Abstract
Lens epithelium-derived growth factor/p75 (LEDGF/p75) is a prominent cellular interaction partner of human immunodeficiency virus-1 (HIV-1) integrase, tethering the preintegration complex to the host chromosome. In light of the development of LEDGF/p75-integrase interaction inhibitors, it is essential to understand the cell biology of LEDGF/p75. We identified pogZ as new cellular interaction partner of LEDGF/p75. Analogous to lentiviral integrase, pogZ, a domesticated transposase, carries a DDE domain, the major determinant for LEDGF/p75 interaction. Using different in vitro and in vivo approaches, we corroborated the interaction between the C terminus of LEDGF/p75 and the DDE domain of pogZ, revealing an overlap in the binding of pogZ and HIV-1 integrase. Competition experiments showed that integrase is efficient in displacing pogZ from LEDGF/p75. Moreover, pogZ does not seem to play a role as a restriction factor of HIV. The finding that LEDGF/p75 is capable of interacting with a DDE domain protein that is not a lentiviral integrase points to a profound role of LEDGF/p75 in DDE domain protein function.
In 2003 we identified LEDGF/p755 as the major cellular interaction partner of HIV-1 integrase (IN) (1). In vitro studies demonstrated that the interaction of LEDGF/p75 is restricted to lentiviral integrases (2–4). In recent years the important role of LEDGF/p75 in HIV replication has been demonstrated. Knock-down (5, 6), knock-out (7), and transdominant inhibition studies (6, 8, 9) all revealed a pivotal role of LEDGF/p75 in HIV-1 integration and replication. These studies also provided a proof-of-principle to block the interaction between LEDGF/p75 and HIV-1 IN as a potential antiviral strategy. By sequencing lentiviral vector integration sites in mouse LEDGF/p75 knock-out cell lines (7) and human LEDGF/p75 knock-down cell lines (10, 11), the co-factor was shown to play a role in integration site selection. Indeed, depletion of LEDGF/p75 induced a shift in lentiviral integration sites from the characteristic distribution in transcription units outside the promoter regions to a more random distribution.
The interaction between LEDGF/p75 and HIV-1 IN is mediated by the IN binding domain (IBD) in the C terminus of LEDGF/p75 (12). A crystal structure of a complex between two IBDs and a dimer of HIV-1 IN catalytic core domains (CCDs) identified amino acid residues in LEDGF/p75 that are essential for the interaction with IN (13). The hydrophobic amino acids Ile-365, Phe-406, and Val-408 and the charged Asp-366 residue all reside in the interhelical loops of the IBD.
As a ubiquitously expressed protein, LEDGF/p75 functions as a transcriptional co-activator, protecting cells from extracellular stress by regulating transcription of stress-related genes (for review, see Ref. 14). By preventing cells from undergoing apoptotic cell death, the protein is also involved in oncogenesis (15–19). Through a link with the mixed-lineage leukemia (MLL) histone methyltransferase, LEDGF/p75 was recently shown to be essential for MLL-dependent transcription and leukemic transformation (20).
To gain more insight into the cellular and virological role of LEDGF/p75, we attempt to identify and validate the cellular interaction partners of LEDGF/p75. Previously, we and others identified JPO2 as a first cellular interaction partner of the C-terminal end of LEDGF/p75 (21, 22). Like HIV-1 IN, JPO2 also interacts with the IBD of LEDGF/p75. Amino acid residues in the IBD that are critical for IN interaction were not crucial for the interaction with JPO2 (21), pointing to differential structural constraints for both interactions.
Here we describe the identification of the interaction between the cellular protein pogZ (pogo transposable element-derived protein with zinc finger) and the C terminus of LEDGF/p75 using yeast two-hybrid screening. Our in silico analysis of the protein sequence indicates that pogZ represents a domesticated transposase related to the Tigger-derived (TIGD) DNA transposases with a DDE domain (23). A DDE domain, also present in lentiviral integrases (24), is characterized by a catalytic site composed of two or three aspartic acid and/or glutamic acid residues with a specific spatial arrangement to allow coordination of Mg2+ cations (25). It allows DNA-modifying reactions such as strand cleaving, nicking, and ligation.
Our characterization of the interaction between pogZ and LEDGF/p75 gives new insight in the role of LEDGF/p75 and suggests a more profound role for LEDGF/p75 in DDE domain protein function. They support speculation on a possible evolutionary relationship between DNA transposons and lentiviral integrases.
EXPERIMENTAL PROCEDURES
Yeast Two-hybrid Screen—A yeast two-hybrid screen was performed using a cell-to-cell mating protocol (26). The experimental setup was designed as described previously (27). The prey consisted of a random-primed cDNA library prepared from CEMC7 cells (human T-cell line). The bait construct comprised the C-terminal domain (aa 341–507) of LEDGF/p75.
Cell Culture—HeLaP4 CCR5 cells, a kind gift from Pierre Charneau, Institut Pasteur, Paris, France, were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (International Medical), 20 μg/ml gentamicin (Invitrogen) (further referred to as Dulbecco's modified Eagle's medium-complete), and 0.5 mg/ml Geneticin at 37 °C and 5% CO2 in a humidified atmosphere. HeLaP4 CCR5 FLAG-LEDGF/p75 cells were treated the same with the addition of 2 μg/ml hygromycin B (Invitrogen) to the growth medium.
Expression Plasmids and Lentiviral Vector Cloning—pEGFP-pogZ was a kind gift from M. Lechner, Drexel University, Philadelphia, PA. A lentiviral vector construct for the expression of mRFP-ΔNpogZ was produced by PCR amplification of the C-terminal coding region of pogZ from pEGFP-pogZ with the primers BamHI 5′-aatggatccatgttacccttgtctatgattgt and SalI 5′-aatgtcgactcaaatctccatcagatc. The PCR product was digested and used to replace JPO2 in the pCHMWS-mRFP-JPO2-IRES-hygro transfer plasmid (21), 3′ to the mRFP-coding region. A bacterial expression plasmid encoding MBP-tagged C-terminal pogK, pK-ΔN, was produced by PCR amplification of the pogK coding sequence from HeLaP4 genomic DNA and insertion into pMalc2e (New England Biolabs) 3′ to the MBP coding sequence. PCR primers used were forward EcoRI, 5′-aatgaattctatgaggtagctcagatgg, and reverse BamHI, 5′-taaggatcctcagttgctctcagccatgc. The bacterial expression plasmid pZ-ΔN was produced by PCR amplification of the C-terminal coding region of pogZ from pEGFP-pogZ and cloning into pMalc2e 3′ to the MBP-coding sequence. PCR primers used were forward EcoRI, 5′-aatgaattcatgttacccttgtctatgattgt, and reverse BamHI, 5′-taaggatcctcaaatctccatcagatc. Bacterial expression plasmids encoding FLAG-tagged LEDGF/p75 and its IBD mutants as well as bacterial expression plasmids encoding p52, LEDGF/p75, HIV-1 IN-His, and MBP-JPO2 were described previously (21). Eukaryotic expression plasmids and lentiviral vector constructs for the expression of mRFP-tagged LEDGF/p75, eGFP-tagged LEDGF/p75, and the nuclear localization signal (NLS)-deficient mutant (K150A) as well as eGFP-tagged Δ325 and the D366A mutant were described previously (6, 9). A lentiviral vector construct co-expressing FLAG-tagged LEDGF/p75 and a hygromycin resistance gene was produced by PCR amplification of FLAG-LEDGF/p75 from the FLAG-LEDGF/p75 bacterial expression plasmid pCP-Nat-FLAG (21) and cloning into pCHMWS-IRES-hygro transfer plasmid 5′ to the IRES, yielding pCHMWS-FLAG-LEDGF/p75 IRES-Hygro. Primers used were BglII forward, gcgagatctatggactacaaagaccatgacg, and SalI reverse, gaattcgtcgacctagttatctagtgtagaatcc.
Purification of Recombinant ΔNpogZ, LEDGF/p75, p52, HIV-1 Integrase, CCD, and ΔNpogK—pZΔN and pKΔN were used to transform Rosetta2 Escherichia coli cells (Novagen, Germany). The transformants were grown at 37 °C to an A600 of 0.6, and protein production was induced by the addition of 0.5 mm isopropyl-β-d-thiogalactopyranoside. After induction, the culture was allowed to grow for 4 h before cells were collected by centrifugation (15 min, 4 °C, 6000 × g). The bacterial pellet was resuspended in lysis buffer (20 mm Tris/HCl, pH 7.4, 200 mm NaCl) and lysed by sonication (MSE 150-watt Ultrasonic Desintegrator). The MBP-ΔNpogZ and MBP-ΔNpogK fusion proteins were purified based on their affinity to amylose resin according to the manufacturer's protocol (New England Biolabs). The protein concentration of the collected fractions was determined with a BCA test (Pierce), and purity was determined by SDS-PAGE followed by Coomassie Brilliant Blue staining (Sigma-Aldrich). 20% glycerol was added to the fractions with the highest concentration and purity. The respective protein samples were stored at -20 °C. His-tagged HIV-1 IN was expressed from pRP1012 and purified as described previously (2). Non-tagged LEDGF/p75 and p52 were expressed and purified as described previously (29). The FLAG-tagged LEDGF/p75 expression and purification was essentially the same as for the non-tagged LEDGF/p75. HIV-1 IN CCD was purified as described previously (30).
Vector Production—Lentiviral vectors were prepared as described previously (31).
Stable Cell Lines—To make the HeLaP4 CCR5 FLAG-LEDGF/p75 stable cell line, HeLaP4 CCR5 cells were seeded in a 24-well plate and transduced with 104 RNA equivalents of CHMWS-FLAG-LEDGF/p75 IRES-Hygro lentiviral vector the following day. After 4 h of incubation, the supernatant was removed, cells were washed with PBS, and 1 ml of Dulbecco's modified Eagle's medium complete medium was added. After 48 h, selection was initiated by adding 2 μg/ml hygromycin B (Invitrogen).
Analysis of Direct Protein-Protein Interaction by AlphaScreen—The AlphaScreen assay was performed according to the manufacturer's protocol (PerkinElmer Life Sciences) and as described before (21). Cross-titration experiments were performed by titrating increasing amounts of one protein interaction partner against different concentrations of a second protein interaction partner.
Co-immunoprecipitation—Nuclear extracts were prepared as described previously (1), and all further manipulations were performed at 4 °C. ANTI-FLAG® M2-agarose affinity beads (Sigma-Aldrich) were washed with PBS and incubated with the lysate for 1 h. The beads were collected by centrifugation (30 s, 1800 × g, 4 °C) and washed 3 times with 400 μl of 400CSK buffer (10 mm Pipes, pH 6.8, 10% (w/v) sucrose, 1 mm dithiothreitol, 1 mm MgCl2, 400 mm NaCl, complete protease inhibitor w/o EDTA (Roche Applied Science). Immunoprecipitated protein was eluted with 40 μl of SDS-PAGE loading buffer. Samples were analyzed by 10% SDS-PAGE and Western blotting using appropriate antibodies.
Western Blotting—Protein samples were separated on 10% SDS-PAGE and electroblotted onto polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked with milk powder in PBS, 0.1% Tween 20, and detection was carried out using mouse anti-LEDGF/p75 antibody (Bethyl, Montgomery, TX), rabbit anti-pogZ antibody (Aviva Systems Biology, San Diego, CA), or rabbit anti-mRFP antibody (Chemicon). Visualization was performed using chemiluminescence (ECL+, Amersham Biosciences) using anti-mouse (α-FLAG) or anti-rabbit (α-pogZ, α-mRFP) antibodies coupled to horseradish peroxidase (Dako).
Fluorescence and Laser Scanning Microscopy—Cells grown in LabTek II glass chamber slides (VWR International) were fixed by incubation with 4% formaldehyde in PBS for 10 min and washed with PBS. The nuclear DNA was stained with 0.5 μg/ml DAPI (Molecular Probes). Immunohistochemistry staining of pogZ was performed using anti-pogZ antibody (Aviva Systems Biology, San Diego, CA). Confocal microscopy was performed using an LSM 510 meta unit (Zeiss, Zaventem, Belgium). All images were acquired in the multi-track mode. eGFP was excited at 488 nm (AI laser), mRFP at 543 nm (HeNe laser), and DAPI at 790 nm (MAI TAI two photon laser). After the main dichroic beam splitter (HFT UV/488/543/633 for eGFP, HFT 700/543 for mRFP, and HFT KP 650 for DAPI), the fluorescence signal was divided by a secondary dichroic beam splitter (NFT 490 for eGFP or NFT 545 for mRFP) and detected in the separate channels using the appropriate filters (BP 500-550 for eGFP, BP 561–615 for mRFP, and BP 435–485 for DAPI).
HIV Infection and Analysis of Transfected HeLaP4 Cells—The day before transient transfection, 200,000 cells were seeded per well in a 6-well plate, and attachment to the plate was allowed overnight. Cells were transfected with 20 nm siRNA following the guidelines of the siFECTamine™ protocol. Synthetic siRNAs were designed as follows and provided by Qiagen (Belgium); sipZ1, (aagaagagagctgttaggaaa), sipZ2 (aaagaacagcgacagtacaaa), siCD-4 targeting the CD4 receptor was described previously (33). Three days after transfection 1.5 × 104 cells were re-seeded in a 24-well plate for 4 h at 37 °C. After attachment, cells were infected with 8.5 × 105 pg p24/ml of HIV-1 in a total volume of 250 μl. At 24 h after infection, a single well was analyzed for β-galactosidase activity (chemiluminescent β-galactosidase reporter gene assay; Roche Applied Science). The β-galactosidase activity was measured according to the manufacturer's protocol. Chemiluminescence was measured with a LumiCount instrument (Packard Instrument Co.). The protein concentration of each sample was determined (BCA protein assay kit; Perbio), and read-outs were normalized for protein content.
RESULTS
Identification of pogZ as a Novel Interaction Partner of LEDGF/p75—To identify novel cellular interaction partners of LEDGF/p75, we performed a Y2H screen. In light of our ongoing drug discovery program, we were primarily interested in the identification of cellular binding partners of LEDGF/p75 that interact with the IBD. Therefore, we used the C-terminal region (aa 341–507) as bait (Fig. 1A). The prey consisted of a CEM-C7 T-cell line cDNA library. Next to the earlier-described LEDGF/p75 binding partner JPO2 (21, 22), the transposase-like protein, pogZ, was identified as putative interaction partner of LEDGF/p75. Ten clones of different length were identified, and alignment of the sequences pinpointed to the C-terminal region of pogZ as the specific interaction domain (SID) for LEDGF/p75 (Fig. 1B).
FIGURE 1.
Schematic representation and alignments of LEDGF/p75, pogZ, and pogK and their respective domains. A, schematic representation of LEDGF/p75. The chromatin-associating domain (PWWP), the DNA binding domain (AT-hooks), and the NLS are indicated. The alternative splice variant, p52, and the LEDGF/p75 specific C-terminal fragment (Δ325) are indicated below. The IBD is located in the C-terminal domain of LEDGF/p75. The fragment of LEDGF/p75 used as bait in the Y2H screen spans amino acid residues 341–507. B, for pogZ and pogK, the predicted DDE domains are indicated, as is the CENB-P HTH domain. The six-zinc-finger array and ZNF280 homology region is indicated for pogZ. The predicted N-terminal KRAB domain is indicated for pogK. The C-terminal regions of both pogZ and pogK, encoded by a single exon (exon 19 and exon 6, respectively), are indicated. The different clones from the Y2H screen are aligned with the protein sequence of pogZ. The SID between amino acid residue 1139 and 1248 is found in the DDE domain of pogZ. C, alignment of partial protein sequences of the DDE domains of human TIGD1, JRK, JRKL, and pogZ. The alignment was created using the ClustalW algorithm and manually refined (28). The DDE domain consensus sequence for the catalytic aspartate and glutamate residues DxD35(E/D) is indicated. The asterisk marks complete conservation, whereas the colon and dot stand for partial conservation of amino acid residues. The protein sequence of the DDE domain of pogK is aligned below. Conserved catalytic residues are indicated by +, whereas non-conserved residues are denoted by -. D, alignment of predicted secondary structure succession of the DDE domain of pogZ and mos-1. The primary amino acid sequences are denoted. The secondary structure components (H, helix; E, strand) are indicated above (pogZ) and below (mos-1). The DD(E/D) residues are indicated. Secondary structure prediction was performed by the JPRED algorithm (32).
In Silico Analysis Reveals pogZ to Contain a DDE Domain, a Helix-turn-helix Domain, and a Six-zinc-finger Array Representing a Domesticated DNA Transposase—PogZ (Uniprot entry Q7Z3K3) was previously identified as a potential interaction partner of the transcription factor sp1 in a Y2H screen (34). However, nothing was known about the cellular function of the protein. PogZ (1410 aa) has a calculated molecular mass of 155 kDa. A sequence homology search using the NCBI-BLAST algorithm for the N-terminal region of pogZ showed 57% homology with the human protein ZNF280D overlapping the zinc finger region (Fig. 1A). A conserved domain scan using the NCBI BLAST algorithm (35) revealed the presence of a DDE domain and a DNA binding helix-turn-helix (HTH) domain in the C-terminal end of pogZ (Fig. 1B). The SID of pogZ with LEDGF/p75 overlapped with the predicted DDE domain (Fig. 1, A and B). This finding is of particular interest as the interaction of HIV-1 IN with LEDGF/p75 is mediated by the IN CCD, essentially a DDE domain (24, 36).
Next, the PSI-BLAST algorithm (37) was used to reveal functionally and evolutionarily important protein similarities for the DDE domain of pogZ (aa 1117–1323). When convergence was reached, PSI-BLAST uncovered sequence homology with TIGD transposases, such as TIGD1, human Jerky homologue (JRK), and human Jerky homologue like (JRKL) (Fig. 1C). Both Jerky and TIGD transposases are remnants of DNA-transposons related to the Tc1/mariner transposons that were active in the primate genome 60–80 million years ago (38, 39).
An earlier report points to the conservation of the catalytic Asp, Asp, and Asp/Glu residues in the DDE domain of TIGD1 by sequence homology with Tc/mariner transposases (39). Alignment of the human JRK, JRKL, and TIGD1 with the DDE domain of pogZ demonstrates the conservation of the catalytic Asp, Asp, and Asp/Glu residues in pogZ and both JRKL and JRK (Fig. 1C). The positioning of the catalytic residues in pogZ follows the DD35(D/E) consensus. In addition, a search of the PDB repository for the closest enzymatically active, structural homologue of the pogZ DDE domain using the PHYRE search engine (40) yielded the DDE domain of mos-1 DNA transposase (PDB entry 2f7t), which has a consensus DDE domain fold. Alignment of the predicted secondary structure of the DDE domains of pogZ and mos-1 revealed a significant conservation in length and position of secondary structure elements (Fig. 1D). Although there is limited sequence homology at the primary structure, the catalytic important DD(E/D) triad of pogZ could be aligned with that of mos-1.
Altogether, these data suggest that the pogZ protein represents a domesticated DNA transposase that was C-terminal-fused to a zinc finger-rich domain. This hypothesis is further supported by the fact that the C terminus of pogZ, including the transposase homology domain and the HTH (Fig. 1A) is encoded by a single exon (exon 19). A similar evolutionary path seems to be taken by a human paralogue of pogZ, pogK. Like pogZ and TIGDs, the C-terminal end of pogK also contains a predicted HTH and a DDE domain (Fig. 1A). Similar to pogZ, this region is also encoded by a single exon for the pogK protein. In pogK, however, this transposase-derived sequence is N-terminal-fused to a predicted KRAB domain (Fig. 1A). Sequence alignment of the DDE domain of pogK points to a loss of the DxD35(E/D) catalytic triad (Fig. 1C).
Confirmation of the Cellular Interaction between LEDGF/p75 and pogZ—Confocal fluorescence microscopy analysis of cells transiently transfected with a plasmid encoding eGFP-tagged pogZ demonstrated its specific nuclear localization. Whereas expression of the pogZ fusion was rather low (data not shown), co-expression of mRFP-tagged LEDGF/p75 resulted in increased eGFP-pogZ expression levels (Fig. 2A). The fluorescent signals for eGFP-pogZ and mRFP-LEDGF/p75 displayed a similar intranuclear localization (Fig. 2A, overlay).
FIGURE 2.
Validation and characterization of the LEDGF/p75-pogZ interaction. A, HeLaP4 CCR5 cells were transfected with two plasmids encoding eGFP-pogZ or mRFP-LEDGF/p75. Confocal fluorescence microscopy analysis of the cells is shown. The merge between the eGFP and mRFP signals is shown in the overlay panel. B, co-immunoprecipitation (CoIP) of endogenous pogZ with FLAG-tagged LEDGF/p75. HeLaP4 CCR5 FLAG-LEDGF/p75 cells (lanes 1–5) and HeLaP4 CCR5 cells (lane 6 and 7) were fractionated into a cytoplasmic (lane 1), a nuclear (lane 2), and a chromatin-associated fraction (lane 3). Co-immunoprecipitation was performed on the nuclear fraction in 400 mm NaCl (lanes 4–6) or 250 mm NaCl (lanes 5–7) using FLAG-M2-agarose resin to immunoprecipitate the FLAG-tagged LEDGF/p75. Samples were separated by SDS-PAGE followed by Western blotting. The presence of pogZ and LEDGF/p75 in the samples was verified using anti-pogZ (upper panel) or anti-LEDGF/p75 (lower panel) antibodies, respectively. Ab, antibody.
To confirm that LEDGF/p75 and pogZ are in the same complex, HeLaP4 CCR5 cells stably overexpressing N-terminal FLAG-tagged LEDGF/p75 (HeLaP4-CCR5 FLAG-LEDGF/p75) were fractionated into a cytoplasmic, a soluble nuclear, and an insoluble high salt-resistant chromatin fraction. Both pogZ and LEDGF/p75 were present in the nuclear fraction. Whereas LEDGF/p75 completely dissociated from the chromatin by applying 400 mm NaCl (Fig. 2B, compare lanes 2 and 3, lower panel), a significant amount of pogZ remained associated with the chromatin (Fig. 2B, lanes 2 and 3, upper panel) possibly because of a strong interaction of the six-zinc-finger array and the HTH domain with the chromatin. The soluble nuclear fraction was used for immunoprecipitation of FLAG-tagged LEDGF/p75. Parental HeLaP4-CCR5 cells were used as control (Fig. 2B, lanes 6 and 7). Endogenous pogZ was efficiently co-immunoprecipitated with FLAG-tagged LEDGF/p75 (Fig. 2B). Dilution of the 400 mm NaCl nuclear fraction to 250 mm NaCl improved pogZ co-immunoprecipitation (Fig. 2B, compare lanes 4 and 5, upper panel). These data indicate the presence of a salt-sensitive nuclear complex containing LEDGF/p75 and pogZ. In a separate set of co-immunoprecipitation experiments we could show that both pogZ and JPO2, like HIV IN, interact with Hrp2, next to LEDGF/p75, the only known human IBD-containing protein (12) (supplemental Fig. 1).
The pogZ-LEDGF/p75 Interaction Is Mediated by the C-terminal Domain of Each Protein—The Y2H data show an interaction between the pogZ DDE domain and the C-terminal part of LEDGF/p75. To verify this finding, co-localization of the fusion of mRFP to the C-terminal part of pogZ (mRFP-ΔNpogZ) (aa 1117–1410, Fig. 1A) with eGFP-LEDGF/p75 was evaluated by confocal microscopy. When expressed in the absence of eGFP-LEDGF/p75, mRFP-ΔNpogZ showed an unspecific cellular distribution, although a slight preference for the nucleus was observed (Fig. 3A, upper panel). Upon co-expression of eGFP-LEDGF/p75 and mRFP-ΔNpogZ, the latter partially relocated to the nucleus (Fig. 3A, middle panel). Co-expression of mRFP-ΔNpogZ and the NLS-defective mutant K150A mutant of eGFP-LEDGF/p75 excluded mRFP-ΔNpogZ from the nucleus (Fig. 3A, lower panel) as was previously shown for HIV-1 IN (5, 6, 12). In addition, expression of mRFP-ΔNpogZ in LEDGF/p75 knock-down cells resulted in lower mRFP-ΔNpogZ expression, suggesting that LEDGF/p75 stabilizes mRFP-ΔNpogZ (data not shown). In contrast, the expression level of full-length pogZ remained unaltered (data not shown). In an effort to narrow down the pogZ domain interacting with LEDGF/p75, the SID (see Fig. 1B) of pogZ was fused to mRFP. However, we could not demonstrate a similar relocation upon co-expression with either eGFP-LEDGF/p75 or its NLS-deficient counterpart. This is possibly because of misfolding or steric hindrance of the fluorescent protein tags (Fig. 3B: compare B to the upper and middle panel of A). The interaction between LEDGF/p75 and the C-terminal end of pogZ was confirmed by co-immunoprecipitation of mRFP-ΔNpogZ from HeLaP4 CCR5 FLAG-LEDGF/p75 cells with anti-FLAG agarose (Fig. 3C, lane 2, lower panel). In this experiment total cell lysates were prepared in 400 mm NaCl and before co-immunoprecipitation diluted to 250 mm NaCl. Of note, mRFP-ΔNpogZ overexpression was able to partially out-compete endogenous pogZ for binding to LEDGF/p75 (Fig. 3C, compare lanes 2–4, upper panel).
FIGURE 3.
Truncation mutants of LEDGF/p75 and pogZ colocalize in the cell. A, mRFP-ΔNpogZ was expressed in HeLaP4 CCR5 cells either alone (upper panel) or together with eGFP-LEDGF/p75 (middle panel) or the NLS-deficient K150A mutant (lower panel), respectively. Confocal fluorescence microscopy analysis of the cells is shown. DAPI staining of DNA is shown (blue). The fluorescent molecules analyzed are indicated above. The merge of eGFP-LEDGF/p75 and mRFP-ΔNpogZ signals is shown in the overlay panel. B, colocalization of mRFP-SID-pogZ with eGFP-LEDGF/p75NLS-. The same experimental setup as in A was used. C, co-immunoprecipitation of mRFP-ΔNpogZ with FLAG-tagged LEDGF/p75. HeLaP4 CCR5 FLAG-LEDGF/p75 cells were transduced with a lentiviral vector overexpressing mRFP-ΔNpogZ (lanes 1 and 2) or left untreated (lanes 3 and 4). Total cell lysate was prepared in 400 mm NaCl to remove LEDGF/p75 from the chromatin and subsequently diluted to 250 mm NaCl to perform the immunoprecipitation using FLAG-M2-agarose resin. Samples were run in a SDS-PAGE gel followed by Western blotting. The presence of mRFP-ΔNpogZ, endogenous pogZ, and LEDGF/p75 in the samples was analyzed using anti-mRFP (lower panel), anti-pogZ (upper panel), or anti-LEDGF/p75 (middle panel) antibodies, respectively. D, mRFP-ΔNpogZ was expressed in HeLaP4 CCR5 cells together with eGFP-Δ325. Confocal fluorescence microscopy analysis of the cells is shown. DAPI staining of DNA is shown (blue). The fluorescent molecules analyzed are indicated above the panels. The merge of eGFP-Δ325 and mRFP-ΔNpogZ signals is shown in the overlay panel.
We and others reported earlier that the C-terminal end of LEDGF/p75 (aa 325–507, eGFP-Δ325) has a predominant nuclear localization and that co-expression of HIV-1 IN or the cellular LEDGF/p75 binding partner, JPO2, relocates both proteins to the cytoplasm (6, 21). Likewise, mRFP-ΔNpogZ and eGFP-Δ325 co-expression resulted in nuclear exclusion of both fusion proteins (Fig. 3D). In addition, it was previously shown that both JPO2 and IN specifically interact with the IBD domain of LEDGF/p75 (22). Co-expression of an eGFP-IBD fusion together with mRFP-ΔNpogZalso resulted in cytoplasmic relocalization, indicating that the IBD is sufficient for the interaction with pogZ (data not shown).
LEDGF/p75 is known to tether JPO2 and HIV-1 IN to the chromosomes during mitosis (22, 29). To analyze whether LEDGF/p75 functions as a chromosome-tethering factor for pogZ, the subcellular localization of endogenous pogZ during mitosis was analyzed by confocal microscopy in HeLaP4 CCR5 cells (Fig. 4A). Surprisingly, pogZ did not associate with condensed chromatin. Overexpression of LEDGF/p75 as an eGFP fusion localized to mitotic chromosomes without tethering mRFP-ΔNpogZ (Fig. 4B). These findings are at odds with the previous suggestion that LEDGF/p75 is a general chromatin-tethering factor (22).
FIGURE 4.
PogZ is not associated with mitotic chromosomes. A, endogenous pogZ in HeLa CCR5 cells was immunohistochemically analyzed using anti-pogZ antibody. DAPI staining was used to stain the DNA (blue). Confocal fluorescence microscopy analysis of mitotic cells is shown. B, mRFP-ΔNpogZ was expressed in HeLaP4 CCR5 cells either alone (upper panel) or together with eGFP-LEDGF/p75 (lower panel). DAPI staining was used to stain the DNA (blue). Confocal fluorescence microscopy analysis of mitotic cells is shown. The fluorescent molecules analyzed are indicated above the panels. The merged signals are shown in the overlay panel.
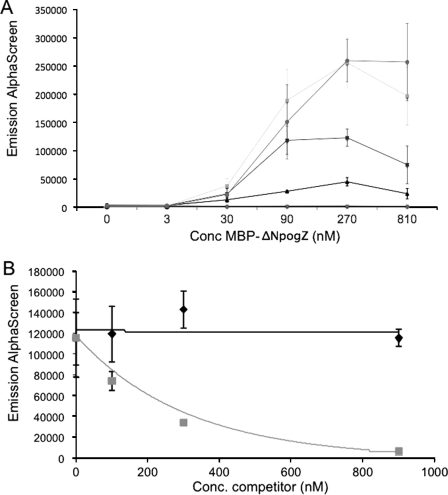
Next, the direct interaction between the LEDGF/p75 IBD and pogZ was studied in more detail using an AlphaScreen™ protein-protein interaction assay. The recombinant MBP-tagged C terminus of pogZ (MBP-ΔNpogZ) was purified from a bacterial lysate, and direct interaction with recombinant FLAG-tagged LEDGF/p75 (FLAG-LEDGF/p75) was demonstrated in a cross-titration experiment (Fig. 5A). For all subsequent competition experiments equimolar amounts (100 nm) of MBP-ΔNpogZ and FLAG-LEDGF/p75 were chosen as base-line concentrations. Increasing amounts of recombinant non-tagged LEDGF/p75 and p52 were added to the interaction mixture containing 100 nm MBP-ΔNpogZ and 100 nm FLAG-LEDGF/p75. The recombinant LEDGF/p75 out-competed the interaction, whereas recombinant p52 did not (Fig. 5B). In addition, the C terminus of the pogZ paralogue, pogK, was purified as a recombinant MBP fusion protein. However, this protein did not show interaction with FLAG-tagged LEDGF/p75 in an AlphaScreen cross-titration interaction assay (data not shown). This experiment confirms the specificity of the LEDGF/p75-pogZ interaction, pinpointing the interaction site to the C-terminal domains of each protein.
FIGURE 5.
In vitro analysis of the interaction between recombinant LEDGF/p75 and pogZ proteins. A, cross-titration for FLAG-LEDGF/p75 and MBP-ΔNpogZ interaction as measured by AlphaScreen. Interaction was measured at different concentrations of FLAG-LEDGF/p75 (♦, 0 nm; ▪, 3 nm; ▴, 30 nm; ▪, 90 nm; ▪, 270 nm; •, 900 nm) and different concentrations of MBP-JPO2 as indicated on the x axis. The experiment was done in triplicate. Data represent the average values ± S.D. for the three measurements. B, non-tagged LEDGF/p75 competes for the binding between FLAG-LEDGF/p75 and MBP-ΔNpogZ, whereas non-tagged p52 does not (n = 3). FLAG-LEDGF/p75 (100 nm) was incubated with MBP-ΔNpogZ (100 nm) in the presence of increasing amounts of recombinant non-tagged p52 (black) or non-tagged LEDGF/p75 (gray). Data represent average values ± S.D. for three independent measurements.
The pogZ Binding Site in LEDGF/p75 Overlaps to a Larger Extent with the HIV-1 IN Binding Site Than Does JPO2—LEDGF/p75 interacts with the HIV-1 IN CCD through its C-terminal IBD. A crystal structure of the IN CCD-IBD interaction was previously resolved, and several amino acid residues crucial for the interaction with HIV-1 IN were identified (13, 41). Although JPO2 also interacts with the IBD, our previous mutational analysis suggested that different contacts in the interface are required (21). In the light of our ongoing drug discovery, we are interested in the relative binding affinities of IBD cellular binding partners versus integrase. This research could also shed light on the mechanism by which IN out-competes the cellular LEDGF/p75 interaction partners. Because pogZ also contains a DDE domain, we investigated whether amino acid residues in the IBD engaged by HIV-1 IN are also used by pogZ. Side-by-side comparison of the IBD mutants in the AlphaScreen assay revealed that neither the D366A nor V408A mutations affected the interaction with pogZ or JPO2, whereas they, respectively, abrogated and severely inhibited the interaction with HIV-1 IN (Fig. 6A). The K360A, I365A, V370A, and F406A mutations on the other hand abrogated or severely inhibited the interaction of LEDGF/p75 with both pogZ and HIV-1 IN (Fig. 6A). As reported before, these mutations differentially affected interaction between LEDGF/p75 and JPO2 (21) (Fig. 6A). These data demonstrate that binding of HIV-1 IN and pogZ to the IBD overlaps more than that of JPO2.
FIGURE 6.
Analysis of differential interaction of HIV-1 IN, JPO2, and pogZ with the IBD of LEDGF/p75. A, interaction of HIV-1 IN, JPO2, and pogZ with LEDGF/p75 IBD mutants. FLAG-LEDGF/p75 or its mutants were present at 100 nm, IN-His was added at 300 nm, and MBP-ΔNpogZ and MBP-JPO2 were each added at 100 nm. Interaction was measured by AlphaScreen. Dark gray bars represent the interaction of wild-type (WT) FLAG-LEDGF/p75 and its mutants with MBP-JPO2. Light gray bars represent the interaction of wild-type FLAG-LEDGF/p75 and its mutants with IN-His. White bars represent the interaction of FLAG-LEDGF/p75 wild-type and its mutants with MBP-ΔNpogZ. The mutant residues are indicated on the x axis. The signals for the interaction between FLAG-LEDGF/p75 and its mutants with IN-His and MBP-JPO2 were normalized to the signal for the interaction between FLAG-LEDGF/p75 and MBP-ΔNpogZ. The average AlphaScreen emission with S.D. is shown (n = 3). B, the HIV-1 IN CCD efficiently interferes with the interaction between LEDGF/p75 and pogZ as evidenced by an AlphaScreen competition assay. FLAG-LEDGF/p75 (100 nm) was incubated with either MBP-ΔNpogZ (100 nm, black line) or MBP-JPO2 (100 nm, gray line) in the presence of increasing amounts of recombinant non-tagged HIV-1 IN CCD (10, 100, 300, 900 nm). The concentration of the HIV-1 IN CCD is indicated on the x axis. The average AlphaScreen emission with the S.D. is shown (n = 3). C, determination of the relative affinities of JPO2, pogZ, and HIV-1 IN CCD for LEDGF/p75. In an AlphaScreen interaction assay 100 nm FLAG-LEDGF/p75 was titrated with increasing amounts of MBP-JPO2 or MBP-ΔNpogZ (0, 5, 10, 50, 100, 150, and 200 nm) or IN-CCD-His (0, 3, 6, 33, 100 nm). The concentration of the titered protein is given on the x axis. The average AlphaScreen emission with the S.D. is shown on the y axis (n = 3). A sigmoid curve was used to fit the kinetics of the interaction, and Kd values were derived as indicated.
The HIV-1 IN Catalytic Core Domain Out-competes the pogZ-LEDGF/p75 Interaction More Efficiently Than the JPO2-LEDGF/p75 Interaction—Because pogZ and HIV-1 IN use an overlapping set of amino acid residues in the IBD, we examined whether HIV-1 IN can out-compete the interaction between LEDGF/p75 and pogZ. Increasing amounts of non-tagged HIV-1 IN CCD were added to an equimolar mixture (100 nm) of FLAG-LEDGF/p75 and MBP-ΔNpogZ or MBP-JPO2 (Fig. 6B). At equimolar concentrations (100 nm), IN CCD efficiently competed with MBP-ΔNpogZ for the interaction, whereas 100 nm MBP-JPO2 was displaced, only in part reducing the signal for less than 10%. To explain these results we calculated the relative affinities of JPO2, pogZ, and HIV-1 IN CCD for LEDGF/p75. Increasing amounts of either His-tagged HIV-1 IN CCD, MBP-JPO2, or MBP-ΔNpogZ were titrated against 100 nm FLAG-LEDGF/p75 in an AlphaScreen interaction assay. The steady-state binding curves were fitted best according to a sigmoidal model (Fig. 6C). Calculated apparent Kd values showed an up-to-2-fold higher affinity of HIV-1 IN CCD for LEDGF/p75 (Kd = 28.6 ± 0.8 nm) as compared with JPO2 (Kd = 62.0 ± 11.8 nm) or pogZ (Kd = 62.6 ± 13.7 nm). The more pronounced inhibition of the LEDGF/p75-pogZ interaction by HIV-1 IN CCD is consistent with a better overlap between IN and pogZ than between IN and JPO2 for binding to LEDGF/p75 (Fig. 6, A–C).
PogZ Does Not Restrict HIV-1 Replication—Because HIV-1 IN and pogZ compete for an overlapping interaction site on LEDGF/p75 and because the interaction between LEDGF/p75 and HIV-1 integrase is of crucial importance for HIV-1 replication in the cell (7–9), we investigated whether the interaction with pogZ plays a role in HIV infection.
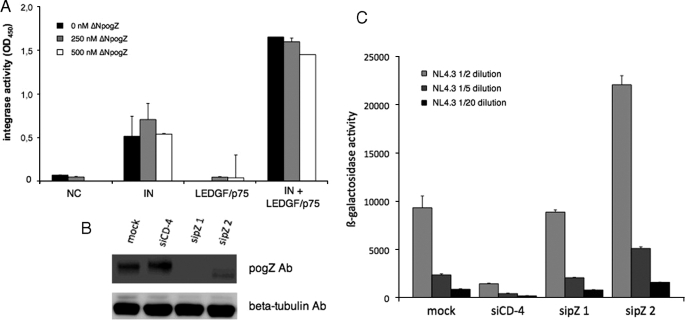
An ELISA-based assay (42) was performed to measure in vitro integrase activity in the presence of MBP-ΔNpogZ. Biotin and digoxigenin-labeled oligonucleotides were incubated with recombinant HIV-1 integrase. As shown before (4), LEDGF/p75 stimulated integrase activity (Fig. 7A). In the absence or presence of LEDGF/p75, the addition of increasing amounts of recombinant MBP-ΔNpogZ did not affect integrase activity. This finding is in agreement with the finding that HIV-1 IN can efficiently out-compete pogZ for interaction with LEDGF/p75 (Fig. 6B).
FIGURE 7.
pogZ does not inhibit LEDGF/p75-based stimulation of the HIV-1 integration reaction in vitro and does not influence HIV-1 replication. A, recombinant HIV-1 integrase (250 nm) was incubated with 40 nm oligonucleotide substrate in the absence (IN) or presence of 250 nm LEDGF/p75 (IN + LEDGF/p75) and/or varying concentrations of MBP-ΔNpogZ (ΔNpogZ: black, 0 nm; gray, 250 nm; white, 500 nm). NC, no LEDGF/p75 and no integrase added. LEDGF/p75, no integrase added. Data represent the average values ± S.D. for three independent measurements. B and C, HeLaP4 cells were transiently transfected with one of two different siRNAs targeting pogZ (sipZ1 and sipZ2) or were mock-transfected. As a positive control HeLaP4 cells were transiently transfected with siRNA targeting the CD-4 receptor (siCD-4). B, knock-down of pogZ was monitored by Western blotting using anti-pogZ antibody (Ab, upper panel) and normalized for β-tubulin concentration (lower panel). C, HeLaP4 cells were infected with a dilution series of HIV-1 NL4.3 (1/2, 1/5, and 1/10 of a 8.5 × 105-pg p24 stock) for 24 h and lysed. β-Galactosidase reporter gene activity was determined as a measure of HIV-1 replication. Data represent the average values ± S.D. for three independent measurements.
Next, we performed transient knock-down experiments to assess a possible role of pogZ in HIV-1 replication. HeLaP4 cells, containing an internal HIV-1 LTR-driven β-galactosidase reporter gene, were transiently transfected with one of two different siRNAs targeting pogZ (sipZ1 and sipZ2) or mock-transfected. As a positive control HeLaP4 cells were transiently transfected with siRNA targeting the CD4 receptor (siCD-4). Knock-down of pogZ was monitored with Western blotting (Fig. 7B). Cells were infected with a dilution series of HIV-1 NL4.3 for 24 h. After 24 h the cells were lysed, and β-galactosidase reporter gene activity was determined (Fig. 7C). Although an incomplete knockdown (sipZ2) induced a 2-fold stimulation, a near complete knockdown (sipZ1) of pogZ expression revealed wild-type levels of HIV-1 replication compared with the mock-transfected control cells. These data are at odds with a major function of pogZ as a restriction factor of HIV-1 replication in this cell line.
DISCUSSION
To gain more insight into the function of LEDGF/p75 in cell biology and during HIV replication, we embarked on the identification of cellular interaction partners. This knowledge is of crucial importance when considering the LEDGF/p75-HIV-1 IN interaction as an antiviral target. One aims at identifying small molecule inhibitors that block the interaction between IBD and HIV-1 IN without affecting the interaction of IBD with cellular binding partners. Alternatively, IBD binding partners could serve as restriction factors competing for the interaction with integrase.
Here we identified and validated pogZ as a novel cellular interaction partner of the IBD in LEDGF/p75. Our in silico analysis revealed that this protein with an unknown cellular function shows a striking homology to the DNA transposase proteins of the TIGD family of which the domesticated CENP-B transposase is also a member (43). pogZ features the hallmarks of a domesticated transposase that was C-terminal-fused to a zinc-finger-rich region. This hypothesis is strengthened by the fact that the entire C-terminal fragment of pogZ, containing the DDE domain and HTH, is encoded by a single exon, whereas the N-terminal part of the gene is encoded by multiple exons. The coding of the entire protein by an extensively spliced mRNA together with the large size of the entire gene and the absence of any obvious terminal inverted repeats indicate that the pogZ gene itself does not possess transposon activity. Domestication or the recruitment of transposase enzymes derived from mobile genetic elements into cellular functions has occurred multiple times in mammalian evolution. Well known examples of such domesticated transposases function in nuclear organization (centromere protein B), recombination (RAG-1), or DNA repair (SETmar) (44).
Via colocalization, co-immunoprecipitation, and direct protein-protein interaction assays, the interaction between LEDGF/p75 and pogZ was validated. We showed that the interaction is mediated by the IBD of LEDGF/p75. The IBD interacts with the C terminus of pogZ, which includes the transposase-derived DDE domain. This finding is of particular interest given that HIV-1 integrase also interacts with the LEDGF/p75 IBD through its DDE domain-containing CCD (13, 45). In contrast to JPO2, HIV-1 integrase and pogZ show a significant overlap in critical IBD amino acid residues required for interaction. IBD mutations I365A, V370A, and F406A equally affected the interactions with HIV-1 IN and pogZ. On the contrary, mutations D366A and V408A had no effect on the interaction between LEDGF/p75 and pogZ, whereas these mutants abrogate the interaction of LEDGF/p75 with HIV-1 IN. Possibly these residues enable integrase to out-compete JPO2 and pogZ for binding to LEDGF/p75, a crucial property that assures hijacking of LEDGF/p75 by the HIV pre-integration complex and consecutive targeting of the virus to the cellular genome (Fig. 6A). As JPO2 and pogZ have a comparable affinity for LEDGF/p75, the more efficient and complete out-competition of the pogZ-LEDGF/p75 interaction by the HIV-1 IN CCD as compared with the JPO2-LEDGF/p75 interaction further corroborates the extensive overlap in IBD residues engaged by both HIV-1 IN and pogZ (Fig. 6B). Elucidation of the structural basis of the differential interactions of the LEDGF/p75 binding proteins with the IBD should confirm this hypothesis. Recently the MLL methyltransferase was shown to be yet another IBD interacting protein (20). Interestingly, a third player, menin, is required for this interaction. This requirement may explain why MLL was not highlighted by our Y2H screen. Given the essential importance of the LEDGF/p75-MLL interaction in leukemogenesis (20), it is mandatory to incorporate MLL in future interaction studies. Together these findings suggest that the C terminus of LEDGF acts as a playground for protein-protein interaction.
Side by side comparison of the positioning of the catalytic triad DD(E/D) amino acid residues in the DDE domain of TIGD1 with those in the DDE domain of the pogZ protein reveals the conservation of these residues. Identification of a putative catalytic activity in a nucleic acid-modifying pathway mediated by pogZ would shed more light on the cellular functions of both LEDGF/p75 and pogZ. The DDE domain of pogK, the human paralogue of pogZ, does not appear to contain these conserved catalytic DD(E/D) residues. Interestingly, we could not detect an interaction between LEDGF/p75 and pogK (data not shown).
Next to a better understanding of the function of LEDGF/p75 in cellular pathways and viral replication, this study also has important implications for future anti-HIV drug development. Subsequent to the validation of LEDGF/p75 as an important HIV-1 cofactor drug discovery, laboratories in academia and industry now exploit the IN-LEDGF/p75 interaction as a new antiviral target (46–48). In light of cellular toxicity, the effect of potential drugs on pogZ and JPO2 binding to LEDGF/p75 should be assessed. The fact that the binding mode of JPO2 and pogZ to LEDGF/p75 does not entirely overlap with the binding of IN to LEDGF/p75 supports the feasibility of specific drug design.
Because LEDGF/p75 links both JPO2 and HIV-1 IN to chromatin (22, 29), LEDGF/p75 has been described as a multifunctional tethering factor. Our finding that neither pogZ nor the C-terminal part of pogZ associate with mitotic chromosomes even when LEDGF/p75 is overexpressed (Fig. 4) indicates that this function of LEDGF/p75 is not applicable to pogZ.
Because pogZ and integrase bind to the same domain of LEDGF/p75, pogZ might potentially act as a restriction factor to viral replication. The well known stimulation of in vitro integrase activity by LEDGF/p75, however, was not inhibited by the addition of MBP-ΔNpogZ. Accordingly, transient knockdown of pogZ showed either no or only a 2-fold stimulation of HIV-1 replication in HeLaP4 cells. These findings indicate no important function for the LEDGF/p75-pogZ interaction in HIV-1 replication but further point to a mechanism whereby HIV-1 IN usurps LEDGF/p75 in the cell during infection.
Binding of LEDGF/p75 to integrases is lentiviral-specific (2, 4). The finding that LEDGF/p75 can bind a domesticated transposase sheds a new light on this finding. Possibly a common ancestor was able to interact with LEDGF/p75, whereas this interaction was lost in most retroviruses and transposons. An evolutionary link between the DDE domains of Tc/mariner DNA transposases, retrotransposons, and retroviruses through a common ancestor has been suggested previously (49, 50). Alternatively, LEDGF/p75 interaction might have occurred several times in a convergent evolution. In any case binding of LEDGF/p75 to a DDE domain-containing protein must have significant functional advantage. Given that the DDE domain of pogZ shows an evolutionary link with the DDE domains of Tc1/mariner DNA transposases through its homology with TIGD transposases, we are currently investigating the possible involvement of LEDGF/p75 in the interaction and functionality of DDE domains of other genetic mobile elements such as human DNA transposons.
Acknowledgments
We acknowledge M. McNeely for critical reading of the manuscript. We thank the KULeuven Cell Imaging Core for use of the confocal microscope and Katrien Busschots for help with the LEDGF/p75 mutagenesis.
This work was supported by grants from the FP6 and FP7 framework of the European Union (TRIoH, LSHB-CT-2003-503480 and THINC, HEALTH-2007-2.3.2-1) and the CellCoVir SBO project of the Institute for the Promotion of Innovation by Science and Technology in Flanders.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Footnotes
The abbreviations used are: LEDGF/p75, lens epithelium-derived growth factor/p75; IN, integrase; HIV-1, human immunodeficiency virus-1; IBD, IN binding domain; CCD, catalytic core domains; MLL, mixed-lineage leukemia; TIGD, Tigger-derived; IRES, internal ribosome entry site; Y2Hs, yeast two-hybrid; SID, specific interaction domain; HTH, helix-turn-helix; JRK, Jerky homologue; JRKL, JRK-like; NLS, nuclear localization signal; aa, amino acids; MBP, maltose-binding protein; eGFP, enhanced green fluorescent protein; mRFP, monomeric red fluorescent protein; PBS, phosphate-buffered saline; Pipes, 1,4-piperazinediethanesulfonic acid; DAPI, 4′,6-diamidino-2-phenylindole; siRNA, small interfering RNA.
References
- 1.Cherepanov, P., Maertens, G., Proost, P., Devreese, B., Van Beeumen, J., Engelborghs, Y., De Clercq, E., and Debyser, Z. (2003) J. Biol. Chem. 278 372-381 [DOI] [PubMed] [Google Scholar]
- 2.Busschots, K., Vercammen, J., Emiliani, S., Benarous, R., Engelborghs, Y., Christ, F., and Debyser, Z. (2005) J. Biol. Chem. 280 17841-17847 [DOI] [PubMed] [Google Scholar]
- 3.Llano, M., Vanegas, M., Fregoso, O., Saenz, D., Chung, S., Peretz, M., and Poeschla, E. M. (2004) J. Virol. 78 9524-9537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherepanov, P. (2007) Nucleic Acids Res. 35 113-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llano, M., Vanegas, M., Hutchins, N., Thompson, D., Delgado, S., and Poeschla, E. M. (2006) J. Mol. Biol. 360 760-773 [DOI] [PubMed] [Google Scholar]
- 6.De Rijck, J., Vandekerckhove, L., Gijsbers, R., Hombrouck, A., Hendrix, J., Vercammen, J., Engelborghs, Y., Christ, F., and Debyser, Z. (2006) J. Virol. 80 11498-11509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shun, M. C., Raghavendra, N. K., Vandegraaff, N., Daigle, J. E., Hughes, S., Kellam, P., Cherepanov, P., and Engelman, A. (2007) Genes Dev. 21 1767-1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llano, M., Saenz, D. T., Meehan, A., Wongthida, P., Peretz, M., Walker, W. H., Teo, W., and Poeschla, E. M. (2006) Science 314 461-464 [DOI] [PubMed] [Google Scholar]
- 9.Hombrouck, A., De Rijck, J., Hendrix, J., Vandekerckhove, L., Voet, A., De Maeyer, M., Witvrouw, M., Engelborghs, Y., Christ, F., Gijsbers, R., and Debyser, Z. (2007) PLoS Pathog. 3 418-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciuffi, A., Llano, M., Poeschla, E., Hoffmann, C., Leipzig, J., Shinn, P., Ecker, J. R., and Bushman, F. (2005) Nat. Med. 11 1287-1289 [DOI] [PubMed] [Google Scholar]
- 11.Marshall, H. M., Ronen, K., Berry, C., Llano, M., Sutherland, H., Saenz, D., Bickmore, W., Poeschla, E., and Bushman, F. D. (2007) PLoS ONE 2 e1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maertens, G., Cherepanov, P., Debyser, Z., Engelborghs, Y., and Engelman, A. (2004) J. Biol. Chem. 279 33421-33429 [DOI] [PubMed] [Google Scholar]
- 13.Cherepanov, P., Ambrosio, A. L., Rahman, S., Ellenberger, T., and Engelman, A. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 17308-17313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganapathy, V., Daniels, T., and Casiano, C. A. (2003) Autoimmun. Rev. 2 290-297 [DOI] [PubMed] [Google Scholar]
- 15.Daniels, T., Zhang, J., Gutierrez, I., Elliot, M. L., Yamada, B., Heeb, M. J., Sheets, S. M., Wu, X., and Casiano, C. A. (2005) Prostate 62 14-26 [DOI] [PubMed] [Google Scholar]
- 16.Daugaard, M., Kirkegaard-Sorensen, T., Ostenfeld, M. S., Aaboe, M., Hoyer-Hansen, M., Orntoft, T. F., Rohde, M., and Jaattela, M. (2007) Cancer Res. 67 2559-2567 [DOI] [PubMed] [Google Scholar]
- 17.Grand, F. H., Koduru, P., Cross, N. C., and Allen, S. L. (2005) Leuk. Res. 29 1469-1472 [DOI] [PubMed] [Google Scholar]
- 18.Hussey, D. J., Moore, S., Nicola, M., and Dobrovic, A. (2001) BMC Genet. 2 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morerio, C., Acquila, M., Rosanda, C., Rapella, A., Tassano, E., Micalizzi, C., and Panarello, C. (2005) Leuk. Res. 29 467-470 [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama, A., and Cleary, M. L. (2008) Cancer Cell 14 36-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholomeeusen, K., De Rijck, J., Busschots, K., Desender, L., Gijsbers, R., Emiliani, S., Benarous, R., Debyser, Z., and Christ, F. (2007) J. Mol. Biol. 372 407-421 [DOI] [PubMed] [Google Scholar]
- 22.Maertens, G. N., Cherepanov, P., and Engelman, A. (2006) J. Cell Sci. 119 2563-2571 [DOI] [PubMed] [Google Scholar]
- 23.Namgoong, S. Y., and Harshey, R. M. (1998) EMBO J. 17 3775-3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyda, F., Hickman, A. B., Jenkins, T. M., Engelman, A., Craigie, R., and Davies, D. R. (1994) Science 266 1981-1986 [DOI] [PubMed] [Google Scholar]
- 25.Allingham, J. S., Pribil, P. A., and Haniford, D. B. (1999) J. Mol. Biol. 289 1195-1206 [DOI] [PubMed] [Google Scholar]
- 26.Fromont-Racine, M., Rain, J. C., and Legrain, P. (2002) Methods Enzymol. 350 513-524 [DOI] [PubMed] [Google Scholar]
- 27.Emiliani, S., Mousnier, A., Busschots, K., Maroun, M., Van Maele, B., Tempe, D., Vandekerckhove, L., Moisant, F., Ben-Slama, L., Witvrouw, M., Christ, F., Rain, J. C., Dargemont, C., Debyser, Z., and Benarous, R. (2005) J. Biol. Chem. 280 25517-25523 [DOI] [PubMed] [Google Scholar]
- 28.Chenna, R., Sugawara, H., Koike, T., Lopez, R., Gibson, T. J., Higgins, D. G., and Thompson, J. D. (2003) Nucleic Acids Res. 31 3497-3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maertens, G., Cherepanov, P., Pluymers, W., Busschots, K., De Clercq, E., Debyser, Z., and Engelborghs, Y. (2003) J. Biol. Chem. 278 33528-33539 [DOI] [PubMed] [Google Scholar]
- 30.Goldgur, Y., Dyda, F., Hickman, A. B., Jenkins, T. M., Craigie, R., and Davies, D. R. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 9150-9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geraerts, M., Michiels, M., Baekelandt, V., Debyser, Z., and Gijsbers, R. (2005) J. Gene Med. 7 1299-1310 [DOI] [PubMed] [Google Scholar]
- 32.Cole, C., Barber, J. D., and Barton, G. J. (2008) Nucleic Acids Res. 36 W187-W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novina, C. D., Murray, M. F., Dykxhoorn, D. M., Beresford, P. J., Riess, J., Lee, S. K., Collman, R. G., Lieberman, J., Shankar, P., and Sharp, P. A. (2002) Nat. Med. 8 681-686 [DOI] [PubMed] [Google Scholar]
- 34.Gunther, M., Laithier, M., and Brison, O. (2000) Mol. Cell. Biochem. 210 131-142 [DOI] [PubMed] [Google Scholar]
- 35.Marchler-Bauer, A., Panchenko, A. R., Shoemaker, B. A., Thiessen, P. A., Geer, L. Y., and Bryant, S. H. (2002) Nucleic Acids Res. 30 281-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plasterk, R. H. (1995) Nat. Struct. Biol. 2 87-90 [DOI] [PubMed] [Google Scholar]
- 37.Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. (1997) Nucleic Acids Res. 25 3389-3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pace, J. K., Jr., and Feschotte, C. (2007) Genome Res. 17 422-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smit, A. F., and Riggs, A. D. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 1443-1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett-Lovsey, R. M., Herbert, A. D., Sternberg, M. J., and Kelley, L. A. (2008) Proteins 70 611-625 [DOI] [PubMed] [Google Scholar]
- 41.Cherepanov, P., Sun, Z. Y., Rahman, S., Maertens, G., Wagner, G., and Engelman, A. (2005) Nat. Struct. Mol. Biol. 12 526-532 [DOI] [PubMed] [Google Scholar]
- 42.Hwang, Y., Rhodes, D., and Bushman, F. (2000) Nucleic Acids Res. 28 4884-4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kipling, D., and Warburton, P. E. (1997) Trends Genet 13 141-145 [DOI] [PubMed] [Google Scholar]
- 44.Volff, J. N. (2006) BioEssays 28 913-922 [DOI] [PubMed] [Google Scholar]
- 45.Busschots, K., Voet, A., De Maeyer, M., Rain, J. C., Emiliani, S., Benarous, R., Desender, L., Debyser, Z., and Christ, F. (2007) J. Mol. Biol. 365 1480-1492 [DOI] [PubMed] [Google Scholar]
- 46.Al-Mawsawi, L. Q., Christ, F., Dayam, R., Debyser, Z., and Neamati, N. (2008) FEBS Lett. 582 1425-1430 [DOI] [PubMed] [Google Scholar]
- 47.Al-Mawsawi, L. Q., and Neamati, N. (2007) Trends Pharmacol. Sci. 28 526-535 [DOI] [PubMed] [Google Scholar]
- 48.Hou, Y., McGuinness, D. E., Prongay, A. J., Feld, B., Ingravallo, P., Ogert, R. A., Lunn, C. A., and Howe, J. A. (2008) J. Biomol. Screen 13 406-414 [DOI] [PubMed] [Google Scholar]
- 49.Capy, P., Langin, T., Higuet, D., Maurer, P., and Bazin, C. (1997) Genetica 100 63-72 [PubMed] [Google Scholar]
- 50.Capy, P., Vitalis, R., Langin, T., Higuet, D., and Bazin, C. (1996) J. Mol. Evol. 42 359-368 [DOI] [PubMed] [Google Scholar]