Abstract
BACKGROUND
A single family has been described in which obesity results from a mutation in the leptin-receptor gene (LEPR), but the prevalence of such mutations in severe, early-onset obesity has not been systematically examined.
METHODS
We sequenced LEPR in 300 subjects with hyperphagia and severe early-onset obesity, including 90 probands from consanguineous families, and investigated the extent to which mutations cosegregated with obesity and affected receptor function. We evaluated metabolic, endocrine, and immune function in probands and affected relatives.
RESULTS
Of the 300 subjects, 8 (3%) had nonsense or missense LEPR mutations — 7 were homozygotes, and 1 was a compound heterozygote. All missense mutations resulted in impaired receptor signaling. Affected subjects were characterized by hyperphagia, severe obesity, alterations in immune function, and delayed puberty due to hypogonadotropic hypogonadism. Serum leptin levels were within the range predicted by the elevated fat mass in these subjects. Their clinical features were less severe than those of subjects with congenital leptin deficiency.
CONCLUSIONS
The prevalence of pathogenic LEPR mutations in a cohort of subjects with severe, early-onset obesity was 3%. Circulating levels of leptin were not disproportionately elevated, suggesting that serum leptin cannot be used as a marker for leptin-receptor deficiency. Congenital leptin-receptor deficiency should be considered in the differential diagnosis in any child with hyperphagia and severe obesity in the absence of developmental delay or dysmorphism.
The assessment of patients with severe early-onset obesity conventionally includes screening for potentially treatable neurologic and endocrine conditions and identifying known genetic conditions so that appropriate genetic counseling and, in some cases, treatment can be instituted.1 Classically, patients with genetic obesity syndromes have been identified in childhood as a result of associated mental retardation and developmental abnormalities.2 However, several monogenic disorders have been identified in which obesity itself is the predominant presenting feature. These disorders result from disruption of the hypothalamic leptin–melanocortin signaling pathway.3-8
Twelve subjects with congenital leptin deficiency due to loss-of-function mutations in the gene encoding leptin have been identified3,4,9,10 (and unpublished data). Characteristic features include hyperphagia, obesity, hypogonadism, and impaired T-cell–mediated immunity. Treatment with recombinant human leptin reverses all aspects of the phenotype.9,11,12 So far, only one mutation in the leptin-receptor gene (LEPR)has been reported, in three severely obese adult siblings from a consanguineous family of Algerian origin.5 This mutation results in abnormal splicing of leptin-receptor transcripts and generates a mutant leptin receptor that lacks both transmembrane and intracellular domains. The mutant receptor circulates at high concentrations, binding leptin and resulting in very elevated serum leptin levels.13 To determine the prevalence of pathogenic mutations in LEPR in severely obese patients, we studied 300 subjects with severe, early-onset obesity.
Methods
Subjects
When we began the study, the Genetics of Obesity Study (GOOS) cohort consisted of 2100 unrelated probands with severe obesity of early onset (before 10 years of age); severe obesity was defined as a standard-deviation score for the body-mass index (BMI) (the weight in kilograms divided by the square of the height in meters) of more than 3. We calculated BMI standard-deviation scores using reference data from the U.K. population.14 The mean (±SD) score in the GOOS cohort is 4.2±0.8. Of the 2100 subjects, 1800 were reported to have a history of hyperphagia. Of these 1800 subjects, 300 were selected to determine the prevalence of leptin-receptor mutations: all 90 subjects in the GOOS cohort from consanguineous families, as well as 210 additional subjects who were impartially selected. The mean BMI standard-deviation score for the group screened was 4.5±1.2. Mutationsin known obesity genes were ruled out with the use of biochemical analysis (mutant leptin and prohormone convertase 1 genes) and direct nucleotide sequencing (of the genes encoding pro-opiomelanocortin and the melanocortin 4 receptor [MC4R]); there were no mutations in additional candidate genes for obesity (SIM1, NHLH2, CPE, MCHR1, and MCHR2).
Subjects with mutations in LEPR and their relatives were invited to participate in clinical studies at the Wellcome Trust Clinical Research Facility at Addenbrooke's Hospital, Cambridge, United Kingdom. All studies were approved by the Anglia and Oxford multiregional ethics committee and the local–regional ethics committee of Cambridge. Each subject, or his or her parent if the subject was a child younger than 16 years, provided written informed consent; the minors provided oral consent. All clinical studies were conducted in accordance with the principles of the Declaration of Helsinki.
In adults, overweight and obesity were defined according to the World Health Organization criteria: a BMI of 25.0 to 29.9 and a BMI of 30.0 or higher, respectively. Because there are no internationally recognized definitions of overweight and obesity in persons under 18 years of age, we used criteria proposed by the International Obesity Task Force and supported by a recent International Consensus on Childhood Obesity15: an age-adjusted BMI above the 91st percentile and an age-adjusted BMI above the 99th percentile, respectively.
DETECTION OF MUTATIONS AND GENOTYPING
Genomic DNA was isolated from leukocytes derived from whole blood, and the coding region of the LEPR gene was amplified with the use of the polymerase chain reaction and was then sequenced (see the Supplementary Appendix, available with the full text of this article at www.nejm.org). We also sequenced LEPR in impartially selected non-obese control subjects: 100 alleles from each of three population-derived cohorts — of white European origin,16 of South Asian origin,17 and of Turkish origin.18 (See the Supplementary Appendix for details of our studies of mutant-receptor function.)
BODY COMPOSITION, GROWTH, AND ENERGY BALANCE
We used anthropometric methods and whole-body dual-energy x-ray absorptiometry (DPX software, Lunar) to determine body composition, as previously described.8,11 We measured resting metabolic rate using indirect calorimetry after a 12-hour overnight fast and using an open-circuit, ventilated, canopy measurement system (Europa Gas Exchange Monitor, Nutren Technology). After adjustment for body composition, the resting metabolic rate was compared with that predicted by age- and sex-specific equations.19,20 Semiquantitative assessment of eating behavior was undertaken in subjects younger than 18 years, as previously described.8,9
METABOLIC AND ENDOCRINE STUDIES
Fasting blood samples were analyzed for levels of leptin, glucose, insulin, thyrotropin, free thyroxine, insulin-like growth factor 1 (IGF-1), follicle-stimulating hormone, luteinizing hormone, estradiol, and testosterone, with the use of standard assays.11
LYMPHOCYTE COUNT AND FUNCTION
Lymphocytes were isolated from fresh whole blood, and cell phenotypes were measured by cytofluorometric (fluorescence-activated cell-sorting) analysis, as reported previously.9 Proliferative responses to antigenic stimuli were measured.9 Lymphocyte counts and proliferative responses in subjects with leptin-receptor deficiency were compared with those in 46 control subjects matched for age (from 8 years to adult).
STATISTICAL ANALYSIS
Clinical data are expressed as means ±SD. Differences between groups were compared with use of the unpaired Student's t-test. All reported P values are from two-sided tests, and P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
DETECTION OF MUTATIONS AND FAMILY STUDIES
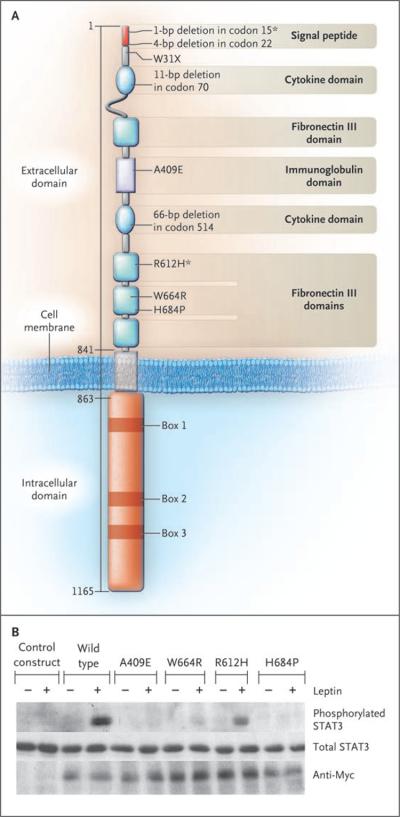
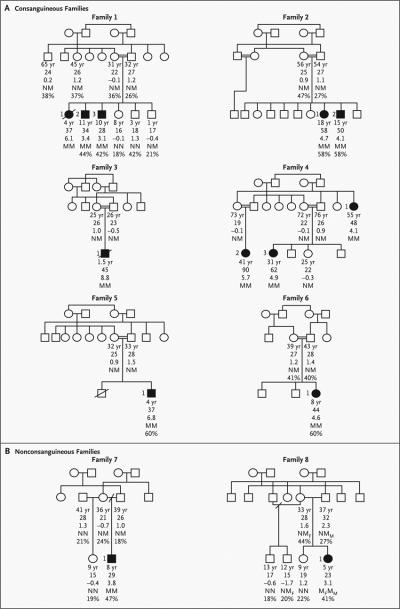
We identified five nonsense and four missense mutations in 8 of the 300 probands (Table 1 and Fig. 1A). Seven probands were homozygous for such mutations, and one proband (Subject 1 in Family 8) was a compound heterozygote for a missense mutation (R612H) and a nonsense mutation. None of these mutations were found in alleles from control subjects. Six of the probands were from consanguineous families (Fig. 2A). In three pedigrees (Families 1, 2, and 4), additional homozygous family members were identified, all of whom had severe, early-onset obesity (Fig. 2A).
Table 1.
Leptin-Receptor Mutations in Subjects with Severe Early-Onset Obesity.
| Mutation | Family No. |
No. of Affected Subjects |
Race or Ethnic Group* |
|---|---|---|---|
| Homozygous frame-shift | |||
| 4-bp deletion in codon 22 | 1 | 3 (1 deceased) | Bangladeshi |
| 11-bp deletion in codon 70 | 2 | 2 | Turkish |
| 66-bp deletion in codon 514 | 3 | 1 (deceased) | Iranian |
| Homozygous nonsense | |||
| W31X | 4 | 3 | Southern European |
| Homozygous missense | |||
| A409E | 5 | 1 | Turkish |
| W664R | 6 | 1 | Norwegian |
| H684P | 7 | 1 | White (United Kingdom) |
| Compound heterozygous | |||
| 1-bp deletion in codon 15 and R612H | 8 | 1 | White (United Kingdom) |
Race or ethnic group was assigned by the physician.
Figure 1. Mutations in the Leptin-Receptor Gene (Panel A) and in Vitro Function of the Leptin-Receptor Mutant Constructs (Panel B).
Panel A shows the positions of the identified mutations in the leptin receptor. We observed homozygous mutations in seven severely obese probands; in an eighth (Subject 1 in Family 8, a compound heterozygote), we observed a nonsense mutation and a missense mutation (asterisks). Amino acid numbers are shown on the left-hand side. Panel B shows the induction of the phosphorylation of signal transducer and activator of transcription 3 (STAT3) by wild-type and mutant leptin receptors in the absence (minus signs) and presence (plus signs) of recombinant human leptin. The control construct was an empty pcDNA3 vector.
Figure 2. Pedigrees of Consanguineous Families and Nonconsanguineous Families with Mutations in the Leptin-Receptor Gene That Segregate with Severe Obesity.
The squares represent male family members, and the circles female family members; open symbols represent unaffected family members, and solid symbols family members with obesity (in adults, defined as a BMI [the weight in kilograms divided by the square of the height in meters] of 30 or more; in children, defined as a BMI above the 99th age-adjusted percentile). A slash through the symbol denotes a subject who has died. Below each symbol, age is given, followed by the BMI value, the BMI standard-deviation score, and the genotype, with N denoting the normal (wild-type) allele and M the mutant allele. In Family 8, Mf denotes the frame-shift mutation, and Mm the missense mutation. For subjects in whom it was available, the percentage of body fat, measured by dual-energy x-ray absorptiometry, is listed below the genotype.
FUNCTIONAL ANALYSIS OF MUTANT RECEPTORS
All frame-shift mutations occurred in the N-terminal domain of the leptin receptor and were predicted to result in the loss of all leptin-receptor isoforms. We examined the signaling properties of receptors with missense mutations (Fig. 1B). Three of the missense mutations (A409E, W664R, and H684P) resulted in a complete loss of signaling, as measured by leptin-stimulated phosphorylation of signal transducer and activator of transcription 3 (STAT3). The other missense mutation, R612H, encodes receptors with some residual ability to phosphorylate STAT3 in response to leptin (Fig. 1B).
CLINICAL PHENOTYPE OF LEPTIN-RECEPTOR DEFICIENCY
We studied the clinical phenotype of leptin-receptor deficiency in 10 subjects (6 probands and 4 affected relatives) with early-onset obesity who were homozygous for complete loss-of-function mutations in LEPR. All 10 had become obese in early childhood; at the time of study, 4 were adults and 6 were children. We compared their phenotype with that of five subjects with congenital leptin deficiency and with that of five subjects who were homozygous for complete loss-of-function mutations in MC4R, whom we had studied previously using identical protocols8,9 (and unpublished data).
Body Composition and Energy Balance
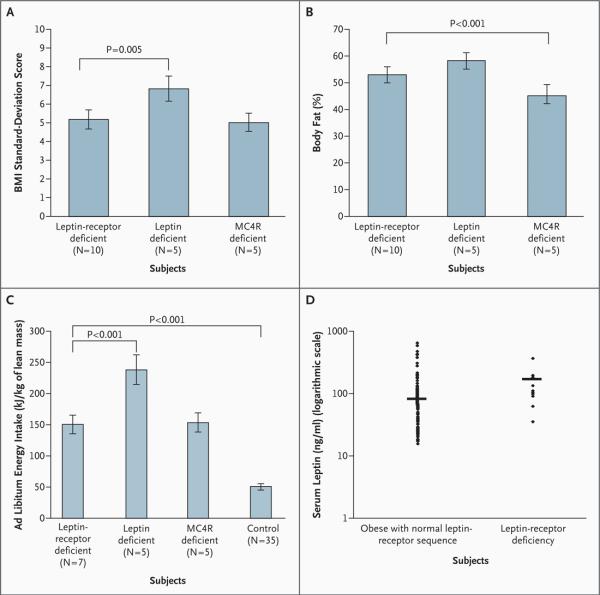
In all cases, the body weight of subjects with LEPR mutations deviated from predicted percentiles within the first year of life (data not shown). The mean BMI standard-deviation score for these subjects was 5.1±1.6, as compared with 6.8±2.1 for subjects with congenital leptin deficiency (P=0.005) and 5.0±1.5 for MC4R-deficient subjects. The mean percentage of body fat among homozygous carriers of LEPR mutations was high (52.8±3.2; normal range, 15 to 25) and was similar to that in the subjects with congenital leptin deficiency (mean, 58.0±3.5) but higher than that in the subjects lacking MC4R (45.2±3.3; P<0.001) (Fig. 3B). The percentage of lean mass was lower in subjects with LEPR mutations than in equally obese subjects without such mutations, although the actual amount of lean mass in kilograms for each subject was within the age-related normal range21,22 (data not shown).
Figure 3. Body-Mass Index (BMI) (Panel A), Percentage of Body Fat (Panel B), Ad Libitum Energy Intake (Panel C), and Serum Leptin Levels (Panel D) in Leptin-Receptor Deficiency.
Panel A shows the BMI standard-deviation score.14 Panel B shows the percentage of body fat as measured by dual-energy x-ray absorptiometry. Panel C shows the ad libitum energy intake at a test meal (adjusted for kilograms of lean mass).8 In Panel D, the horizontal line indicates the geometric mean. The data in Panels A, B, and C are means ±SD. Subjects 1, 2, and 3 in Family 4 were not able to travel to the United Kingdom, so data for 7 (rather than 10) subjects who were homozygous for LEPR mutations are presented in Panels B and C. The BMI is calculated as the weight in kilograms divided by the square of the height in meters. MC4R denotes melanocortin 4 receptor.
All subjects had a history of increased food-seeking behavior in childhood, which continued into later life in the adult subjects. During an ad libitum test meal, the probands with LEPR mutations consumed almost three times the amount of energy that control subjects consumed (Fig. 3C). This increased intake was similar to that of subjects lacking MC4R but considerably less than that of subjects with congenital leptin deficiency. The basal metabolic rate was greater in leptin-receptor-deficient, obese subjects than in subjects of normal weight, as one would expect. However, the basal metabolic rate is usually adjusted for lean mass (in kilograms) to allow for differences in body weight (and thus lean mass) among subjects. The adjusted basal metabolic rate per kilogram of lean mass in the subjects with LEPR mutations was similar to that predicted for persons with this body composition on the basis of accepted age- and sex-specific calculations23 (see Fig. 1A in the Supplementary Appendix).
Metabolic and Endocrine Function
Most of the subjects with LEPR mutations had normal glucose concentrations (Table 2), although the two oldest adults (Subjects 1 and 2 in Family 4) had type 2 diabetes, which was managed with oral hypoglycemic medication. All subjects had hyperin-sulinemia (Table 2) to a degree consistent with the degree of obesity (see Fig. 1B in the Supplementary Appendix).
Table 2.
Metabolic and Endocrine Features of Leptin-Receptor Deficiency in Subjects Homozygous for LEPR Mutations.*
| Variable | Subject | Normal Range† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family no. | 5 | 6 | 7 | 1 | 1 | 2 | 2 | 4 | 4 | 4 | |
| Subject no. | 1 | 1 | 1 | 3 | 2 | 2 | 1 | 3 | 2 | 1 | |
| Age (yr) |
4 | 8 | 8 | 10 | 11 | 15 | 18 | 31 | 41 | 55 | |
| Sex | M | F | M | M | M | M | F | F | F | F | |
| Glucose (mg/dl) |
76 | 76 | 81 | 88 | 83 | 94 | 72 | 99 | 157 | 130 | 75–115 |
| Insulin (μU/ml) |
4 | 14 | 17 | 28 | 81 | 26 | 29 | 15 | Diabetes mellitus |
Diabetes mellitus |
5–20 |
| Standard-deviation score for height |
1.8 | 4.2 | 2.1 | 1.3 | 1.8 | −1.7 | −2.0 | −2.0 | −2.0 | — | |
| IGF-1 (U/ml) |
4 | 29.1 | 27.3 | 19.5 | 16.5 | 8.4 | 8.0 | 8.5 | 5.7 | — | Age-specific |
| FSH (IU/L) |
0.3 | 0.3 | 0.2 | 0.2 | 1.3 | 3.7 | 1.8 | 4.2 | 7.7 | 13.7 | Age-specific |
| Luteinizing hormone (IU/L) |
0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 1.8 | 0.9 | 4.8 | 6.8 | 7.6 | Age-specific |
| Estradiol (pg/ml) |
— | 18.3 | — | — | — | — | 17.2 | 54.8 | 50.4 | 13.6 | Age-specific |
| Testosterone (ng/ml) |
— | 0.058 | 0.058 | 0.058 | 0.26 | — | — | — | — | Age-specific | |
| Thyrotropin (mU/L) |
3.3 | 1.6 | 4.1 | 0.8 | 2.1 | 0.9 | 0.9 | 2.9 | 4.2 | 3.8 | 0.6–4.6 |
| Free thyroxine (ng/dl) |
1.1 | 1.0 | 0.9 | 1.1 | 1.1 | 1.1 | 1.1 | 0.9 | 1.2 | 1.0 | 0.7–1.7 |
| Serum leptin (ng/ml) |
36 | 194 | 14 | 97 | 110 | 178 | 365 | 180 | 133 | 90 |
To convert values for glucose to millimoles per liter, multiply by 0.05551; to convert values for insulin to picomoles per liter, multiply by 6.95; to convert values for estradiol to picomoles per liter, multiply by 3.671; to convert values for testosterone to nanomoles per liter, multiply by 3.467; and to convert values for thyroxine to picomoles per liter, multiply by 12.87.
The age-specific normal ranges for insulin-like growth factor 1 (IGF-1) are 2.5 to 20.0 U per liter for 0.0 to 6.9 years, 4.5 to 37.5 for 7.0 to 9.9 years, 7.0 to 50.0 for 10.0 to 10.9 years, 8.5 to 60.0 for 11.0 to 11.9 years, 10.0 to 75.0 for 12.0 to 12.9 years, and 7.0 to 50.0 for 13.0 years or older; for follicle-stimulating hormone (FSH), less than 0.2 U per liter for clinically prepubertal subjects, 2.9 to 8.4 for women in the follicular phase of the menstrual cycle, 21.0 to 140.0 for postmenopausal women, and 1.0 to 10.0 for men; for luteinizing hormone (LH), less than 0.2 U per liter for clinically prepubertal subjects, 1.3 to 8.4 for women in the follicular phase, 16.0 to 75.0 for postmenopausal women, and 1.5 to 6.3 for men; for estradiol, less than 22 pg per milliliter for clinically prepubertal persons, 27 to 204 for women in the follicular phase, and less than 30 for postmenopausal women; and for testosterone, less than 14.3 ng per deciliter for clinically prepubertal persons and 229.0 to 1086.0 for men.
In childhood, linear growth was normal, and the standard-deviation scores for height of children with LEPR mutations (Table 2) were similar to those of equally obese children without LEPR mutations.8 Serum levels of IGF-1 were appropriate for the age of the children (Table 2), and growth hormone was secreted in a pulsatile fashion (data not shown). However, final height was reduced in adults with LEPR mutations, owing to the lack of a pubertal growth spurt. This is reflected in the short statures of the 15-year-old male subject with a standard-deviation score for height of −1.7 and the four female subjects with a score of −2.0 (Table 2).
All four adults (all of whom were female) had clinical evidence of hypogonadism, with lack of a pubertal growth spurt and reduced expression of secondary sexual characteristics; three had no menses until after 20 years of age. The 18-year-old female (Subject 1 in Family 2) and the 15-year-old male (Subject 2 in Family 2), both of whom were clinically prepubertal, had low sex-steroid levels and low follicle-stimulating hormone and luteinizing hormone levels, indicative of hypogonadotropic hypogonadism. In addition, Subject 2 in Family 1 had a complete loss of luteinizing hormone pulsatility (see Fig. 1C in the Supplementary Appendix). Notably, the females who were 31, 41, and 55 years of age had irregular menses after the age of 20 years and had estradiol, luteinizing hormone, and follicle-stimulating hormone levels that were consistent with their age. Free thyroxine and thyrotropin levels were with in the normal range in all subjects (Table 2).
Serum leptin levels in subjects with LEPR mutations (Table 2) were similar to those in equally obese subjects with a normal leptin-receptor gene sequence (Fig. 3D). Serum leptin levels correlated with the fat mass in subjects with LEPR mutations and in age- and BMI-matched subjects(see Fig. 1D in the Supplementary Appendix).
Immune Function
All the children with LEPR mutations had more frequent childhood infections (predominantly of the upper respiratory tract) than did their siblings with wild-type LEPR. The premature deaths of two obese children in these families were associated with acute respiratory tract infections. Since our group previously found that leptin-deficient subjects have a marked reduction in the CD4+ T-cell count and reduced T-cell proliferation,9 we analyzed the immunophenotype and T-cell responses in subjects with LEPR mutations and compared these data with those of age-matched control subjects. We observed a modest reduction in the absolute CD4+ T-cell counts in the leptin-receptor–deficient subjects (mean, 988±186 cells per cubic millimeter; 42±13%), as compared with those in control subjects (mean, 1100±892 cells per cubic millimeter; 44±7%), although the CD4+:CD8+ ratios were similar(2±2 and 2±1). However, leptin-receptor–deficient subjects had a significant compensatory increase in the CD19+ cell (B-cell) count (mean, 460±238 cells per cubic millimeter; 18±6%) as compared with control subjects (mean, 280±328 cells per cubic millimeter; 11±3%)(P=0.006). T cells from subjects with LEPR mutations showed reduced proliferative responses to a variety of polyclonal stimuli specific to T cells, such as muromonab-CD3 (OKT3), phytohemagglutinin, phorbol myristate acetate (PMA) or ionomycin (Iono), and the recall antigen purified protein derivative (PPD) (see Fig. 1E in the Supplementary Appendix). The cytokine pattern of the subjects with leptin-receptor deficiency was less impaired than that of the leptin-deficient subjects, with only a modest reduction in secretion of the proinflammatory cytokine interferon-γ as compared with age-matched controls — especially during OKT3 and PMA/Iono stimulation — and increased secretion of the inhibitory cytokine interleukin-10 during stimulation with purified protein derivative (see Fig. 1F and 1G in the Supplementary Appendix), but no significant change in interleukin-4 secretion (see Fig. 1H in the Supplementary Appendix).
Heterozygote Phenotype
We assessed the level of obesity in the 22 family members who were heterozygous and the 6 who were homozygous for wild-type LEPR. Heterozygous subjects were not severely obese, and their mean BMI standard-deviation score (0.6±0.8) was similar to that of their relatives who were homozygous for wild-type LEPR (0.6±1.0). We compared the measured percentage of body fat and that predicted according to height and weight; the absolute difference was significantly greater in the 14 of the 22 heterozygotes for whom fat-mass data were available than in their relatives with wild-type LEPR6 (mean, 8.2% vs. 2.1%; P=0.009).
DISCUSSION
The prevalence of pathogenic LEPR mutations in our subjects with hyperphagia and severe early-onset obesity was 3%. The prevalence of LEPR mutations in this highly selected cohort is unlikely to reflect that in unrelated populations of obese subjects or in populations in which the age at the onset of obesity is more heterogeneous.24,25 Six of the probands were from consanguineous families, but two probands (including the compound heterozygote) were whites in the United Kingdom whose parents were not known to be related. Although the prevalence of LEPR mutations is likely to be higher in ethnic groups in which consanguinity is common, LEPR deficiency should be considered in all patients with hyperphagic obesity of early onset.
None of the subjects with LEPR mutations characterized in this study, including those with nonsense mutations that were predicted to result in the loss of all isoforms, had disproportionately elevated serum leptin levels. Thus, serum leptin levels are not a generally useful marker of leptin-receptor deficiency — contrary to a previous suggestion.13
Congenital leptin-receptor deficiency is characterized by severe, early-onset obesity associated with selective deposition of fat mass, as seen in subjects with leptin deficiency.9 All of our subjects had hyperphagia from an early age, and we demonstrated that the ad libitum energy intake was greatly increased in children with leptin-receptor deficiency, with no evidence of a major deficit in basal energy expenditure.
Children with leptin-receptor deficiency had normal linear growth during childhood and had normal IGF-1 levels. However, because of the lack of a pubertal growth spurt, the final height of adult subjects was reduced. In the one previously described family, short stature and abnormal serum growth hormone levels and IGF-binding protein 3 levels were noted in childhood.5 However, assessment of the growth hormone–IGF axis is difficult in obese children and adults, since obesity is itself associated with abnormal results of basal and of dynamic tests of this axis.26,27 We conclude that impaired linear growth does not appear to be a common characteristic of patients with this disease.
Adults with leptin-receptor deficiency have hypogonadotropic hypogonadism and do not undergo puberty. Irregular menses developed in the third and fourth decades in the three oldest women in our study, as reported previously for one woman with leptin deficiency.28 It is plausible that the excess mass of adipose tissue leads to the production of sufficient estrogen (owing to the action of aromatase) to result in uterine development and irregular menses in the absence of fully developed secondary sexual characteristics. However, this may not be the only explanation, since luteinizing hormone and follicle-stimulating hormone levels in these three subjects were within the normal range for the follicular phase of the menstrual cycle, suggesting that even in the absence of leptin activity, some activation of the hypothalamic–pituitary–gonadal axis is possible, albeit temporally delayed.
Subjects with LEPR mutations tended to have a lower CD4+ T-cell count and a significantly greater compensatory B-cell count than age-matched control subjects — findings that are consistent with the known effects of leptin on immune function.29 Lymphocytes in the affected subjects showed decreased proliferation and altered cytokine release in response to nonspecific and antigen-specific stimuli. In two families, very obese children died after an infection in the first decade of life. It is likely that this immune dysfunction, perhaps together with impaired respiratory reserve as a result of severe obesity, contributed to these early deaths.
Heterozygotes who were leptin-receptor-deficient but not obese had an increased fat mass, a finding consistent with our observation that heterozygote carriers of a leptin mutation had 23% more fat than was predicted with anthropometric methods.17 These findings are consistent with those of Chung et al., who found an increase in the fat mass of mice that were heterozygous for deletion of leptin or of the leptin receptor (ob+/− or db+/−).30
Our data suggest that several phenotypic features seen in subjects with leptin-receptor deficiency are not as severe as those in subjects with leptin deficiency.9,11 This is surprising, given the fact that the LEPR protein product is the only known receptor for leptin and given the phenotypic similarity between mice lacking leptin (ob/ob) and mice lacking the leptin receptor (db/db) that share the same genetic background.31 The differences seen between the two groups of subjects may relate to the fact that our leptin-receptor–deficient subjects were, on average, older than the leptin-deficient subjects we studied previously.9 Also, the leptin-deficient subjects were of Pakistani origin, whereas the leptin-receptor–deficient subjects were from various ethnic groups. However, the differences between these two groups are striking in magnitude and consistency and raise the possibility that in humans, the canonical leptin receptor may not be the only receptor that mediates the actions of leptin, at least when serum leptin levels are high.
Congenital leptin-receptor deficiency cannot be ruled out by measuring serum leptin levels, and this diagnosis should be considered in all patients with severe obesity and hyperphagia in the absence of developmental delay and dysmorphic features. This diagnosis has implications for the care of these patients, both in terms of genetic counseling of the affected families and in terms of future prospects for treatment, since these patients would be predicted to have a favorable response to drugs targeted at pathways downstream of the leptin receptor.
Supplementary Material
Acknowledgments
Supported by grants from the Wellcome Trust (to Drs. Farooqi, Collins, Bottomley, Barroso, and O'Rahilly), the Medical Research Council (to Dr. O'Rahilly), the Norwegian Foundation for Health and Rehabilitation and the Eastern Regional Health Authorities (to Dr. Undlien), Fondo de Investigaciones Sanitarias (FIS PI02/0544, to Dr. Lopez-Fernandez) and Fundacion Canaria de Investigacióny Salud (FUNCIS PI 4800, to Dr. Lopez-Fernandez) in Spain, the Juvenile Diabetes Research Foundation–Telethon–Italy (GJT04008, to Dr. Matarese), and the European Union FP6 (LSHM-CT-2003-503041, to Drs. Barroso and O'Rahilly).
We thank Beate Skinningsrud, Daniala Aufiero, and Allan Daly for technical assistance; Mrs. Juana Ledesma and colleagues from Servicio de Endocrinologia y Nutricion, Hospital Universitario de Canarias; Dr. Emilia Llanos and the people of Alojera village in La Gomera (Canary Islands) for help with the family studies; the subjects and their families for their participation; and the physicians involved in GOOS.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kiess W, Galler A, Reich A, et al. Clinical aspects of obesity in childhood and adolescence. Obes Rev. 2001;2:29–36. doi: 10.1046/j.1467-789x.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med. 2005;56:443–58. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- 3.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 4.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–5. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 5.Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 6.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–6. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 8.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 9.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson WT, Farooqi IS, Moreau M, et al. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab. 2004;89:4821–6. doi: 10.1210/jc.2004-0376. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 12.Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101:4531–6. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahlou N, Clement K, Carel JC, et al. Soluble leptin receptor in serum of subjects with complete resistance to leptin: relation to fat mass. Diabetes. 2000;49:1347–52. doi: 10.2337/diabetes.49.8.1347. [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–9. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speiser PW, Rudolf MC, Anhalt H, et al. Childhood obesity. J Clin Endocrinol Metab. 2005;90:1871–87. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- 16.Williams DR, Wareham NJ, Brown DC, et al. Undiagnosed glucose intolerance in the community: the Isle of Ely Diabetes Project. Diabet Med. 1995;12:30–5. doi: 10.1111/j.1464-5491.1995.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 17.Farooqi IS, Keogh JM, Kamath S, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 18.Semple RK, Achermann JC, Ellery J, et al. Two novel missense mutations in G protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–55. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- 19.Hayter JE, Henry CJ. Basal metabolic rate in human subjects migrating between tropical and temperate regions: a longitudinal study and review of previous work. Eur J Clin Nutr. 1993;47:724–34. [PubMed] [Google Scholar]
- 20.Molnar D, Jeges S, Erhardt E, Schutz Y. Measured and predicted resting metabolic rate in obese and nonobese adolescents. J Pediatr. 1995;127:571–7. doi: 10.1016/s0022-3476(95)70114-1. [DOI] [PubMed] [Google Scholar]
- 21.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35(Suppl):1169–75. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 22.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–29. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham JJ. Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54:963–9. doi: 10.1093/ajcn/54.6.963. [DOI] [PubMed] [Google Scholar]
- 24.Echwald SM, Sorensen TD, Sorensen TI, et al. Amino acid variants in the human leptin receptor: lack of association to juvenile onset obesity. Biochem Biophys Res Commun. 1997;233:248–52. doi: 10.1006/bbrc.1997.6430. [DOI] [PubMed] [Google Scholar]
- 25.Heo M, Leibel RL, Fontaine KR, et al. A meta-analytic investigation of linkage and association of common leptin receptor (LEPR) polymorphisms with body mass index and waist circumference. Int J Obes Relat Metab Disord. 2002;26:640–6. doi: 10.1038/sj.ijo.0801990. [DOI] [PubMed] [Google Scholar]
- 26.Casanueva FF, Dieguez C. Interaction between body composition, leptin and growth hormone status. Baillieres Clin Endocrinol Metab. 1998;12:297–314. doi: 10.1016/s0950-351x(98)80024-4. [DOI] [PubMed] [Google Scholar]
- 27.Kelestimur F, Popovic V, Leal A, et al. Effect of obesity and morbid obesity on the growth hormone (GH) secretion elicited by the combined GHRH + GHRP-6 test. Clin Endocrinol (Oxf) 2006;64:667–71. doi: 10.1111/j.1365-2265.2006.02525.x. [DOI] [PubMed] [Google Scholar]
- 28.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [Erratum, J Clin Endocrinol Metab 2000;85:416.] [DOI] [PubMed] [Google Scholar]
- 29.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 30.Chung WK, Belfi K, Chua M, et al. Heterozygosity for Lep(ob) or Lep(rdb) affects body composition and leptin homeostasis in adult mice. Am J Physiol. 1998;274:R985–R990. doi: 10.1152/ajpregu.1998.274.4.R985. [DOI] [PubMed] [Google Scholar]
- 31.Bray GA, Fisler JS, York DA. Neuroendocrine control of the development of obesity: understanding gained from studies of experimental models of obesity. Front Neuroendocrinol. 1990;4:128–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.