Abstract
The Asian parasitoid, Binodoxys communis (Gahan) (Hymenoptera: Braconidae), is a candidate for release against the exotic soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae), in North America. In this study, we examined preferences by B. communis for the different developmental stages of A. glycines and investigated consequences of these preferences for parasitoid fitness. We also determined to what extent aphid defensive behaviours mediate such preferences. We found that B. communis readily attacks and successfully develops in the different A. glycines developmental stages. Binodoxys communis development time gradually increased with aphid developmental stage, and wasps took longest to develop in alates. An average (±SE) of 54.01±0.08% of parasitized A. glycines alatoid nymphs transformed into winged adult aphids prior to mummification. No-choice assays showed a higher proportion of successful attacks for immature apterous A. glycines nymphs compared to adults and alatoid nymphs. Also, choice trials indicated avoidance and lower attack and oviposition of adults and alatoid nymphs. The different aphid stages exhibited a range of defensive behaviours, including body raising, kicking and body rotation. These defenses were employed most effectively by larger aphids. We discuss implications for the potential establishment, spread and biological control efficacy of A. glycines by B. communis in the event that it is released in North America.
Keywords: biological control, host quality, host selection, koinobiont parasitoids, fitness, life history, phoresy
Introduction
Biological control introductions are being considered against the soybean aphid, Aphis glycines Matsumura, an invasive species from Asia that is a destructive pest of soybeans in North America (Heimpel et al., 2004; Ragsdale et al., 2004; Wu et al., 2004; Wyckhuys & Heimpel, 2007; Wyckhuys et al., 2007a,b). A strain of Binodoxys communis (Gahan) (Hymenopera: Braconidae) that was collected from soybean aphid in China is one of the most promising natural enemies for release against A. glycines. This species appears to be very well adapted to A. glycines in laboratory studies and exhibits fairly high levels of host specificity (Wyckhuys et al., 2007b; N. Desneux, personal communication).
The immature stages of insect parasitoids, such as B. communis, depend on their host for nutrients, and so adult female parasitoids must accurately assess the suitability of hosts for progeny development (e.g. Sequeira & Mackauer, 1992a; Colinet et al., 2005; Henry et al., 2005). Many parasitoids preferentially attack certain sizes, ages or stages of a given host species (Mackauer, 1973; Hopper & King, 1984; Liu et al., 1984; Wang & Liu, 2002; Lin & Ives, 2003). These preferences influence developmental rate, survival and reproductive capacities of parasitoid offspring (Lewis & Redlinger, 1969; Nechols & Kikuchi, 1985; Hopper, 1986; Sequeira & Mackauer, 1993; Godfray, 1994; Lacoume et al., 2006). Host-stage preferences can also strongly influence host behaviour and population growth (Hopper & King, 1984; Murdoch et al., 1987; Murdoch et al., 2003; Lin & Ives, 2003) and may affect parasitoid efficiency in biological control of aphids (Hågvar & Hofsvang, 1991).
All aphid parasitoids are endoparasitic koinobionts, meaning that eggs are laid within the host and the host continues developing after parasitism. Thus, the host stage used for oviposition generally precedes the host stage(s) used for larval development, complicating oviposition strategies (e.g. Kouamé & Mackauer, 1991; Sequeira & Mackauer, 1992b; Rivero, 2000; Li & Mills, 2004; Jenner & Kuhlmann, 2006). Koinobiosis also may decouple host stages that engage in defensive behaviour from those that support parasitoid development, in some cases allowing development on hosts that would otherwise be inaccessible (Liu et al., 1984; Gerling et al., 1990; Weisser, 1994, 1995; Chau & Mackauer, 2000). For example, although large aphids contain more nutritional resources than small aphids, they also defend themselves more effectively than smaller ones (Gerling et al., 1990; Chau & Mackauer, 1997; Losey & Denno, 1998; Chau & Mackauer, 2000).
Differences in host-stage use also have implications for parasitoid dispersal and establishment in novel environments. Most aphid species, including A. glycines, exhibit a polyphenism whereby individuals exhibit both winged (alate) and wingless (apterous) morphs (Dixon, 1998; Ragsdale et al., 2004; Hodgson et al., 2005). Alates generally leave their natal host plant and colonize new plants, eventually in different environments. Successful parasitism of winged or alatoid morphs could then lead to situations where parasitoid eggs or larvae are carried for considerable distances within their host (Kelly 1917; Hight et al., 1972; Rogers et al., 1972; Rauwald & Ives, 2001). Such phenomena could be of great importance to establishment, spread and efficacy of potential biological control agents such as B. communis.
Here, we investigate B. communis parasitism of different developmental stages of the soybean aphid, A. glycines, under choice and no-choice settings. Parasitism differences are related to A. glycines defensive behaviour, B. communis handling costs and oviposition success on each of the A. glycines stages. We also assess parasitoid fitness on each of the A. glycines instars by measuring key life-history traits, such as development time, survival and sex ratio (e.g. Roitberg et al., 2001). This research increases basic knowledge of this host-parasitoid association and helps predict B. communis establishment, spread and biological control success upon release in North America.
Materials and methods
Study insects
We established a colony of A. glycines from individuals that were collected in 2003 from a soybean field in St Paul, Minnesota, USA. This colony has subsequently been maintained in the Minnesota Department of Agriculture/Minnesota Agricultural Experiment Station (MDA/MAES) Quarantine Facility in St Paul with periodic supplementation of aphids from the field. As aphid colonies were never started from one single aphid and no genetic characterization was done, no estimates are available of the number of clones of which the A. glycines colony consisted. The A. glycines colony was kept on soybean plants (cultivar M96-D133151), which were grown under greenhouse conditions (25±5°C, 60-80% RH and L16:D8).
A Chinese strain of Binodoxys communis was initiated with seven males and 33 females from collections of parasitized A. glycines by K. Hoelmer, K. Chen and W. Meikle made in several soybean fields in late August 2002 near Harbin (45°41'27” N, 126°37'42” E) and in Suihua County (45°36'28” N, 126°57'49” E) in the Chinese province of Heilongjiang. Voucher specimens of progeny from the material collected in China are stored frozen in molecular grade ethanol at the USDA-ARS Beneficial Insect Introduction Research Laboratory in Newark, Delaware, USA. We maintained B. communis in three subpopulations on A. glycines with a geometric mean of 66-68 adult parasitoids for each subpopulation for 26 generations. The parasitoid colony at the MDA/MAES Quarantine Facility was initiated from a total of 102 mummies in 2003, and has since been maintained on A. glycines. For experiments, we collected B. communis mummies from soybean plants and isolated these in clear gelatin capsules (size 0; Drum Point Pharmacy, Brick, NJ, USA). Adult female wasps were mated within 24 hr of emergence and kept in capsules with a droplet of mixed-flower honey prior to their use in experiments.
No-choice parasitism trials
In the first experiment, we determined B. communis fitness on each of the A. glycines developmental stages by measuring overall parasitism levels as well as a set of life-history traits. Hodgson et al. (2005) reported A. glycines to have a total of four different immature instars, and winged or wingless adult stages. Third and 4th instar nymphs of winged morphs possess wing-pads and are termed alatoid nymphs; of these two morphs, we included 4th instar alatoids in our experiment, yielding a total of seven A. glycines types to be tested.
We planted soybean plants in the greenhouse and used them at the V1 stage for our experiments. The V1 developmental stage is characterized by a fully developed first trifoliate leaf and expanded unifoliate leaves (McWilliams et al., 2004). In the laboratory, individually potted live plants were covered with a 0.5 l transparent PETE plastic cup (Solo Cup Company, Urbana, Illinois, USA) from which the bottom was removed. The top of the plastic cups was fitted with a fine nylon mesh, and the entire unit will be referred to as an `experiment cage'. We placed a total of 40 individuals of a given A. glycines stage onto each soybean plant using a fine brush. The different aphid stages were determined using an identification key developed by Hodgson et al. (2005). We allowed aphids to establish on plants for 1-2 hours before the introduction of parasitoids. Mated female B. communis were subsequently transferred to the cages and allowed to parasitize aphids for 4 h. The 4 h period ensured that high numbers of aphid offspring were not produced during the experiment, thereby likely distorting parasitism rates on each of the different aphid stages. Parasitoids were introduced into the cages between 12:00 and 14:00 and were removed after 4 h. The cages were maintained at 25°C, 75% RH and 16:8 L:D and checked on a daily basis for the presence of parasitoid mummies. Mummies were counted upon formation, and the number of days until mummy formation was recorded. Mummies were subsequently placed singly in clear gelatin capsules (size 0) and the number of days until parasitoid emergence was recorded. The sex of emerged parasitoids was determined and sex ratio is expressed as the proportion of adults that were male. We report the parasitism rate as the number of mummies divided by the starting number of aphids (i.e. 40). Although this measure does not distinguish parasitoid acceptance of hosts from host physiological suitability for parasitoid development, it provides a useful assessment of the net effect of parasitoid choice and host suitability on overall parasitism success (Li & Mills, 2004; Colinet et al., 2005). For each of the seven A. glycines developmental stages, we carried out a total of ten replicates.
To compare B. communis life history traits on the different A. glycines developmental stages, we used a Kruskal-Wallis test or One way analysis of variance (ANOVA) with Fisher's protected LSD as post-hoc analysis, according to the normality of the data set.
No-choice assay of host acceptance
A second experiment was done to quantify B. communis acceptance of each of the A. glycines developmental stages to determine the nature and extent of defensive behaviour of these stages. The behavioural arena consisted of a single leaflet that was removed from one of the fully expanded leaves of an uninfested V3-5 soybean plant and placed upside down within a 5.8 cm dia. Petri dish filled with moist sand. The leaflet had a diameter of >5 cm and commonly occupied the entire space within the Petri dish. The V3-5 soybean developmental stages are characterized by fully developed and expanded third-fifth trifoliate leaves (McWilliams et al., 2004). The Petri dish was then placed under a Leica GZ6E dissecting microscope. On this leaflet, we placed one individual of a given A. glycines stage and allowed it to settle for 5 min. Aphids were collected with a fine brush from A. glycines colonies on soybean plants of identical phenological stage and (visually) similar quality (Stadler et al., 1994). Next, a one-day-old, mated B. communis female was gently introduced into a 1 cm dia.×0.65 cm high clear plastic dome. This dome was then placed over the individual aphid within the Petri dish. The observation was started when the parasitoid first encountered the aphid.
We observed both aphid and parasitoid behaviour until a successful oviposition occurred but not longer than 5 min. For B. communis, we recorded the time elapsed until oviposition, the number of encounters and the number of probing events. An encounter was defined as the parasitoid making contact with the aphid after having walked away from it for > 5 s for re-encounters. All intervals were timed with a stopwatch to the nearest second. For the different A. glycines stages, we recorded defensive behaviour as `kick', `rotate', `walk away' or `cornicle secretion'. Kicking was defined as the aphid raising its body and then contacting the parasitoid with one of its legs. Exposures were replicated 25 times for each aphid stage. For every observation, a different B. communis female was used.
For analysis, we computed the number of probing events and the number of encounters as frequencies over the allotted time (i.e. maximum of 5 min or until successful oviposition) (Desneux et al., 2004). We compared these frequencies and the time until oviposition for the various aphid developmental stages using a non-parametric Kruskal-Wallis test. Next, pair-wise Mann-Whitney U tests were carried out following a Bonferroni correction for multiple comparisons. Proportional measures of attack and successful oviposition were compared between the different A. glycines stages using a Chi-square test. The same analysis was used to compare the proportion of aphids exhibiting defensive behaviours among the various stages.
Choice assay of host acceptance
A third experiment was done to determine whether B. communis prefers certain A. glycines stages over others and if such preference changes as a parasitoid forages within a patch of aphids of various stages. As in the previous assay, a soybean leaflet was placed upside down within a 5.8 cm dia. Petri dish filled with moist sand and placed under a dissecting microscope. On this leaflet (which will be referred to as the `patch'), we placed a total of five individuals of each of the seven different developmental stages of A. glycines, totaling 35 aphids. We allowed the aphids to establish for 5-10 min and then introduced one B. communis female. Upon introduction of the parasitoid, the Petri dish was covered with a plastic lid 5.1 cm in diameter and 1.3 cm in height.
We noted the sequence of aphids that were encountered and recorded parasitoid attack and oviposition on each aphid attacked. The observation was terminated when the parasitoid stayed outside the patch for longer than 1 min or when 30 min had elapsed. As B. communis did not appear to discriminate against previously-parasitized aphids (see Results), we did not replace parasitized aphids during the course of the observations or treat them differently in subsequent data analysis. However, for each replicate, we composed a new patch using only unparasitized aphids collected from the A. glycines colony. The exposure was replicated 25 times.
Procedures for statistical analysis of this experiment were modified from Weisser (1994). Instar preference was measured using the formula of Manly (1974), whereby preference is scored as a deviation of the number of individuals of a given developmental stage selected for a particular behaviour (i.e. encounter, attack and oviposition) from the number of this stage eligible for the action (e.g. number present in the patch, number attacked, etc.). Let Ai be the number of individuals of a given stage i eligible for a particular action by the parasitoid (Ai=N= total number eligible for this action), and let xi be the number of stage i that have been selected for this particular action and ri the number that have not been selected (so that xi+ri=Ai). We considered the case in which an aphid already selected for an interaction is still eligible for this action (Weisser, 1994). Then
is Manly's Beta of the jth stage for this particular action (with a total of seven different stages being considered). If βj is greater than 1/7 for any given developmental stage j, then the parasitoid prefers this given stage for the action under consideration. If βj is less than 1/7 then the parasitoid avoids this interaction with stage j and, finally, if βj=1/7, then the parasitoids accept any eligible stage for the action under consideration. This formula is used to determine whether the different stages are encountered, attacked and parasitized in the same proportion as they are present within the patch. To determine whether B. communis preference changes with respect to the sequence of aphid attacks, we computed Manly's Beta values for different intervals over the course of the experiment (encounters 0-20, 20-40 and 40-60). We then compared these values between intervals for a select set of actions on each aphid stage (i.e. encounters, attacks or oviposition).
We compared Manly's Beta values for the different A. glycines stages using a Kruskal-Wallis test. For data that were normally distributed or could be successfully transformed, a One-way ANOVA was used, followed by Fisher's protected LSD as post-hoc analysis. All statistical analyses were executed using SPSS software (Landau & Everitt, 2004). For datasets that yielded non-significant differences, we performed a power analysis using GPower 3.0.4. (Faul et al., 2007).
Results
No-choice parasitism trials
Binodoxys communis was able to successfully parasitize and develop on each of the seven stages of A. glycines. The number of mummies formed in each of the stages did not show any significant differences (table 1; ANOVA, F6,62=1.05, P=0.40). However, the achieved power of this analysis was 0.46. Emergence rates of B. communis on the different A. glycines stages also did not show significant differences (ANOVA, F6,60=0.91, P=0.50). The power of this analysis was also low, equalling 0.37. Among the 4th instar alatoid nymphs that developed into mummies, 54.01±0.08% (average±SE) had transformed into winged adults prior to mummification, and the B. communis mummies produced from these also possessed wings.
Table 1.
Life history traits of Binodoxys communis progeny emerging from the various developmental stages of its aphid host, Aphis glycines. Only one aphid stage was exposed to each adult female parasitoid in this experiment.
| Host instar | Life history trait |
|||||
|---|---|---|---|---|---|---|
| Number of mummies formed | Proportion (of mummified aphids) emerged | Sex ratio (proportion males) | Days until mummification | Female development time | Male development time | |
| 1 | 7.22±2.13a | 0.51±0.10a | 0.56±0.14ab | 7.00±0.12ab | 11.25±0.44a | 10.15±0.08a |
| 2 | 10.66±1.85a | 0.62±0.06a | 0.69±0.09b | 7.25±0.16b | 11.45±0.33ab | 11.23±0.28b |
| 3 | 12.60±1.62a | 0.73±0.07a | 0.59±0.11ab | 6.79±0.12a | 11.77±0.29ab | 11.95±0.22cd |
| 4 | 16.40±2.81a | 0.68±0.06a | 0.42±0.11ab | 7.07±0.14ab | 12.12±0.24b | 12.16±0.23d |
| Apterous adult | 10.56±2.90a | 0.61±0.10a | 0.34±0.08a | 6.94±0.11ab | 11.27±0.13a | 11.41±0.13bc |
| Alatoid 4th instar | 9.80±2.14a | 0.53±0.09a | 0.48±0.12ab | 6.80±0.12a | 12.59±0.29bc | 12.88±0.44de |
| Alate adult | 15.70±5.37a | 0.59±0.06a | 0.56±0.11ab | 8.40±0.15c | 13.00±0.26c | 13.03±0.26e |
Mean±SE; values within the same column followed by identical letters are not significantly different (P>0.05, one-way ANOVA with Fisher's protected LSD).
The sex ratio of B. communis was highly male-biased on 2nd instar aphids, while female-biased on apterous A. glycines adults (table 1). Development time to mummification varied significantly with A. glycines stage (Kruskal-Wallis statistic=92.72, P<0.001) with mummification taking longest for alate adults. Time to emergence of both female and male B. communis also differed among A. glycines stages (Kruskal-Wallis statistic=40.41, P<0.001; KW statistic=64.27, P<0.001, respectively). In the various A. glycines stages, parasitoid development time gradually increased with aphid stage up until 4th instar. Parasitoids took longest to develop on alatoid nymphs and alate adults.
No-choice assays of host acceptance
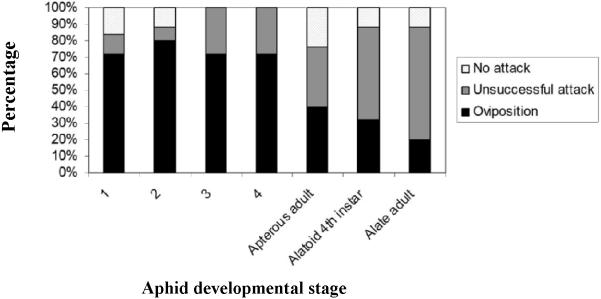
Although attack by B. communis females did not differ among the various A. glycines stages in no-choice assays (fig. 1; likelihood ratio χ2=1.23, P=0.97), parasitoid oviposition success did vary with stage (likelihood ratio χ2=15.65, P=0.02). Attacks on apterous adult, alate and alatoid nymphal stages were less likely to succeed than attacks on the apterous nymphal stages. Based on all replicates, we found B. communis to encounter 1st instars more frequently than other stages and alate adults less frequently than 2nd instars (table 2; Kruskal-Wallis statistic=16.26, P=0.01). Also, parasitoids probed immature A. glycines stages more frequently than alatoids and adults (Kruskal-Wallis statistic=18.284, P=0.006). First instar A. glycines were probed more frequently than other stages. The preferred location for oviposition was the aphid thorax, receiving 70.1% of successful ovipositions, compared to 17.5% for the head region and 12.4% for the abdomen. Aphid instars 1-4 received 72, 65, 72 and 66% of ovipositions in the thorax and 0, 5, 22 and 33% in the head region, respectively. Apterous adults, alatoids and alates received 80, 50 and 100% of oviposition in the thorax and 20, 50 and 0% in the head region, respectively.
Fig. 1.
Outcome of B. communis encounters with the various A. glycines developmental stages in a no-choice experiment. The graph represents the proportion of aphids (out of 25) of a given stage that were attacked or accepted for oviposition by B. communis.
Table 2.
Acceptance behaviour of B. communis upon encounter with different A. glycines developmental stages in a no-choice experiment. Behavioural parameters are reported for all replicates (N=25) and for wasps that successfully oviposited in the aphids presented. Number of probing events and number of encounters are indicated as frequencies over the allotted time (i.e. 5 min or until successful oviposition). Parameters include the total number of probing events or encounters and the total time until oviposition.
| All replicates (N=25) | ||
|---|---|---|
| Host stage | Number of encounters (min-1) | Number of probing events (min-1) |
| 1 | 3.27±0.47a* | 3.88±0.60a |
| 2 | 2.34±0.26b | 3.49±0.58ab |
| 3 | 2.27±0.33b | 3.99±0.60a |
| 4 | 2.16±0.23bc | 3.70±0.57ab |
| Apterous adult | 1.79±0.31bc | 2.24±0.53bc |
| Alatoid 4th instar | 1.77±0.19bc | 2.24±0.64bc |
| Alate adult | 1.39±0.12c | 1.70±0.32c |
| Observations where successful oviposition was recorded | ||||
|---|---|---|---|---|
| Host stage | N | Number of encounters (min-1) | Number of probing events (min-1) | Time until oviposition (s) |
| 1 | 18 | 3.97±0.55a | 2.25±0.12a | 34.00±8.04a |
| 2 | 20 | 2.69±0.26b | 1.98±0.14a | 58.90±12.21a |
| 3 | 18 | 2.81±0.38b | 2.08±0.17a | 71.83±18.37a |
| 4 | 18 | 2.50±0.27b | 2.10±0.15a | 70.22±12.93a |
| Apterous adult | 10 | 2.91±0.59ab | 2.12±0.17a | 69.10±26.13ab |
| Alatoid 4th instar | 8 | 2.25±0.31b | 2.19±0.31a | 129.75±25.66c |
| Alate adult | 5 | 1.65±0.42b | 1.83±0.08a | 136.80±32.42bc |
Mean±SE; values within the same column followed by identical letters are not significantly different (P>0.05, Mann-Whitney U test
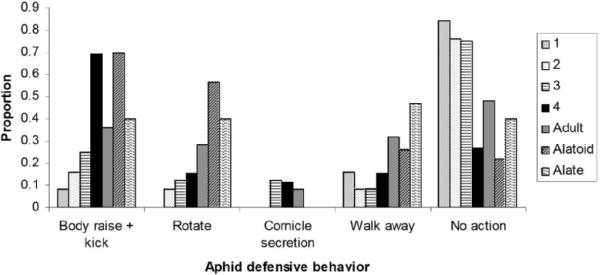
The various A. glycines stages differed in their defensive behaviour upon attack by B. communis (fig. 2). Many of the immature aphid stages did not exhibit any defensive behaviour, and the frequency of inaction varied among A. glycines stages (Chi-square χ2=16.348, P=0.012<0.05). Kicking was the most frequently recorded behaviour (seen in 38% of the aphids). The frequency of kicking or body rotation varied among aphid stages (Chi-square χ2=21.818, P=0.001; χ2=24.718, P<0.001, respectively). Some aphids exhibited more than one type of defensive behaviour, commonly combining kicking with body rotation.
Fig. 2.
Aphid defensive behaviours associated with B. communis attacking the various developmental stages of the soybean aphid, Aphis glycines, in a no-choice experiment. The proportion of aphids (out of 25) of a given instar exhibiting certain behaviours is indicated.
Choice assay of host acceptance
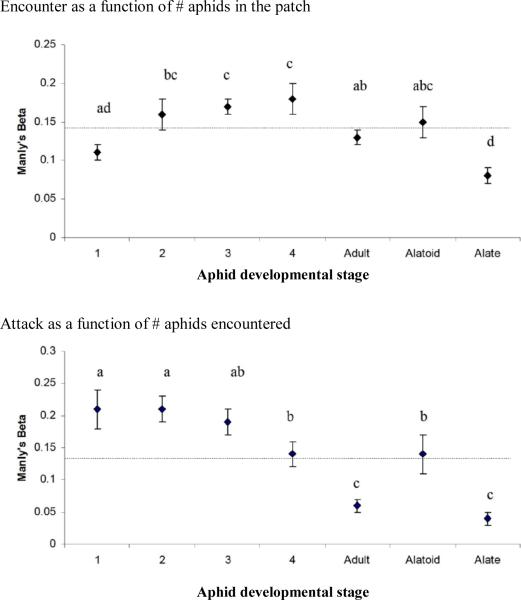
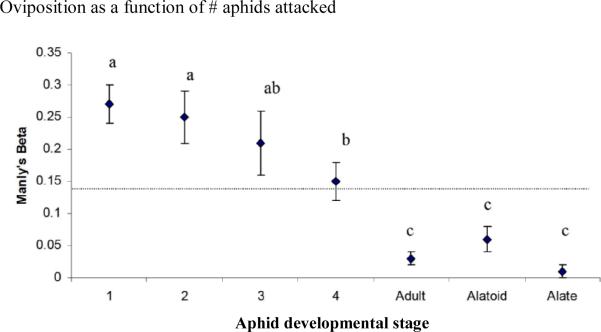
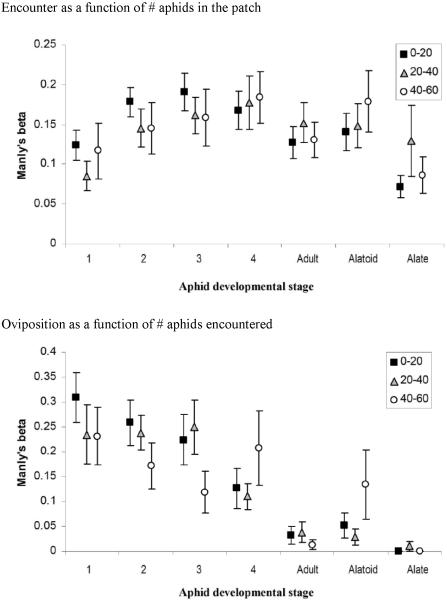
Parasitoid wasps stayed within the patch for 21.64±1.40 min and encountered 43.80±3.67 (average±SE) aphids, indicating that aphids were frequently encountered more than once. Binodoxys communis encountered the different A. glycines stages to varying extents (fig. 3; ANOVA F6,168=5.71, P<0.001). There were also significant differences in attack rates among stages that were encountered and in oviposition rates among stages that were attacked (fig. 3; ANOVA F6,168=9.76, P<0.001; Kruskal-Wallis χ2=74.61, P<0.001, respectively). Attack rates were highest for the young instars and lowest for alate and apterous adults, given their respective encounter rates. Lastly, 1st and 2nd instar A. glycines were also oviposited to highest extent, given their respective rates of attack. Parasitoids did not alter their preference for oviposition of certain aphid stages during the course of the experiment. Manly's Beta values for this action did not differ between the three intervals (Kruskal-Wallis statistic=1.88, P=0.39; Kruskal-Wallis statistic=1.60, P=0.45; Kruskal-Wallis statistic=3.95, P=0.14; Kruskal-Wallis statistic=0.53, P=0.77; Kruskal-Wallis statistic=0.30, P=0.86; Kruskal-Wallis statistic=1.98, P=0.37; Kruskal-Wallis statistic=1.86, P=0.39 for 1st, 2nd, 3rd, 4th instar, adult, alatoid and alate stages, respectively). Also, B. communis did not modify its preference for encounter or attack of any of the aphid stages over the allotted 30 min period (fig. 4).
Fig. 3.
Average (±SE) Manly's Beta values for B. communis behaviour in patches with a total of 35 A. glycines individuals, equally distributed among all seven different developmental stages. Within each figure, the line represent the baseline value (1/7) against which Manly's Beta values for each stage are compared. Different letters indicate significantly different Manly's Beta values for the subsequent aphid developmental stages (P>0.05, one-way ANOVA with Fisher's protected LSD post hoc analysis).
Fig. 4.
Variation in B. communis preference for the different A. glycines developmental stages over the course of a 30 min choice experiment. Parasitoid preference for a given stage is represented as Manly's Beta values (±SE), which indicate whether the various stages are either encountered in the same proportion as they are present within the patch or oviposited in the same proportion as they are encountered. Values are computed for the following intervals: encounters 0-20, 20-40 and 40-60.
Discussion
Many parasitoids in the braconid subfamily, Aphidiinae, preferentially parasitize small or intermediate host instars (Liu et al., 1984; Sequeira & Mackauer, 1987, 1992a; Weisser, 1994; Mackauer et al., 1996; Ives et al., 1999; Sharmila & Rajendra, 1999; Chau & Mackauer, 2000, 2001; Perdikis et al., 2004). Our research confirms this general pattern for B. communis, a parasitoid of the soybean aphid, A. glycines. No-choice assays showed a high proportion of successful attacks on all apterous nymphal A. glycines instars, while choice trials indicated lower encounter, attack and oviposition of apterous and alate adults, as well as alatoid nymphs. Nevertheless, parasitism trials with exposures over a longer time revealed similar B. communis parasitization of the various A. glycines stages. This disparity could hint at a lower suitability of young A. glycines instars for development of B. communis, as indicated below.
Parasitism of the various A. glycines stages possibly has major implications for fitness of B. communis offspring. Parasitism levels, mummy emergence and parasitoid sex ratio showed little differences among the various aphid stages. However, B. communis showed a higher rate of acceptance of young A. glycines instars compared to adults or alatoid nymphs. Thus, younger instars may have experienced greater mortality following parasitism (Rakhshani et al., 2004; Colinet et al., 2005). Alternative explanations are that super-parasitism levels of preferred younger instars is high or that host-stage preferences are not expressed in patches containing a single host stage, particularly for naïve parasitoids. Lastly, additional time of exposure (4 h) during parasitism trials could lead to higher parasitism rates of older stages despite short-term behavioural avoidance. With the exception of the A. glycines apterous adult stage, development time of both B. communis sexes increased with aphid stage. Various relationships exist between parasitoid development time and host age at oviposition (Hopper, 1986; Colinet et al., 2005), with positive associations being occasionally reported (Vinson, 1972; Lawrence et al., 1976). Rapid parasitoid development in 1st instar A. glycines shows that these hosts provide minimum required nutrient levels for B. communis (Henry et al., 2005) although parasitoids emerging from young hosts may be smaller. The gradual increase in development time on later A. glycines stages may reflect changes in nutritional value of the host, increased aphid resistance and competition of parasitoid larvae with the developing host embryos (Walker & Hoy, 2003; Colinet et al., 2005) or increased time necessary for development of a larger parasitoid. No evidence was found of delayed parasitoid development in younger or smaller hosts, a common pattern in koinobiont parasitoids (Harvey, 2005). Younger A. glycines instars were much smaller than later developmental stages (Hodgson et al., 2005; K. Wyckhuys, personal observation). The interaction between B. communis and A. glycines is also mediated by host behaviour, particularly aphid defense. In no-choice assays, A. glycines exhibited a variety of defensive behaviours, all of which are commonly observed among aphid species (e.g. Gerling et al., 1990; Hågvar & Hofsvang, 1991; De Farias & Hopper, 1999; Villagra et al., 2002). In no-choice assays, B. communis did not refrain from attacking larger or older aphid stages or aphids that exhibited stronger defenses. This could reflect either a low response threshold of B. communis for oviposition (Mackauer et al., 1996) or acceptance decisions resulting from its lack of previous experience (Henry et al., 2005). Binodoxys communis females encountered and probed larger aphid stages at a lower frequency and with many probing attempts unsuccessful. Like other members of the genera Trioxys and Binodoxys, B. communis uses a pair of terminal abdominal prongs to grasp the host prior to oviposition (Völkl & Mackauer, 2000), and this grasping is thought to be more effective on smaller instars. Also, as older A. glycines stages were less frequently oviposited in and exhibited more body rotation and walking behaviours, thus these defensive behaviours may deter B. communis attack. However, 4th instar apterous A. glycines exhibited kicking behaviour as frequently as 4th instar alatoid nymphs; but the former were oviposited in as often as younger instar apterous nymphs, suggesting that this defense does not always work (see fig. 1).
In choice assays, B. communis females encountered alate morphs and 1st instars less often than other stages and morphs, while encountering 3rd and 4th instars at a higher rate than other stages and morphs. Although most parasitoids have poor ability to assess host suitability from a distance, they sometimes evaluate aphid shape, size or movement (Battaglia et al., 1995; Le Ralec et al., 2005). Our results suggest that B. communis might employ aphid size as an initial criterion to determine host suitability, while increased movement of A. glycines alates may act as a release stimulus for attack.
Because B. communis successfully develops on all A. glycines developmental stages, field releases do not need to target specific phases of aphid infestation. Also, considering that young aphid instars are generally more abundant than older stages in field populations (Hughes, 1963; Chau & Mackauer, 1997; Losey & Denno, 1998), parasitoids are very likely to successfully establish irrespective of A. glycines colony composition. Parasitoid preference for younger stages can significantly affect host population growth (e.g. Lin & Ives, 2003), while a sustained attack of older and larger A. glycines stages, along with its induction of costly defenses could reduce reproductive capacity of B. communis (Nelson & Rosenheim, 2005).
Successful B. communis parasitism of A. glycines alatoid nymphs and alates and its increased development time on winged aphid hosts suggests the existence of a phoretic association. Such association was suggested by Hoelmer & Kirk (2005), who reported the presence of B. communis in association with A. glycines at early stages of their colonization of soybean fields in China and hypothesized parasitoid arrival as eggs carried within winged aphids (see also Liu et al., 2004). Also, the finding that B. communis does not disrupt the development of wings of A. glycines alatoid nymphs (see Demmon et al., 2004; Christiansen-Weniger & Hardie, 2000) indicates that flight of parasitized aphids might be possible. Our findings can have implications for parasitoid establishment, dispersal capability and biological control success upon release in novel environments.
Acknowledgements
We thank Zeynep Sezen for helpful comments that improved the quality of the manuscript, and Jo Barta, Jon Malespy and Erika Commers for help with parasitoid and aphid colony maintenance. This work was funded in part by the multi-state USDA-RAMP project, in part by the North Central Soybean Research Council and in part by the Minnesota Agricultural Experiment Station.
References
- Battaglia D, Pennacchio F, Romano A, Tranfaglia A. The role of physical cues in the regulation of host recognition and acceptance behavior of Aphidius ervi Haliday (Hymenoptera: Braconidae) Journal of Insect Behavior. 1995;8:739–750. [Google Scholar]
- Chau A, Mackauer M. Dropping of pea aphids from feeding site: a consequence of parasitism by the wasp, Monoctonus paulensis. EntomologiaExperimentalis et Applicata. 1997;83:247–252. [Google Scholar]
- Chau A, Mackauer M. Host-instar selection in the aphid parasitoid Monoctonus paulensis (Hymenoptera: Braconidae, Aphidiinae): a preference for small aphids. European Journal of Entomology. 2000;97:347–353. [Google Scholar]
- Chau A, Mackauer M. Host-instar selection in the aphid parasitoid Monoctonus paulensis (Hymenoptera: Braconidae, Aphidiinae): assessing costs and benefits. Canadian Entomologist. 2001;133:549–564. [Google Scholar]
- Christiansen-Weniger P, Hardie J. The influence of parasitism on wing development in male and female pea aphids. Journal of Insect Physiology. 2000;46:861–867. doi: 10.1016/s0022-1910(99)00192-4. [DOI] [PubMed] [Google Scholar]
- Colinet H, Salin C, Boivin G, Hance Th. Host age and fitness-related traits in a koinobiont aphid parasitoid. Ecological Entomology. 2005;30:473–479. [Google Scholar]
- De Farias AMI, Hopper KR. Oviposition behavior of Aphelinus asychis and Aphidius matricariae and defense behavior of their host Diuraphis noxia. Environmental Entomology. 1999;28:858–862. [Google Scholar]
- Demmon AS, Nelson HJ, Ryan PJ, Ives AR, Snyder WE. Aphidius ervi (Hymenoptera: Braconidae) increases its adult size by disrupting host wing development. Environmental Entomology. 2004;33:1523–1527. [Google Scholar]
- Desneux N, Wajnberg E, Fauverge X, Privet S, Kaiser L. Oviposition behavior and patch-time allocation in two aphid parasitoids exposed to deltamethrin residues. Entomologia Experimental et Applicata. 2004;112:227–235. [Google Scholar]
- Dixon AFG. Aphid Ecology. Chapman & Hall; London, UK: 1998. p. 288. [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Gerling D, Roitberg BD, Mackauer M. Instar-specific defense of the pea aphid Acyrthosiphon pisum: influence on oviposition success of the parasite Aphelinus asychis (Hymenoptera: Aphelinidae) Journal of Insect Behavior. 1990;3:501–514. [Google Scholar]
- Godfray HCJ. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press; Princeton, NJ, USA: 1994. p. 488. [Google Scholar]
- Hågvar EB, Hofsvang T. Aphid parasitoids (Hymenoptera: Aphidiidae): biology, host selection and use in biological control. Biocontrol News and Information. 1991;12:13–41. [Google Scholar]
- Harvey JA. Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomologia Experimentalis et Applicata. 2005;117:1–13. [Google Scholar]
- Heimpel GE, Ragsdale DW, Venette R, Hopper KR, O'Neil RJ, Rutledge CE, Wu Z. Prospects for importation biological control of the soybean aphid: anticipating potential costs and benefits. Annals of the Entomological Society of America. 2004;97:249–258. [Google Scholar]
- Henry LM, Gillespie DR, Roitberg BD. Does mother really know best? Oviposition preference reduces reproductive performance in the generalist parasitoid Aphidius ervi. Entomologia Experimentalis et Applicata. 2005;116:167–174. [Google Scholar]
- Hight SC, Eikenbary RD, Miller RJ, Starks KJ. The greenbug and Lysiphlebus testaceipes. Environmental Entomology. 1972;1:205–209. [Google Scholar]
- Hodgson EW, Venette RC, brahamson M, Ragsdale DW. Alate production of soybean aphid (Homoptera: Aphididae) in Minnesota. Environmental Entomology. 2005;34:1456–1463. [Google Scholar]
- Hoelmer KA, Kirk AA. Selecting arthropod biological control agents against arthropod pests: can the science be improved to decrease the risk of releasing ineffective agents? Biological Control. 2005;34:255–264. [Google Scholar]
- Hopper KR. Preference, acceptance and fitness components of Microplitis croceipes (Hymenoptera: Braconidae) attacking various instars of Heliothis virescens (Lepidoptera: Noctuidae) Environmental Entomology. 1986;15:274–280. [Google Scholar]
- Hopper KR, King EG. Preference of Microplitis croceipes (Hymenoptera: Braconidae) for instars and species of Heliothis (Lepidoptera: Noctuidae) Environmental Entomology. 1984;13:1145–1150. [Google Scholar]
- Hughes RD. Population dynamics of the cabbage aphid, Brevicoryne brassicae (L.) Journal of Animal Ecology. 1963;32:393–424. [Google Scholar]
- Ives AR, Schooler SS, Jagar VJ, Knuteson SE, Grbic M, Settle WH. Variability and parasitoid foraging efficiency: a case study of pea aphids and Aphidius ervi. American Naturalist. 1999;154:652–673. doi: 10.1086/303269. [DOI] [PubMed] [Google Scholar]
- Jenner W, Kuhlmann U. Significance of host size for a solitary endoparasitoid: a trade-off between fitness parameters. Basic and Applied Ecology. 2006;7:461–471. [Google Scholar]
- Kelly EOG. The green-bug (Toxoptera graminum Rond.) outbreak of 1916. Journal of Economic Entomology. 1917;10:233–248. [Google Scholar]
- Kouamé KL, Mackauer M. Influence of aphid size, age and behaviour on host choice by the parasitoid wasp Ephedrus californicus: a test of host-size models. Oecologia. 1991;88:197–203. doi: 10.1007/BF00320811. [DOI] [PubMed] [Google Scholar]
- Lacoume S, Bressac C, Chevrier C. Effect of host size on male fitness in the parasitoid wasp Dinarmus basalis. Journal of Insect Physiology. 2006;52:249–254. doi: 10.1016/j.jinsphys.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Landau S, Everitt BS. A Handbook of Statistical Analyses Using SPSS. Chapman & Hall/CRC Press; Boca Raton, FL, USA: 2004. p. 354. [Google Scholar]
- Lawrence PO, Baranowski RM, Greany PD. Effect of host age on development of Biosteres longicaudatus, a parasitoid of the Carribean fruitfly, Anastrepha suspense. Florida Entomologist. 1976;59:33–39. [Google Scholar]
- Le Ralec A, Curty C, Wajnberg K. Inter-specific variation in the reactive distance of different aphid-parasitoid associations: analysis from automatic tracking of the walking path. Applied Entomology and Zoology. 2005;40:413–420. [Google Scholar]
- Lewis WJ, Redlinger LJ. Suitability of eggs of the almond moth Cadra cautella of various ages for parasitism by Trichogramma evanescens. Annals of the Entomological Society of America. 1969;62:1482–1484. [Google Scholar]
- Li BP, Mills N. The influence of temperature on size as an indicator of host quality for the development of a solitary koinobiont parasitoid. Entomologia Eperimentalis et Applicata. 2004;110:249–256. [Google Scholar]
- Lin LA, Ives AR. The effect of parasitoid host size preference on host population growth rates: an example of Aphidius colemani and Aphis glycines. Ecological Entomology. 2003;28:542–550. [Google Scholar]
- Liu J, Wu KM, Hopper KR, Zhao KJ. Population dynamics of Aphis glycines (Homoptera: Aphididae) and its natural enemies in soybean in northern China. Annals of the Entomological Society of America. 2004;97:235–239. [Google Scholar]
- Liu SS, Morton R, Hughes RD. Oviposition preferences of a hymenopterous parasite for certain instars of its aphid host. Entomologia Experimentalis et Applicata. 1984;35:249–254. [Google Scholar]
- Losey JE, Denno RF. The escape response of pea aphids to foliar-foraging predators: factors affecting dropping behaviour. Ecological Entomology. 1998;23:53–61. [Google Scholar]
- Mackauer M. Host selection and host suitability in Aphidius smithi. In: Lowe AD, editor. Perspectives in Aphid Biology. Entomological Society of New Zealand; Christchurch, New Zealand: 1973. pp. 20–29. [Google Scholar]
- Mackauer M, Michaud JP, ölkl W. Host choice by aphidiid parasitoids (Hymenoptera: Aphidiidae): host recognition, host quality and host value. Canadian Entomologist. 1996;128:959–980. [Google Scholar]
- Manly BFJ. A model for certain types of selection experiments. Biometrics. 1974;30:281–294. [Google Scholar]
- McWilliams DA, Berglund DR, Endres GJ. Soybean growth and management. North Dakota State University; 2004. Extension Publication No. A-1174. http://www.ag.ndsu.edu/pubs/plantsci/rowcrops/a1174/a1174w.htm. Accessed on March 2, 2007. [Google Scholar]
- Murdoch WW, Nisbet RM, Blythe SP, Gurney WSC, Reeve JD. An invulnerable age class and stability in delay-differential parasitoid-host models. American Naturalist. 1987;129:263–282. [Google Scholar]
- Murdoch WW, Briggs CJ, Nisbet RM. Consumer-Resource Dynamics. Monographs in Population Biology. Princeton University Press; Princeton, NJ, USA: 2003. p. 456. [Google Scholar]
- Nechols JR, Kikuchi RS. Host selection of the spherical mealybug (Homoptera: Pseudococidae) by Anagyrus indicus (Hymenoptera: Encyrtidae): influence of host age on parasitoid oviposition, development, sex ratio and survival. Environmental Entomology. 1985;14:32–37. [Google Scholar]
- Nelson EH, Rosenheim JA. Encounters between aphids and their predators: the relative frequencies of disturbance and consumption. Entomologia Experimentalis et Applicata. 2005;118:211–219. [Google Scholar]
- Perdikis DC, Lkouressis DR, Gaantonakis NG, Iatrou SA. Instar preference and parasitization of Aphis gossypii and Myzus persicae (Hemiptera: Aphididae) by the parasitoid Aphidius colemani (Hymenoptera: Aphididae) European Journal of Entomology. 2004;101:333–336. [Google Scholar]
- Ragsdale DW, Voegtlin DJ, O'Neil RJ. Soybean aphid biology in North America. Annals of the Entomological Society of America. 2004;97:204–208. [Google Scholar]
- Rakhshani E, Talebim AA, Kavllieratos N, Fathipour Y. Host stage preference, juvenile mortality and functional response of Trioxys pallidus. Biologia. 2004;59:197–203. [Google Scholar]
- Rauwald KS, Ives AR. Biological control in disturbed agricultural systems and the rapid recovery of parasitoid populations. Ecological Applications. 2001;11:1224–1234. [Google Scholar]
- Rivero A. The relationship between host selection behaviour and offspring fitness in a koinobiont parasitoid. Ecological Entomology. 2000;25:467–472. [Google Scholar]
- Rogers CE, Jackson HB, Eikenbary RD, Starks KJ. Host-parasitoid interaction of Aphis helianthi on sunflowers with introduced Aphelinus asychis, Ephedrus plagiator, and Praon gallicum, and native Aphelinus nigritus and Lysiphlebus testaceipes. Annals of the Entomological Society of America. 1972;65:38–41. [Google Scholar]
- Roitberg BD, Boivin G, Vet LEM. Fitness, parasitoids and biological control: an opinion. Canadian Entomologist. 2001;133:429–438. [Google Scholar]
- Sequeira R, Mackauer M. Host instar preference of the aphid parasite Praon pequodorum (Hymenoptera: Aphidiidae) Entomologia Generalis. 1987;12:259–265. [Google Scholar]
- Sequeira R, Mackauer M. Covariance of adult size and development time in the parasitoid wasps Aphidius ervi in relation to its host, Acyrthosiphum pisum. Evolutionary Ecology. 1992a;6:34–44. [Google Scholar]
- Sequeira R, Mackauer M. Nutritional ecology of an insect host parasitoid association - the pea aphid Aphidius ervi system. Ecology. 1992b;73:183–189. [Google Scholar]
- Sequeira R, Mackauer M. The nutritional ecology of a parasitoid wasp, Ephedrus californicus Baker (Hymenoptera: Aphidiidae) Canadian Entomologist. 1993;125:423–430. [Google Scholar]
- Sharmila P, Rajendra S. Host size induced variation in progeny sex ratio of an aphid parasitoid Lysiphlebia myrzai. Entomologia Experimentalis et Applicata. 1999;90:61–67. [Google Scholar]
- Stadler B, Weisser WW, Houston AI. Defense reactions in aphids - the influence of state and future reproductive success. Journal of Animal Ecology. 1994;63:419–430. [Google Scholar]
- Villagra CA, Ramirez CC, Niemeyer H. Anti-predator responses of aphids to parasitoids change as function of aphid physiological state. Animal Behaviour. 2002;64:677–683. [Google Scholar]
- Vinson SB. Effect of the parasitoid Campoletis sonorensis on the growth of its host, Heliothis virescens. Journal of Insect Physiology. 1972;18:1509–1514. [Google Scholar]
- Völkl W, Mackauer M. Oviposition behaviour of aphidiine wasps (Hymenoptera: Braconidae, Aphidiinae): morphological adaptations and evolutionary trends. Canadian Entomologist. 2000;132:197–212. [Google Scholar]
- Walker AM, Hoy MA. Responses of Lipolexis oregmae (Hymenoptera: Aphidiidae) to different instars of Toxoptera citricida (Homoptera: Aphididae) Journal of Economic Entomology. 2003;96:1685–1692. doi: 10.1093/jee/96.6.1685. [DOI] [PubMed] [Google Scholar]
- Wang XG, Liu SS. Effects of host age on the performance of Diadromus collaris, a pupal parasitoid of Plutella xylostella. Biocontrol. 2002;47:293–307. [Google Scholar]
- Weisser WW. Age-dependent foraging behaviour and host instar preference of the aphid parasitoid Lysiphlebus cardui. Entomologia Experimentalis et Applicata. 1994;76:133–141. [Google Scholar]
- Weisser WW. Within-patch foraging behaviour of the aphid parasitoid Aphidius funebris: plant architecture, host behaviour and individual variation. EntomologiaExperimentalis et Applicata. 1995;76:133–141. [Google Scholar]
- Wu Z, Hopper KR, O'Neil RJ, Voegtlin DJ, Prokrym DR, Heimpel G. Reproductive compatibility and genetic variation between two strains of Aphelinus albipodus (Hymenoptera: Aphelinidae), a parasitoid of the soybean aphid, Aphis glycines (Homoptera: Aphididae) Biological Control. 2004;31:311–319. [Google Scholar]
- Wyckhuys KAG, Heimpel GE. Response of the soybean aphid parasitoid Binodoxys communis to olfactory cues from target and non-target host-plant complexes. Entomologia Experimentalis et Applicata. 2007;123:149–158. [Google Scholar]
- Wyckhuys KAG, Koch RL, Heimpel GE. Physical and ant-mediated refuges from parasitism: implications for non-target effects in biological control. Biological Control. 2007a;40:306–313. [Google Scholar]
- Wyckhuys KAG, Hopper KR, Wu KM, Straub C, Gratton C, Heimpel GE. Predicting potential ecological impact of soybean aphid biological control introductions. CABI Biocontrol News and Information. 2007b;28:30N–34N. [Google Scholar]