Summary
Hereditary medullary thyroid carcinoma (MTC) is caused by specific autosomal dominant gain-of function mutations in the RET proto-oncogene. Genotype-phenotype correlations exist that help predict the presence of other associated endocrine neoplasms as well as the timing of thyroid cancer development. MTC represents a promising model for targeted cancer therapy, as the oncogenic event responsible for initiating malignancy has been well characterized. The RET proto-oncogene has become the rational target for molecularly designed drug therapy. Tyrosine kinase inhibitors targeting activated RET are currently in clinical trials for the treatment of patients with MTC. This review will provide a brief overview of MTC and the associated RET oncogenic mutations, as well as a summary of the therapies designed to strategically interfere with pathologic activation of the RET oncogene.
Keywords: Multiple endocrine neoplasia, tyrosine kinase inhibitors, thyroid cancer, oncogenes, clinical trial
Introductory overview of the disease: epidemiology and pathophysiology
Thyroid cancer represents approximately 1% of malignancies occurring in the United States, accounting for an estimated 33,550 cancer diagnoses and 1,530 cancer deaths per year. Of these cancers, 2% to 3% are due to medullary thyroid cancer (MTC) [1]. MTC is a rare calcitonin-producing tumor that arises from the thyroid gland parafollicular C cells, which are derived from the embryonic neural crest. While the majority of patients with MTC have sporadic disease, 25% to 30% of cases are due to hereditary forms of MTC [2]. The presentation of disease in hereditary MTC is usually bilateral and multicentric, compared with a single, unilateral thyroid tumor found in sporadic cases [3].
Hereditary MTC is classified according to three distinct clinical subtypes (Table 1). The most common of these subtypes MEN2A, accounts for 70% to 80% of individuals with hereditary MTC. MEN2A is characterized by MTC, pheochromocytoma, and primary hyperparathyroidism [4]. Two rare variants of MEN2A have been identified, one with Hirshsprung’s disease and the other with cutaneous lichen amyloidosis [2]. MTC is frequently the first neoplastic manifestation in MEN2A patients, with MTC occurring as early as in the first 5 years of life. In families without an established diagnosis of MEN2A, patients typically present with a neck mass between the ages of 15 and 20 years [2]. The second inherited subtype of MTC, MEN2B, accounts for only 5% of hereditary MTC cases. MEN 2B is characterized by clinically aggressive MTC, pheochromocytoma, a Marfanoid body habitus, mucosal (and other) neuromas, and intestinal tumors (mostly ganglioneuromas); these patients typically do not manifest hyperparathyroidism [4]. The third inherited subtype of MTC is familial MTC (FMTC). This subtype accounts for 10% to 20% of hereditary MTC cases; only the thyroid gland is affected typically in these patients, although rare large families with both MEN2A and FMTC also exist. In general, FMTC patients present later with MTC than those with MEN2A or MEN2B, usually between 20 and 40 years of age [5].
Table 1.
Inherited subtypes of medullary thyroid cancer
| Syndrome | Characteristic Features |
|---|---|
| MEN 2A | Medullary thyroid cancer Pheochromocytoma Primary hyperparathyroidism (may include Hirshsprung’s disease and cutaneous lichen amyloidosis) |
| MEN 2B | Medullary t hyroid cancer Pheochromocytoma Marfanoud habitus Intestinal and mucosal ganglioneuromatosis |
| FMTC | Medullary thyroid cancer |
MTC is the most common cause of death in patients with MEN2A, MEN2B, and FMTC [6]. Carriers of all variants of inherited MTC have a high penetrance for developing thyroid cancer: 90% of carriers of such predisposition eventually are diagnosed with MTC and present with a palpable thyroid nodule or elevation of calcitonin levels [7].
Summary of current optimal therapeutic practices
MTC is relatively unresponsive to radiation therapy and to standard chemotherapeutic regimens [8,9]. Various chemotherapeutic regimens, including alkylating agents, antimetabolites, and anthracyclines have shown no proven benefit in patients with metastatic MTC [10–12]. Radiation therapy has only a palliative role in the treatment of MTC [13]. Surgery remains the only standard treatment for patients with MTC: total thyroidectomy that is performed before MTC grows or spreads beyond the gland is currently the only curative therapy.
Once MTC metastasizes, it has a tendency to spread to local and regional lymph nodes, and more distantly to lung, liver, and bone [8]. Although MTC is often widely metastatic, it tends to be a slow growing tumor. Patients with metastatic disease continue to have 5 and 10 year survival rates of 80% and 70%, respectively [14]. However, and despite its slow growth, the average survival for MTC is lower than that for other more common types of thyroid cancer, such as papillary and follicular carcinoma which have a 90% to 94% 5-year survival rate, respectively [15]. Decreased survival in MTC can be correlated with the stage of diagnosis. Survival also varies by the extent of local disease, with 10-year survival rates as high as 95% for patients with disease confined to the thyroid gland, and rates as low as 40% for those with distant metastases [16].
In the early 1990’s, mutations in the RET proto-oncogene were found to cause MEN 2A, MEN 2B, and FMTC [17–19]. Following this discovery, it became possible to identify relatives of patients with these syndromes who have inherited a mutated RET allele and in whom MTC is almost certain to develop. Because MTC is not curable once it metastasizes beyond the thyroid, it is now recommended that young members of kindreds with MEN 2A and 2B or FMTC have genetic screening performed to determine if they are carriers of a RET mutation [4]. Current practice recommends performing prophylactic thyroidectomy prior to the development of MTC in at-risk patients [6,20]. Specific genotype-phenotype correlations have been established that associate the clinical aggressiveness of MTC with the mutated codon of the RET protein [4]. Therefore, the timing and extent of prophylactic surgery is dictated by germline analysis of mutations [21]. For those patients identified by screening to have inherited a mutated RET allele who go on to have prophylactic thyroidectomy, 5 and 10 year survival rates approach 100% [6,8,14]. In patients with sporadic MTC, however, genetic screening has rarely been applied; these patients often present with metastatic disease [8,14].
Structure and function of RET; mutations and recommendations
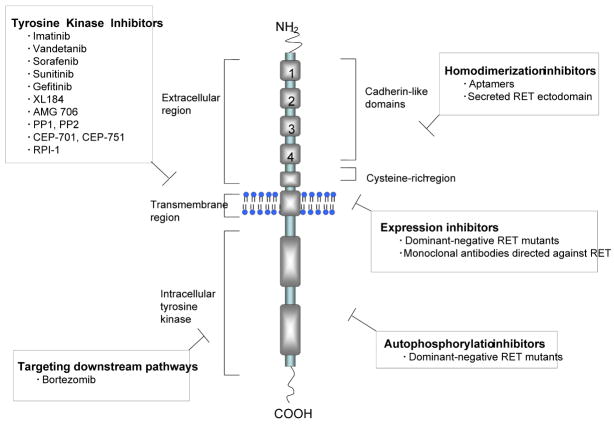
The RET proto-oncogene is located on chromosome 10q11.2 and includes 21 exons. RET (REarranged during Transfection) was first identified and by Takahashi et al. in 1985 as a proto-oncogene able to undergo activation after a genetic rearrangement [22]. RET encodes a receptor tyrosine kinase with key roles in cell growth, differentiation, and survival; it is a transmembrane receptor that consists of three functional domains, an extracellular domain with four cadherin-like repeats, a cysteine-rich region, and an intracellular tyrosine kinase region (Figure 1). Following ligand binding to the extracellular region, receptor dimerization is mediated by activation of the cysteine-rich region that lies adjacent to the plasma membrane [23]. Receptor dimerization leads to autophosphorylation of intracellular tyrosine residues, which subsequently activate downstream pathways of signal transduction [23]. Ligands for RET include those in the glial cell-line derived neurotrophic factor (GDNF) family, including persepherin, artemin, and neurturin [24].
Figure 1. Structure of the RET tyrosine kinase receptor and strategies for targeting RET activation.
The RET receptor tyrosine kinase consists of three functional domains: an extracellular domain with four cadherin-like repeats, a cysteine rich region, and an intracellular tyrosine kinase region. Various strategies for targeting RET activation include tyrosine kinase inhibitors, targeting downstream pathways, homodimerization inhibitors, expression inhibitors, and autophosphorylation inhibitors.
RET signals through multiple downstream pathways. One such pathway, the RAS/MEK/ERK pathway promotes cell cycle progression; another downstream pathway, P13K/AKT/NF-κB, leads to increased cell motility, survival, and progression through the cell cycle [25]. RET activation also stimulates p38, MAPK, JAK/STAT, and protein kinase C [24,26]. RET is expressed widely in neural-crest derived tissues such as noradrenergic and dopamanergic neurons and neuroendocrine tissues including thyroid C cells and the adrenal medulla [27]. RET is also involved in the development of the enteric nervous system and the kidney [28].
The role of the RET oncogene in the development of MTC has been well characterized. Activating RET germline mutations have been identified as the primary cause of all the hereditary MTC syndromes: approximately a quarter to a third of all sporadic MTC cases and up to 98% of MEN 2 cases have a germline RET mutation leading to constitutive activation of the RET receptor; somatic RET mutations account for another quarter to half of all sporadic MTCs [29].
The specific site of the particular mutated residue within the RET protein has been correlated to phenotypic differences among patients with inherited MTC. For example, patients with MEN2A characteristically have missense mutations in exon 10 (codons 609, 610, 611, 618, 620) and exon 11 (codon 634) [4]. These mutations affect one of the six cysteine residues present in the RET extracellular domain [30]. Mutations in these cysteine residues lead to receptor homodimerization via the formation of disulfide bonds, rendering the receptor activated regardless of the presence of ligand. In the case of MEN2B, more than 95 percent of patients have a mutation at exon 16 (codon 918), in the tyrosine kinase domain of the protein. This mutation renders the receptor activated in its monomeric state, and leads to increased phosphorylation of intracellular tyrosine residues [31,32]. Patients with FMTC harbor mutations in exons 10, 11, 13 (codon 768), and 14 (codons 804, 806) [4].
Total thyroidectomy is indicated in patients with inherited RET mutations, regardless of the plasma calcitonin level. The International RET Mutation Consortium correlated patient genotypes with clinical aggressiveness of hereditary MTC, and created guidelines for the timing of prophylactic thyroidectomy: the patients at highest risk include those with MEN 2B and RET mutations in codons 883 or 918 [4,29]. The guidelines call for prophylactic thyroidectomy in these patients within the first year of life. Patients with MEN 2A or FMTC who have mutations in codons 611, 618, 620, and 634 are at high risk and thyroidectomy should be undertaken prior to the age of five years, whereas the timing of surgery of patients with other mutations can be individualized; such a decision, however, should never be left for later than early childhood [4, 6, 29].
Targeted drug therapy in y thyroid cancer: Recent significant advances
Rationally designed small molecular compounds that affect tyrosine kinase-dependent oncogenic pathways, tyrosine kinase inhibitors or TKIs, are promising potential treatments for patients with MTC. One of the first such drugs to demonstrate efectiveness, imatinib, targets the oncogenic tyrosine kinases BCR-ABL, KIT and other molecules; it has been shown to be very effective in the treatment of chronic myeloid leukemia as well as gastrointestinal stromal tumors [33]. Targeting activated RET has become a key strategy in the treatment of MTC (Figure 1). RET-dependent pathways are ideal targets for molecularly engineered cancer therapy: agents that specifically interfere with targets aberrant in MTC are ideal in that they potentially provide a relatively high therapeutic window with low toxicity as compared to conventional cytotoxic chemotherapy. Various classes of small molecule TKIs have shown anti-RET activity in preclinical studies, including pyrazolopyridimidine inhibitors PP1 and PP2, indolocarbazole derivatives CEP-701 and -751, 2-indolinone derivative RPI-1, and anilinoquinazoline ZD6474 [34–36]. Among this group of compounds, the clinical development of ZD6474 (vandetanib) is the most advanced.
Vandetanib is an orally available TKI that targets VEGF-dependent tumor angiogenesis and EGFR and RET-dependent tumor cell proliferation [36]. Like other small molecule TKIs in this class of anticancer agents, vandetanib competes with ATP and blocks autophosphorylation and signal transduction. Pre-clinical studies showed that vandetanib inhibits RET with a 50% inhibitory concentration of 100 nanomolar. In addition, vandetanib was shown to inhibit growth in RET-transformed thyroid cell xenografts [37]. The ability of vandetanib to inhibit RET at relatively low concentrations is an important feature when comparing this drug to other TKIs. Imatinib, for example, is only able to inhibit RET activation and MTC growth at very high doses. When used clinically in phase II trial, imatnib showed no response in 15 patients with MTC [38]. Phase 1 studies have shown vandetanib to be well tolerated at does of ≤ 300 mg/day with once-daily administration. Its long half life (>120 h) supports once-daily dosing, and steady-state levels are achieved in approximately 28 days. Common adverse events included diarrhea, nausea, rash, hypertension, and asymptomatic QTc prolongation [36].
A number of phase II studies of vandetanib in patients with locally-advanced or metastatic medullary thyroid cancer are ongoing. A phase II, open-label study to assess the efficacy and tolerability of vandetanib monotherapy in patients with locally advanced or metastatic hereditary MTC is underway. Preliminary results in the first 20 patients accrued show that after a median of 6.5 months of treatment, a greater than 50% decrease in plasma calcitonin was observed in over 80% of the patients. Nearly a third of the patients showed an objective remission (RECIST criteria), and stable disease was documented in another 50% of patients [39,40]. Vandetanib appears to have anti-tumor activity in some patients; however, the data on progression-free survival are still being collected. Currently, an international, phase II, randomized, double-blinded, placebo-controlled, multi-center study to assess the efficacy of vandetanib versus placebo in subjects with unresectable, locally advanced or metastatic MTC is ongoing. We are currently conducting a phase I/II trial of vandetanib in children and adolescents with hereditary MTC at the National Institutes of Health.
Because of its potent activity against RET receptor tyrosine kinase, vandetanib is being investigated inpatients with other types of thyroid cancers in which RET-activating mutations may be found. Constitutively active RET proto-oncogenes are also involved, for example, in the development of papillary thyroid cancer (PTC). PTC is much more common than MTC, accounting for 80–90% of all thyroid carcinomas [41]. It is frequently associated with chromosomal rearrangements that align the C-terminal (RET tyrosine kinase-encoding domain) with the promoter and N-terminal portion of unrelated genes, usually from other chromosomes. These chromosomal translocations lead to the expression of a constitutively active chimeric form of the RET tyrosine kinase receptor with its associated oncogenic activity [23]. RET-involving chromosomal translocations are found more frequently in sporadic pediatric PTCs and in radiation-induced PTCs, in particular related to the Chernobyl accident [42]. While first-line therapy for PTC is surgical resection and radioiodine therapy, TKIs may have a role in patients with metastatic disease. A parallel-group, randomized, double blind, placebo controlled, multicenter study is in progress to assess the efficacy and safety of vandetanib in patients with metastatic PTC or follicular thyroid cancer (FTC).
Other oncogenic kinases implicated in the development of thyroid cancer have been targets for molecularly designed therapies. BRAF (B-type RAF kinase) is a serine/threonine kinase involved in the MAP kinase pathway. Activating mutations involving the oncogene BRAF are found in 29 to 83% of all thyroid cancers, especially PTC. The compound BAY 43-9006 (sorafenib) binds to the Raf kinase domain, thereby causing inactivation [43]. Sorafenib has been used clinically in patients with metastatic PTC, FTC, as well as MTC. Preclinical studies of sorafenib show that it inhibits RET phosphorylation and thus may also be useful in patients with MTC: cell lines harboring RET mutations at codon 804, which typically confer resistance to ZD6474, were susceptible to sorafenib [44,45]. Potentially, sorafenib will be a useful therapy in MTC patients who are resistant to other targeted molecular therapies. A phase II trial of sorafenib in patients with metastatic MTC is ongoing [46]. Sunitinib is another TKI with moderate specificity for RET that is currently under investigation for use in thyroid cancer patients, including MTC patients. Table 2 summarizes the therapies for treatment of MTC currently in clinical trials.
Table 2.
Therapies for treatment of medullary thyroid carcinoma currently in clinical trials *
| Drug | Trial Phase | Class | Mechanism of action |
|---|---|---|---|
| NGR-TNF | I | Angiogenesis inhibitor | CNGRC is a peptide fused to the cyokine TNF-α; CNGRC binds to endothelial cells, subsequently TNF-αinhibits angiongenesis [55]. |
| Sunitinib | II | TKI | Blocks VEGFR2, PDGFRb and c-kit, thereby inhibiting angiogenesis and cell proliferation [56]. |
| Sorafenib | II | TKI | Blocks RAF kinase, a component of the RAF/MEK/ERK signaling patway that controls cell division and proliferation; also inhibits VEGFR-2/PDGFR-βthereby blocking angiogenesis [46]. |
| Vandetanib | II | TKI | Selectively inhibits VEGF2 and endothelial proliferation, blocks EGFR and angiogenesis, blocks activated RET [40]. |
| XL184 | I | TKI | Inhibits VEGF2, MET, and RET kinases implicated in tumor formation. growth, and migration [57]. |
| AMG 706 | II | TKI | Multi-receptor TK inhibitor, targets VEGFR, PDGFR, kit, and RET [58,59]. |
| Gefitinib | II | TKI | Inhibits EGFR, induces cell cycle arrest and angiogenesis [60]. |
| Bevacizumab and Sorafenib | I | Monoclonal antibody and TKI | Bevacizumab is a humanized monoclonal antibody directed against VEGF, it inhibits VEGF receptor binding. Combined with angiogenesis inhibitor described above [61]. |
| Imatinib with Dacarbazine and Capeciabine | I and II | TKI, alkylating agent, and antimetabolite | TKI (described above) in combination with alkylating agent to induce apoptosis and antimetabolite to inhibit DNA synthesis |
| Axitinib | II | TKI | Small molecule inhibitor of VEGF receptors [62]. |
| In-111 Pentetreotide | I | Radioisotope | Radioisotope used to target malignancies expressing somatostatin receptors [63]. |
| Irinotecan | II | Topoisomerase inhibitor | S phase specific agent, inhibits topoisomerase I activity, resulting in DNA breaks that lead to apoptosis [35]. |
| 17-allylamino-demethoxygeldamycin | II | HSP90 inhibitor | 17-DMAG binds to HSP90, a chaperone protein that aids in the folding of proteins, leading to degradation of kinases involved in signal transduction and cell cycle regulation [64–66]. |
| Bispecific antibody and di-DTPA-131I | II | Radioimmnotherapy | Anti-CEAxanti-DTPA and di-DTPA-131I peptide to target radiation to MTC tumor cells [67]. |
Data derived from ClinicalTrials.gov and www.cancer.gov/clinical trials by selecting medulllary thyroid carcinoma for trials posted prior to November 15, 2007. Abbreviations: TNF, tumor necrosis factor, VEGFR, vascular endothelial growth factor receptor, PDGFR, platelet-derived growth factor receptor, EGFR, epidermal growtfh factor receptor; HSP90- Heat shock protein 90; TKI-Tyrosine kinase inhibitor
Targeting pathways involved in signaling downstream of the RET proto-oncogene is another rational approach to therapy of MTC. One such pathway, P13K/AKT/NF-κB, is activated by RET and is associated with increased cell cycle progression. The proteasome inhibitor bortezomib has been shown in-vitro to be effective in causing cell death in MTC cell lines at low concentrations [47].
Finally, the use of highly selective oligonucleotide ligands, or aptamers, is another method of targeting activated RET. The pre-clinical development of specific aptamers that disrupt RET receptor dimerization is underway [48].
Ongoing challenges and unmet needs
Drug resistance to TKIs will likely emerge as one of the upcoming challenges in the field of MTC treatment, just as is the case in other cancers where TKis have been used. In patients receiving Imatinib for chronic myelogenous leukemia, for example, resistance emerges often [49]. Further understanding of the mechanisms involved in drug resistance will be crucial to the effective treatment of patients with MTC. It is likely that specific RET mutations will prove to have different sensitivities to TKIs. Knowledge regarding the development of resistance could potentially aid in the design of more effective small molecule inhibitors. The possible ability to predict which patients harboring specific RET mutations respond best to specific RET inhibitors may aid in the selection of patients to be treated. In the future combination agents may be used to treat patients with resistance to particular RET inhibitors and it may become possible to select specific combination therapies based on the unique characteristics of the patient’s malignancy. Preclinical models have examined the use of TKIs in combination with cytotoxic drugs that increase apoptosis, such as irinotecan, and an additive effect has been shown [35].
Two possible additional mechanisms for targeting the RET proto-oncogene include molecular mimicry and dominant-negative RET mutants. Molecular mimicry has been shown using in vitro models to inhibit constitutively active RET [50]. Dominant negative RET mutants may have a future role in the treatment of MTC: these mutant proteins can dimerize with oncogenic RET molecules, resulting in the retention of the protein complex in the endoplasmic reticulum, blocking transduction and/or ultimately leading to increased apoptosis [51].
Another possible future direction for RET tyrosine kinase inhibition involves monoclonal antibodies directed against RET. In breast cancer, the monoclonal antibody trastuzumab, that targets the HER2/neu protein, has had remarkable success [52]. While several anti-ret antibodies have been reported, none have been used clinically to date [53,54].
Summary and Conclusion
The only current cure for patients with MTC is total thyroidectomy performed at an early stage, when the disease is confined to the thyroid gland. Standard chemotherapy and radiation have not shown to be effective. MTC is a promising disease for the field of targeted drug therapy, and the RET proto-oncogene is an excellent target for selective inhibition in MTC, as well as a subset of patients with other, more common types of thyroid cancer. Inhibitors of the RET proto-oncogene are promising potential therapies for patients with MTC and other thyroid cancers where surgery, conventional chemo- and radiotherapy have failed.
Key issues
MTC is a rare calcitonin-producing tumor that arises from the parafolicular C cells of the thyroid gland
Hereditary and most sporadic MTCs as well as several sporadic PTCs are caused by genetic defects of the RET proto-oncogene
MTC is relatively unresponsive to radiation therapy and to standard chemotherapeutic regimens
The RET proto-oncogene is an excellent target for small molecule inhibitors because of its role in the development of several thyroid cancers
TKIs and other kinase inhibitors are promising agents in the medical treatment of advanced thyroid cancer.
Five-Year View
A number of new agents for the treatment of MTC are in clinical development. Results from a variety of clinical trials will likely become available in the near future. Many of these new agents are small molecule inhibitors targeting the RET proto-oncogene. Combinations of currently available TK inhibitors with second-generation TK inhibitors or with standard chemotherapy may be beneficial to treat patients in whom drug resistance emerges. Treatment options for MTC will likely expand in the next few years, ideally providing more therapies for those patients with disease beyond the confines of the thyroid gland.
Acknowledgments
Dr. Lodish’s fellowship is supported by the Intramural Program on Pediatric Endocrinology, National Institutes of Child Health & Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD; funds for this work are in part supported by NICHD, NIH intramural project Z01-HD-000642-04 to Dr. C. A. Stratakis.
Reference List
• of interest
•• of considerable interest
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Kouvaraki MA, Shapiro SE, Perrier ND, et al. RET proto-oncogene, a review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid. 2005;15(6):531–544. doi: 10.1089/thy.2005.15.531. [DOI] [PubMed] [Google Scholar]
- 3.Block MA, Jackson CE, Greenawald KA, Yott JB, Tashjian AH., Jr Clinical characteristics distinguishing hereditary from sporadic medullary thyroid carcinoma. Treatment implications. Arch Surg. 1980;115(2):142–148. doi: 10.1001/archsurg.1980.01380020012004. [DOI] [PubMed] [Google Scholar]
- 4.••.Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. doi: 10.1210/jcem.86.12.8070. Consensus statement on management of MEN1 and MEN2. [DOI] [PubMed] [Google Scholar]
- 5.Farndon JR, Leight GS, Dilley WG, et al. Familial medullary thyroid carcinoma without associated endocrinopathies, a distinct clinical entity. Br J Surg. 1986;73(4):278–281. doi: 10.1002/bjs.1800730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.••.Skinner MA, Moley JA, Dilley WG, Owzar K, Debenedetti MK, Wells SA., Jr Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. 2005;353(11):1105–1113. doi: 10.1056/NEJMoa043999. Landmark study showing that thyroidectomy in young patients identified as carriers of RET mutations resulted in no evidence of persistent or recurrent MTC 5 years after surgery. [DOI] [PubMed] [Google Scholar]
- 7.Ponder BA, Ponder MA, Coffey R, et al. Risk estimation and screening in families of patients with medullary thyroid carcinoma. Lancet. 1988;1(8582):397–401. doi: 10.1016/s0140-6736(88)91191-9. [DOI] [PubMed] [Google Scholar]
- 8.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma, clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88(5):1139–1148. doi: 10.1002/(sici)1097-0142(20000301)88:5<1139::aid-cncr26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Quayle FJ, Moley JF. Medullary thyroid carcinoma, including MEN 2A and MEN 2B syndromes. J Surg Oncol. 2005;89(3):122–129. doi: 10.1002/jso.20184. [DOI] [PubMed] [Google Scholar]
- 10.Wu LT, Averbuch SD, Ball DW, de BA, Baylin SB, McGuire WP., III Treatment of advanced medullary thyroid carcinoma with a combination of cyclophosphamide, vincristine, and dacarbazine. Cancer. 1994;73(2):432–436. doi: 10.1002/1097-0142(19940115)73:2<432::aid-cncr2820730231>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Di BM, Bajetta E, Bochicchio AM, et al. A phase II trial of dacarbazine, fluorouracil and epirubicin in patients with neuroendocrine tumours. A study by the Italian Trials in Medical Oncology (I.T.M.O.) Group. Ann Oncol. 1995;6(1):77–79. doi: 10.1093/oxfordjournals.annonc.a059049. [DOI] [PubMed] [Google Scholar]
- 12.Nocera M, Baudin E, Pellegriti G, Cailleux AF, Mechelany-Corone C, Schlumberger M. Treatment of advanced medullary thyroid cancer with an alternating combination of doxorubicin-streptozocin and 5 FU-dacarbazine. Groupe d’Etude des Tumeurs a Calcitonine (GETC) Br J Cancer. 2000;83(6):715–718. doi: 10.1054/bjoc.2000.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brierley J, Tsang R, Simpson WJ, Gospodarowicz M, Sutcliffe S, Panzarella T. Medullary thyroid cancer, analyses of survival and prognostic factors and the role of radiation therapy in local control. Thyroid. 1996;6(4):305–310. doi: 10.1089/thy.1996.6.305. [DOI] [PubMed] [Google Scholar]
- 14.Modigliani E, Cohen R, Campos JM, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma, results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin Endocrinol (Oxf) 1998;48(3):265–273. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 15.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83(12):2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma, demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107(9):2134–2142. doi: 10.1002/cncr.22244. [DOI] [PubMed] [Google Scholar]
- 17.Donis-Keller H, Dou S, Chi D, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2(7):851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 18.Mulligan LM, Kwok JB, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 19.Carlson KM, Dou S, Chi D, et al. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A. 1994;91(4):1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells SA, Jr, Chi DD, Toshima K, et al. Predictive DNA testing and prophylactic thyroidectomy in patients at risk for multiple endocrine neoplasia type 2A. Ann Surg. 1994;220(3):237–247. doi: 10.1097/00000658-199409000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner MA. Management of hereditary thyroid cancer in children. Surg Oncol. 2003;12(2):101–104. doi: 10.1016/s0960-7404(03)00033-1. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42(2):581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 23.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16(4–5):441–467. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Drosten M, Putzer BM. Mechanisms of Disease, cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nat Clin Pract Oncol. 2006;3(10):564–574. doi: 10.1038/ncponc0610. [DOI] [PubMed] [Google Scholar]
- 25.Ichihara M, Murakumo Y, Takahashi M. RET and neuroendocrine tumors. Cancer Lett. 2004;204(2):197–211. doi: 10.1016/S0304-3835(03)00456-7. [DOI] [PubMed] [Google Scholar]
- 26.Schuringa JJ, Wojtachnio K, Hagens W, et al. MEN2A-RET-induced cellular transformation by activation of STAT3. Oncogene. 2001;20(38):5350–5358. doi: 10.1038/sj.onc.1204715. [DOI] [PubMed] [Google Scholar]
- 27.Ball DW. Medullary thyroid cancer, therapeutic targets and molecular markers. Curr Opin Oncol. 2007;19(1):18–23. doi: 10.1097/CCO.0b013e32801173ea. [DOI] [PubMed] [Google Scholar]
- 28.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 29.Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276(19):1575–1579. [PubMed] [Google Scholar]
- 30.Asai N, Iwashita T, Matsuyama M, Takahashi M. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol. 1995;15(3):1613–1619. doi: 10.1128/mcb.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro M, Carlomagno F, Romano A, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267(5196):381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 32.Salvatore D, Melillo RM, Monaco C, et al. Increased in vivo phosphorylation of ret tyrosine 1062 is a potential pathogenetic mechanism of multiple endocrine neoplasia type 2B. Cancer Res. 2001;61(4):1426–1431. [PubMed] [Google Scholar]
- 33.Ren R. Mechanisms of BCR-ABL in the pathogenesis of CML. Nature Rev Cancer. 2005;5(3):172–83. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 34.Cuccuru G, Lanzi C, Cassinelli G, et al. Cellular effects and antitumor activity of RET inhibitor RPI-1 on MEN2A-associated medullary thyroid carcinoma. J Natl Cancer Inst. 2004;96(13):1006–1014. doi: 10.1093/jnci/djh184. [DOI] [PubMed] [Google Scholar]
- 35.Strock CJ, Park JI, Rosen DM, et al. Activity of irinotecan and the tyrosine kinase inhibitor CEP-751 in medullary thyroid cancer. J Clin Endocrinol Metab. 2006;91(1):79–84. doi: 10.1210/jc.2005-1882. [DOI] [PubMed] [Google Scholar]
- 36.Herbst RS, Heymach JV, O’Reilly MS, Onn A, Ryan AJ. Vandetanib (ZD6474), an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs. 2007;16(2):239–249. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- 37.Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62(24):7284–7290. [PubMed] [Google Scholar]
- 38.de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, et al. A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(9):3466–3469. doi: 10.1210/jc.2007-0649. [DOI] [PubMed] [Google Scholar]
- 39.You YN, Lakhani V, Wells SA., Jr New directions in the treatment of thyroid cancer. J Am Coll Surg. 2007;205(4 Suppl):S45–S48. doi: 10.1016/j.jamcollsurg.2007.06.323. [DOI] [PubMed] [Google Scholar]
- 40.Wells SA, Gosnell JE, Gagel RF, et al. Vandetanib in metastatic hereditary medullary thyroid cancer: Follow-up results of an open-label phase II trial. ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 6018. [Google Scholar]
- 41.Sherman SI, Angelos P, Ball DW, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2007;5(6):568–621. doi: 10.6004/jnccn.2007.0052. [DOI] [PubMed] [Google Scholar]
- 42.Williams D. Cancer after nuclear fallout, lessons from the Chernobyl accident. Nat Rev Cancer. 2002;2(7):543–549. doi: 10.1038/nrc845. [DOI] [PubMed] [Google Scholar]
- 43.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 44.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98(5):326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 45.Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004;23(36):6056–6063. doi: 10.1038/sj.onc.1207810. [DOI] [PubMed] [Google Scholar]
- 46.Kober F, Hermann M, Handler A, Krotia G. Effect of sorafenib in symptomatic metastatic medullary thyroid cancer. ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 14065. [Google Scholar]
- 47.Mitsiades CS, McMillin D, Kotoula V, et al. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab. 2006;91(10):4013–4021. doi: 10.1210/jc.2005-2472. [DOI] [PubMed] [Google Scholar]
- 48.Cerchia L, Duconge F, Pestourie C, et al. Neutralizing aptamers from whole-cell SELEX inhibit the RET receptor tyrosine kinase. PLoS Biol. 2005;3(4):e123. doi: 10.1371/journal.pbio.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Mauro MJ. Defining and managing imatinib resistance. Hematology. 2006:219–225. doi: 10.1182/asheducation-2006.1.219. [DOI] [PubMed] [Google Scholar]
- 50.Cerchia L, Libri D, Carlomagno MS, De FV. The soluble ectodomain of RetC634Y inhibits both the wild-type and the constitutively active Ret. Biochem J. 2003;372(Pt 3):897–903. doi: 10.1042/BJ20021530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drosten M, Stiewe T, Putzer BM. Antitumor capacity of a dominant-negative RET proto-oncogene mutant in a medullary thyroid carcinoma model. Hum Gene Ther. 2003;14(10):971–982. doi: 10.1089/104303403766682232. [DOI] [PubMed] [Google Scholar]
- 52.Plosker GL, Keam SJ. Trastuzumab, a review of its use in the management of HER2-positive metastatic and early-stage breast cancer. Drugs. 2006;66(4):449–475. doi: 10.2165/00003495-200666040-00005. [DOI] [PubMed] [Google Scholar]
- 53.Yano L, Shimura M, Taniguchi M, et al. Improved gene transfer to neuroblastoma cells by a monoclonal antibody targeting RET, a receptor tyrosine kinase. Hum Gene Ther. 2000;11(7):995–1004. doi: 10.1089/10430340050015301. [DOI] [PubMed] [Google Scholar]
- 54.Salvatore G, Nagata S, Billaud M, Santoro M, Vecchio G, Pastan I. Generation and characterization of novel monoclonal antibodies to the Ret receptor tyrosine kinase. Biochem Biophys Res Commun. 2002;294(4):813–817. doi: 10.1016/S0006-291X(02)00560-0. [DOI] [PubMed] [Google Scholar]
- 55.Corti A, Ponzoni M. Tumor vascular targeting with tumor necrosis factor alpha and chemotherapeutic drugs. Ann N Y Acad Sci. 2004;1028:104–112. doi: 10.1196/annals.1322.011. [DOI] [PubMed] [Google Scholar]
- 56.Chow LQ, Eckhardt SG. Sunitinib, from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 57.Salgia R, Hong DS, Camacho LH, et al. A phase 1 dose-escalation study of the safety and pharmacokinetics (PK) of XL184, a VEGFR and MET kinase inhibitor, administered orally to patients with advanced malignancies. ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 14031. [Google Scholar]
- 58.Sherman SI, Schlumberger MJ, Droz J, et al. Initial results from a phase II trial of motesnab diphosphate (AMG 706) in patients with differentiated thyroid cancer (DTC). ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 6017. [Google Scholar]
- 59.Rosen LS, Kurzrock R, Mulay M, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25(17):2369–2376. doi: 10.1200/JCO.2006.07.8170. [DOI] [PubMed] [Google Scholar]
- 60.Pennell NA, Daniels GH, Haddad RI, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 6020. [Google Scholar]
- 61.Salnikov AV, Heldin NE, Stuhr LB, et al. Inhibition of carcinoma cell-derived VEGF reduces inflammatory characteristics in xenograft carcinoma. Int J Cancer. 2006;119(12):2795–2802. doi: 10.1002/ijc.22217. [DOI] [PubMed] [Google Scholar]
- 62.Cohen EE, Vokes EE, Rosen LS, et al. A phase II study of axitinib (AG-013736 [AG] in patients with advanced thyroid cancers. ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 6008. [Google Scholar]
- 63.Buscombe JR, Caplin ME, Hilson AJ. Long-term efficacy of high-activity 111in-pentetreotide therapy in patients with disseminated neuroendocrine tumors. J Nucl Med. 2003;44(1):1–6. [PubMed] [Google Scholar]
- 64.Xiao L, Lu X, Ruden DM. Effectiveness of hsp90 inhibitors as anti-cancer drugs. Mini Rev Med Chem. 2006;6(10):1137–1143. doi: 10.2174/138955706778560166. [DOI] [PubMed] [Google Scholar]
- 65.Vaishampayan UN, Sausville EA, Horiba MN, et al. Phase I trial of intravenous 17-allylaminogeldanamycin (A) and oral sorafenib (B) in pretreated advanced malignancy: plasma Hsp90 induction correlates with clinical benefit. ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 3531. [Google Scholar]
- 66.Murgo AJ, Kummar S, Gardner ER, et al. Phase 1 trial of 17-dimethylaminoethylamino-17demethoxygeldanamycin (17-DMAG) administered twice weekly. ASCO Annual Meeting Proceedings Part 1. J Clin Oncol. 2007;25(18S) Abstract 3566. [Google Scholar]
- 67.Chatal JF, Campion L, Kraeber-Bodere F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy, a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24(11):1705–1711. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]