Abstract
Background
Nursing home residents with advanced dementia are at high risk of infections and antimicrobial exposure near the end of life. Detailed studies quantifying antimicrobial prescribing practices among these residents have not been performed.
Methods
A cohort of 214 residents with advanced dementia from 21 Boston-area nursing homes were followed up prospectively for 18 months or until death. We analyzed antimicrobial use, including type, indication, and quantity, by days of therapy per 1000 resident-days.
Results
During an average of 322 days of follow-up, 142 residents (66.4%) with advanced dementia received at least 1 course of antimicrobial therapy (mean [SD] number of courses per resident, 4.0 [3.7]). The mean (SD) number of days of therapy per 1000 resident-days for the entire cohort was 53.0 (4.3). Quinolones and third-generation cephalosporins were the most commonly prescribed antimicrobials, accounting for 38.3% and 15.2%, respectively, of 540 prescribed antimicrobial therapy courses. A respiratory tract infection was the most common indication (46.7% of all antimicrobial therapy courses). Among 99 decedents, 42 (42.4%) received antimicrobials during the 2 weeks before death, of which 30 of 72 courses (41.7%) were administered via the parenteral route. The number of decedents receiving antimicrobials (P< .001), the number of antimicrobials prescribed (P=.01), and the days of therapy per 1000 resident-days (P< .001) increased significantly as subjects approached death.
Conclusions
Persons with advanced dementia are frequently exposed to antimicrobials, especially during the 2 weeks before death. The implications of this practice from the perspective of the individual treatment burden near the end of life and its contribution to the emergence of antimicrobial resistance in the nursing home setting need further evaluation.
Approximately 70% of the more than 5 million Americans with dementia will reside in nursing homes during the final stage of their disease.1 Recurrent infections and febrile episodes typically occur during this stage.2,3 Therefore, nursing home residents with advanced dementia are at high risk for exposure to antimicrobial agents. However, it remains unclear whether treatment of infections confers any life-prolonging or symptomatic benefit in this population.4,5 At the same time, administration of antimicrobials to frail older patients who are near the end of life is also a potentially burdensome intervention.6
From a broader public health perspective, antimicrobial use is the primary factor leading to the emergence of antimicrobial-resistant bacteria. Antibiotic resistance among bacteria implicated in the most common infections is rising exponentially throughout the world. Infections caused by antimicrobial-resistant bacteria are associated with up to 5 times higher mortality rates and lead to more frequent and prolonged hospitalizations compared with infections caused by antimicrobial-susceptible bacteria.7–9 These issues are particularly relevant for older patients, who harbor relatively high rates of antimicrobial-resistant bacteria, and in nursing homes, where antimicrobials are the most frequently prescribed pharmaceutical agents.10–12
Taken together, the potential for widespread use of antimicrobial agents in advanced dementia raises concerns not only from the perspective of individual benefits and burdens but also from a public health standpoint with respect to the emergence and spread of antimicrobial resistance.
A few earlier reports have described patterns of antimicrobial use in patients with terminal cancer.13–15 However, to the best of our knowledge, these data have not been reported in end-stage dementia. Therefore, the main objective of this study was to better examine how infections in advanced dementia are currently being managed. To achieve this objective, the report describes the quantity, type, and pattern of antimicrobials prescribed and the indication for therapy in a cohort of nursing home residents with advanced dementia living in 21 Boston-area facilities who underwent repeated assessment and prospective follow-up for 18 months or until death. These data are needed as a first step toward providing benchmarks for antimicrobial use in advanced dementia and understanding how this use may contribute to the development and spread of antimicrobial resistance.
METHODS
STUDY POPULATION AND FACILITIES
Nursing home residents with advanced dementia living in 21 Boston-area facilities were recruited from February 1, 2003, until September 30, 2006, as part of the ongoing prospective cohort Choices, Attitudes and Strategies for Care of Advanced Dementia at the End-of-Life (CASCADE) study.16 The overriding goal of the CASCADE study was to describe multiple facets of the end-of-life experience of nursing home residents with advanced dementia and their families. Data available for this report were derived from the first 241 subjects recruited into the study.
Details of the complete CASCADE study protocol are provided elsewhere.16 Briefly, to identify a cohort with advanced dementia, the residents’ most recent Minimum Data Set assessments were used to identify those with a Cognitive Performance Scale score of 5 or 6, indicating severe to very severe cognitive impairment.17 Once identified, the charts of residents with a Cognitive Performance Scale score of 5 or 6 underwent screening for full eligibility, which included (1) age of 60 years or older, (2) length of stay of 30 days or longer, (3) cognitive impairment due to dementia, (4) Global Deterioration Scale score of 7,18 and (5) an appointed health care proxy who could communicate in English. The diagnosis of dementia was confirmed with the resident’s physician if it was ambiguous in the record. At a Global Deterioration Scale score of 7, patients with dementia are characterized by very severe cognitive decline, minimal to no verbal communication, dependence for eating and toileting, incontinence of urine and stool, and loss of the ability to walk.18 Residents were excluded if they were in a subacute or short-term rehabilitative unit; had cognitive impairment due to stroke, traumatic brain injury, tumor, or a chronic psychiatric condition; or were in a coma. Finally, participant facilities were required to have at least 60 beds and be located within a 60-mile radius of Boston.
DATA COLLECTION AND RESIDENT ASSESSMENTS
Data presented in this report were abstracted from the subjects’ medical records and a brief mental status examination. Assessments were conducted at baseline and quarterly thereafter for a maximum of 18 months. If the resident died during the follow-up period, an assessment was obtained within 14 days of death.
The subjects’ age, sex, and race (white vs other) were obtained at the baseline assessment. Additional descriptive variables collected at baseline included whether the subjects lived in a special-care dementia unit, their length of nursing home stay (in days), and the cause of their dementia (Alzheimer disease, vascular, or other). Cognitive disability was determined by direct clinical examination of the resident using the Test for Severe Impairment (range of 0–24, with lower scores indicating greater cognitive impairment).19
ANTIMICROBIAL USE
At each quarterly and death assessment, the number of courses of antibiotic therapy prescribed for the resident since the prior assessment was obtained from the medicine administration records. The following details were determined for each course: the name of the antibiotic, start and stop dates of therapy, route of administration, and indication. Indication was categorized according to the suspected diagnosis documented by a physician as 1 of the following infections: respiratory tract; skin; urogenital; ear, nose, or throat; gastrointestinal tract; and others (ie, prophylaxis for pacemaker placement and dental procedures). The days of therapy (DOT) value was defined as the total number of days that a single antimicrobial (parenteral or oral) was administered, regardless of dosage.20 The DOT was further specified per 1000 resident-days of observation.
To describe the pattern of antimicrobial use specifically among the subjects who died, the period before death was categorized by 14-day intervals, a common duration for a course of antimicrobial therapy.
STATISTICAL ANALYSIS
We used descriptive statistics to present the subject characteristics and patterns of antimicrobial use, with means (SDs) for continuous variables and proportions for categorical variables. Among the decedents, the Spearman correlation coefficient was used to analyze the association between the total number of antimicrobial therapy courses prescribed per resident and the number of days before death. Similarly, we used the Spearman rank correlation coefficient to examine the association between the proportion of subjects who received antimicrobials and the number of days before death. The association between the antimicrobial indication and the timing of administration (ie, 2-week intervals) before death was analyzed using the χ2 test for linear trend. We used Stata statistical software (release 7.0; Stata Corp, College Station, Texas) for all statistical analyses.
RESULTS
STUDY POPULATION
A total of 240 subjects with advanced dementia were recruited into the study at the time of this report. The study is ongoing, and 26 subjects were excluded from these analyses because only a single baseline evaluation was completed and no follow-up data regarding antibiotic use were available. Among the remaining 214 subjects included in the analyses, 99 (46.3%) died during the observation period.
The mean age of the subjects was 85.2 (7.9) years; 184 (86.0%) were female; and 189 (88.3%) were white (Table 1). The causes of the subjects’ dementia were as follows: Alzheimer disease (149 subjects [69.6%]), vascular (39 [18.2%]), and other (30 [14.0%]) (4 subjects had more than 1 cause). Among the cohort, 100 subjects (46.7%) resided in special-care dementia units, and the median length of stay was 41.2 months (Table 1). Subjects were severely cognitively impaired, with 162 (75.7%) scoring 0 on the Test for Severe Impairment. The entire cohort of 214 subjects contributed a total of 68 861 follow-up days (mean, 322 [191]; range, 11–603 days) to the analyses.
Table 1.
Baseline Characteristics of 214 Nursing Home Residents With Advanced Dementia
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 85.2 (7.9) |
| Female | 184 (86.0) |
| White | 189 (88.3) |
| Cause of dementia | |
| Alzheimer | 149 (69.6) |
| Vascular | 39 (18.2) |
| Other | 30 (14.0) |
| Special-care dementia units | |
| Reside in unit | 100 (46.7) |
| Median length of stay, mo | 88 (41.2) |
| Test for Severe Impairment score of 0 | 162 (75.7) |
ANTIMICROBIAL USE AMONG ALL SUBJECTS
Table 2 presents the use of antimicrobials in the entire cohort (N=214). During the follow-up period, 142 of the 214 subjects (66.4%) received at least 1 dose of antimicrobials. A total of 540 courses were prescribed (mean number of courses per subject who received antimicrobials, 4.0 [3.7]; range, 1–20). The mean DOT per 1000 resident-days for all antimicrobials was 53.0 (4.3). The areas of infection for which the 540 courses of antimicrobial therapy were prescribed were as follows: respiratory tract (252 infections [46.7%]); urinary tract (192 [35.6%]); skin (71 [13.1%]); ear, nose, or throat (12 [2.2%]); gastrointestinal tract (8 [1.5%]); and other (5 [0.9%]).
Table 2.
Antimicrobial Use Among 214 Nursing Home Residents With Advanced Dementia (N=214)
| Antimicrobial Group | No. (%) of Subjects Receiving at Least 1 Antimicrobial Therapy Course | No. (%) of Antimicrobial Therapy Courses | No. of Courses per Subject, Mean (SD) | DOT per 1000 Resident-Days, Meana |
|---|---|---|---|---|
| Any antimicrobial | 142 (66.4) | 540 (100.0) | 4.0 (3.7) | 53.0 (4.3) |
| Quinolones | 96 (44.9) | 207 (38.3) | 2.2 (1.8) | 16.9 |
| Third-generation cephalosporins | 47 (22.0) | 82 (15.2) | 1.7 (1.4) | 4.6 |
| First-generation cephalosporins | 39 (18.2) | 61 (11.3) | 1.6 (0.7) | 6.3 |
| Penicillins | 27 (12.6) | 44 (8.1) | 1.6 (1.0) | 4.6 |
| Macrolides | 25 (11.7) | 30 (5.6) | 1.2 (0.5) | 2.7 |
| Nitrofurantoin | 16 (7.5) | 25 (4.6) | 1.6 (1.2) | 5.6 |
| Trimethoprim-sulfamethoxazole | 15 (7.0) | 25 (4.6) | 1.7 (1.3) | 6.0 |
| Metronidazole | 15 (7.0) | 24 (4.4) | 1.6 (1.1) | 2.5 |
| Tetracyclines | 10 (4.7) | 14 (2.6) | 1.4 (0.5) | 1.2 |
| Vancomycin hydrochloride | 6 (2.8) | 8 (1.5) | 1.3 (0.8) | 0.9 |
| Second-generation cephalosporins | 6 (2.8) | 8 (1.5) | 1.3 (0.5) | 0.9 |
| Clindamycin | 4 (1.9) | 4 (0.7) | 1.3 (0.5) | 0.3 |
| Gentamicin sulfate | 2 (0.9) | 2 (0.4) | 1.0 (0.0) | 0.2 |
Abbreviation: DOT, days of therapy.
The standard deviation could be generated for “any antimicrobial” only, not for individual antimicrobials.
Quinolones were the most commonly prescribed agent, accounting for 207 of all antimicrobial therapy courses (38.3%), with 96 subjects (44.9%) receiving at least 1 course of quinolone therapy. The DOT per 1000 resident-days for quinolones was 16.9. Among all quinolones, levofloxacin (171 courses [82.6%]) was the most commonly prescribed antimicrobial, followed by ciprofloxacin (33 [16.0%]) and gatifloxacin (3 [1.4%]). Third-generation cephalosporins were the second most common antimicrobials prescribed, accounting for 82 antimicrobial therapy courses (15.2%), with 47 residents (22.0%) receiving at least 1 dose. The DOT per 1000 resident-days for third-generation cephalosporins was 4.6. Ceftriaxone was the most commonly prescribed third-generation cephalosporin, accounting for 80 of 82 therapy courses (97.6%). The third most common antimicrobials were first-generation cephalosporins, accounting for 61antimicrobial therapy courses (11.3%), with 39 residents (18.2%) receiving at least 1 course. The DOT per 1000 resident-days for first-generation cephalosporins was 6.3. Cephalexin, cefazolin, and cefadroxil accounted for 53 (86.9%), 6 (9.8%), and 2 (3.3%) of all 61 first-generation cephalosporin therapy courses, respectively.
ANTIMICROBIAL USE AMONG DECEDENTS BEFORE DEATH
Among the 99 subjects who died, those available for analysis during the 14-day intervals before death included 99 at 0 to 14 days, 94 at 15 to 28 days, 88 at 29 to 42 days, and 83 at 43 to 56 days.
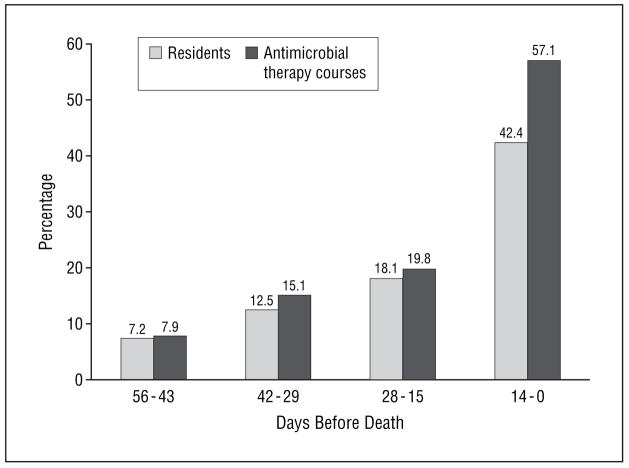
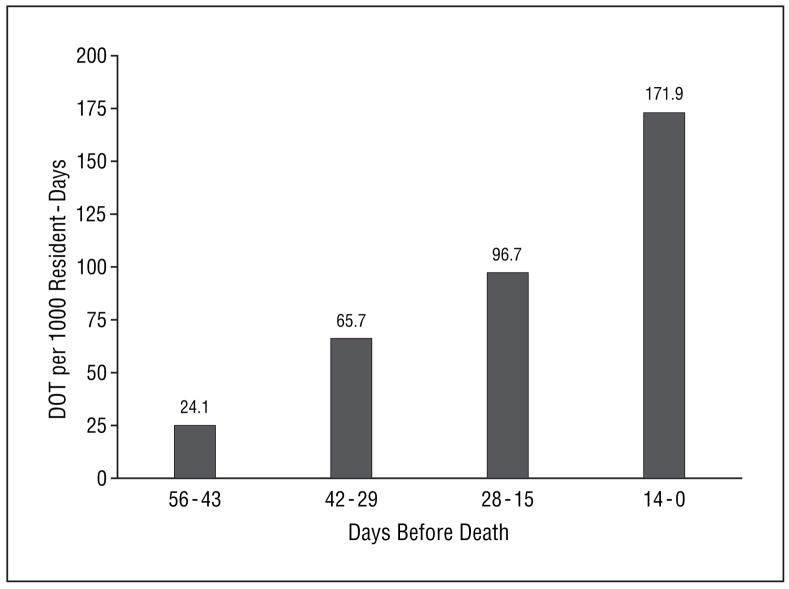
A total of 52 decedents (51.5%) received at least 1 dose of antimicrobials within the 8 weeks before death. As subjects approached death, the proportion of subjects receiving at least 1 antimicrobial (P<.001), the number of antimicrobial therapy courses per resident prescribed (P=.01), and the DOT per 1000 resident-days (P<.001) all increased significantly (Figure 1 and Figure 2). The proportions of antimicrobial therapy courses administered parenterally (total number of residents receiving antimicrobials parenterally/total number of residents receiving antimicrobials, interval before death) were as follows: 20.0% (2/10, 43–56 days), 26.3% (5/19, 29–42 days), 28.0% (7/25, 15–28 days), and 41.7% (30/72, 0–14 days) (P=.07). The 3 most commonly prescribed antimicrobials during the 8 weeks before death were quinolones, third-generation cephalosporins, and first-generation cephalosporins (Table 3). The number of antimicrobial therapy courses, the number of residents receiving at least 1 antimicrobial agent, and the DOT per 1000 resident-days increased for all other individual antimicrobial groups as the subjects approached death (data not shown).
Figure 1.
Percentages of nursing home residents with advanced dementia receiving antimicrobials and of antimicrobial therapy courses, among a total of 126 courses prescribed, during the last 8 weeks of life. The total number of residents and antimicrobial courses during each 14-day interval before death were as follows: 99 and 10 at 0 to 14 days; 94 and 19 at 15 to 28 days; 88 and 25 at 29 to 42 days; and 83 and 72 at 43 to 56 days, respectively.
Figure 2.
Days of therapy (DOT) per 1000 resident-days for any antimicrobial received by nursing home residents with advanced dementia during the last 8 weeks of life. The total number of subjects at each 14-day interval before death was as follows: 99 at 0 to 14 days; 94 at 15 to 28 days; 88 at 29 to 42 days; and 83 at 43 to 56 days.
Table 3.
Antimicrobial Exposure, Therapy Courses, and DOT per 1000 Resident-Days for the 3 Most Commonly Prescribed Antimicrobials Among Nursing Home Residents With Advanced Dementia During the 8 Weeks Before Death
| Days Before Death |
||||
|---|---|---|---|---|
| Antimicrobials | 0–14 (n=99) | 15–28 (n=94) | 29–43 (n=88) | 43–56 (n=83) |
| Quinolones | ||||
| No. (%) of residents | 23 (23.2) | 10 (10.6) | 3 (3.4) | 3 (3.6) |
| Total No. of courses | 27 | 11 | 3 | 3 |
| DOT per 1000 resident-days | 62.4 | 37.8 | 16.2 | 6.0 |
| Third-generation cephalosporins | ||||
| No. (%) of residents | 18 (18.2) | 5 (5.3) | 3 (3.4) | 1 (1.2) |
| Total No. of courses | 24 | 5 | 6 | 1 |
| DOT per 1000 resident-days | 36.3 | 14.4 | 21.1 | 0.9 |
| First-generation cephalosporins | ||||
| No. (%) of residents | 7 (7.1) | 4 (4.3) | 3 (3.4) | 2 (2.4) |
| Total No. of courses | 7 | 4 | 3 | 2 |
| DOT per 1000 resident-days | 21.0 | 18.1 | 9.7 | 5.2 |
Abbreviation: DOT, days of therapy.
Among the 126 courses of antimicrobial therapy prescribed for all 99 decedents during the 8 weeks before death, the sites of infection prompting treatment were as follows: respiratory tract (80 courses [63.5%]), urogenital (22 [17.5%]), skin (21 [16.7%]), and gastrointestinal tract (3 [2.4%]). There was a statistically significant increase in the diagnosis of respiratory tract illness as the indication for antimicrobial treatment as the decedents approached death (P=.01) (Table 4).
Table 4.
Indications for Administration of 126 Courses of Antimicrobial Therapy Among Nursing Home Residents With Advanced Dementia During the 8 Weeks Before Death
| No. (%) of Decedents by Days Before Death |
||||
|---|---|---|---|---|
| Site of Infection | 0–14 (n=99) | 15–28 (n=94) | 29–42 (n=88) | 43–56 (n=83) |
| Respiratory tract | 56 (77.8) | 13 (52.0) | 8 (42.1) | 3 (30.0) |
| Urogenital | 8 (11.1) | 5 (20.0) | 6 (31.6) | 3 (30.0) |
| Skin | 6 (8.3) | 6 (24.0) | 5 (26.3) | 4 (40.0) |
| Gastrointestinal tract | 2 (2.8) | 1 (4.0) | 0 | 0 |
| All Sites | 72 | 25 | 19 | 10 |
COMMENT
This prospective cohort study demonstrates that antimicrobial exposure among nursing home residents with advanced dementia is extensive and steadily increases toward the end of life. During the follow-up period (mean follow-up, 322 days), two-thirds of the subjects were prescribed at least 1 course of antimicrobial therapy and, on average, a total of 4 courses. Among the residents who died, 42.4% received antimicrobials during the last 2 weeks of life, often via a parenteral route. The proportion of residents taking antimicrobials was 7 times greater in the last 2 weeks of life compared with 6 to 8 weeks before death. This extensive use of antimicrobials and pattern of antimicrobial management in advanced dementia raises concerns not only with respect to individual treatment burden near the end of life but also with respect to the development and spread of antimicrobial resistance in the nursing home setting.
To our knowledge, this is the first comprehensive, prospective study to describe the quantity, type, and pattern of antimicrobials prescribed and the indication for therapy among patients with advanced dementia in the long-term care setting. Earlier studies that focused on the treatment of specific infections (ie, pneumonia) in advanced dementia were retrospective or cross-sectional in design, examined hospitalized patients, or studied only a single institution.2–5,21,22 Despite these differences, our study corroborates that antimicrobial agents are commonly prescribed in advanced dementia and extends these findings by demonstrating a marked increase in antimicrobial use as death becomes imminent. Terminally ill patients with cancer in palliative care settings also frequently receive antimicrobial therapy.13,14 White et al13 reported that approximately 30% of hospice recipients with advanced cancer are prescribed antibiotics. Although a urinary tract infection was the most frequent indication for treatment among patients with cancer who were dying, a respiratory tract infection was the most common diagnosis in our cohort with end-stage dementia.
Treatment decisions for infections in advanced dementia can be difficult for family members and care-givers. The 2 purported reasons to administer antimicrobials are life prolongation and symptom control. Limited observational studies4,5,22 have failed to demonstrate that antimicrobial treatment achieves either outcome in this frail population; however, randomized trials have not been conducted. Our findings further support that antimicrobials may not meaningfully extend the life of patients with advanced dementia for whom infections are frequently a terminal event. Palliation is often the main goal of care in this condition.23 It is difficult to assess the extent to which infections cause suffering in patients with advanced dementia. Previous work demonstrates that pneumonia is an uncomfortable experience for these patients and suggests that antimicrobial therapy may improve symptoms.5 However, it remains unclear whether antimicrobial therapy promotes symptomatic relief beyond what can be achieved by high-quality palliative treatment with more conservative modalities (eg, oxygen and acetaminophen). Finally, it is also important to minimize inappropriate antimicrobial exposure. For example, up to one-third of antimicrobials prescribed in nursing homes are for asymptomatic bacteriuria, for which treatment is not indicated.24
Antimicrobial administration has associated risks in the frail elderly population that merit consideration. Older persons are particularly susceptible to the adverse effects of antimicrobials owing to altered pharmacokinetics, polypharmacy, dosing errors, and an increased risk of Clostridium difficile infections.25–27 Moreover, parenteral administration, which was common in our cohort, can be an uncomfortable procedure in advanced dementia.6 Thus, from the individual patient’s perspective, the balance of advantages and disadvantages of antimicrobial treatment of infections in advanced dementia remains unclear, regardless of the primary goal of care.
On a broader level, the emergence and spread of antimicrobial-resistant bacteria is a major public health concern. Older persons account for one of the largest patient reservoirs of these organisms.10,28 In particular, up to 40% of residents living in nursing homes harbor at least 1 species of antimicrobial-resistant bacteria.29–31 Once admitted to the hospital, these nursing home residents contribute substantially to the influx and spread of antibiotic-resistant bacteria.28,29 Exposure to antibiotics is strongly associated with the development of antibiotic resistance. Quinolones and third-generation cephalosporins were the most frequently prescribed antimicrobials in our cohort. Several studies have reported that more than 50% of isolates recovered from nursing home residents are resistant to these 2 classes of drugs.31–33 These observations and the extensive use of antibiotics found in this study raise the serious concern that nursing home residents with advanced dementia may be contributing to the emergence and spread of antimicrobial-resistant bacteria, posing health risks that extend beyond the individual being treated.
Future initiatives aimed at optimizing antimicrobial use will require standardized units of measurement. In this study, a DOT value was used to quantify antimicrobial utilization. The DOT quantifies the mean duration of therapy adjusted for the total time that the population was observed and does not take into consideration the actual dose administered. The DOT value was developed as an alternative to the defined daily dose measure.20,34 The daily defined dose assumes that all patients receive standard antimicrobial doses, and therefore does not take into consideration unique patient characteristics that require dosage adjustments. This limitation is especially relevant to elderly patients, who often require alternative dosing to adjust for low body mass and renal insufficiency.25 Measures of antimicrobial DOT have not been previously reported for patients with advanced dementia. Thus, the DOT values found in this study provide a benchmark for future research in this area and for comparing prescribing practices between institutions.
This study has limitations that warrant comment. First, this prospective cohort study did not include a comparison group. Therefore, we do not know whether the pattern of antimicrobial use observed in our subjects with advanced dementia differs from that of other long-term care residents. Second, data were not collected to determine the presence of antimicrobial-resistant bacteria. Thus, although our study raises concerns that nursing home residents with advanced dementia are reservoirs of antimicrobial-resistant organisms, this supposition remains to be proved. Finally, this observational study was not designed to study the outcomes of antimicrobial therapy in advanced dementia, despite the fact that many subjects died shortly after receiving treatment.
Infections and febrile episodes are a hallmark of end-stage dementia. The extensive antimicrobial use demonstrated in this study is concerning given the lack of demonstrable benefits and the potential burdens of treatment in this terminally ill population for whom the goal of care is often palliation.23 Moreover, we believe that the widespread use of antibiotics in advanced dementia may pose a potential public health risk through the emergence of antibiotic resistance. This hypothesis requires further research. Meanwhile, from individual and societal perspectives, our study supports the development of programs and guidelines designed to reduce the use of antimicrobial agents in advanced dementia.
Acknowledgments
Funding/Support: This study was supported by grant R01 AG024091 from the National Institute on Aging, National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Study concept and design: D’Agata and Mitchell. Acquisition of data: Mitchell. Analysis and interpretation of data: D’Agata and Mitchell. Drafting of the manuscript: D’Agata and Mitchell. Critical revision of the manuscript for important intellectual content: D’Agata and Mitchell. Statistical analysis: D’Agata and Mitchell. Obtained funding: Mitchell. Administrative, technical, and material support: Mitchell.
References
- 1.Mitchell SL, Teno JM, Miller SC, Mor V. A national study of the location of death for older persons with dementia. J Am Geriatr Soc. 2005;53(2):299–305. doi: 10.1111/j.1532-5415.2005.53118.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen JH, Lamberg JL, Chen Y, et al. Occurrence and treatment of suspected pneumonia in long-term care residents dying with advanced dementia. J Am Geriatr Soc. 2006;54(2):290–295. doi: 10.1111/j.1532-5415.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 3.van der Steen JT, Kruse RL, Ooms ME, et al. Treatment of nursing home residents with dementia and lower respiratory tract infection in the United States and the Netherlands: an ocean apart. J Am Geriatr Soc. 2004;52(5):691–699. doi: 10.1111/j.1532-5415.2004.52204.x. [DOI] [PubMed] [Google Scholar]
- 4.Fabiszewski KJ, Volicer B, Volicer L. Effect of antibiotic treatment on outcome of fevers in institutionalized Alzheimer patients. JAMA. 1990;263(23):3168–3172. [PubMed] [Google Scholar]
- 5.van der Steen JT, Ooms ME, van der Wal G, Ribbe MW. Pneumonia: the demented patient’s best friend? discomfort after starting or withholding antibiotic treatment. J Am Geriatr Soc. 2002;50(10):1681–1688. doi: 10.1046/j.1532-5415.2002.50460.x. [DOI] [PubMed] [Google Scholar]
- 6.Morrison RS, Ahronheim JC, Morrison GR, et al. Pain and discomfort associated with common hospital procedures and experiences. J Pain Symptom Manage. 1998;15(2):91–101. [PubMed] [Google Scholar]
- 7.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove SE, Kaye KS, Eliopoulous GM, Carmeli Y. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med. 2002;162(2):185–190. doi: 10.1001/archinte.162.2.185. [DOI] [PubMed] [Google Scholar]
- 9.Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2002;162(19):2223–2228. doi: 10.1001/archinte.162.19.2223. [DOI] [PubMed] [Google Scholar]
- 10.Flamm RK, Weaver MK, Thornsberry C, Jones ME, Karlowsky JA, Sahm DF. Factors associated with relative rates of antibiotic resistance in Pseudomonas aeruginosa isolates tested in clinical laboratories in the United States from 1999 to 2002. Antimicrob Agents Chemother. 2004;48(7):2431–2436. doi: 10.1128/AAC.48.7.2431-2436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren JW, Palumbo FB, Fiserman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc. 1991;39(10):963–972. doi: 10.1111/j.1532-5415.1991.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 12.Crossley K, Henry K, Irvine P, Willenbring K. Antibiotic use in nursing homes: prevalence, cost, and utilization review. Bull N Y Acad Med. 1987;63(6):510–518. [PMC free article] [PubMed] [Google Scholar]
- 13.White PH, Kuhlenschmidt HL, Vancura BG, Navari RM. Antimicrobial use in patients with advanced cancer receiving hospice care. J Pain Symptom Manage. 2003;25(5):438–443. doi: 10.1016/s0885-3924(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 14.Pereira J, Watanabe S, Wolch G. A retrospective review of the frequency of infections and patterns of antibiotic utilization on a palliative care unit. J Pain Symptom Manage. 1998;16(6):374–381. doi: 10.1016/s0885-3924(98)00093-1. [DOI] [PubMed] [Google Scholar]
- 15.Oneschuk D, Fainsinger R, Demoissac D. Antibiotic use in the last week of life in three different palliative care settings. J Palliat Care. 2002;18(1):25–28. [PubMed] [Google Scholar]
- 16.Mitchell SL, Kiely DK, Jones RN, Prigerson H, Volicer L, Teno JM. Advanced dementia research in the nursing home: the CASCADE Study. Alzheimer Dis Assoc Disord. 2006;20(3):166–175. doi: 10.1097/00002093-200607000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 18.Reisberg B, Ferris SH, De Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 19.Albert M, Cohen C. The Test for Severe Impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40(5):449–453. doi: 10.1111/j.1532-5415.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 20.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44(5):664–670. doi: 10.1086/511640. [DOI] [PubMed] [Google Scholar]
- 21.van der Steen JT, Ooms ME, Ader HJ, Ribbe MW, van der Wal G. Withholding antibiotic treatment in pneumonia patients with dementia. Arch Intern Med. 2002;162(15):1753–1760. doi: 10.1001/archinte.162.15.1753. [DOI] [PubMed] [Google Scholar]
- 22.Morrison RS, Siu AL. Survival in end-stage dementia following acute illness. JAMA. 2000;284(1):47–52. doi: 10.1001/jama.284.1.47. [DOI] [PubMed] [Google Scholar]
- 23.Luchins DJ, Hanrahan P. What is appropriate health care for end-stage dementia? J Am Geriatr Soc. 1993;41(1):25–30. doi: 10.1111/j.1532-5415.1993.tb05943.x. [DOI] [PubMed] [Google Scholar]
- 24.Loeb M, Simor A, Landry L, et al. Antibiotic use in facilities that provide chronic care. J Gen Intern Med. 2001;16(6):376–383. doi: 10.1046/j.1525-1497.2001.016006376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faulkner CM, Cox HL, Williamson JC. Unique aspects of antimicrobial use in older adults. Clin Infect Dis. 2005;40(7):997–1004. doi: 10.1086/428125. [DOI] [PubMed] [Google Scholar]
- 26.Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161(13):1629–1634. doi: 10.1001/archinte.161.13.1629. [DOI] [PubMed] [Google Scholar]
- 27.Kyne L, Merry C, O’Connell B, Kelly A, Keane C, O’Neill D. Factors associated with prolonged symptom and severe diseases due to Clostridium difficile. Age Ageing. 1999;28(2):107–113. doi: 10.1093/ageing/28.2.107. [DOI] [PubMed] [Google Scholar]
- 28.Pop-Vicas AE, D’Agata EMC. The rising influx of multi-drug resistant gram-negative bacteria into a tertiary care hospital. Clin Infect Dis. 2005;40(12):1792–1798. doi: 10.1086/430314. [DOI] [PubMed] [Google Scholar]
- 29.Wiener J, Quinn JP, Bradford PA, et al. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA. 1999;281(6):517–523. doi: 10.1001/jama.281.6.517. [DOI] [PubMed] [Google Scholar]
- 30.Trick WE, Weinstein RA, DeMarais PL, et al. Colonization of skilled-care facility residents with antimicrobial-resistant pathogens. J Am Geriatr Soc. 2001;49(3):270–276. doi: 10.1046/j.1532-5415.2001.4930270.x. [DOI] [PubMed] [Google Scholar]
- 31.Viray M, Linkin D, Maslow JN, et al. Longitudinal trends in antimicrobial susceptibilities across long-term-care facilities: emergence of fluoroquinolone resistance. Infect Control Hosp Epidemiol. 2005;26(1):56–62. doi: 10.1086/502487. [DOI] [PubMed] [Google Scholar]
- 32.Muder RR, Brennen C, Drenning SD, Stout JE, Wagener MM. Multiply antibiotic-resistant gram-negative bacilli in a long-term-care facility: a case-control study of patient risk factors and prior antibiotic use. Infect Control Hosp Epidemiol. 1997;18(12):809–813. [PubMed] [Google Scholar]
- 33.Maslow JN, Lautenbach E, Glaze T, Bilker W, Johnson J. Colonization with extraintestinal pathogenic Escherichia coli among nursing home residents and its relationship to fluoroquinolone resistance. Antimicrob Agents Chemother. 2004;48(9):3618–3620. doi: 10.1128/AAC.48.9.3618-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zagorski BM, Trick WE, Schwartz DN, et al. The effect of renal dysfunction on antimicrobial use measurements. Clin Infect Dis. 2002;35(12):1491–1497. doi: 10.1086/344753. [DOI] [PubMed] [Google Scholar]