Abstract
Objective
Lower serotonin transporter (5-HTT) binding (BPP= = fPBavail/KD) is reported during a major depressive episode (MDE) compared to healthy controls. Higher 5-HTT binding in the diencephalon has previously been associated with acute response to antidepressant treatment. We assessed baseline 5-HTT binding as a predictor of one-year remission from a MDE, examining binding in brain regions implicated in the pathophysiology of major depressive disorder (MDD).
Methods
5-HTT binding was quantified using positron emission tomography (PET) with [11C]McN5652 in 19 currently depressed subjects with MDD and 41 healthy controls. Depressed subjects received open, naturalistic antidepressant treatment. Remission status was determined one year after PET scan and treatment initiation.
Results
Significant differences in 5-HTT binding among the three groups (healthy controls, remitters, and non-remitters) were observed in a linear mixed-effects model. Post-hoc, non-remitters had lower 5-HTT binding than controls in midbrain, amygdala, and anterior cingulate. Remitters did not differ significantly from controls or non-remitters in 5-HTT binding. Remitters did not differ from non-remitters in clinical characteristics apart from greater family history of depression among non-remitters. A logistic regression model fit to determine the capacity of baseline 5-HTT binding to predict remission status at one year yielded a coefficient that was suggestive but not significant (p=0.057).
Limitations
The small sample size and heterogeneous treatments received reduced statistical power to detect differences in binding based on clinical outcome.
Conclusions
Lower pretreatment 5-HTT binding may be predictive of non-remission from major depression following one year of naturalistic antidepressant treatment. Future studies using standardized treatment are warranted.
Keywords: serotonin transporter, 5-HTT, depression, positron emission tomography, remission, prediction
Objectives of the Study
Psychiatrists lack tools to select treatments for major depression based on the likelihood of efficacy in individual patients. Markers identified in previous studies are not used clinically due to overlap between responder and non-responder groups and non-standardized definitions of response or remission (Joyce & Paykel, 1989).
The serotonin transporter (5-HTT) has been implicated in the pathophysiology of depression, and is a target of antidepressant action (Owens & Nemeroff, 1994). We found lower 5-HTT binding (BPP, proportional to the total number of available transporters) in midbrain and amygdala in vivo in major depressive disorder (MDD) using positron emission tomography (PET) with [11C]McN5652 (Parsey et al., 2006b), in agreement with some (Lehto et al., 2006; Malison et al., 1998; Newberg et al., 2005), but not all (Ichimiya et al., 2002; Meyer et al., 2004a), previous reports.
A SPECT study of depressed patients using [123I]β-CIT found that higher 5-HTT binding in the diencephalon predicts better acute antidepressant response to selective serotonin reuptake inhibitors (SSRIs) (Kugaya et al., 2004). They also observed a similar trend between brainstem 5-HTT and treatment response.
We hypothesized that 5-HTT binding would predict one-year remission following antidepressant treatment in MDD. We performed PET scanning with [11C]McN5652 to study relevant brain regions previously unexamined as predictors of outcome. We hypothesized that low 5-HTT binding would favor a lower remission rate. We also compared remitters and non-remitters to 41 healthy controls.
Materials and Methods
Subjects
This is a follow-up to a previous study of 5-HTT binding in depression, which contains details regarding methods (Parsey et al., 2006b). Nineteen subjects in a SCID-diagnosed DSM-IV MDE (APA, 1994) and 41 healthy volunteers completed a 24-item Hamilton Depression Rating Scale (HAMD-24) and PET scans while medication-free (Table 1).
Table 1.
Characteristics of Patients with Major Depression (remitters and non-remitters) and Healthy Volunteers.
| Remitters N (%) | Non-Remitters N (%) | Controls N (%) | Remitters vs. Non-remitters (t-test or fisher’s P-value) | Non-remitters vs. Controls (t- test or fisher’s P-value) | Remitters vs. Controls (t-test or fisher’s P-value) | |
|---|---|---|---|---|---|---|
| Total N | 7 | 12 | 41 | |||
| Female | 5 (71.4) | 10 (83.3) | 20 (48.8) | .60 | .05 | .42 |
| Prior Meds | 5 (71.4) | 8 (66.7) | 0 (0) | 1 | <.00001 | <.0001 |
| Age | 38.9 ± 11.4 | 41.7 ± 15.1 | 39.0 ± 16.1 | .68 | .60 | 1.00 |
| # of prior suicide attempters | 3 (42.9) | 5 (41.7) | 0 (0) | 1 | .0003 | .0020 |
| HAMD1 24 Baseline | 25.0 ± 8.5 | 25.7 ± 7.9 | 0.61 ± 0.89 | .86 | <.0001 | <.0001 |
| HAMD1 24 1 year | 4.1 ± 3.4 | 23.8 ± 8.8 | n/a | <.0001 | n/a | n/a |
| Family hx of MDD | 1 (14.3) | 9 (75) | 0 | .02 | .15 | |
| # of 1st degree relatives w/MDD | 0.14 ± 0.38 | 1.33 ± 1.07 | 0 ± 0 | .012 | <.00001 | .013 |
| Ethnicity | .38 | .16 | .82 | |||
| Asian | 1 (14.3) | 1 (8.3) | 6 (14.6) | |||
| Black | 2 (28.6) | 0 (0) | 6 (14.6) | |||
| Hispanic | 1 (14.3) | 4 (33.3) | 6 (14.6) | |||
| White | 3 (42.9) | 6 (50) | 23 (56.1) | 12 | .752 | .692 |
| >1 Race | 0 (0) | 1 (8.3) | 0 (0) | |||
| Comorbid Axis I | 3 (42.9) | 6 (50) | 0 | 1 | <.0001 | .0020 |
| Comorbid Panic Disorder | 1 (14.3) | 3 (25) | 0 | 1 | .0094 | .15 |
| Injected dose (mCi) | 15.10 ± 2.17 | 13.48 ± 4.55 | 11.97 ± 4.12 | .39 | .28 | .06 |
| Injected mass(μg) | 4.33 ± 1.15 | 4.09 ± 1.38 | 4.50 ± 1.47 | .24 | .89 | .78 |
Hamilton Depression Rating Scale
comparing to all other groups pooled given low sample size.
MDD subjects met the following inclusion criteria: (1) age 18 to 65 years; (2) DSM-IV criteria for a current MDE; (3) ≥ two week medication-free period prior to PET scanning (four weeks for oral neuroleptics, six weeks for fluoxetine, and an exception of three days for short-acting benzodiazepines); (4) absence of current or lifetime history of alcohol or other drug abuse or dependence; (5) no lifetime exposure to 3,4-methylenedioxymethamphetamine (MDMA); (6) absence of significant current medical conditions; (7) absence of pregnancy; and (8) capacity to provide informed consent. Study criteria for healthy volunteers were similar except for the absence of a psychiatric history or a history of a mood or psychotic disorder in any first-degree relative. The Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbauh, 1961), Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960), and Global Assessment Scale (GAS) (Endicott, Spitzer, Fleiss, & Cohen, 1976) assessed subjective and objective depression severity and functional impairment. Eight of 19 depressed subjects had made at least one prior suicide attempt. Nine depressed subjects (47%) had current co-morbid Axis I disorders, all of which were anxiety disorders.
Following baseline assessment and PET scans, depressed patients received open, non-standardized antidepressant treatment. All subjects received outpatient treatment; 63% of subjects additionally received initial inpatient treatment. Remission, defined as 50% reduction of HAMD-24 score from baseline and final HAMD-24 score <10, was assessed at one year. The Antidepressant Treatment History Form (ATHF) was used to assess treatment adequacy (Oquendo et al., 2003). This study was approved by the Institutional Review Board of The New York State Psychiatric Institute. All subjects gave written informed consent after explanation of the study.
Radiochemistry
[11C](+)-McN 5652, (+)-McN butyryl thioester tartrate, was produced as previously described (Frankle et al., 2004). The injected dose and mass of [11C]McN5652 did not differ between remitters and non-remitters (Table 1).
Image Analysis and Modeling
PET and magnetic resonance imaging (MRI) data acquisition, analysis, and measurement of metabolite corrected arterial input functions were performed as previously described (Parsey et al., 2006a; Parsey et al., 2006b; Parsey et al., 2000). After a ten-minute transmission scan, [11C]McN5652 was injected intravenously and emission data acquired for 130 minutes. Regions of interest (ROIs) were traced on MRIs obtained for each individual subject using brain atlases (Duvernoy, 1991; Talairach & Tournoux, 1988) and published reports (Kates, Abrams, Kaufmann, Breiter, & Reiss, 1997; Killiany, Moss, Nicholson, Jolesz, & Sandor, 1997). Six ROIs previously associated with serotonergic abnormalities in depression were included in this study: the anterior cingulate, amygdala, putamen, hippocampus, midbrain, and thalamus (Parsey et al., 2006b). Derivation of [11C]McN5652 regional distribution volumes (VT) was performed using likelihood estimation in graphical approach (LEGA) (Ogden, 2003; Ogden, Parsey, & Mann, 2002; Parsey, Ogden, & Mann, 2003). VT is the sum of the specific (VS) and non-displaceable (free plus nonspecific binding = VND) distribution volumes. Binding potential (BPP) = VT – VND. The abbreviation BPP is consistent with a recent consensus statement (Innis RB, 2007). We utilized a 12.1 ± 1.5 mL sample of the cerebellar gray matter as a measure of VND (Parsey et al., 2006b).
Statistics
Considering six ROIs at once, data from all three groups (healthy controls, remitters, and non-remitters) were analyzed using linear mixed effects models with brain region and group as fixed effects and subject as the random effect. To stabilize the variance and ensure modeling assumptions were met, analysis was performed on the natural log of the data (after first adding a quantity (2.5) to all measures to ensure positivity). The log transform was necessary because of two features of our BPP data: skewness, and unequal standard deviation (SD) of measurements across groups with different binding levels (with SD roughly proportional to the binding level). We and others have used this or related statistical approaches to allow for valid statistical analysis of PET data with these characteristics (Meltzer et al., 2004; Oquendo et al., 2006; Parsey et al., 2006a; Parsey et al., 2006b; Parsey et al., 2006c; Parsey et al., 2006d; Rabiner et al., 2002; Sullivan et al., 2005). Graph of binding potential (Figures 1 and 2) uses actual (not log-transformed) BPP values. Logistic regression was performed on the data from the depressed subjects with remitter status as the binary outcome and mean (across the six regions considered) BPP as the predictor. Also, linear regression models were fit with final HAMD-24 as outcome and binding measures and baseline HAMD-24 as predictors. For all analyses, reported p-values correspond to two-sided alternatives, not adjusted for multiple comparisons. Linear mixed effects models of binding, logistic and linear regression analysis, and Fisher’s exact test were performed in R 2.1.0 (http://cran.r-project.org). Student’s t-test and Chi-squared test were performed in Excel (Microsoft, 2003) or SPSS for Windows Version 12.0 (SPSS, Chicago, IL) to examine clinical and demographic variables. Statistics are presented as (test statistic, DF, p-value).
Figure 1.
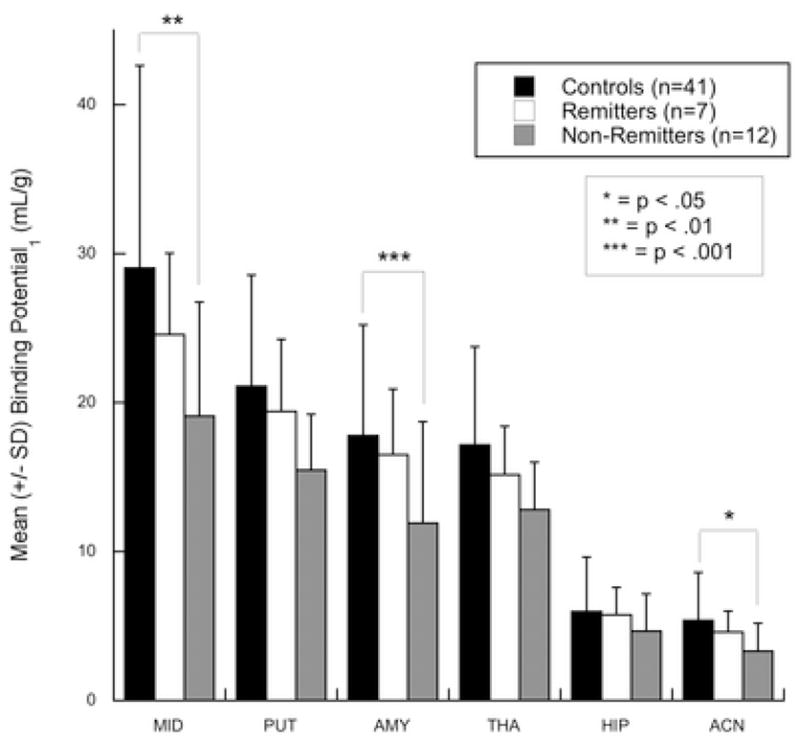
Differences in baseline 5-HTT BPP between controls and subjects with major depression who remit or do not remit by one-year follow-up. Non-remitters have lower 5-HTT BPP than healthy controls in the linear mixed-effects model, comparing all regions simultaneously (p=.005). Reported levels of significance are for post-hoc tests of group-by-region effects. Regions of interest: anterior cingulate (ACN), amygdala (AMY), dorsal putamen (PUT), hippocampus (HIP), midbrain (MID), thalamus (THA). Error bars represent one standard deviation from mean values. * = p<.05, ** = p<.01, *** = p<.001.
Figure 2.
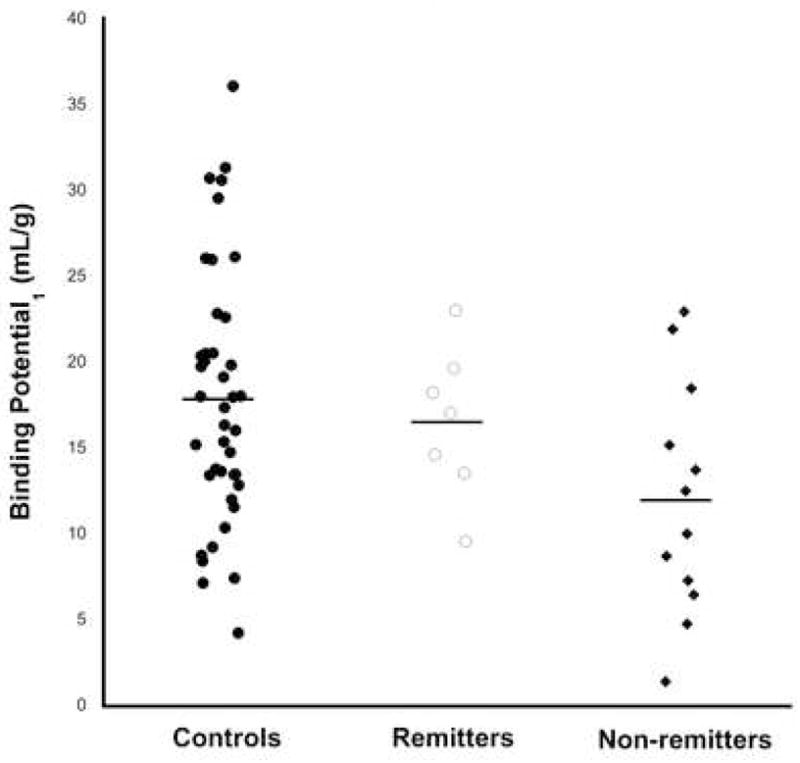
Scatter plot of individual subjects’ amygdala 5-HTT BPP by group, with means plotted as horizontal lines.
Results
Clinical Outcomes
36.8% of depressed subjects (7 of 19) were in remission at 1-year. There were no differences between remitters and non-remitters in baseline HAMD-24 values or other clinical or demographic variables including gender, ethnicity, prior suicide attempt, prior psychiatric medication use, co-morbid anxiety disorders, or number of prior MDEs (Table 1). Non-remitters had more first-degree relatives with a history of MDD than non-remitters.
The adequacy of antidepressant treatment during the one year period did not differ between remitters and non-remitters as assessed by the ATHF (Table 2). There were no differences between remitters and non-remitters in percentage of each group treated with various classes of antidepressant medications, including SSRIs, during the one-year follow-up period (Table 2).
Table 2.
Details of antidepressant treatments among non-remitters and remitters prior to and during study period.
| Remitters N (%) | Non-Remitters N (%) | Fisher’s exact or t-test | |
|---|---|---|---|
| # previously treated with antidepressant medications | 5 (71.4) | 8 (66.7) | 1 |
| Med-free days prior to enrollment for non-med-naïve subjects | 23.6 ± 8.8 | 72 ± 119.1 | .39 |
| Treatment intensity (ATHF1) | 2.71 ± 1.7 | 3.08 ± 1.3 | .60 |
| On meds at 1 year | 6 (85.7) | 8 (61.5) | .60 |
| Exposure to: | |||
| Selective serotonin reuptake inhibitor | 4 (57.1) | 9 (75) | .62 |
| Tricyclic antidepressant | 1 (14.3) | 1 (7.7) | 1 |
| Monoamine oxidase inhibitor | 0 (0) | 1 (8.3) | 1 |
| Bupropion | 2 (28.6) | 4 (33.3) | 1 |
| Venlafaxine | 1 (14.3) | 3 (25) | 1 |
| Lithium | 3 (42.9) | 1 (8.3) | .12 |
| Thyroid | 1 (14.3) | 1 (8.3) | 1 |
| ECT | 0 (0) | 0 (0) | 1 |
| Psychotherapy | 8 (67.7) | 5 (71.4) | 1 |
antidepressant treatment history form
Baseline Binding Potential Determinations in Remitters, Non-Remitters and Healthy Controls
5-HTT BPP differed between the three groups (Figure 1; F=3.29, df=2,57, p=0.044). Post-hoc, non-remitters had lower 5-HTT BPP than controls (F=6.59, df=1,57, p=0.013). There was a region-by-group interaction and post-hoc testing revealed lower 5-HTT BPP in amygdala (Figure 2; t=−3.37, df=289, p=0.00087), midbrain (t=−2.89, df=289, p=0.0041), and anterior cingulate (t=−2.43, df=289, p=0.016). Non-remitters had lower mean 5-HTT BPP compared with remitters in every brain region examined, but this effect was not statistically significant (F=1.89, df=1,57, p=0.18). Incorporating family history of MDD into logistic regression or mixed linear effects models did not affect these findings (data not shown).
Considering only the depressed subjects, a logistic regression model to determine the predictive capacity of 5-HTT BPP (mean across all 6 regions) for remitter status was suggestive, with a p-value of 0.057 in a two-tailed test (χ2=3.6, df=1, p=0.057).
We also performed linear regression analysis to examine the predictive capacity of baseline 5-HTT BPP on a continuous measure of clinical outcome, final HAMD-24 score at one year. In all of these analyses, baseline HAMD-24 score was included as a regressor to account for baseline severity as a potential confounder. Using mean BPP across the 6 regions as a predictor yielded a coefficient in the regression analysis with a corresponding p-value of 0.059 (F=4.13, df=1,16, p=0.059). As differences between non-remitters and controls were most significant in the amygdala, we modeled baseline amygdala BPP as a predictor of final HAMD-24 score, which was significantly predictive (F=5.32, df=1, p=0.035).
There was no significant difference in 5-HTT BPP between remitters and controls (F=0.18, df=1,57, p=0.67). There was no group difference in non-specific binding quantified in cerebellar VT (F=0.69, df=2,58, p=0.51). Using an alternative outcome measure, BPND (equal to fNDBavail/KD), the difference in 5-HTT binding between the three groups was nearly significant (F=3.11, df=2,56, p=0.052), and non-remitters still had lower 5-HTT binding than controls (F=6.11, df=1,56, p=0.017).
Discussion
We found lower 5-HTT BPP at baseline in amygdala, midbrain, and anterior cingulate in depressed subjects who did not remit after one year of open antidepressant treatment compared with healthy volunteers. Differences in baseline BPP between remitters and non-remitters when compared using linear mixed effects modeling did not reach statistical significance. Similarly, fitting a logistic regression model on the depressed subjects resulted in an effect of mean BPP that was nearly significant. This may reflect a lack of difference between the two groups, or may be attributed to the relatively small sample size and heterogeneous treatments administered in this study. The direction of our findings is consistent with a previous report of higher 5-HTT binding predicting a better acute antidepressant response (Kugaya et al., 2004). We extend these previous results by finding differences between non-remitters and controls in previously unexamined brain structures, amygdala and anterior cingulate. Rostral anterior cingulate hypometabolism at baseline is reported in antidepressant non-responders compared to controls (Mayberg et al., 1997), providing a partial convergence of our neurochemical data with the metabolic literature. Of note, the clinical features of remitters and non-remitters did not differ apart from family history of depression in this study, suggesting that in vivo 5-HTT quantification with PET may detect some additional feature of the illness important in determining clinical outcome.
The higher family history of MDD we observed in non-remitters compared to remitters is consistent with previous reports of poor long-term outcome among depressed patients with a history of severe psychiatric illness (Duggan, Sham, Minne, Lee, & Murray, 1998). In addition, family history of depression was recently associated with recurrence of depressive episodes in the STAR*D sample (Hollon et al., 2006).
Our data are not directly comparable to a recent report by Cavanagh et al, as they examined the relationship of treatment response to serotonin transporter occupancy by medication (Cavanagh et al., 2006), whereas we assessed binding prior to treatment as a predictor of remission. The doses of antidepressants used by Cavanagh et al are comparable those presented by Meyer et al, who demonstrated approximately 80% occupancy of 5-HTT by therapeutic doses of SSRIs (Meyer et al., 2004b).
Lower 5-HTT binding in non-remitters compared to controls may reflect a deficit of serotonergic neurons in the dorsal raphe nucleus, of projections from these neurons to their terminal field, or of 5-HTT in terminal projections. Any of these alterations could contribute to non-remission by making the serotonergic system less responsive to treatments that are mediated by 5-HTT, including SSRIs, which were administered to 68% of depressed subjects. If the observed deficits in 5-HTT are related to an early environmental stressor or a genetic predisposition, they may reflect a persistent trait, rather than a state, and may indicate likely refractoriness to antidepressant treatments. This hypothesis is consistent with reports of a trait serotonin system deficiency that may predict poorer antidepressant responses (Malone et al., 1993), and may reflect the predisposition to recurrent depression.
A functional promotor polymorphism in the 5-HTT gene (5-HTTLPR) (Lesch et al., 1996) has previously been associated with antidepressant response (Serretti, Benedetti, Zanardi, & Smeraldi, 2005), and 5-HTTLPR genotype may be independent of 5-HTT binding (Parsey et al., 2006a; Shioe et al., 2003; Van Dyck et al., 2004). While the sample size of the current study was not sufficient for a genetic association study, we include genetic data obtained from this sample in the discussion, which should be considered exploratory. We assessed 5-HTTLPR genotype as a predictor of one-year remission status in this same sample, using both a biallelic (Lesch et al., 1996) and more recent tri-allelic categorization (Hu et al., 2005). There was a trend toward a difference in tri-allelic frequency between remitters and non-remitters (remitters: LA=7 (50%), LG=5 (35.7%), S=2 (14.3%); non-remitters: LA=12 (50%), LG=2 (8.3%), S=10 (41.7%); Fisher’s, p=0.073). Remitters had a higher frequency of the biallelic L allele than controls (85.7% vs. 51.3%; Fisher’s, p=0.02), but did not differ from non-remitters (non-remitters: 58.3%; Fisher’s, p=0.15). It is not clear that 5-HTTLPR genotype is predictive of adult human brain 5-HTT binding (Parsey et al., 2006a; Shioe et al., 2003; Van Dyck et al., 2004), so these trends may be due to an independent effect of genotype on clinical outcome. Incorporating 5-HTTLPR genotype with 5-HTT binding into the logistic regression model did not improve its predictive power.
The primary limitations of this study are its modest sample size, the heterogeneous antidepressant treatments administered, and the technical limitations of the [11C]McN5652 ligand. The greater family history of depression among non-remitters raises the possibility of genetic factors unrelated to 5-HTT influencing treatment outcome that the current study was underpowered to detect. 45% of the depressed subjects in our study had comorbid anxiety disorders, and panic disorder has been reported to be associated with lower 5-HTT binding in the midbrain, temporal lobes and thalamus (Maron et al., 2004). However, the rates of panic disorder did not differ significantly between the remitters and non-remitters. Furthermore, there was no difference in 5-HTT binding between depressed patients with and without panic disorder in any region examined (data not shown), and including comorbid axis I diagnosis as a covariate did not affect the comparison of BPP between remitters and non-remitters (p=0.18).
Future studies are needed in larger samples, using better PET tracers such as [11C]DASB, with standardized treatment, to determine whether these measures predict clinical outcomes with specific classes of medications. We have previously found that 5-HT1A receptor binding is elevated in non-remitters compared to remitters and controls, and demonstrated an association between a polymorphism in the 5-HT1A promoter and remission status (Parsey et al., 2006c). Combining imaging and genetic variables for 5-HTT and 5-HT1A in a statistical model may allow us to account for a greater degree of the variance in treatment outcome. Such an analysis was not possible with the current sample due to the limited number of subjects with measures of both 5-HTT and 5-HT1A. Analogous use of multiple predictors in a statistical model has recently been performed effectively with respect to first-episode psychosis (Wood, 2006). The recent development of novel statistical tools using voxel-level parametric images as predictors in regression models may aid further in this endeavor (Reiss, 2006).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbauh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Patterson J, Pimlott S, Dewar D, Eersels J, Dempsey MF, Wyper D. Serotonin transporter residual availability during long-term antidepressant therapy does not differentiate responder and nonresponder unipolar patients. Biol Psychiatry. 2006;59:301–8. doi: 10.1016/j.biopsych.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Duggan C, Sham P, Minne C, Lee A, Murray R. Family history as a predictor of poor long-term outcome in depression. Br J Psychiatry. 1998;173:527–30. doi: 10.1192/bjp.173.6.527. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. Surface, three-dimensional sectional anatomy and MRI. New York: Sringer-Verlag Wien; 1991. The human brain. [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med. 2004;45:682–94. [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psych. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, Shelton RC, Wisniewski S, Warden D, Biggs MM, Friedman ES, Husain M, Kupfer DJ, Nierenberg AA, Petersen TJ, Shores-Wilson K, Rush AJ. Presenting characteristics of depressed outpatients as a function of recurrence: preliminary findings from the STAR*D clinical trial. J Psychiatr Res. 2006;40:59–69. doi: 10.1016/j.jpsychires.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, Inoue M, Yasuno F, Takano A, Maeda J, Shibuya H. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry. 2002;51:715–22. doi: 10.1016/s0006-3223(01)01351-8. [DOI] [PubMed] [Google Scholar]
- Innis RBCV, Delforge J, Fujita M. Consensus Nomenclature for Binding Potential and Related Terms Used in Radioligand Imaging. J Cereb Blood Flow & Metabolism. 2007 doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Joyce PR, Paykel ES. Predictors of drug response in depression. Arch Gen Psychiatry. 1989;46:89–99. doi: 10.1001/archpsyc.1989.01810010091014. [DOI] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiat Res Neuroimag. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Nicholson T, Jolesz F, Sandor T. An interactive procedure for extracting features of the brain from magnetic resonance images: The lobes. Human Brain Mapping. 1997;5:355–363. doi: 10.1002/(SICI)1097-0193(1997)5:5<355::AID-HBM4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G, Staley JK, Malison RT, Bozkurt A, Khan S, Anand A, Van Dyck CH, Baldwin RC, Seibyl JP, Charney DS, Innis RB. Brain Serotonin Transporter Availability Predicts Treatment Response to Selective Serotonin Reuptake Inhibitors. Biol Psychiatry. 2004;56:497–502. doi: 10.1016/j.biopsych.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lehto S, Tolmunen T, Joensuu M, Saarinen PI, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J. Midbrain binding of [(123)I]nor-beta-CIT in atypical depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006 doi: 10.1016/j.pnpbp.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–8. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Malone KM, Thase ME, Mieczkowski T, Myers JE, Stull SD, Cooper TB, Mann JJ. Fenfluramine challenge test as a predictor of outcome in major depression. Psychopharmacol Bull. 1993;29:155–61. [PubMed] [Google Scholar]
- Maron E, Kuikka JT, Shlik J, Vasar V, Vanninen E, Tiihonen J. Reduced brain serotonin transporter binding in patients with panic disorder. Psychiatry Res. 2004;132:173–81. doi: 10.1016/j.pscychresns.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–65. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J, Wilson AA. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004a;61:1271–9. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, Ginovart N, Spencer EP, Cheok A, Houle S. Serotonin Transporter Occupancy of Five Selective Serotonin Reuptake Inhibitors at Different Doses: An [(11)C]DASB Positron Emission Tomography Study. Am J Psychiatry. 2004b;161:826–35. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N, Ploessl K, Swanson RL, Shults J, Alavi A. 123I-ADAM Binding to Serotonin Transporters in Patients with Major Depression and Healthy Controls: A Preliminary Study. 2005:973–977. [PubMed] [Google Scholar]
- Ogden RT. On estimation of kinetic parameters in graphical analysis of PET imaging data. Statistics in Medicine. 2003;22:3557–68. doi: 10.1002/sim.1562. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Parsey RV, Mann JJ. Likelihood approach to parameter estimation in Logan graphical analaysis. Neuroimage. 2002;16:S73. [Google Scholar]
- Oquendo MA, Baca-Garcia E, Kartachov A, Khait V, Campbell CE, Richards M, Sackeim HA, Prudic J, Mann JJ. A computer algorithm for calculating the adequacy of antidepressant treatment in unipolar and bipolar depression. J Clin Psychiatry. 2003;64:825–33. doi: 10.4088/jcp.v64n0714. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Hastings RS, Huang Y, Simpson N, Ogden RT, Arango V, Van Heertum RL, Mann JJ, Parsey RV. Lower brain serotonin transporter in depressed bipolar patients using Positron Emission Tomography. Arch Gen Psychiatry. 2006 doi: 10.1001/archpsyc.64.2.201. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–95. [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006a;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006b;163:52–8. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M. In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med. 2000;41:1465–77. [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Mann JJ. Determination of Volume of Distribution using Likelihood Estimation in Graphical Analysis: Elimination of Estimation Bias. Journal of Cerebral Blood Flow and Metabolism. 2003;23:1471–8. doi: 10.1097/01.WCB.0000099460.85708.E1. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006c;31:1745–9. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006d;59:106–13. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, de Haes JU, de Vries M, Grasby PM. 5-Hydroxytryptamine1A Receptor Occupancy by Novel Full Antagonist 2-[4-[4-(7-Chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1,2-benzisothiazol-3-(2H)-one-1,1-dioxide: A [11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclohexanecarboxamide Trihydrochloride (WAY-100635) Positron Emission Tomography Study in Humans. 2002:1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- Reiss PT. Biostatistics. New York: Columbia University; 2006. Regression With Signals and Images as Predictors; p. 166. [Google Scholar]
- Serretti A, Benedetti F, Zanardi R, Smeraldi E. The influence of Serotonin Transporter Promoter Polymorphism (SERTPR) and other polymorphisms of the serotonin pathway on the efficacy of antidepressant treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1074–84. doi: 10.1016/j.pnpbp.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, Hirano M, Shinohara M, Kagami M, Okubo Y, Nankai M, Kanba S. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–8. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58:947–54. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-dimensional proportional system: an approach of cerebral imaging. New York: Theime Medical Publisher; 1988. Co-planar stereotactic atlas of the human brain. [Google Scholar]
- Van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Central Serotonin Transporter Availability Measured With [(123)I]beta-CIT SPECT in Relation to Serotonin Transporter Genotype. Am J Psychiatry. 2004;161:525–31. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Lambert M, Conus P, Velakoulis D, Stuart G, Desmond P, McGorry PD, Pantelis C. Prediction of Functional Outcome 18 Months After a First Psychotic Episode. Arch Gen Psychiatry. 2006;63:969–976. doi: 10.1001/archpsyc.63.9.969. [DOI] [PubMed] [Google Scholar]


