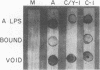
Abstract
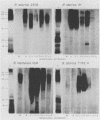
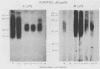
Monoclonal antibodies which bind Brucella A lipopolysaccharide (LPS)-specific, M LPS-specific, or cross-reactive epitopes were used as reagents in quantitative dot blot, Western blot (immunoblot), and immunoprecipitation analysis of Brucella whole cells, whole-cell extracts, and purified LPS preparations. This set of monoclonal antibodies detected four unique epitopes on Brucella LPS. The specificity of monoclonal antibodies reactive with Brucella unique (A and M) and common (C and C/Y) LPS epitopes was demonstrated by blot analysis. The serotype specificity of monoclonal antibodies for A LPS of B. abortus 1119.3 or M LPS of Brucella melitensis 16M was confirmed. Type C monoclonal antibodies recognized epitopes on Brucella A and M LPS and did not cross-react with Yersinia enterocolitica O:9. In Western blots, type C monoclonal antibodies were bound by epitopes on Brucella A and M LPSs ranging in Mrs from 30,000 to 70,000, relative to marker proteins. Type C/Y monoclonal antibodies were cross-reactive with Y. enterocolitica O:9 and recognized Brucella A LPS epitopes with a restricted Mr ranging only from 40,000 to 50,000, relative to marker proteins. Type C/Y monoclonal antibodies also displayed a more restricted pattern of binding to Brucella M LPS. The monoclonal antibodies were able to detect 5 to 50 pg of a purified A LPS preparation in dot blots. The limits of detection by the monoclonal antibodies of a purified M LPS preparation ranged from 0.05 to 50 pg. Monoclonal antibody analysis of whole-cell preparations also demonstrated quantitative differences in the presence of the respective epitopes. The binding profiles of the monoclonal antibodies to Brucella whole cells varied between acetone- and chloroform-killed organisms as well as between species and strains. The lower limit of detection of any whole-cell preparation by the dot blot technique was 10(5) CFU. Binding profiles in Western blots and endotoxin activity of immunoprecipitates obtained with these monoclonal antibodies further defined the Brucella LPS antigens. These monoclonal antibodies and the techniques described may be useful in monitoring the antigenic content of Brucella vaccines and diagnostics.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascoul S., Cannat A., Huguet M. F., Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978 Aug;35(2):213–221. [PMC free article] [PubMed] [Google Scholar]
- Bosseray N., Plommet M. Antagonism between two immunogens extracted from Brucella (cell wall peptidoglycan and lipopolysaccharide fractions) and inactivity of the brucellin allergen in immunization of the mouse. Ann Microbiol (Paris) 1980 Mar-Apr;131A(2):157–169. [PubMed] [Google Scholar]
- Brada D., Roth J. "Golden blot"--detection of polyclonal and monoclonal antibodies bound to antigens on nitrocellulose by protein A-gold complexes. Anal Biochem. 1984 Oct;142(1):79–83. doi: 10.1016/0003-2697(84)90518-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Caroff M., Perry M. B. The lipopolysaccharides of Brucella abortus and B. melitensis. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):92–98. doi: 10.1016/0769-2609(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. The structure of the lipopolysaccharide O-chain (M antigen) and polysaccharide B produced by Brucella melitensis 16M. FEBS Lett. 1987 Jun 1;216(2):261–264. doi: 10.1016/0014-5793(87)80702-0. [DOI] [PubMed] [Google Scholar]
- Butler J. E., Seawright G. L., McGivern P. L., Gilsdorf M. Preliminary evidence for a diagnostic immunoglobulin G1 antibody response among culture-positive cows vaccinated with Brucella abortus strain 19 and challenge exposed with strain 2308. Am J Vet Res. 1986 Jun;47(6):1258–1264. [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur J Biochem. 1984 Feb 15;139(1):195–200. doi: 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Diaz R., Garatea P., Jones L. M., Moriyon I. Radial immunodiffusion test with a Brucella polysaccharide antigen for differentiating infected from vaccinated cattle. J Clin Microbiol. 1979 Jul;10(1):37–41. doi: 10.1128/jcm.10.1.37-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. T., Naka S. O., Lee J. W. Development of an ELISA for detection of antibody in leprosy. Int J Lepr Other Mycobact Dis. 1984 Mar;52(1):19–25. [PubMed] [Google Scholar]
- Douglas J. T., Palmer D. A. Use of monoclonal antibodies to identify the distribution of A and M epitopes on smooth Brucella species. J Clin Microbiol. 1988 Jul;26(7):1353–1356. doi: 10.1128/jcm.26.7.1353-1356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Limet J. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):27–37. doi: 10.1016/0769-2609(87)90051-2. [DOI] [PubMed] [Google Scholar]
- Fernandex-Lago L., Diaz R. Demonstration of antibodies against Brucella melitensis 16M lipopolysaccharide and native hapten in human sera by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Jul;24(1):76–80. doi: 10.1128/jcm.24.1.76-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lago L., Moriyon I., Toyos J., Diaz R. Immunological identity of brucella native hapten, polysaccharide B, and yersinia enterocolitica serotype 9 native hapten. Infect Immun. 1982 Nov;38(2):778–780. doi: 10.1128/iai.38.2.778-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman P. J., Adams L. G., Hunter D. M., Heck F. C., Nielsen K. H., Wagner G. G. Derivation of monoclonal antibodies against Brucella abortus antigens. Vet Immunol Immunopathol. 1983 Jul;4(5-6):603–614. doi: 10.1016/0165-2427(83)90068-5. [DOI] [PubMed] [Google Scholar]
- Hurvell B., Lindberg A. A., Carlsson H. E. Differentiation of antibodies against Brucella abortus and Yersinia enterocolitica by enzyme-linked immunosorbent assay. Contrib Microbiol Immunol. 1979;5:73–79. [PubMed] [Google Scholar]
- Limet J., Plommet A. M., Dubray G., Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- Maze M., Gray G. M. Intestinal brush border aminooligopeptidases: cytosol precursors of the membrane enzyme. Biochemistry. 1980 May 27;19(11):2351–2358. doi: 10.1021/bi00552a011. [DOI] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Borowiak D., Mayer H. Brucella lipopolysaccharides and polysaccharides. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):102–105. doi: 10.1016/0769-2609(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Moreno E., Mayer H., Moriyon I. Characterization of a native polysaccharide hapten from Brucella melitensis. Infect Immun. 1987 Nov;55(11):2850–2853. doi: 10.1128/iai.55.11.2850-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Speth S. L., Jones L. M., Berman D. T. Immunochemical characterization of Brucella lipopolysaccharides and polysaccharides. Infect Immun. 1981 Jan;31(1):214–222. doi: 10.1128/iai.31.1.214-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983 Jul;41(1):97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Amdams L. G., Pugh R. Immunochemical and partial chemical characterization of fractions of membrane-bound smooth lipopolysaccharide-protein complex from Brucella abortus. Mol Cell Biochem. 1987 Jun;75(2):93–102. doi: 10.1007/BF00229897. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Mackenzie N. E. Structural and immunochemical characterization of the O-haptens of Brucella abortus lipopolysaccharides from strains 19 and 2308. Mol Cell Biochem. 1987 Jun;75(2):103–111. doi: 10.1007/BF00229898. [DOI] [PubMed] [Google Scholar]
- Zygmunt M. S., Dubray G. Preparation by ultrafiltration and control by high-performance liquid chromatography of the native hapten of Brucella abortus for use in radial immunodiffusion diagnostic test. J Clin Microbiol. 1987 Oct;25(10):1860–1863. doi: 10.1128/jcm.25.10.1860-1863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]