-5 was replaced by glycine or alanine, only the S168A mutation
yielded a moderately higher
as a driving ion
(Fig. 5J). When
Ser-168 of ClC-5 was replaced by glycine or alanine, only the S168A mutation
yielded a moderately higher
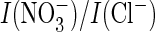 current ratio (Fig.
5K). This contrasts with AtClC-a, where glycine could
substitute for proline without compromising its
current ratio (Fig.
5K). This contrasts with AtClC-a, where glycine could
substitute for proline without compromising its
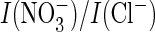 ratio (Fig. 4K) and
its efficient
ratio (Fig. 4K) and
its efficient
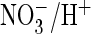 exchange. Hence, a single mutation that replaced a serine by proline, which is
found at that position in AtClC-a, strongly increased the preference of ClC-5
for
exchange. Hence, a single mutation that replaced a serine by proline, which is
found at that position in AtClC-a, strongly increased the preference of ClC-5
for 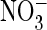 and converted it into an
efficient
and converted it into an
efficient
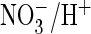 exchanger.
exchanger.
The uncoupling E211A mutation in the ClC-5 gating glutamate slightly
increased its relative nitrate conductance
(Fig. 5K), whereas the
equivalent mutation in AtClC-a quite drastically lowered its nitrate
versus chloride conductance (Fig.
4F). Exchanging serine 168 for proline in the uncoupled
ClC-5 mutant increased relative conductance in the presence of
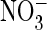 (Fig. 5K), whereas a
similar exchange in the uncoupled AtClC-a mutant failed to change the
(Fig. 5K), whereas a
similar exchange in the uncoupled AtClC-a mutant failed to change the
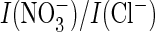 current ratio (Fig.
4F).
current ratio (Fig.
4F).
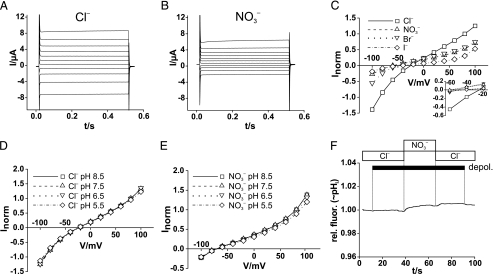
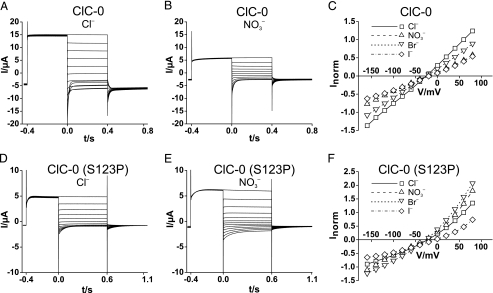
We finally asked whether also CLC Cl– channels gain higher
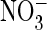 conductance with an equivalent
mutation and used the Torpedo Cl– channel ClC-0
(35) as a widely used model
channel. When expressed in Xenopus oocytes, the S123P mutant gave
robust currents that were, however, ∼4-fold smaller than those from WT
ClC-0 (Fig. 6, A and
D). Whereas the WT channel conducts Cl–
much better than nitrate (Fig. 6,
A–C),
conductance with an equivalent
mutation and used the Torpedo Cl– channel ClC-0
(35) as a widely used model
channel. When expressed in Xenopus oocytes, the S123P mutant gave
robust currents that were, however, ∼4-fold smaller than those from WT
ClC-0 (Fig. 6, A and
D). Whereas the WT channel conducts Cl–
much better than nitrate (Fig. 6,
A–C), 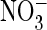 conductance was larger than Cl– conductance in the S123P
mutant (Fig. 6,
D–F). The selectivity was also changed for other
anions, as evident in the large increase of Br– conductance
(Fig. 6F). In
addition, the mutation drastically slowed the fast protopore gate (as evident
from current relaxations after stepping to negative voltages,
Fig. 6, A and
D) and introduced an open-pore outward rectification
(Fig. 6, C and
F). The concomitant change of pore and gating properties
reflects the tight coupling of permeation and gating in CLC
Cl– channels
(39).
conductance was larger than Cl– conductance in the S123P
mutant (Fig. 6,
D–F). The selectivity was also changed for other
anions, as evident in the large increase of Br– conductance
(Fig. 6F). In
addition, the mutation drastically slowed the fast protopore gate (as evident
from current relaxations after stepping to negative voltages,
Fig. 6, A and
D) and introduced an open-pore outward rectification
(Fig. 6, C and
F). The concomitant change of pore and gating properties
reflects the tight coupling of permeation and gating in CLC
Cl– channels
(39).
FIGURE 6.
Modification of ClC-0 Cl– channel currents by the S123P
mutation. A and B, typical voltage-clamp traces of an
oocyte expressing WT ClC-0 and measured in Cl– saline
(A) and 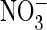 saline
(B). After ∼8 s at –120 mV (to activate the slow
hyperpolarization-activated gate of ClC-0), the oocytes were clamped
consecutively for 1 s to –120 mV (to keep the “slow” common
gate open), 400 ms to +80 mV to open the fast protopore gate. At t =
0, they were clamped (for 400 ms) to test voltages between +80 and –160
mV in steps of 20 mV, followed by a constant pulse to –100 mV for 400
ms. C, normalized I/V curves of WT ClC-0 in the
presence of different extracellular anions. Values were obtained from
exponential fits to t = 0 and represent open channel currents because
both slow and fast gates were opened by hyperpolarizing and depolarizing
prepulses, respectively. Averaged currents from five oocytes of two batches
were normalized for each individual oocyte to the current in
Cl– at V =+60 mV, where mean I was 13.8
± 2.2 μA. D and E, typical traces from an oocyte
expressing the S123P mutant of ClC-0. Voltage-clamp protocol was similar to
A, but the test voltages were applied for 600 ms, followed by a
500-ms pulse to –100 mV. F, normalized open-channel
I/V for the mutant obtained as in C. Means are from
seven oocytes of two batches (normalized to current in Cl– at
+60 mV, where mean I was 4.41 ± 0.90 μA).
saline
(B). After ∼8 s at –120 mV (to activate the slow
hyperpolarization-activated gate of ClC-0), the oocytes were clamped
consecutively for 1 s to –120 mV (to keep the “slow” common
gate open), 400 ms to +80 mV to open the fast protopore gate. At t =
0, they were clamped (for 400 ms) to test voltages between +80 and –160
mV in steps of 20 mV, followed by a constant pulse to –100 mV for 400
ms. C, normalized I/V curves of WT ClC-0 in the
presence of different extracellular anions. Values were obtained from
exponential fits to t = 0 and represent open channel currents because
both slow and fast gates were opened by hyperpolarizing and depolarizing
prepulses, respectively. Averaged currents from five oocytes of two batches
were normalized for each individual oocyte to the current in
Cl– at V =+60 mV, where mean I was 13.8
± 2.2 μA. D and E, typical traces from an oocyte
expressing the S123P mutant of ClC-0. Voltage-clamp protocol was similar to
A, but the test voltages were applied for 600 ms, followed by a
500-ms pulse to –100 mV. F, normalized open-channel
I/V for the mutant obtained as in C. Means are from
seven oocytes of two batches (normalized to current in Cl– at
+60 mV, where mean I was 4.41 ± 0.90 μA).
DISCUSSION
We have achieved for the first time the functional expression of a plant
CLC transporter in animal cells. This enabled us to study the currents and
proton transport of wild-type and mutant AtClC-a, a
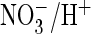 exchanger that serves to accumulate the plant nutrient
exchanger that serves to accumulate the plant nutrient
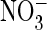 in vacuoles
(16,
37). We have studied the role
in AtClC-a of key glutamate residues that are important for anion/proton
coupling in bacterial and mammalian CLC isoforms and have shown that a
proline-serine exchange in a highly conserved stretch (GSGIPE, the “CLC
signature sequence”) strongly affects nitrate/proton transport in
AtClC-a and ClC-5.
in vacuoles
(16,
37). We have studied the role
in AtClC-a of key glutamate residues that are important for anion/proton
coupling in bacterial and mammalian CLC isoforms and have shown that a
proline-serine exchange in a highly conserved stretch (GSGIPE, the “CLC
signature sequence”) strongly affects nitrate/proton transport in
AtClC-a and ClC-5.
In our previous study, we were unable to detect plasma membrane currents in Xenopus oocytes injected with AtClC-a–AtClC-d (12). Currents reported previously for the oocyte-expressed tobacco CLC NtClC (40) resemble endogenous oocyte currents (12). Hence, the present study may represent the first functional characterization of plant CLC proteins in animal cells. This renders AtClC-a accessible to structure-function analysis by mutagenesis. The most important technical differences from our previous study (12) are the injection of about twice the amount of RNA and a longer time of expression. Whereas we previously measured oocytes 3 days after injection, we now found that AtClC-a currents rise above background levels only after 5 days. Because AtClC-a is physiologically expressed in the plant vacuole (16), the presence of plasma membrane currents may indicate a misrouting of AtClC-a in overexpressing oocytes.
The currents reported here for oocyte-expressed AtClC-a resemble in many
aspects the currents from Arabidopsis vacuoles that were studied by
patch clamp in the whole vacuole configuration and that were absent in strains
with AtClC-a gene deletions
(16). Both currents showed
similar rectification and proton coupling. However, there are also conspicuous
differences. When studied under asymmetric ionic conditions with
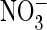 in the pipette (vacuole) and
Cl– in the bath (cytosol), which resembles our oocyte
experiments with external
in the pipette (vacuole) and
Cl– in the bath (cytosol), which resembles our oocyte
experiments with external 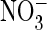 , vacuolar
currents showed prominent, depolarization-induced activation that remained
incomplete after several seconds
(16). Such current relaxations
were clearly absent from our recordings. Moreover, whereas our currents
increased with extracellular
, vacuolar
currents showed prominent, depolarization-induced activation that remained
incomplete after several seconds
(16). Such current relaxations
were clearly absent from our recordings. Moreover, whereas our currents
increased with extracellular 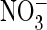 only
∼1.6-fold, this increase was much more drastic with vacuolar currents
(16). Several explanations may
be invoked. Current properties may be influenced by different lipid
compositions of membranes from oocytes and plant vacuoles, or AtClC-a might
endogenously form complexes with other proteins. These other proteins may
include structurally unrelated ancillary β-subunits (as known for some
mammalian CLC proteins) (10,
41), other AtClC isoforms
(12) (CLC proteins function as
dimers (22,
25,
30)), or anchoring
proteins.
only
∼1.6-fold, this increase was much more drastic with vacuolar currents
(16). Several explanations may
be invoked. Current properties may be influenced by different lipid
compositions of membranes from oocytes and plant vacuoles, or AtClC-a might
endogenously form complexes with other proteins. These other proteins may
include structurally unrelated ancillary β-subunits (as known for some
mammalian CLC proteins) (10,
41), other AtClC isoforms
(12) (CLC proteins function as
dimers (22,
25,
30)), or anchoring
proteins.
The outward rectification of AtClC-a is less strong than that of ClC-4 and
-5, both of which do not transport measurably at negative voltages
(26). This difference is not
due to the presence of proline instead of serine in the signature sequence of
AtClC-a, as revealed by our mutagenesis experiments. The weaker rectification
of AtClC-a may be crucial for the proton-driven uptake of
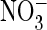 into vacuoles that may have a
lumen-positive voltage. The much stronger rectification of ClC-4 and -5 is
a priori difficult to reconcile with their presumed role in
electrical compensation of H+-ATPase currents
(11).
into vacuoles that may have a
lumen-positive voltage. The much stronger rectification of ClC-4 and -5 is
a priori difficult to reconcile with their presumed role in
electrical compensation of H+-ATPase currents
(11).
The heterologous expression of AtClC-a allowed the first structure-function
analysis of a plant CLC protein. We began by studying two glutamate residues
that are conserved in all confirmed CLC exchangers. The gating glutamate is
important for anion/proton coupling in CLC exchangers, as well as for gating
in CLC Cl– channels. Its replacement in AtClC-a by alanine
uncoupled anion flux from protons as in other CLC transporters
(17,
21). Similar to ClC-4 and
ClC-5, this mutation also drastically changed current rectification, with both
inward and outward rectification being observed. Unlike equivalent mutations
in ClC-4 and -5, this uncoupling AtClC-a mutation also drastically changed the
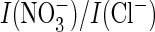 current ratio. This change did not depend on the presence of a proline in the
signature sequence.
current ratio. This change did not depend on the presence of a proline in the
signature sequence.
In CLC anion/proton exchangers, the pathways of Cl– and H+ diverge at a point approximately half-way through the membrane and reach the cytoplasm at different points (27). A proton glutamate at the cytoplasmic CLC surface is thought to bind protons, handing them over to the gating glutamate using a poorly defined path. In both mammalian and E. coli CLC exchangers, this glutamate could be replaced by other titratable amino acids without abolishing anion/proton coupling (21, 27, 42). When mutated to nontitratable residues, however, Cl– and H+ transport were below detection levels in ClC-4 and -5 (21), whereas small, uncoupled anion currents were observed with EcClC-1 (42). This can be rationalized by a blockade of anion/proton exchange at the gating glutamate, when the supply of protons ceases (21). The uncoupled but reduced anion flux in EcClC-1 might be owed to “slippage” past the central exchange site (42). This model is strongly supported by double mutants in ClC-4 and -5, in which the uncoupling gating glutamate mutation rescued the uncoupled anion flux (21). Exactly this situation was found here with AtClC-a.
It was puzzling that anion transport of ClC-4, -5, and EcClC-1 is largely
uncoupled from proton transport with the polyatomic anion
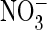 (21), whereas AtClC-a
efficiently couples
(21), whereas AtClC-a
efficiently couples 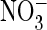 to proton
countertransport (16), an
essential property for its role in accumulating this plant nutrient in
vacuoles. Using sequence comparison, we identified a proline in the CLC
signature sequence as a likely candidate for this difference. In all animal
CLC proteins, this position is occupied by serine. Indeed, when Pro-160 in
AtClC-a was mutated to serine, the plant transporter performed the
Cl–/H+ exchange with similar efficiency as the
to proton
countertransport (16), an
essential property for its role in accumulating this plant nutrient in
vacuoles. Using sequence comparison, we identified a proline in the CLC
signature sequence as a likely candidate for this difference. In all animal
CLC proteins, this position is occupied by serine. Indeed, when Pro-160 in
AtClC-a was mutated to serine, the plant transporter performed the
Cl–/H+ exchange with similar efficiency as the
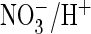 exchange, rather than preferring
exchange, rather than preferring 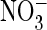 as
in the WT. Importantly, when the equivalent serine of ClC-5 was mutated to
proline, ClC-5 mediated an efficient
as
in the WT. Importantly, when the equivalent serine of ClC-5 was mutated to
proline, ClC-5 mediated an efficient
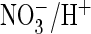 exchange
both in the present study as well as in parallel work by Zifarelli and Pusch
(38). A novel method
(43) to measure proton
transport allowed these authors to show that ClC-5(S168P) had gained an
exchange
both in the present study as well as in parallel work by Zifarelli and Pusch
(38). A novel method
(43) to measure proton
transport allowed these authors to show that ClC-5(S168P) had gained an
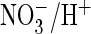 coupling
ratio of ∼2 at voltages between +40 and +60 mV, indistinguishable from
that for Cl–/H+ exchange
(38). Our data on other ClC-5
mutants at this position (Fig.
5K) largely agree with their results
(38) but show interesting
differences with data obtained for AtClC-a
(Fig. 4F). The
substitution of the critical proline by glycine in AtClC-a did not interfere
with its efficient
coupling
ratio of ∼2 at voltages between +40 and +60 mV, indistinguishable from
that for Cl–/H+ exchange
(38). Our data on other ClC-5
mutants at this position (Fig.
5K) largely agree with their results
(38) but show interesting
differences with data obtained for AtClC-a
(Fig. 4F). The
substitution of the critical proline by glycine in AtClC-a did not interfere
with its efficient
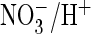 exchange, possibly suggesting that a helix breaker might be sufficient to
support such an exchange. However, glycine at the equivalent position in ClC-5
does not increase currents in the presence of
exchange, possibly suggesting that a helix breaker might be sufficient to
support such an exchange. However, glycine at the equivalent position in ClC-5
does not increase currents in the presence of
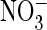 nor does it enable efficient
nor does it enable efficient
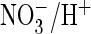 exchange
(data not shown) (38).
exchange
(data not shown) (38).
Only four of seven Arabidopsis CLC proteins have a proline in
their signature sequence, with the remaining three displaying a serine like
all known animal CLC proteins. This suggests that all these four
proline-containing AtClC proteins function as
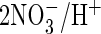 exchangers. A more definitive assignment of their physiological roles,
however, is not yet possible. In this respect, it is interesting to note that
only AtClC-c and AtClC-d were reported to complement
(12,
32) growth phenotypes of a
Saccharomyces cerevisiae strain deleted for the single yeast CLC
(ScClC or Gef) (33). Whether
this is related to the fact that AtClC-c and -d, just like ScClC, carry serine
in their signature sequence and hence probably prefer chloride over nitrate
remains unclear.
exchangers. A more definitive assignment of their physiological roles,
however, is not yet possible. In this respect, it is interesting to note that
only AtClC-c and AtClC-d were reported to complement
(12,
32) growth phenotypes of a
Saccharomyces cerevisiae strain deleted for the single yeast CLC
(ScClC or Gef) (33). Whether
this is related to the fact that AtClC-c and -d, just like ScClC, carry serine
in their signature sequence and hence probably prefer chloride over nitrate
remains unclear.
The interpretation of conductance ratios of ClC-5, and by extension of
AtClC-a, is complicated by results from a noise analysis that indicates that
ClC-5 switches from transporting to nontransporting modes of operation
(21). Such a gating of
transport activity probably underlies the time-dependent current relaxation
upon depolarization of ClC-5 and might also explain the slow
depolarization-induced activation of vacuolar currents observed by De Angeli
et al. (16).
Zifarelli and Pusch (38)
recently concluded from their noise analysis that the increase in ClC-5
currents with 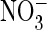 is not due to an
increased “unitary conductance” of the “turned on”
transporter (which would include slippage of the anion) but rather to a higher
“open probability” of the transporter. However, it seems unlikely
that the difference in
is not due to an
increased “unitary conductance” of the “turned on”
transporter (which would include slippage of the anion) but rather to a higher
“open probability” of the transporter. However, it seems unlikely
that the difference in
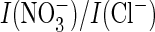 ratios between WT AtClC-a and mutant P160S is solely due to an effect on
gating. This is because this mutation not only modified conductance ratios but
also changed the apparent permeability (reversal potentials). We therefore
conclude that the proline-serine exchange affects the ion selectivity of the
exchange process. This conclusion is indirectly bolstered by the
single-channel analysis of the ClC-0(S123T) mutant, which demonstrated changes
in ion selectivity and other pore properties
(25) and by the changed ion
selectivity of the ClC-0(S123P) mutant described here. Unfortunately, we
cannot draw similar conclusions for ClC-5 because its strong rectification
precludes the determination of reversal potentials.
ratios between WT AtClC-a and mutant P160S is solely due to an effect on
gating. This is because this mutation not only modified conductance ratios but
also changed the apparent permeability (reversal potentials). We therefore
conclude that the proline-serine exchange affects the ion selectivity of the
exchange process. This conclusion is indirectly bolstered by the
single-channel analysis of the ClC-0(S123T) mutant, which demonstrated changes
in ion selectivity and other pore properties
(25) and by the changed ion
selectivity of the ClC-0(S123P) mutant described here. Unfortunately, we
cannot draw similar conclusions for ClC-5 because its strong rectification
precludes the determination of reversal potentials.
In the crystal of EcClC-1, the equivalent serine participates in the
coordination of a Br– ion (used as a Cl–
substitute in crystallography) in the central binding site
(22). Several mutations in
Tyr-445, another residue involved in this coordination
(22), uncoupled chloride from
proton fluxes (44). Such
mutations were associated with a reduced presence or complete absence of
anions at the central binding site
(44). Likewise, crystals
obtained in the presence of 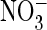 revealed that this anion, which uncouples Cl– from
H+ transport in EcClC-1, cannot be detected at this position
(18). Thus, anion/proton
coupling seems to require that an anion occupies this site. It was proposed
that this anion serves as an intermediate binding site for protons on their
way from the proton glutamate to the gating glutamate, leading to the
seemingly outlandish proposal of HCl as a proton transport intermediate
(45). We suggest that the
replacement of serine by proline in the GSGIPE sequence enables
revealed that this anion, which uncouples Cl– from
H+ transport in EcClC-1, cannot be detected at this position
(18). Thus, anion/proton
coupling seems to require that an anion occupies this site. It was proposed
that this anion serves as an intermediate binding site for protons on their
way from the proton glutamate to the gating glutamate, leading to the
seemingly outlandish proposal of HCl as a proton transport intermediate
(45). We suggest that the
replacement of serine by proline in the GSGIPE sequence enables
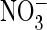 to occupy the central anion binding
site, thereby leading to efficient proton coupling.
to occupy the central anion binding
site, thereby leading to efficient proton coupling.
In summary, a breakthrough in heterologous expression of AtClC-a has
allowed us to extend the structure-function analysis of anion/proton exchange
to plant CLC proteins. Mutagenesis of critical gating and proton glutamates
resulted in changes of proton coupling and rectification that bear close
resemblance to results from mammalian endosomal CLC proteins. However, there
were also significant differences in effects on
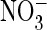 conductance. We further identified
an important proline residue in the CLC signature sequence of AtClC-a that is
crucial for its efficient
conductance. We further identified
an important proline residue in the CLC signature sequence of AtClC-a that is
crucial for its efficient
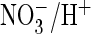 exchange
activity and that conferred more efficient
exchange
activity and that conferred more efficient
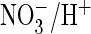 coupling
on the mammalian ClC-5 exchanger and an increase in
coupling
on the mammalian ClC-5 exchanger and an increase in
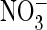 conductance on the Torpedo
Cl– channel ClC-0. Our work provides a basis for future
studies of other plant CLC proteins and their comparison to mammalian
counterparts.
conductance on the Torpedo
Cl– channel ClC-0. Our work provides a basis for future
studies of other plant CLC proteins and their comparison to mammalian
counterparts.
Acknowledgments
We thank A. Fast, N. Kroenke, P. Seidler, and S. Zillmann for technical assistance.
This work was supported by a Deutsche Forschungsgemeinschaft grant (to A. A. Z. and T. J. J.) and by the Leibniz Graduate School of Biophysics.
Footnotes
The abbreviations used are: AtCIC-n, member n of the CLC
family of Cl– channels and transporters in the plant
Arabidopsis thaliana; CIC-n, member n of the CLC
family of chloride channel and transporters (in animals);
pHi, intracellular pH; pH0,
extracellular pH; 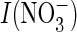 and
I(Cl–), current in the presence of
and
I(Cl–), current in the presence of
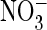 and Cl–,
respectively, which in CLC exchangers also involves an H+
component; WT, wild-type; MES, 2-(N-morpholino)ethanesulfonic acid;
BCECF, 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein.
and Cl–,
respectively, which in CLC exchangers also involves an H+
component; WT, wild-type; MES, 2-(N-morpholino)ethanesulfonic acid;
BCECF, 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein.
References
- 1.Jentsch, T. J. (2008) Crit. Rev. Biochem. Mol. Biol. 43, 3–36 [DOI] [PubMed] [Google Scholar]
- 2.Bösl, M. R., Stein, V., Hübner, C., Zdebik, A. A., Jordt, S. E., Mukhophadhyay, A. K., Davidoff, M. S., Holstein, A. F., and Jentsch, T. J. (2001) EMBO J. 20, 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon, D. B., Bindra, R. S., Mansfield, T. A., Nelson-Williams, C., Mendonca, E., Stone, R., Schurman, S., Nayir, A., Alpay, H., Bakkaloglu, A., Rodriguez-Soriano, J., Morales, J. M., Sanjad, S. A., Taylor, C. M., Pilz, D., Brem, A., Trachtman, H., Griswold, W., Richard, G. A., John, E., and Lifton, R. P. (1997) Nat. Genet. 17, 171–178 [DOI] [PubMed] [Google Scholar]
- 4.Matsumura, Y., Uchida, S., Kondo, Y., Miyazaki, H., Ko, S. B., Hayama, A., Morimoto, T., Liu, W., Arisawa, M., Sasaki, S., and Marumo, F. (1999) Nat. Genet. 21, 95–98 [DOI] [PubMed] [Google Scholar]
- 5.Rickheit, G., Maier, H., Strenzke, N., Andreescu, C. E., De Zeeuw, C. I., Muenscher, A., Zdebik, A. A., and Jentsch, T. J. (2008) EMBO J. 27, 2907–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmeyer, K., Klocke, R., Ortland, C., Gronemeier, M., Jockusch, H., Gründer, S., and Jentsch, T. J. (1991) Nature 354, 304–308 [DOI] [PubMed] [Google Scholar]
- 7.Piwon, N., Günther, W., Schwake, M., Bösl, M. R., and Jentsch, T. J. (2000) Nature 408, 369–373 [DOI] [PubMed] [Google Scholar]
- 8.Kornak, U., Kasper, D., Bösl, M. R., Kaiser, E., Schweizer, M., Schulz, A., Friedrich, W., Delling, G., and Jentsch, T. J. (2001) Cell 104, 205–215 [DOI] [PubMed] [Google Scholar]
- 9.Kasper, D., Planells-Cases, R., Fuhrmann, J. C., Scheel, O., Zeitz, O., Ruether, K., Schmitt, A., Poët, M., Steinfeld, R., Schweizer, M., Kornak, U., and Jentsch, T. J. (2005) EMBO J. 24, 1079–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange, P. F., Wartosch, L., Jentsch, T. J., and Fuhrmann, J. C. (2006) Nature 440, 220–223 [DOI] [PubMed] [Google Scholar]
- 11.Jentsch, T. J. (2007) J. Physiol. (Lond.) 578, 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hechenberger, M., Schwappach, B., Fischer, W. N., Frommer, W. B., Jentsch, T. J., and Steinmeyer, K. (1996) J. Biol. Chem. 271, 33632–33638 [DOI] [PubMed] [Google Scholar]
- 13.Marmagne, A., Vinauger-Douard, M., Monachello, D., de Longevialle, A. F., Charon, C., Allot, M., Rappaport, F., Wollman, F. A., Barbier-Brygoo, H., and Ephritikhine, G. (2007) J. Exp. Bot. 58, 3385–3393 [DOI] [PubMed] [Google Scholar]
- 14.von der Fecht-Bartenbach, J., Bogner, M., Krebs, M., Stierhof, Y. D., Schumacher, K., and Ludewig, U. (2007) Plant J. 50, 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Angeli, A., Monachello, D., Ephritikhine, G., Frachisse, J. M., Thomine, S., Gambale, F., and Barbier-Brygoo, H. (2009) Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Angeli, A., Monachello, D., Ephritikhine, G., Frachisse, J. M., Thomine, S., Gambale, F., and Barbier-Brygoo, H. (2006) Nature 442, 939–942 [DOI] [PubMed] [Google Scholar]
- 17.Accardi, A., and Miller, C. (2004) Nature 427, 803–807 [DOI] [PubMed] [Google Scholar]
- 18.Nguitragool, W., and Miller, C. (2006) J. Mol. Biol. 362, 682–690 [DOI] [PubMed] [Google Scholar]
- 19.Scheel, O., Zdebik, A., Lourdel, S., and Jentsch, T. J. (2005) Nature 436, 424–427 [DOI] [PubMed] [Google Scholar]
- 20.Picollo, A., and Pusch, M. (2005) Nature 436, 420–423 [DOI] [PubMed] [Google Scholar]
- 21.Zdebik, A. A., Zifarelli, G., Bergsdorf, E.-Y., Soliani, P., Scheel, O., Jentsch, T. J., and Pusch, M. (2008) J. Biol. Chem. 283, 4219–4227 [DOI] [PubMed] [Google Scholar]
- 22.Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T., and MacKinnon, R. (2002) Nature 415, 287–294 [DOI] [PubMed] [Google Scholar]
- 23.Dutzler, R., Campbell, E. B., and MacKinnon, R. (2003) Science 300, 108–112 [DOI] [PubMed] [Google Scholar]
- 24.Gründer, S., Thiemann, A., Pusch, M., and Jentsch, T. J. (1992) Nature 360, 759–762 [DOI] [PubMed] [Google Scholar]
- 25.Ludewig, U., Pusch, M., and Jentsch, T. J. (1996) Nature 383, 340–343 [DOI] [PubMed] [Google Scholar]
- 26.Friedrich, T., Breiderhoff, T., and Jentsch, T. J. (1999) J. Biol. Chem. 274, 896–902 [DOI] [PubMed] [Google Scholar]
- 27.Accardi, A., Walden, M., Nguitragool, W., Jayaram, H., Williams, C., and Miller, C. (2005) J. Gen. Physiol. 126, 563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, X., Wang, T., Zhao, Z., and Weinman, S. A. (2002) Am. J. Physiol. 282, C1483–C1491 [DOI] [PubMed] [Google Scholar]
- 29.Zuñiga, L., Niemeyer, M. I., Varela, D., Catalán, M., Cid, L. P., and Sepúlveda, F. V. (2004) J. Physiol. (Lond.) 555, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middleton, R. E., Pheasant, D. J., and Miller, C. (1996) Nature 383, 337–340 [DOI] [PubMed] [Google Scholar]
- 31.Weinreich, F., and Jentsch, T. J. (2001) J. Biol. Chem. 276, 2347–2353 [DOI] [PubMed] [Google Scholar]
- 32.Gaxiola, R. A., Yuan, D. S., Klausner, R. D., and Fink, G. R. (1998) Proc. Natl. Acad. Sci. U. S. A. 95, 4046–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene, J. R., Brown, N. H., DiDomenico, B. J., Kaplan, J., and Eide, D. J. (1993) Mol. Gen. Genet. 241, 542–553 [DOI] [PubMed] [Google Scholar]
- 34.Steinmeyer, K., Schwappach, B., Bens, M., Vandewalle, A., and Jentsch, T. J. (1995) J. Biol. Chem. 270, 31172–31177 [DOI] [PubMed] [Google Scholar]
- 35.Jentsch, T. J., Steinmeyer, K., and Schwarz, G. (1990) Nature 348, 510–514 [DOI] [PubMed] [Google Scholar]
- 36.Lorenz, C., Pusch, M., and Jentsch, T. J. (1996) Proc. Natl. Acad. Sci. U. S. A. 93, 13362–13366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geelen, D., Lurin, C., Bouchez, D., Frachisse, J. M., Lelièvre, F., Courtial, B., Barbier-Brygoo, H., and Maurel, C. (2000) Plant J. 21, 259–267 [DOI] [PubMed] [Google Scholar]
- 38.Zifarelli, G., and Pusch, M. (2009) EMBO J. 28, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pusch, M., Ludewig, U., Rehfeldt, A., and Jentsch, T. J. (1995) Nature 373, 527–531 [DOI] [PubMed] [Google Scholar]
- 40.Lurin, C., Geelen, D., Barbier-Brygoo, H., Guern, J., and Maurel, C. (1996) Plant Cell 8, 701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estévez, R., Boettger, T., Stein, V., Birkenhäger, R., Otto, M., Hildebrandt, F., and Jentsch, T. J. (2001) Nature 414, 558–561 [DOI] [PubMed] [Google Scholar]
- 42.Lim, H. H., and Miller, C. (2009) J. Gen. Physiol. 133, 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zifarelli, G., Soliani, P., and Pusch, M. (2007) Biophys. J. 94, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Accardi, A., Lobet, S., Williams, C., Miller, C., and Dutzler, R. (2006) J. Mol. Biol. 362, 691–699 [DOI] [PubMed] [Google Scholar]
- 45.Miller, C., and Nguitragool, W. (2009) Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]