Abstract
Oncogene-induced senescence is a tumor-suppressive defense mechanism triggered upon activation of certain oncogenes in normal cells. Recently, the senescence response to oncogene activation has been shown to act as a bona fide barrier to cancer development in vivo. Multiple previous studies have implicated the importance of the p38 MAPK pathway in oncogene-induced senescence. However, the contribution of each of the four p38 isoforms (encoded by different genes) to senescence induction is unclear. In the current study, we demonstrated that p38α and p38γ, but not p38β, play an essential role in oncogenic ras-induced senescence. Both p38α and p38γ are expressed in primary human fibroblasts and are activated upon transduction of oncogenic ras. Small hairpin RNA-mediated silencing of p38α or p38γ expression abrogated ras-induced senescence, whereas constitutive activation of p38α and p38γ caused premature senescence. Furthermore, upon activation by oncogenic ras, p38γ stimulated the transcriptional activity of p53 by phosphorylating p53 at Ser33, suggesting that the ability of p38γ to mediate senescence is at least partly achieved through p53. However, p38α contributed to ras-inducted senescence via a p53-indepdendent mechanism in cells by mediating ras-induced expression of p16INK4A, another key senescence effector. These findings have identified p38α and p38γ as essential components of the signaling pathway that regulates the tumor-suppressing senescence response, providing insights into the molecular mechanisms underlying the differential involvement of the p38 isoforms in senescence induction.
The ras proto-oncogenes encode small GTP-binding proteins that transduce growth signals from cell surface (1–3). Aberrant activation of ras is a crucial step in tumor formation. Constitutive activation of ras genes, either through point mutations or overexpression, is associated with a wide variety of human tumors at high frequency and contributes to the initiation and maintenance of multiple tumorigenic phenotypes in these cancers (4–11). However, in early-passage primary human and rodent cells, activated ras causes a permanent proliferative arrest known as premature senescence, because of its phenotypic similarities to replicative senescence observed in late-passage cells (12). Other oncogenes, such as E2F1 and raf, or inactivation of certain tumor suppressor genes, also induce senescence in normal human cells (13–15). The existence of the premature senescence response to oncogene activation implies that like apoptosis, oncogene-induced senescence serves as an anti-tumorigenic defense mechanism. Indeed, it has been well documented that cellular transformation by ras requires cooperation from immortalizing oncogenes that overcome the senescence response, such as those inactivating p53 (8, 16, 17). Recent studies have also demonstrated that senescent cells can be detected in early-stage premalignant lesions of lung, pancreas, skin, and prostate in both human cancer patients and mouse tumor models and that disruption of senescence accelerates the development of malignant tumors(18–23). These findings indicate that oncogene-induced senescence occurs in vivo and serves as a barrier to tumorigenesis.
Although the downstream effectors of the oncogenic activity of ras have been studied extensively, relatively little is known about the signaling pathways that mediate the ras-induced senescence response. Studies have indicated that the ability of ras to induce senescence depends on activation of the Raf/MEK/ERK MAPK pathway (13, 24) and is accompanied by up-regulation of several inhibitors of cell proliferation, including p16INK4A, p53, p14/p19ARF, and p21WAF1 (12, 25), and silencing of E2F target genes (26). In some cells, senescence is triggered as a result of ras-induced production of reactive oxygen species (27). In addition, it has been reported that oncogene induced senescence is mediated by DNA damage responses generated by aberrant DNA replication (28, 29). Recently, studies from our laboratory and others have shown that ras-induced senescence relies on activation of the p38 MAPK3 (30–33). p38 and its upstream MAPK kinases MKK3 and MKK6 (34, 35) are activated by oncogenic ras as a result of persistent MEK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase) activation in senescent cells. Constitutive activation of p38 causes premature senescence, whereas pharmacological inhibition of p38 prevents ras-induced senescence (30).
The requirement of p38 for oncogene-induced senescence suggests that the p38 pathway has a tumor-suppressing function, in addition to its previously known roles in inflammatory and stress responses (36–38). Indeed, target deletion of p38α or PRAK, a downstream substrate kinase of p38, accelerates cancer development in mouse models (23, 39, 40). Moreover, deletion of Wip1, a p38 phosphatase frequently amplified in human breast tumors, leads to p38 activation and reduced mammary tumorigenesis in mice (41, 42). Therefore, the p38 pathway is likely to play an important role in tumor suppression by mediating the senescence response to oncogene activation.
Four mammalian isoforms of p38 (α, β, δ, and γ), each encoded by a different gene, have been identified; they differ in tissue-specific expression and affinity for the upstream regulatory MAPK kinases (43–49). Among these isoforms, only p38α has been shown essential for inflammatory and stress responses by genetic analysis in murine models (50), whereas the physiological roles of the other p38 isoforms in inflammation or other cellular functions are still unclear (51, 52). We have shown previously that SB203580, a chemical compound that inhibits p38α and p38β, prevents ras-induced senescence in primary cells (30), indicating that p38α/β or both might be required for senescence induction. However, this compound also inhibits the activity of other p38 isoforms and other protein kinases although with lower affinity. The specific involvement of each p38 isoform in senescence has never been investigated. In the current study, we examined the role of the p38 isoforms in oncogenic ras-induced senescence in primary human cells. Our data demonstrate that p38α and p38γ, but not p38β, are essential components of the signaling pathway that mediates ras-induced senescence and that p38α and p38γ contribute to senescence induction through different mechanisms. Whereas p38γ mediates ras-induced senescence at least partly by stimulating the transcriptional activity of p53 through direct phosphorylation, p38α appears to regulate senescence in a p53-independent, p16INK4A-dependent manner.
EXPERIMENTAL PROCEDURES
Cell Culture—BJ human foreskin fibroblasts were maintained in minimum essential medium supplemented with 10% fetal calf serum, non-essential amino acids, glutamine, and antibiotics. WI38 and IMR90 human fibroblasts and LinX-A retroviral packaging cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, glutamine, and antibiotics.
Plasmids—The Ha-RasV12 expression vectors were obtained from Dr. Scott Lowe. Retroviral vectors for FLAG-tagged wild type p38 isoforms were constructed by subcloning the respective cDNA into WZLHygro. Retroviral vectors for hemagglutinin-tagged wild type and intrinsically active mutants of p38 isoforms (53) were constructed by subcloning the respective cDNA into pBabePuro. Oligonucleotides for shRNA targeting p38α-758 (AAATTCTCCGAGGTCTAAT), p38γ-550 (GCGCTAAGGTGGCCATCAA), p38γ-1023 (GCGTGTTACTTACAAAGAG) and GFP (54) were cloned into pSUPER.retro according to the published protocol (55). Oligonucleotides for shRNA targeting p38α-577 (CGCGGTTACTTAAACATATGAA), p38α-756 (CACCAAATTCTCCGAGGTCTAA), p38β-319 (CCCCTGATGGGCGCCGACCTGA), and p38β-661 (ACCCTCTTCCCGGGAAGCGACT) were cloned into pSM2C according to the published protocol (56). Retroviral vectors for MKK3E and MKK6E (30), and retroviral p53-reporter PG-Luc and its non-p53-binding control, MG-Luc (57), have been reported previously.
Retrovirus-based Gene Transduction—This was carried out as described previously (58). Transduced cells were purified with 120 μg/ml hygromycin B, 400 μg/ml G418, 5 μg/ml blasticidin, and/or 1.2 μg/ml puromycin.
Analysis of Senescence—This was performed in cell cultures by measuring the rate of proliferation and the expression of the senescence-associated β-galactosidase (SA-β-gal) senescence marker as described previously (30). Population doublings (PD) were calculated with the formula PD = log(N2/N1)/log2, where N1 is the number of cells seeded and N2 is the number of cells recovered (59). To quantify SA-β-gal positives, at least 200 cells were counted in random fields in each of the duplicated wells. Each experiment was performed in triplicates or duplicates.
Western Blot Analysis—Western blot analysis was performed with lysates prepared 7–10 days after transduction of Ras or MKK3/6E from subconfluent cells as described (30). Primary antibodies were from Covance (HA-11), Sigma (FLAG-M5, FLAG-F7425, and actin), Santa Cruz Biotechnology (Ras C-20, MKK3 C-19, p53 FL-393, p21WAF1 C-19), Cell Signaling (phospho-p38-Thr180/Tyr182, phospho-p53-Ser15 and -Ser33, and phospho-ATF2-Thr71). Antibodies against p38α,-β,-γ, and -δ were generated previously in our laboratory. Signals were detected using enhanced chemiluminescence and captured by using the FluorChem™-8900 imaging system (AlphaInnotech).
p53 Reporter Assays—BJ cells were stably transduced with a retroviral luciferase reporter driven by a promoter containing multiple copies of a functional p53-binding sites (PG-Luc) or a mutant p53-binding site (MG-Luc) (57). These cells were transduced with shRNA for GFP, p38α, or p38γ at PD28–32 and subsequently with Ha-RasV12 or vector at PD30–34. Cells were split into 12-well plates on day 7 or 8 post-ras transduction and lysed on day 8 or 9. Luciferase activity was determined using a luciferase assay system (Promega) according to the manufacturer's instructions and normalized to protein concentrations as determined by the Bradford assay. Each experiment was performed in triplicates or duplicates.
Recombinant Proteins—Recombinant GST-ATF2, GST-MKK6E, and His-p38 isoforms were prepared as described previously (60, 61). Wild type and mutant hp53 (1–61), co-expressed with the ZZTAZ2 domain of CREB-binding protein from a bicistronic vector to enhance protein stability, were purified as described (62). Myelin basic protein (MBP) was purchased from Sigma.
Immunoprecipitation-coupled Kinase Assays for p38—BJ cells were lysed at PD30–40 on day 6–8 post-ras/MKK3/6E transduction in a buffer containing 50 mm HEPES, pH 7.5, 2.5 mm EGTA, 1 mm EDTA, 1% Triton X-100, 150 mm NaCl, 10% glycerol, 1 mm phenylmethylsulfonyl fluoride, 50 mm NaF, 1 mm sodium vanadate, 1 mm β-glycerophosphate, 1 mm dithiothreitol, and Complete protease inhibitors. 100–300 μg of lysate were incubated with 60 μl of agarose-conjugated anti-FLAG antibody M2 (Sigma) at 4 °C for 2 h. The beads were washed three times with 1 ml of lysis buffer and three times with 1× kinase buffer (50 mm HEPES, pH 7.5, 0.5 mm EGTA, 10 mm MgCl2, 0.1 mm phenylmethylsulfonyl fluoride, 1 mm NaF, 0.1 mm sodium vanadate, 0.1 mm β-glycerophosphate, and 1 mm dithiothreitol). The reactions were performed in 20 μl of 1× kinase buffer (above) with 10 μm ATP, 0.5 μl of [γ32P]ATP, and 10 μg of hp53 (1–61) or 2 μg of GST-ATF2 at 30 °C for 45 min. The reactions were stopped by 7 μl of 4× Laemmli buffer, heated at 95 °C, and separated by SDS-PAGE. Radioactive signals were detected by using a PhosphorImager. Part of the immunoprecipitates, as well as the total protein lysates, were subjected to Western blot analysis to ensure equal efficiency of immunoprecipitation and equal input of proteins.
Kinase Assays with Recombinant p38—Assays with recombinant kinases were performed in two sequential steps, with the first step being the phosphorylation of His-p38 by MKK6E and the second the phosphorylation of substrates by p38. The first step was carried out at 30 °C for 10 min in 14 μl of 1× kinase buffer (20 mm Tris-HCl, pH 7.5, 20 mm NaCl, 10 mm MgCl2,1 mm dithiothreitol, 20 μm cold ATP, and 1 mm NaF) containing 0.4 μg of His-p38 with or without 50 ng of GST-MKK6. Subsequently, 6 μl of substrate mix in 1× kinase buffer (same as above) containing 20 μg of hp53 (1–61) (wild type or S33A or S46A mutant) or 10 μg of MBP and 2 μCi of [γ-32P]ATP was added to each reaction. The resulting 20 μl of reaction was incubated at 30 °C for 30 min, stopped by the addition of 7 μl of 4× Laemmli buffer, and heated at 95C° for 10 min. The reactions were separated on 4–20% gradient SDS-polyacrylamide gels. Radioactive signals were detected by PhosphorImager.
Northern Blot Analysis—Total RNA was isolated from cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. 10 μg of RNA was separated on a 1% agarose gel containing 3.7% formaldehyde in 1× MOPS buffer (20 mm MOPS, 5 mm NaOAc, and 1 mm EDTA, pH 7.0), transferred to Hybond N+ nylon membranes in 10× SSC (1.5 m NaCl and 150 mm Na3 citrate, pH 7.0), and hybridized at 65 °C in Church-Gilbert buffer (1% bovine serum albumin, 400 mm NaPO4, pH 7.0, 15% formamide, 1 mm EDTA, and 7% SDS) to a 800-base pair human p16 cDNA probe labeled with [α-32P]dATP and [α-32P]dCTP by random priming. After extensive washing with 0.2× SSC/0.1% SDS buffer at 65 °C, the signals were visualized and quantitated by phosphorimaging.
RESULTS
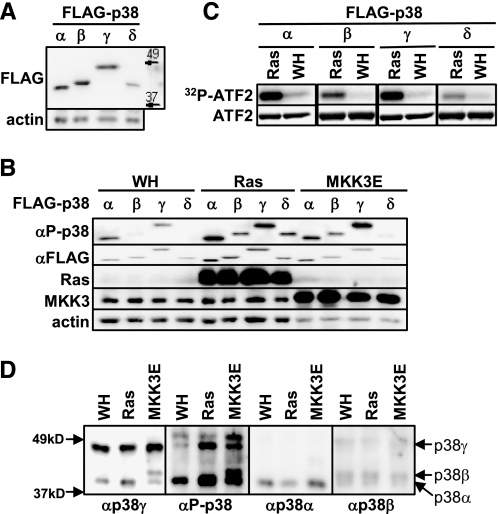
Expression and Activation of the p38 Isoforms during Oncogene-induced Senescence in Primary Human Fibroblasts—We showed previously that oncogenic ras fails to induce senescence in primary BJ human fibroblast cells treated with the p38α- and p38β-specific inhibitor SB203580 (30), suggesting that at least one of these two isoforms might be required for senescence induction. However, this compound also inhibits other p38 isoforms and even other protein kinases, although with a lower affinity. To investigate the specific involvement of each p38 isoforms, in the present work we initially examined the expression and activity of these isoform in senescent cells. Although all four p38 isoforms contain similar numbers of amino acid residues, they displayed distinct rates of mobility on SDS-PAGE. Whereas the other isoforms had apparent molecular mass of 38–42 kDa, p38γ migrated closely to the 49-kDa marker (Fig. 1A). p38α had a slightly faster mobility than p38β and p38δ. These differences allowed us to differentiate these isoforms from each other.
FIGURE 1.
The p38α,-β, and -γ isoforms are expressed in primary human fibroblast cells and activated by oncogenic ras during senescence induction. A, Western blot analysis of BJ cells (PD26) transduced with FLAG-tagged p38 isoforms detecting FLAG-p38 and actin. B, Western blot analysis of BJ cells transduced with FLAG-p38 isoforms and Ha-RasV12 (Ras), MKK3E or vector (WH), detecting phospho-p38, FLAG, Ras, MKK3, and actin. Cells were lysed on day 8 post-Ras transduction at PD30. C, induction of the kinase activity of the FLAG-p38 isoforms toward ATF2 by ras. FLAG-p38 isoforms were immunoprecipitated from BJ cells transduced with FLAG-p38 and Ha-RasV12 or vector at PD30 on day 8 post-Ras transduction (the same lysates as in B) using an agarose-conjugated anti-FLAG M2 antibody and incubated with GST-ATF2 in the presence of [γ-32P]ATP. Phosphorylated ATF2 were detected by autoradiography. The input of ATF2 was determined by staining with Coomassie Brilliant Blue R. D, Western blot analysis of BJ cells transduced with Ha-RasV12, MKK3E, or vector, detecting phospho-p38, p38α, p38β, and p38γ using specific antibodies. Cells were lysed on day 8 post-Ras transduction at PD25. Identical sets of lysates, each set containing lysates from BJ cells transduced with vector, Ha-RasV12, or MKK3E were resolved side-by-side on the same SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was cut into pieces, each containing one set of lysates. These pieces of membrane were then hybridized to the antibody against phospho-p38, -p38α, -p38β, and -p38γ, respectively. The chemiluminescence signals were captured after the membranes were re-aligned into the original position. The positions of p38α, p38β, and p38γ are marked by arrows.
Upon transduction of oncogenic ras (HarasV12) or active mutant of MKK3 (MKK3E), the phosphorylation of ectopically expressed p38α, p38β, p38γ, and p38δ in their activation loop was greatly enhanced in BJ cells as detected by a phospho-specific antibody (Fig. 1B). These p38 isoforms also displayed increased protein kinase activity toward ATF2 in vitro when immunoprecipitated from BJ cells transduced with oncogenic ras as compared with those from control cells (Fig. 1C). These results indicate that all four of the p38 isoforms can be activated during ras-induced senescence. Using isoform-specific antibodies, we were able to show that p38α, p38β, and p38γ were expressed in primary BJ human fibroblasts (Fig. 1D). In addition, both Ras and MKK3E induced the activating phosphorylation of the endogenous p38 proteins that co-migrated with p38α and p38γ (Fig. 1D). We confirmed that the protein bands detected by the phospho-specific antibody and co-migrating with p38α and p38γ indeed represent the phosphorylated p38α and p38γ isoforms, respectively, because these bands were abolished in cells expressing p38α (Fig. 2A) or p38γ shRNA (Fig. 3A). Therefore, these findings indicate that oncogenic ras activates not only the endogenous p38α, as we demonstrated previously (30), but also the endogenous p38γ during senescence induction. Interestingly, in BJ human fibroblasts, oncogenic ras activated p38γ through phosphorylation without altering its expression level (Fig. 1D). This is in contrast to a previous finding that oncogenic ras induces p38γ expression but not its phosphorylation in rat intestinal epithelial cells (IEC-6) (63). This raises the possibility that Ras may stimulate the activity of p38γ through different mechanisms in a species- or cell type-dependent manner.
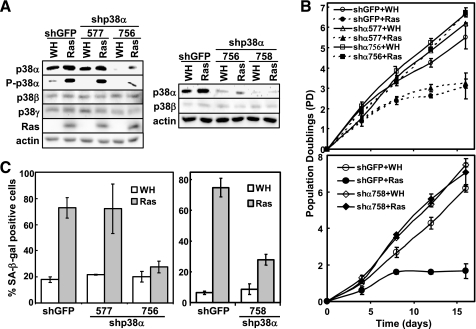
FIGURE 2.
p38α is essential for ras-induced senescence but not for phosphorylation of p53 at Ser33 or induction of p21WAF1 expression. A, BJ cells transduced with shRNA against GFP (shGFP) or p38α (shp38α-577, -756, or -758) and Ha-RasV12 (Ras) or vector (WH) were subjected to Western blot analysis detecting the indicated proteins. Cells were lysed on day 10 post-Ras transduction at PD34 (left panel) or PD35 (right panel). B, the population doublings of BJ cells transduced with shGFP or shp38α-577, -756, or -758 and Ha-RasV12 or vector were followed over a period of 16 days, starting at day 5 post-Ras transduction at PD34 (top panel) or PD35 (bottom panel). Values are mean ± S.D. for duplicates. C, BJ cell lines (described in B) were stained for the SA-β-gal senescence marker on day 15 post-Ras transduction. Values are mean ± S.D. for duplicates.
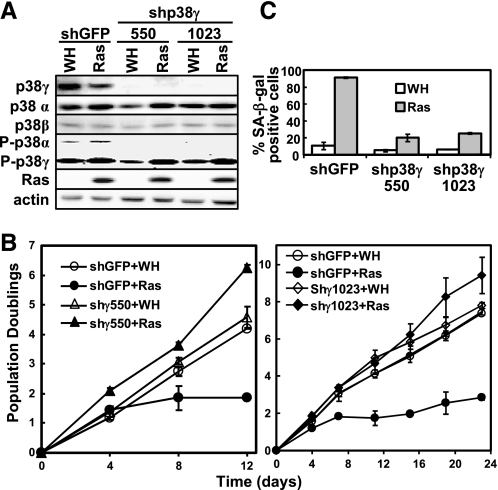
FIGURE 3.
p38γ is essential for ras-induced senescence, phosphorylation of p53 at Ser33, and induction of p21WAF1 expression. A, BJ cells transduced with shGFP or shp38γ-550 or -1023 and Ha-RasV12 (Ras) or vector (WH) were subjected to Western blot analysis detecting the indicated proteins. Cells were lysed on day 8 post-Ras transduction at PD36. B, the population doublings of BJ cells transduced with shGFP or shp38γ-550 or -1023 and Ha-RasV12 or vector were followed over a period of 12 (left panel) or 23 (right panel) days, starting at day 5 post-Ras transduction at PD36 (top panel) or PD35 (bottom panel). Values are mean ± S.D. for duplicates. C, BJ cell lines (described in B) were stained for the SA-β-gal senescence marker on day 17 post-Ras transduction. Values are mean ± S.D. for duplicates.
Although p38β was expressed in primary human fibroblasts, we failed to detect an obvious phospho-p38 band co-migrating with p38β (Fig. 1D). However, based on the induction of phosphorylation and kinase activity of ectopically expressed p38β by oncogenic ras (Fig. 1, B and C), we reasoned that p38β was activated during ras-induced senescence. Because the mobility of p38β was only slightly slower than that of p38α on SDS-PAGE, it is possible that the signal for phospho-p38β was obscured by that of phospho-p38α because of the relatively lower abundance of p38β as compared with p38α. Moreover, although the ectopically expressed p38δ could be activated by both oncogenic ras and MKK3E (Fig. 1, B and C), the level of p38δ was barely detectable in primary human fibroblasts (data not shown). Thus, our study focused on p38α, -β, and -γ isoforms.
p38α and p38γ, but Not p38β, Are Essential for Oncogenic ras-induced Senescence—The detectable expression and activation of p38α, -β, and -γ in senescent cells prompted us to examine the requirement of these p38 isoforms for oncogene-induced senescence. Three p38α shRNA (shp38α-577, -756, and -758), two p38β shRNA (shp38β-319 and -661), and two p38γ shRNA (shp38γ-550 and -1023) were constructed. When stably transduced into BJ cells via retrovirus, shp38α-577 failed to inhibit the expression levels of p38α (Fig. 2A), whereas all of the other shRNAs efficiently silenced the expression of appropriate p38 isoforms without affecting the other isoforms (Figs. 2A and 3A).
When stably expressed in BJ cells, the p38α-shRNAs (shp38α-756 and -758), which efficiently knocked down p38α, prevented oncogenic ras-induced growth arrest (Fig. 2B) and accumulation of SA-β-gal, a biomarker for senescence (Fig. 2C). In contrast, shRNA for GFP or the p38α shRNA (shp38α-577), which failed to silence p38α expression, had no effect on senescence induction (Fig. 2, B and C). Furthermore, two shRNAs that silenced p38γ expression (shp38γ-550 and -1023) also blocked the ability of ras to induce growth arrest (Fig. 3B) and greatly inhibited ras-induced expression of SA-β-gal (Fig. 3C) as compared with the GFP shRNA. On the other hand, the p38β shRNA that effectively silenced p38β expression did not disrupt oncogenic ras-induced senescence (data not shown). These results were reproduced in WI38 primary human fibroblast cells derived from normal embryonic lung tissue, in which ras-induced senescence was inhibited by shRNA for p38α and p38γ but not by shRNA for p38β (supplemental Fig. S1). ShRNA for p38γ also delayed the onset of ras-induced senescence in the IMR90 primary human lung fibroblasts (data no shown). These results demonstrate that both p38α and p38γ are essential for ras-induced senescence, whereas p38β is dispensable for senescence induction.
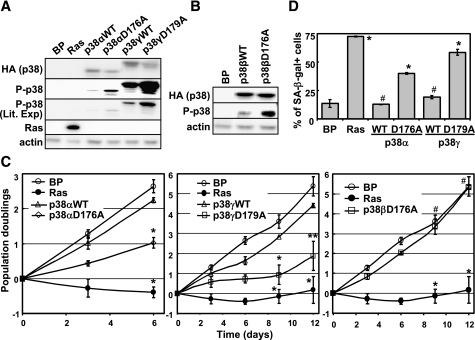
Constitutive Activation of p38α or p38γ, but Not p38β, Leads to Premature Senescence—To analyze the effect of p38 activation on senescence induction, we took advantage of the intrinsically active mutants of the p38 isoforms recently constructed in Drs. Engelberg and Livnah's groups (Hebrew University of Jerusalem (53, 53)). These mutants have acquired spontaneous protein kinase activity in vitro and in vivo and maintain specificity toward substrates and inhibitors similar to that of the wild type p38 isoforms. When transduced into BJ cells via retrovirus, the active form of p38α (p38α-D176A), p38β (p38β-D176A), or p38γ (p38γ-D179A) and its wild type counterpart were expressed at comparable levels (Fig. 4, A and B). However, the active mutants displayed a much higher level of autophosphorylation in the activation loop than the corresponding wild type proteins as detected by the phospho-specific antibody in Western blot analysis (Fig. 4, A and B). These results were consistent with previous reports in other cell lines and indicated that these mutants of p38 isoforms were indeed constitutively active.
FIGURE 4.
Constitutively active p38α and p38γ, but not p38β, induce premature senescence in primary human fibroblast cells. A and B, Western blot analysis of BJ cells transduced with vector control (Babe Puro, BP). Ha-RasV12 (Ras), hemagglutinin-tagged wild type p38α, p38γ, or p38β (WT), or their active mutants (p38αD176A, p38γD179A, or p38βD176A) was performed to detect the indicated proteins. Cells were lysed on day 8 post-transduction at PD33. C, the population doublings of the BJ cell lines described in A and B were followed for 6 (left panel) or 12(middle and right panels) days, starting on day 5 post-transduction at PD32. Values are mean ± S.D. for duplicates. *, p < 0.001; **, p < 0.01; #, p > 0.05, versus vector control by Student's t test. D, BJ cell lines (described in A) were stained for the SA-β-gal senescence marker on day 14 post-transduction. Values in are mean ± S.D. for duplicates. *, p < 0.01; #, p > 0.05, versus vector control by Student's t test.
Moreover, similar to oncogenic ras, expression of p38α-D176A or p38γ-D179A led to growth arrest (Fig. 4C, top and middle panels) and accumulation of the SA-β-gal marker (Fig. 4D) in BJ cells, whereas wild type p38α and p38γ did not significantly inhibit cell proliferation or induce SA-β-gal expression. In contrast, the active mutant of p38β (p38β-D176A) did not cause inhibition of proliferation in primary BJ fibroblasts (Fig, 4C, bottom panel). Therefore, constitutive activation of p38α or p38γ, but not p38β, is sufficient to induce premature senescence in primary human fibroblasts.
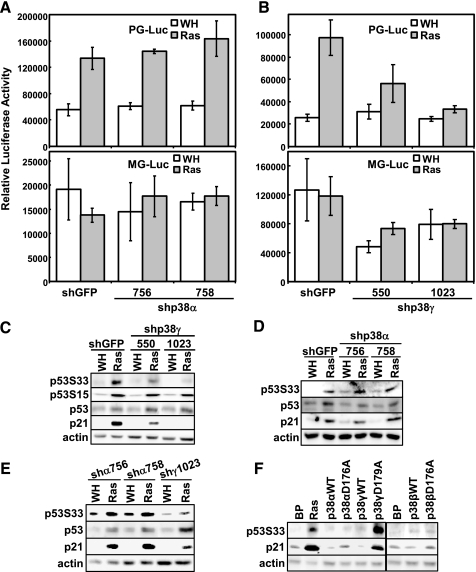
p38γ, but Not p38α, Is Required for ras-induced Activation of p53 in Senescent Cells—In an attempt to investigate the molecular mechanism underlying the essential roles of p38α and p38γ in senescence, we examined the ability of these isoforms to regulate the activity of p53, a key effector of ras-induced senescence. For this purpose, we used a retrovirus-based, stable, luciferase reporter system for p53 (57). In BJ cells stably transduced with this stable p53 reporter (PG-Luc), luciferase activity was stimulated significantly (3–4-fold) in the presence of Ha-RasV12 (Fig. 5, A and B, top panels), confirming the induction of p53 transcriptional activity during senescence. However, ras-induced p53 activity was greatly reduced in BJ cells expressing the effective p38γ shRNA (shp38γ-550 and -1023) (Fig. 5B, top panel), whereas the p38α shRNA had no obvious effect on the p53-dependent luciferase activity in senescent cells. Neither ras nor the p38α or p38γ shRNA significantly altered the transcription of MG-Luc, a control reporter containing mutant p53-binding sites (57) (Fig. 5A and B, bottom panels), indicating that the effects we observed were specific for p53. These data indicate that p38γ, but not p38α, is required for the activation of p53 during ras-induced senescence.
FIGURE 5.
p38γ, but not p38α, is essential for oncogenic ras-induced transcriptional activity of p53. A, BJ cells stably transduced with a retroviral luciferase reporter driven by a promoter containing multiple copies of a functional p53-binding site (PG-Luc, top panel) or a mutant p53-binding site (MG-Luc, bottom panel) were transduced with retroviruses encoding shGFP or shp38γ-756 or -758 at PD33 and with Ha-RasV12 (Ras) or vector (WH) at PD35. Cells were lysed on day 8 post-Ras transduction. B, BJ cells stably transduced with a retroviral luciferase reporter driven by a promoter containing multiple copies of a functional p53-binding site (PG-Luc, top panel) or a mutant p53-binding site (MG-Luc, bottom panel) were transduced with retroviruses encoding shGFP or shp38γ-550 or -1023 at PD28 and with Ha-RasV12 or vector at PD30. Cells were lysed on day 8 post-Ras transduction. In A and B, luciferase activity was measured and normalized to protein concentration. Values are mean ± S.D. for triplicates. Note that the luciferase values are not comparable between cells with PG-Luc and those with MG-Luc, as the luciferase activities were measured with different volumes of lysates under different settings of sensitivity of the luminometer. C and D, Western blot analysis of BJ cells transduced with shGFP or shp38γ-550 or -1023 and Ha-RasV12 or vector (C) or with shGFP or shp38α-756 or -758 and Ha-RasV12 or vector (D), detecting the indicated proteins. Cells were lysed on day 8 (C) or day 10 (D) posttransduction at PD36 (C) or PD35 (D). E, Western blot analysis of IMR90 cells transduced with shRNA for p38α (shα756, -758) or p38γ (shγ1023) and Ha-RasV12 or vector control detecting the indicated proteins. Cell lysates were prepared 11 days post-infection with ras at PD36. F, Western blot analysis of BJ cells transduced with vector control (Babe Puro, BP). Ha-RasV12, hemagglutinin-tagged wild type p38α, p38γ, or p38β (WT), or their active mutants (p38αD176A, p38γD179A, or p38βD176A), detecting the indicated proteins. Cells were lysed on day 8 post-transduction at PD33.
Consistent with the essential role of p38γ in p53 activation and in senescence induction, shRNA for p38γ inhibited ras-mediated induction of p21WAF1, an endogenous transcriptional target of p53 and a key effector of senescence (Fig. 5C). By contrast, although the effective p38α shRNA (shp38α-756 and -758) blocked ras-induced senescence (Fig. 2, B and C), it did not reduce the p21WAF1 induction by ras, as compared with the GFP shRNA (Fig. 5D). A similar observation was made in IMR90 cells (Fig. 5E). Moreover, although the constitutively active mutants of both p38α (p38α-D176A) and p38γ (p38γ-D179A) induced senescence (Fig. 4, C and D), only p38γ-D179A, but not p38α-D176A, increased the expression of p21WAF1 (Fig. 5F). The active mutant of p38β also failed to stimulate the level of p21WAF1 (Fig. 5F), consistent with the inability of this mutant to induce premature senescence. Taken together with the results from the luciferase reporter assays, these data demonstrate that although the role of p38γ in ras-induced senescence correlates with its ability to stimulate p53 activity and subsequently to induce the expression of p21WAF1, p38α mediates senescence induction through a p53/p21WAF1-independent mechanism.
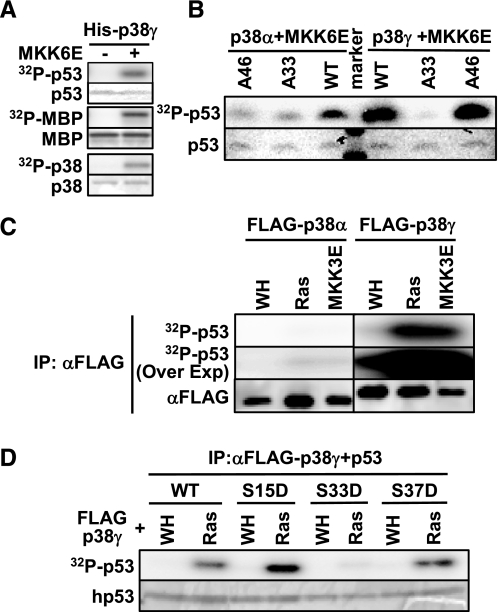
p38γ, but Not p38α, Mediates Phosphorylation of p53 in Vivo during Senescence Induction by Oncogenic ras—The activity of p53 is regulated through phosphorylation of its N-terminal transcriptional activation domain. The differential effects of p38α and p38γ on ras-induced p53 activity suggest that these isoforms may play different roles in p53 phosphorylation during senescence induction. It has been shown that Ser33 and Ser46 of p53 are direct substrates of p38α in vitro and that phosphorylation of these sites contributes to the activation of p53 upon DNA damage (64). The ability of the other p38 isoforms to phosphorylate p53 was unknown. We found that in vitro, recombinant p38γ phosphorylated the N-terminal transcriptional activation domain of p53, as well as the positive control, MBP, in an MKK6E-dependent manner (Fig. 6A), indicating that p53 is a substrate of activated p38γ. Mutation of Ser33 to Ala essentially abolished the phosphorylation of p53 by p38γ, whereas mutation of Ser46 to Ala had little or no effect on p53 phosphorylation (Fig. 6B). In contrast, mutation of either Ser33 or Ser46 to Ala greatly diminished phosphorylation of p53 by p38α (Fig. 6B). Therefore, p38γ phosphorylates p53 mainly at Ser33 in vitro, whereas p38α phosphorylates p53 at both Ser33 and Ser46, as demonstrated previously.
FIGURE 6.
Phosphorylation of p53 by recombinant or immunoprecipitated p38α and p38γ in vitro. A, recombinant p38γ phosphorylates p53. His-p38γ was first incubated with GST-MKK6E (+) or buffer (-) and cold ATP and then with the substrate MBP or p53 (1–61) in the presence of [γ-32P]ATP. B, recombinant p38α phosphorylates p53 at Ser33 and Ser46, whereas recombinant p38γ phosphorylates Ser33 only. His-p38α or -p38γ was first incubated with GST-MKK6E and cold ATP and then with p53 (1–61) (WT, wild type) or p53 (1–61) carrying the S33A or S46A mutation in the presence of [γ-32P]ATP. C, p38γ immunoprecipitated from senescent cells displays much higher kinase activity toward p53 than p38α does. FLAG-p38α or -p38γ was immunoprecipitated from BJ cells transduced with FLAG-p38α or -p38γ and Ha-RasV12 (Ras), MKK3E, or vector (WH) at PD30 on day 8 post-Ras transduction using an agarose-conjugated anti-FLAG M2 antibody and incubated with p53 (1–61) in the presence of [γ-32P]ATP. The same cell lysates as described in the legend for Fig. 1B were used for immunoprecipitation. Part of the immunoprecipitates was subjected to Western blot analysis to detect FLAG-p38. D, p38γ immunoprecipitated from senescent cells phosphorylates p53 at Ser33. FLAG-p38γ immunoprecipitates from control (WH) or Ras-expressing BJ cells (Ras), as described in C, were incubated with wild type or mutant (S15D, S33D, or S46D) p53 (1–61) in the presence of [γ-32P]ATP. A–D, the reactions were separated by SDS-PAGE. Phosphorylated MBP, p53, and p38 were detected by using a PhosphorImager. The input of the substrates was determined by staining with Coomassie Brilliant Blue R.
To investigate the phosphorylation of p53 by p38γ during senescence induction, we compared the p53 kinase activities of p38α and p38γ immunoprecipitated from control and senescent BJ cells. Correlating with its increased phosphorylation in the activation loop (Fig. 1B), p38γ immunoprecipitated from Ras- and MKK3E-expressing, senescent BJ cells phosphorylated p53 at much higher levels as compared with that from control cells (Fig. 6C, right panel). On the other hand, although the phosphorylation of p38α in the activation loop was induced in Ras- or MKK3E-expressing cells to levels comparable with those of p38γ (Fig. 1B), p38α barely phosphorylated p53 when immunoprecipitated from these cells (Fig. 6C, compare left and right panels, which were derived from the same exposure). The phosphorylation of p53 by p38α immunoprecipitated from Ras- and MKK3E-expressing cells could still be detected upon overexposure, but the signals were almost negligible when compared with the signals derived from p38γ on the same exposure (Fig. 6C, 32P-p53 overexposure (Over Exp)). These results demonstrate that whereas oncogenic Ras and MKK3E activate both p38α and p38γ during senescence, only activated p38γ, but not p38α, is able to phosphorylate p53.
The Ras-induced kinase activity of p38γ toward p53 was almost completely abolished when Ser33 was mutated, but it was unaltered by the mutation of Ser15 or Ser46 (Fig. 6D). Thus, upon activation in senescent cells, p38γ phosphorylates p53 mainly at Ser33, consistent with the results obtained with recombinant p38γ activated by MKK6E in vitro (Fig. 6B).
We further investigated the effect of p38α and p38γ on phosphorylation of the endogenous p53 protein at Ser33 in vivo during senescence induction by ras. As with activated ras, intrinsically active p38γ (p38γ-D179A) induced phosphorylation of p53 at Ser33 in BJ cells, whereas active p38α (p38α-D176A) did not increase p53-Ser33 phosphorylation, although it induced senescence (Fig. 5F). Wild type p38α and p38γ had little effect on p53-Ser33 phosphorylation. Wild type and constitutively active p38β also failed to cause phosphorylation of p53-Ser33 (Fig. 5F), which correlates with their inability to induce senescence. Furthermore, the effective p38γ shRNA (shp38γ-550 and -1023) greatly diminished ras-induced phosphorylation of p53 at Ser33 but not the phosphorylation of p53-Ser15, a site that is not a p38 substrate (Fig. 5C). However, the effective p38α shRNA (shp38α-756 and -758) blocked ras-induced senescence but not p53-Ser33 phosphorylation (Fig. 5D). Consistent with these findings in BJ cells, oncogenic ras-induced phosphorylation of p53-Ser33 and the increase in p21WAF1 expression were also inhibited by the p38γ shRNA, but not by the p38α shRNA, in IMR90 primary human fibroblast cells (Fig. 5E). Taken together, our data indicate that oncogenic ras activates both p38α and p38γ, which in turn mediate senescence induction through different mechanisms. Upon activation by oncogenic ras, p38γ induces p53 activity by directly phosphorylating Ser33, a residue that is required for p53 to mediate ras-induced senescence (23). Activation of p53 by p38γ leads to increased expression of a key senescence effector, p21WAF1. By contrast, p38α contributes to senescence induction through a mechanism independent of p53.
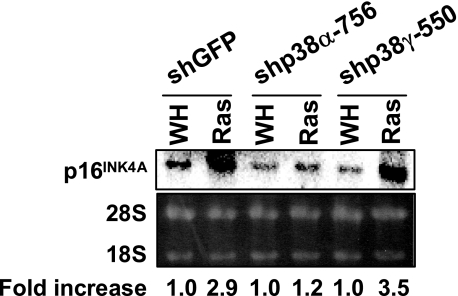
p38α, but Not p38γ, Is Essential for ras-induced Expression of p16INK4A—We demonstrated previously that oncogenic ras stimulates the transcript level of p16INK4A, another major effector of senescence, through activation of the p38 pathway (30). To gain insights into the p53-independent role of p38α in senescence, we examined the requirement of p38α and p38γ for p16INK4A expression in senescent cells. As shown previously (30), oncogenic ras induces a 3-fold increase in the mRNA level of p16INK4A in BJ cells (Fig. 7). However, this induction was abolished by the p38α shRNA but not the p38γ shRNA. Thus, p38α and p38γ mediate oncogene-induced senescence by inducing two major senescence effectors, p53 and p16INK4A, respectively.
FIGURE 7.
p38α, but not p38γ, is essential for oncogenic ras-induced increase in p16INK4A mRNA levels. Total RNA was isolated from BJ cells transduced with shGFP, shp38α-756, or shp38γ-550 and Ha-RasV12 (Ras) or vector (WH) on day 8 after transduction with Ras, separated on an agarose gel, transferred to nylon membrane, and hybridized to a human p16INK4A cDNA probe labeled by random priming. The signals were visualized and quantified with a PhosphorImager. The numbers represent the relative intensities of p16INK4A signals from Ras cells after being normalized to the signals from vector control cells.
DISCUSSION
The existence of multiple p38 isoforms with differences in tissue distribution and affinity for upstream regulators suggests that these isoforms may have distinct functions. Although the p38α isoform has been shown to be required for inflammatory and stress responses in vivo (50, 65–67), the physiology roles of the other p38 isoforms have been unclear. Using SB203580, a pharmacological inhibitor with relatively higher affinity for p38α and p38β as compared with p38γ and p38δ, we were able to demonstrate a key role of p38 in oncogene-induced senescence. However, the relative contribution of the specific p38 isoforms to senescence had never been defined. In the present study, we have shown that p38α and p38γ, but not p38β, mediate senescence induction by oncogenic ras. These studies have identified a novel function of p38γ in the regulation of oncogene-induced senescence. Taking these findings together with our previous report demonstrating the involvement of p38γ in γ-radiation-induced G2 cell cycle arrest and DNA damage checkpoint control (68), we conclude that a major function of p38γ may be to suppress tumorigenesis and maintain genome stability.
The requirement of both p38α and p38γ suggests that the functions of these p38 isoforms are not redundant during senescence induction and that p38α and p38γ may target different downstream substrates in the senescence pathway. Indeed, our data demonstrate that p38α and p38γ contribute to senescence induction through different mechanisms, with p38γ transducing the senescence signal via the p53-p21WAF1 pathway and p38α via a p53-independent, but p16INK4A-dependent, route. The p53-p21WAF1 circuit is one of the key effector pathways known to be essential for almost all types of senescence. The ability of p38γ shRNA to disrupt ras-induced p53-Ser33 phosphorylation, p53 transcriptional phosphorylation and activity, indicates that p38γ mediates senescence induction at least partly by regulating the p53-p21WAF1 pathway. The p16INK4A-Rb pathway is the other major effector of senescence. We previously demonstrated that constitutive activation of p38 by active MKK3 or MKK6 leads to increased expression of p16INK4A at both protein and mRNA levels (30). The results from our current study indicate that ras-induced increase in p16INK4A expression is mediated by p38α.
It has been reported that recombinant p38α phosphorylates p53-Ser33 in vitro (64). We confirmed this finding and further demonstrated that activated recombinant p38γ also phosphorylated p53 at Ser33 in vitro as efficiently as p38α. However, when immunoprecipitated from senescent cells, only p38γ, but not p38α, could phosphorylate p53-Ser33. In addition, ras-induced phosphorylation of p53-Ser33 in senescent cells was greatly reduced by p38γ shRNA but not by p38α shRNA. Constitutively active p38γ, but not active p38α, consistently induced p53-Ser33 phosphorylation in cells. These findings indicate that in cells, phosphorylation of p53-Ser33 is mainly mediated by p38γ, but not p38α, upon senescence induction. The mechanism underlying this discrepancy between the in vitro and in vivo activities of p38 toward p53 is currently unknown. It is possible that during ras-induced senescence in vivo, the kinase activity of p38α toward p53 is repressed as a result of posttranslational modification or binding to an inhibitory protein. Alternatively, the p53 kinase activity of p38γ may be enhanced by posttranslational modification or an associated protein in senescent cells.
In vitro, recombinant p38α and p38γ seem to have different affinities for the substrate sites on a same protein. Whereas p38α phosphorylates both Ser33 and Ser46 of p53, p38γ phosphorylates only Ser33. It has been shown previously that p38α and p38γ have different substrate selectivity in vitro. MAPKAPK2, MAPKAPK3, and PRAK are preferred substrates of p38α over p38γ, whereas p38γ has higher kinase activity toward the microtubule-associated protein Tau and scaffold proteins SAP90 and SAP97 than does p38α (60, 69). These differences in substrate selectivity for p53 and other proteins are consistent with the fact that these two isoforms belong to different subgroups within the p38 MAPK family (35, 69). P38γ shares lower identity in amino acid sequence with p38α than other isoforms, and the structure of the ATP-binding pocket differs between the α and γ isoforms.
Although our study has suggested the importance of the p38γ/p53-Ser33/p21WAF1 cascade in ras-induced senescence, there are almost certainly other pathways that act in a parallel or partially overlapping fashion to mediate senescence induction. Supporting this notion, we found that constitutively active p38γ increased phosphorylation of p53-Ser33 to a significantly higher level than oncogenic ras but that the p21 level was induced more robustly by ras as compared with the active p38γ (Fig. 4A). It is highly likely that besides phosphorylation of Ser33 by p38γ, oncogenic ras induces additional posttranslational modifications on p53, leading to a further increase in the p53 activity and p21WAF1 expression. Oncogenic ras may also induce p53-independent signaling pathways that contribute to increased p21WAF1 expression. It has been shown that senescence induction is accompanied by phosphorylation of other sites on p53 in addition to Ser33, such as Ser15 and Ser37 (23, 25). We found that all of these sites (Ser15, Ser33, and Ser37) are required for p53 to be able to mediate senescence (23) and for the ras-induced activation of p53 (data not shown). Therefore, it is likely that p53 needs to be phosphorylated at multiple sites to be fully activated during senescence and to function as a senescence effector.
The key role of the p38 pathway in inflammation has prompted efforts to develop anti-inflammatory drugs targeting this pathway. Most such drug candidates currently under development inhibit p38α. However, the essential role of p38α in the tumor-suppressing senescence response to activated oncogenes, as demonstrated in this study, suggests that these drugs would potentially increase the risk of initiating cancer. It is thus imperative to determine the functional specificity of the signaling components of the p38 pathway so that the anti-inflammatory drugs can be designed to target the signaling molecules that are specifically involved in inflammation but not in tumor suppression.
Supplementary Material
Acknowledgments
We thank Dr. Hannon for the pSM2C plasmid, Dr. Agami for the pSUPERretro plasmid, Drs. Engelberg and Livnah for the constitutively active mutants of the p38 isoforms, and Drs. Maria Martinez-Yamout, Josephine Ferreon, and Peter Wright for the recombinant p53 proteins.
This work was supported, in whole or in part, by Grant CA106768 from the National Institutes of Health (to P. S.). This is Scripps Manuscript 19841.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: MAPK, mitogen-activated protein kinase; PRAK, p38-regulated/activated protein kinase; shRNA, small hairpin RNA; GFP, green fluorescent protein; shGFP, shRNA against GFP; SA-β-gal, senescence-associated β-galactosidase; PD, population doubling; GST, glutathione S-transferase; MBP, myelin basic protein; MOPS, 4-morpholinepropanesulfonic acid.
References
- 1.Barbacid, M. (1987) Annu. Rev. Biochem. 56 779-827 [DOI] [PubMed] [Google Scholar]
- 2.Medema, R. H., and Bos, J. L. (1993) Crit. Rev. Oncog. 4 615-661 [PubMed] [Google Scholar]
- 3.Cahill, M. A., Janknecht, R., and Nordheim, A. (1996) Curr. Biol. 6 16-19 [DOI] [PubMed] [Google Scholar]
- 4.Bos, J. L. (1989) Cancer Res. 49 4682-4689 [PubMed] [Google Scholar]
- 5.Bos, J. L. (1988) Mutat. Res. 195 255-271 [DOI] [PubMed] [Google Scholar]
- 6.Weinberg, R. A. (1989) Cancer Res. 49 3713-3721 [PubMed] [Google Scholar]
- 7.Ruley, H. E. (1990) Cancer Cells 2 258-268 [PubMed] [Google Scholar]
- 8.Hahn, W. C., Counter, C. M., Lundberg, A. S., Beijersbergen, R. L., Brooks, M. W., and Weinberg, R. A. (1999) Nature 400 464-468 [DOI] [PubMed] [Google Scholar]
- 9.Elenbaas, B., Spirio, L., Koerner, F., Fleming, M. D., Zimonjic, D. B., Donaher, J. L., Popescu, N. C., Hahn, W. C., and Weinberg, R. A. (2001) Genes Dev. 15 50-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter, T. (1991) Cell 64 249-270 [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein, B., and Kinzler, K. W. (1993) Trends Genet. 9 138-141 [DOI] [PubMed] [Google Scholar]
- 12.Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D., and Lowe, S. W. (1997) Cell 88 593-602 [DOI] [PubMed] [Google Scholar]
- 13.Zhu, J., Woods, D., McMahon, M., and Bishop, J. M. (1998) Genes Dev. 12 2997-3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen, C. L., Gardie, B., Yaswen, P., and Stampfer, M. R. (2002) Oncogene 21 6328-6339 [DOI] [PubMed] [Google Scholar]
- 15.Dimri, G. P., Itahana, K., Acosta, M., and Campisi, J. (2000) Mol. Cell. Biol. 20 273-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Land, H., Parada, L. F., and Weinberg, R. A. (1983) Nature 304 596-602 [DOI] [PubMed] [Google Scholar]
- 17.Seger, Y. R., Garcia-Cao, M., Piccinin, S., Cunsolo, C. L., Doglioni, C., Blasco, M. A., Hannon, G. J., and Maestro, R. (2002) Cancer Cell 2 401-413 [DOI] [PubMed] [Google Scholar]
- 18.Narita, M., and Lowe, S. W. (2005) Nat. Med. 11 920-922 [DOI] [PubMed] [Google Scholar]
- 19.Collado, M., Gil, J., Efeyan, A., Guerra, C., Schuhmacher, A. J., Barradas, M., Benguria, A., Zaballos, A., Flores, J. M., Barbacid, M., Beach, D., and Serrano, M. (2005) Nature 436 642. [DOI] [PubMed] [Google Scholar]
- 20.Michaloglou, C., Vredeveld, L. C., Soengas, M. S., Denoyelle, C., Kuilman, T., van der Horst, C. M., Majoor, D. M., Shay, J. W., Mooi, W. J., and Peeper, D. S. (2005) Nature 436 720-724 [DOI] [PubMed] [Google Scholar]
- 21.Braig, M., Lee, S., Loddenkemper, C., Rudolph, C., Peters, A. H., Schlegelberger, B., Stein, H., Dorken, B., Jenuwein, T., and Schmitt, C. A. (2005) Nature 436 660-665 [DOI] [PubMed] [Google Scholar]
- 22.Chen, Z., Trotman, L. C., Shaffer, D., Lin, H. K., Dotan, Z. A., Niki, M., Koutcher, J. A., Scher, H. I., Ludwig, T., Gerald, W., Cordon-Cardo, C., and Pandolfi, P. P. (2005) Nature 436 725-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun, P., Yoshizuka, N., New, L., Moser, B. A., Li, Y., Liao, R., Xie, C., Chen, J., Deng, Q., Yamout, M., Dong, M. Q., Frangou, C. G., Yates, J. R., III, Wright, P. E., and Han, J. (2007) Cell 128 295-308 [DOI] [PubMed] [Google Scholar]
- 24.Lin, A. W., Barradas, M., Stone, J. C., Van Aelst, L., Serrano, M., and Lowe, S. W. (1998) Genes Dev. 12 3008-3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferbeyre, G., de Stanchina, E., Lin, A. W., Querido, E., McCurrach, M. E., Hannon, G. J., and Lowe, S. W. (2002) Mol. Cell. Biol. 22 3497-3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narita, M., Nunez, S., Heard, E., Narita, M., Lin, A. W., Hearn, S. A., Spector, D. L., Hannon, G. J., and Lowe, S. W. (2003) Cell 113 703-716 [DOI] [PubMed] [Google Scholar]
- 27.Lee, A. C., Fenster, B. E., Ito, H., Takeda, K., Bae, N. S., Hirai, T., Yu, Z. X., Ferrans, V. J., Howard, B. H., and Finkel, T. (1999) J. Biol. Chem. 274 7936-7940 [DOI] [PubMed] [Google Scholar]
- 28.Di, M. R., Fumagalli, M., Cicalese, A., Piccinin, S., Gasparini, P., Luise, C., Schurra, C., Garre, M., Nuciforo, P. G., Bensimon, A., Maestro, R., Pelicci, P. G., and d'Adda di Fagagna, F. (2006) Nature 444 638-642 [DOI] [PubMed] [Google Scholar]
- 29.Bartkova, J., Rezaei, N., Liontos, M., Karakaidos, P., Kletsas, D., Issaeva, N., Vassiliou, L. V., Kolettas, E., Niforou, K., Zoumpourlis, V. C., Takaoka, M., Nakagawa, H., Tort, F., Fugger, K., Johansson, F., Sehested, M., Andersen, C. L., Dyrskjot, L., Orntoft, T., Lukas, J., Kittas, C., Helleday, T., Halazonetis, T. D., Bartek, J., and Gorgoulis, V. G. (2006) Nature 444 633-637 [DOI] [PubMed] [Google Scholar]
- 30.Wang, W., Chen, J. X., Liao, R., Deng, Q., Zhou, J. J., Huang, S., and Sun, P. (2002) Mol. Cell. Biol. 22 3389-3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwasa, H., Han, J., and Ishikawa, F. (2003) Genes Cells 8 131-144 [DOI] [PubMed] [Google Scholar]
- 32.Haq, R., Brenton, J. D., Takahashi, M., Finan, D., Finkielsztein, A., Damaraju, S., Rottapel, R., and Zanke, B. (2002) Cancer Res. 62 5076-5082 [PubMed] [Google Scholar]
- 33.Nicke, B., Bastien, J., Khanna, S. J., Warne, P. H., Cowling, V., Cook, S. J., Peters, G., Delpuech, O., Schulze, A., Berns, K., Mullenders, J., Beijersbergen, R. L., Bernards, R., Ganesan, T. S., Downward, J., and Hancock, D. C. (2005) Mol. Cell 20 673-685 [DOI] [PubMed] [Google Scholar]
- 34.Cohen, P. (1997) Trends Cell Biol. 7 353-361 [DOI] [PubMed] [Google Scholar]
- 35.Ono, K., and Han, J. (2000) Cell. Signal. 12 1-13 [DOI] [PubMed] [Google Scholar]
- 36.Han, J., and Sun, P. (2007) Trends Biochem. Sci. 32 364-371 [DOI] [PubMed] [Google Scholar]
- 37.Nebreda, A. R., and Porras, A. (2000) Trends Biochem. Sci. 25 257-260 [DOI] [PubMed] [Google Scholar]
- 38.Johnson, G. L., and Lapadat, R. (2002) Science 298 1911-1912 [DOI] [PubMed] [Google Scholar]
- 39.Hui, L., Bakiri, L., Mairhorfer, A., Schweifer, N., Haslinger, C., Kenner, L., Komnenovic, V., Scheuch, H., Beug, H., and Wagner, E. F. (2007) Nat. Genet. 39 741-749 [DOI] [PubMed] [Google Scholar]
- 40.Ventura, J. J., Tenbaum, S., Perdiguero, E., Huth, M., Guerra, C., Barbacid, M., Pasparakis, M., and Nebreda, A. R. (2007) Nat. Genet. 39 750-758 [DOI] [PubMed] [Google Scholar]
- 41.Bulavin, D. V., Demidov, O. N., Saito, S., Kauraniemi, P., Phillips, C., Amundson, S. A., Ambrosino, C., Sauter, G., Nebreda, A. R., Anderson, C. W., Kallioniemi, A., Fornace, A. J., Jr., and Appella, E. (2002) Nat. Genet. 31 210-215 [DOI] [PubMed] [Google Scholar]
- 42.Bulavin, D. V., Phillips, C., Nannenga, B., Timofeev, O., Donehower, L. A., Anderson, C. W., Appella, E., and Fornace, A. J., Jr. (2004) Nat. Genet. 36 343-350 [DOI] [PubMed] [Google Scholar]
- 43.Han, J., Lee, J. D., Bibbs, L., and Ulevitch, R. J. (1994) Science 265 808-811 [DOI] [PubMed] [Google Scholar]
- 44.Jiang, Y., Chen, C., Li, Z., Guo, W., Gegner, J. A., Lin, S., and Han, J. (1996) J. Biol. Chem. 271 17920-17926 [DOI] [PubMed] [Google Scholar]
- 45.Li, Z., Jiang, Y., Ulevitch, R. J., and Han, J. (1996) Biochem. Biophys. Res. Commun. 228 334-340 [DOI] [PubMed] [Google Scholar]
- 46.Jiang, Y., Gram, H., Zhao, M., New, L., Gu, J., Feng, L., Di Padova, F., Ulevitch, R. J., Han, J., Han, J., Lee, J. D., Bibbs, L., and Ulevitch, R. J. (1997) J. Biol. Chem. 272 30122-30128 [DOI] [PubMed] [Google Scholar]
- 47.Enslen, H., Brancho, D. M., and Davis, R. J. (2000) EMBO J. 19 1301-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanoue, T., Yamamoto, T., Maeda, R., and Nishida, E. (2001) J. Biol. Chem. 276 26629-26639 [DOI] [PubMed] [Google Scholar]
- 49.Shi, Y., and Gaestel, M. (2002) Biol. Chem. 383 1519-1536 [DOI] [PubMed] [Google Scholar]
- 50.Kang, Y. J., Chen, J., Otsuka, M., Mols, J., Ren, S., Wang, Y., and Han, J. (2008) J. Immunol. 180 5075-5082 [DOI] [PubMed] [Google Scholar]
- 51.Beardmore, V. A., Hinton, H. J., Eftychi, C., Apostolaki, M., Armaka, M., Darragh, J., McIlrath, J., Carr, J. M., Armit, L. J., Clacher, C., Malone, L., Kollias, G., and Arthur, J. S. (2005) Mol. Cell. Biol. 25 10454-10464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabio, G., Arthur, J. S., Kuma, Y., Peggie, M., Carr, J., Murray-Tait, V., Centeno, F., Goedert, M., Morrice, N. A., and Cuenda, A. (2005) EMBO J. 24 1134-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avitzour, M., Diskin, R., Raboy, B., Askari, N., Engelberg, D., and Livnah, O. (2007) FEBS J. 274 963-975 [DOI] [PubMed] [Google Scholar]
- 54.Brummelkamp, T. R., Bernards, R., and Agami, R. (2002) Science 296 550-553 [DOI] [PubMed] [Google Scholar]
- 55.Brummelkamp, T. R., Bernards, R., and Agami, R. (2002) Cancer Cell 2 243-247 [DOI] [PubMed] [Google Scholar]
- 56.Paddison, P. J., Cleary, M., Silva, J. M., Chang, K., Sheth, N., Sachidanandam, R., and Hannon, G. J. (2004) Nat. Methods 1 163-167 [DOI] [PubMed] [Google Scholar]
- 57.Deng, Q., Li, Y., Tedesco, D., Liao, R., Fuhrmann, G., and Sun, P. (2005) Cancer Res. 65 8298-8307 [DOI] [PubMed] [Google Scholar]
- 58.Sun, P., Dong, P., Dai, K., Hannon, G. J., and Beach, D. (1998) Science 282 2270-2272 [DOI] [PubMed] [Google Scholar]
- 59.Shay, J. W., and Wright, W. E. (1989) Exp. Cell Res. 184 109-118 [DOI] [PubMed] [Google Scholar]
- 60.New, L., Jiang, Y., Zhao, M., Liu, K., Zhu, W., Flood, L. J., Kato, Y., Parry, G. C., and Han, J. (1998) EMBO J. 17 3372-3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.New, L., Jiang, Y., and Han, J. (2003) Mol. Biol. Cell 14 2603-2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Legge, G. B., Martinez-Yamout, M. A., Hambly, D. M., Trinh, T., Lee, B. M., Dyson, H. J., and Wright, P. E. (2004) J. Mol. Biol. 343 1081-1093 [DOI] [PubMed] [Google Scholar]
- 63.Tang, J., Qi, X., Mercola, D., Han, J., and Chen, G. (2005) J. Biol. Chem. 280 23910-23917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bulavin, D. V., Saito, S., Hollander, M. C., Sakaguchi, K., Anderson, C. W., Appella, E., and Fornace, A. J., Jr. (1999) EMBO J. 18 6845-6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamura, K., Sudo, T., Senftleben, U., Dadak, A. M., Johnson, R., and Karin, M. (2000) Cell 102 221-231 [DOI] [PubMed] [Google Scholar]
- 66.Allen, M., Svensson, L., Roach, M., Hambor, J., McNeish, J., and Gabel, C. A. (2000) J. Exp. Med. 191 859-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adams, R. H., Porras, A., Alonso, G., Jones, M., Vintersten, K., Panelli, S., Valladares, A., Perez, L., Klein, R., and Nebreda, A. R. (2000) Mol. Cell 6 109-116 [PubMed] [Google Scholar]
- 68.Wang, X., McGowan, C. H., Zhao, M., He, L., Downey, J. S., Fearns, C., Wang, Y., Huang, S., and Han, J. (2000) Mol. Cell. Biol. 20 4543-4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuenda, A., and Rousseau, S. (2007) Biochim. Biophys. Acta 1773 1358-1375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.