INTRODUCTION
Association of temporomandibular joint (TMJ) disc integrity and position with internal derangement of the TMJ, joint pain, and dysfunction has been studied since the early 1970's.1-4 Various treatment procedures have been advocated to reposition, repair, remove or replace damaged discs.5-10 Surgical procedures have included TMJ arthroscopy, arthrocentesis, discectomy and disc repair procedures, and total joint replacement.5,11-19 When discectomy or removal of the disc was performed, autologous grafts as well as alloplastic TMJ interpositional implants (TMJ IP) were used to provide interposing tissue between the condyle and the temporal bone and increase joint stability.5,7,20-22 More than 20,000 TMJ IP may have been implanted from 1974 until 1993.7,20,21,25 The most common implanted devices were Proplast/Teflon® (Vitek, Inc., Houston, TX) and silicone rubber or Silastic® (Dow Corning Corp., Midland, MI) since late 1960s.5,20,25-30
Proplast® is a porous form of polytetrafluoroethylene (PTFE) with an admixture of fibers of either vitreous carbon (Proplast I) or aluminum oxide (Proplast II or PTFE-Al2O3). A porous sheet of Proplast® is laminated to Teflon®, a dense smooth form of PTFE, to form Proplast/Teflon®. Proplast/Teflon® implants have a high melting point (above 250°C), unusual toughness, insolubility in all common solvents, resistance to chemical attack, anti-frictional properties; and a modulus of elasticity (E=0.5 GPa) resembling that of bone and fibrous tissue.20,28,37 Certain investigators 20,21,28,37 considered that the porous PTFE offered more stability than nonporous silicone implants, and that other desirable properties were present such as freedom from adverse reactions, toxicological safety, rapid tissue ingrowth, and biocompatibility. Initial studies reported that Proplast/Teflon® implants seemed to produce pain relief and improve TMJ function.22,26,27,29,38
In the late 1980s, Proplast/Teflon® TMJ IP were found to brake or be rejected in many patients, because of the high biomechanical forces placed on them.20,21,25,33,36,39-42 The breakdown lead to production of fragmented particles which resulted in an immune foreign body response21,25,39-42, causing problems ranging from severe cutaneous inflammatory reaction in the pre-auricular and cheek areas43; to severe degenerative joint disease with resorption and erosion of the TMJ bony structures, perforation into the middle cranial fossa44,45,46, chronic pain, increased joint noises, TMJ hypomobility, and malocclusion. The result was a dramatic clinical spectrum of failures for these implants.20,21,25,42-50 The December 1991 issue of the US Foods and Drugs Administration Bulletin recommended immediate removal of all previous TMJ Proplast/Teflon® IPs, due to the mechanical failures, many resulting in progressive bone degeneration.31,33 The American Academy of Oral and Maxillofacial Surgery (AAOMS), in a 1992 workshop, mandated the discontinuation of Proplast/Teflon®IPs.31
Silicone elastomers have been extensively studied and clinically used as implants for a wide range of purposes in various anatomic locations, such as finger, wrist, elbow, shoulder, and metatarsal joints.20,42,51-54 Silastic® is a flexible and inert polydimethylsiloxane (silicone rubber) with resilient properties, easily carved, readily adaptable to the TMJ, and it does not allow tissue ingrowth.55 Silastic® implants can have 1 to 2 mm thickness and be reinforced with Dacron (or polyethylene terephthalate) fibers.20,21 Studies25,42 advocating its use have mentioned its ability to form a fibrous connective tissue capsule that might act as a disc substitute. Silastic® was introduced to the medical community in 1968 as an interpositional material for the reconstruction of arthritic or severely damaged joints in the hand after experimental evidence showed its biocompatibility.42 Researchers37,42,51 demonstrated that different forms of Silastic® implants became surrounded by a fibrous capsule. It was thought that solid silicone fulfilled many of the requirements of an ideal implant material even though occasional intracellular particles of silicone elastomer were observed inside tissue macrophages. Silicone implants showed less mechanical stability, but 50% less gross tearing than Proplast II implants.20,21 In 1981, silicone rubber replacement after TMJ discectomy became popular and foreign-body giant-cell reaction (FBGCR) to the material was rarely reported.25,42,52,53 However, reports published after 1982, demonstrated long-term instability of this material in the TMJ, FBGCR around fragmented silicone particles, peri-implant lymph nodes containing silicone particles, and a severe reactive synovitis, sometimes resulting in destructive arthritis of the condyle.20,21,42,56-60 In 1992, the AAOMS issued recommendations on the use of Silastic® TMJ IPs. They were only to be used in preventing joint ankylosis.31 These implants were subsequently removed from the market in January 1993.
A number of research studies21,25,38-41 have previously evaluated wear debris and particle breakdown of Silastic® and Proplast/Teflon® implants, however, no systematic study has focused on relating implant structural failure patterns to patient clinical outcomes. There is a lack of direct evidence associating clinical failure and implant material failure. The National Institute of Health, in its Technology Assessment conference #19 61, proposed further studies to determine whether the clinical failure of alloplastic implanted devices is related to the implant material.36 Information required to evaluate and improve TMJ implants must come from a combination of clinical data and device retrieval analysis.
The goal of this pilot study was to perform an analysis of surgically explanted Silastic® and Proplast/Teflon® IP, to determine what breakdown factors contributed to their clinical failure, and compare this with patient outcomes. The hypotheses for this study were to test whether the presence of perforation, fracture length, and the type of implant was associated with poor clinical outcomes.
EXPERIMENTAL DESIGN & RESEARCH METHODS
This study was reviewed and approved by the University of Minnesota Institutional Review Board for Human Subjects Research (#0502E67398). Implants, tissue samples and clinical data, de-identified from all demographic data, were provided by the National Institute of Dental and Craniofacial Research's Temporomandibular Joint Implant Registry and Repository (NIDCR's TIRR, N01-DE-22635). The research project was compliant with the “Ethical principles for medical research involving Human subjects” enunciated in the “World Medical Association Declaration of Helsinki”.
Biological Specimens
Ten TMJ IP implants were randomly selected from surgically retrieved and archived implants in NIDCR's TIRR. Three Silastic® and seven Proplast/Teflon® TMJ IP were obtained from eight patients who had them unilaterally, and two who had them bilaterally. Clinical outcome data was available in the database system of NIDCR's TIRR for these implants.
Specimens were stored based on the quantity/quality of each implant/tissue fragment (soft or hard tissue properties). Formalin-fixed specimens were archived at room temperature, paraformaldehyde and glutaraldehyde specimens were refrigerated at -20°C, fresh specimens were stored frozen at -80°C, and dried specimens were stored in a vacuum desiccator. Fixed implant specimens were thoroughly washed with distilled water prior to observation with the stereo zoom microscope.
A Leica S6D stereo zoom microscope with the magnification of 0.63X to 4X was used to evaluate the morphology of the failed implants. Each implant was divided into 2 regions: peripheral and central areas. Surface perforations, including their position, and fragmentation were documented along with the presence or absence of surface scratches. Fragmented surfaces were examined with the help of a penetrating light source. The length of each implant fracture was measured with a graded paper of millimeter squares (2.5mm per square) placed under a clear plastic Petri dish. The lengths were recorded with a digital camera (Nikon Coolpix 8900) and added for each implant.
Soft tissue specimens underwent routine histologic processing and embedding in paraffin. A rotary microtome was used to cut 4-5μm sections for routine hematoxylin and eosin (H&E) staining and histological analysis by light microscopy (Olympus BX 50 from Leeds Precision Instruments, Inc., Minneapolis, MN). Two hard tissue specimens containing implant material, stored in 4% paraformaldeyde at -20°C, were embedded in paraffin, and cut at 50μm. The sections were then stained with toluidine blue (Stevenel's blue) and basic fuchsin (Van Gieson's picric fuchsin stain) and examined under the light microscope.
Electron Microprobe Analysis
The five implants with granulomatous tissue were chosen to evaluate their structural and chemical morphology using an electron microprobe. One section approximately 1-2mm in width was cut from each of the implants. Each section was then placed in a 0.1 mol/L phosphate buffer solution, dehydrated using increasing ethanol concentrations, and finally left for 24 hours inside a desiccator. One additional tissue section, showing a foreign body giant cell reaction (FBGCR) surrounding implant fragments, was selected. This was sent to the electron microprobe facility for qualitative chemical analyses to detect what surface elements might be present and related to the foreign body response. Standardized mounting procedures to perform the microprobe analysis, were employed and are described elsewhere.39,40,57,62 The prepared microprobe mounts were examined, analyzed and photographed with a JEOL JXA-8600 Electron Probe Microanalyzer.
For qualitative analyses, a wavelength dispersive spectrometer spectrum was collected to identify the chemical elements present in the implants surrounded by the FBGCR. In order to show the distribution of the elements across the surface of the implant/soft tissue samples, a series of element maps were collected. Backscattered electron images were produced to show variations in the number of electrons being ejected from the analyzed sample, reflecting variations in the average atomic number. The higher the atomic number at a location, the greater the number of backscattered electron that will be ejected.
Clinical Data
The Symptom Severity Index (SSI) and Jaw Function Questionnaire (JFQ) from the Temporomandibular Disorder Research Diagnostic Criteria (TMD RDC) - Axis II were selected as the clinical outcomes of interest.63-66 These questionnaires were completed by the patients 1 to 3 months prior to the surgical procedure. Patient age was calculated at the time of implant removal. Primary clinical outcome measures collected were the SSI and JFQ The SSI is a reliable and valid self-report measure that was designed to assess multidimensional symptoms in patients with chronic pain, and it was chosen because it includes the symptom domains that are most likely to change with time and treatment. The SSI is composed of 5 single-item scales: 2 Likert rating scales for pain frequency and duration and 3 visual analog scales for sensory intensity, affective intensity, and intolerability of pain. The JFQ was designed to measure the impairment in function and chewing ability of the stomatognatic system. It contains items to assess how much TMD-related pain interferes with mandibular function, i.e., chewing, swallowing, as well as chewing ability. The JFQ was used since it has been tested for reliability and validity. The SSI is an appropriate measure to assess the patient's subjective pain symptoms, and the JFQ is one of the most suitable to appraise the function and chewing ability of the implant patient's masticatory system.63-66
Statistical Analysis
Statistical analysis of the clinical outcome data (SSI, JFQ) and implant failure data was performed. Student t-tests were used for categorical independent variables and linear regression was employed for continuous variables for either outcomes (SSI and JFQ). The tests were adjusted for age and gender variables. A multivariate linear regression model was employed to test if type of implant, presence of perforation, implant fracture length, age and gender are related to SSI and JFQ. The independent variables were the implant type (Silastic® vs Proplast/Teflon®), presence of implant perforation (present versus absent), length of the implant fracture (in millimeters), age and gender. Dependent variables were the SSI and JFQ. Every test (T-test and linear regression model with/without age and gender adjustment) was employed again with each SSI pain component (pain sensory intensity, affective intensity, tolerability of pain, frequency and duration) as the dependent variable.
RESULTS
The study group, composed of nine females and one male, had a mean age of 49.4 (± 11) years. (Table 1 and 2). TMJ implants analyzed had been in place for anywhere from 15 to 25 years prior to explantation. Implant specimens were either intact (most of the cases) or fragmented into numerous pieces. Some samples came with attached fragments of soft blood-tinged tissues with suspected foreign body granuloma, or fragments of hard tissue. The Proplast/Teflon® implant samples were composed of Proplast II and all displayed a characteristic porous bright-colored surface. Silastic® implant samples were unique with two similar shiny surfaces and silicone gel reinforced by a Dacron matrix.
Legend Table 1.
Outcome data from study sample which includes: Type of interpositional implant retrieved (*PTE - Proplast/Teflon); Patients' Age and Gender distribution (**F - female; ***M - male); SSI questionnaire (1.What are your chief complaint symptoms and what is their location?; 2.How intense on average is your level of pain in the past month?; 3.How disturbing or unpleasant is your usual level of pain in the past month?; 4.How difficult is it to endure these pains in the past month?; 5.When these pain(s) has ocurred in the past month, how long have they lasted?; 6. How often has the pain complaint occurred in the past month?); Jaw function questionnaire score (7. Jaw Function Questionnaire from RDC - Research Diagnostic Criteria Axis II); Implant Failure Measures.
| Implant # | Type of TMJ IP Implant | Pt Gender | Pt Age | Symptom Severity Index (SSI) | JFQ7 (0-12) | Implant Failure Outcome Measures | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain Symptoms and Location1 | Sensory Pain Intensity2 | Affective Pain Intensity3 | Pain Intolerability4 | Duration5 | Frequency6 | Fracture Length (mm) | Presence of Perforation (-/+) | |||||
| (0-10) | (0-10) | (0-10) | (0-27) | (0-27) | ||||||||
| 1 | PTE | F* | 72 | Jaw pain Temple pain | 8 | 5 | 8 | 27 | 27 | 5 | 0 | + |
| 2 | PTE | F | 50 | Jaw pain | 8 | 8 | 8 | 27 | 27 | 6 | 15.0 | + |
| 3 | PTE | F | 39 | Temple pain | 4 | 4 | 2 | 27 | 27 | 3 | 25.1 | - |
| 4 | Silastic Spacer | F | 56 | Temple pain | 0 | 5 | 0 | 27 | 27 | 0 | 3.5 | - |
| 5 | PTE | F | 46 | Temple pain | 3 | 8 | 0 | 27 | 27 | 1 | 17.5 | - |
| 6 | Silastic | M** | 41 | Jaw joint pain | 3 | 3 | 1 | 3 | 9 | 5 | 48.1 | - |
| 7 | PTE | F | 38 | Temple pain | 6 | 8 | 6 | 13 | 16 | 7 | 18.5 | + |
| 8 | Silastic | F | 47 | Jaw joint pain | 5 | 5 | 1 | 14 | 8 | 1 | 15.0 | - |
| 9 | PTE | F | 43 | Jaw joint, jaw & temple pain | 3 | 3 | 7 | 27 | 27 | 0 | 40.0 | - |
| 10 | PTE | F | 62 | No complaints | 2 | 2 | 2 | 1 | 1 | 0 | 13.8 | - |
Table 2.
Age (at time of explantation) and Gender distribution (in percentage), and means and standard deviations (SD) for outcome measures by implant group
| Implant Group | Age Mean (SD) | Gender Female Prevalence | Symptom Severity Index Mean (SD) | Jaw Function Questionnaire Mean (SD) |
|---|---|---|---|---|
| Proplast/Teflon® (PTE) N=7 | 50 (±13) | 100% N=7 | 0.62 (0.24) | 2.9 (2.6) |
| Silastic® (Si)* N=3 | 48 (±8) | 66.7% N=2 | 0.40 (0.14) | 2.5 (3.5) |
NOTE: SD: Standard deviation.
The Silastic® implant encompasses the temporary/spacer and permanent types.
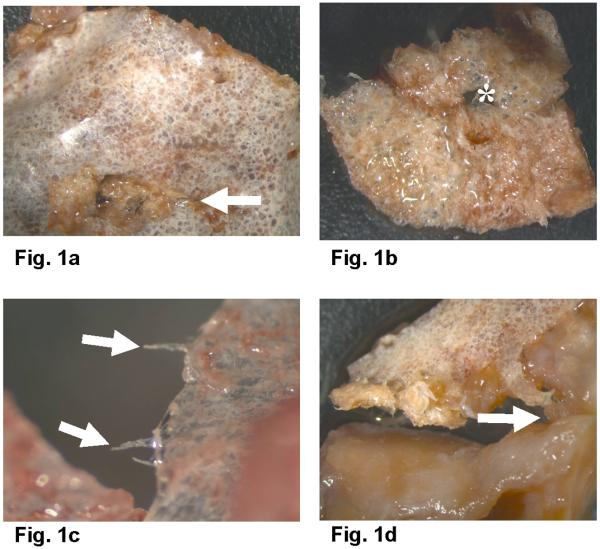
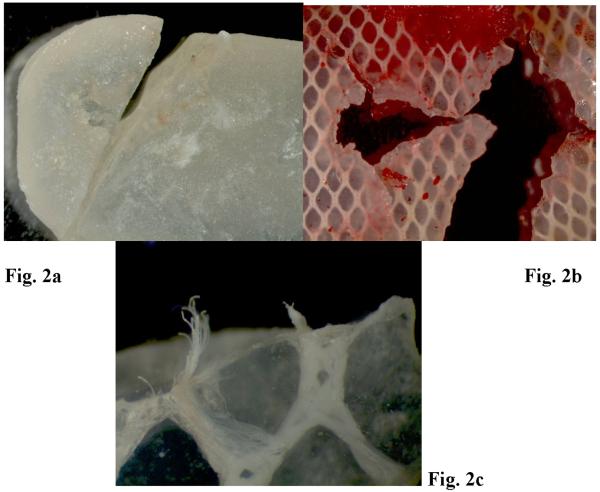
Stereo zoom microscopy of Proplast/Teflon® IP exhibited perforations on the central aspects of the implants. Friction traces (Fig. 1a, Table 1) were present in every direction on the smooth side. Some edges exhibited extruded fibers. (Fig. 1c) Surface evaluation showed ductile failure behavior and fracture/fragmentation features were commonly noted. The size of the perforations was found to reach more than 5 mm in diameter. (Fig. 1b) Occasionally, the porous surface appeared to be attached to red granulomatous tissue; and tearing and breakdown of the two implant layers was observed with TMJ soft tissue observed in between. (Fig.1d) Implant center perforations appeared to progressively open fracture lines to the periphery (Fig. 1a) and also showed fiber extrusion. Stereo zoom microscopy of the Silastic®non-reinforced Dacron implant showed a line of fracture and failure pattern along its width. (Fig. 2a) Two other Silastic® implants appeared to have a large perforation in the center with multiple breakdown lines spreading to the periphery and extrusion of Dacron fibers. (Figs. 2b and 2c)
Figures 1.
TMJ PTE implant fragments observed in the stereo zoom microscope; a.Condylar smooth fluorinated ethylene propylene (FEP) surface of a PTE implant (arrow indicates traces of friction) (Magnification: 0.63X); b. Porous PTFE glenoid fossa surface with (*) perforation in the center (Magnification: 0.63X); c. PTE implant with extruded fibers (arrows) (Magnification: 4X); d. PTE fragment, smooth PTFE surface with attached osseous tissue (arrow) (Magnification: 0,.63 X). PTFE: Polytetrafluorethylene.
Figures 2.
TMJ Silastic implant fragments observed in the stereo zoom microscope; a. TMJ Silastic implant with a fracture line along its width (Magnification: 0.63X); b. TMJ Silastic implant with several fracture lines (Magnification: 2X); c. TMJ Silastic implant with Dacron fiber extrusion (Magnification: 4X).
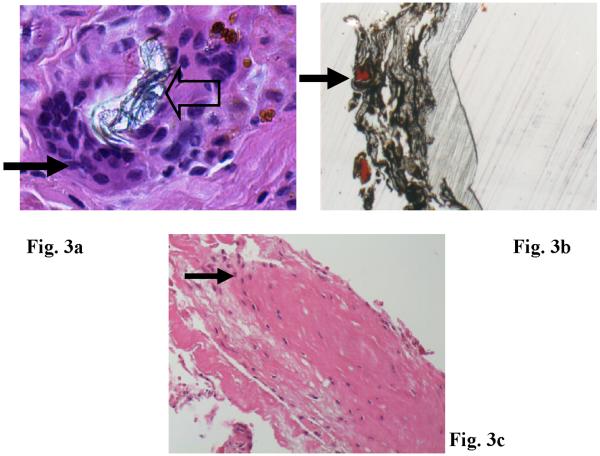
Light microscopy of tissues attached to Proplast/Teflon® IP (H&E sections) revealed portions of fibrous connective tissue containing implant wear debris and an associated prominent FBGCR. Giant cells were observed surrounding large implant pieces and ingesting smaller particles. Dense areas of collagen were present in certain areas (Figs. 3a). Undecalcified sections of implant and tissue were stained for histomorphometric evaluation and the black materials seen in Fig. 3b are Proplast/Teflon® and the red stained areas represent bone ingrowth. Tissue ingrowth can also be seen in the Proplast® surface layer. (Fig. 3b) For the Silastic® IP, tissue sections revealed a small portion of fibrous connective tissue with scattered small deposits of an embedded translucent foreign material. Foreign body reaction composed was not associated with this material. (Fig. 3c)
Figures 3.
a. H&E histological sections of TMJ soft tissue attached to PTE implants. b. PTE implant fragments (large arrow) surrounded by giant cells (small arrow) (H & E, Magnification: 200X); Top Right: PTE implant fragments (large arrow) with bone ingrowth (arrow) on porous Proplast surface (stained with Stevenel's blue and Van Gieson's picric fuchsin. Polarized light with Magnification: 100X). c. H & E stained tissue sections from Silastic implant samples with inflammatory infiltration (arrow). (Magnification: 200X)
Electron Microprobe Analysis
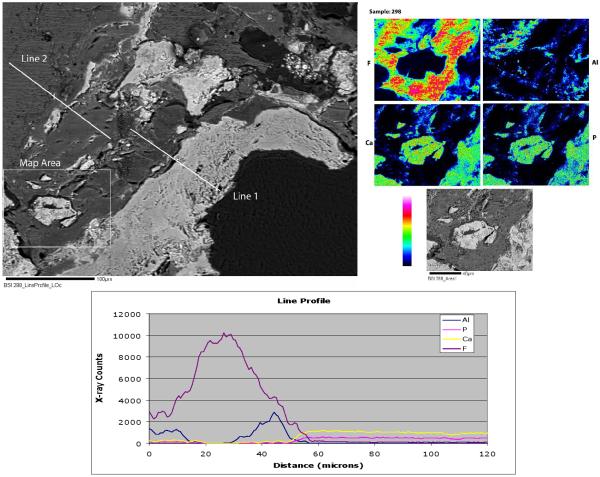
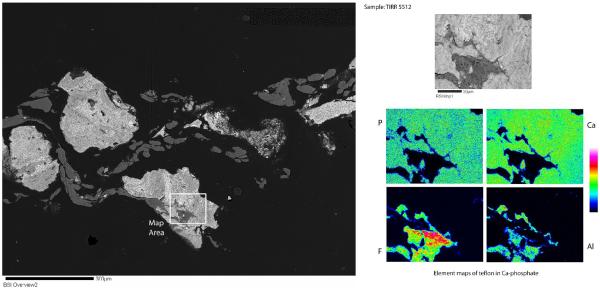
Electron microprobe analysis (EMA) of Proplast/Teflon® IP sections revealed two different phases. There was a large gray area of F-rich (presumably Teflon) and a Ca-phosphate phase, which showed up as the white areas on the backscattered electron image. (Fig. 4) Element mapping and a line profile was done in specific areas to differentiate the two phases. (Fig. 4) The element mapping revealed large areas of F-rich material and small bright areas of Ca-Phosphate. (Fig. 4) The line profile confirmed that the gray material is a F-rich phase, presumably Teflon, intergrown with an Al-rich phase, and poor in Ca-phosphate; and the white areas were Ca and P-rich phases, possibly ceramic, with residual F and Al phases. (Fig. 4) Grooves and ridges were rarely observed in the element maps images and they might be a result of the high intensity levels of the electron beam that damaged the surface.
Figures 4.
Backscattered electron image of PTE implant sample #2 with a labeled area for element mapping and two line profiles (upper left). Element mapping images (upper right). Line profile 1 graph with Aluminum, Phosphorus, Calcium and Fluor x-ray counts (bottom).
A different section contained fragments of the ceramic (compound of Ca-phosphate) material intermixed with the Teflon (areas rich in fluorine - F). (Fig.5) The sample had white irregular shaped fragments - ceramic, which were surrounded by the flattened out gray material - Teflon. The Teflon could be identified by its high F content. Small inclusions composed of a Fe and Cr phase and a Bi and Al phase were also identified with EDS. (images not published)
Figures 5.
BSI of PTE implant section #3 with selected area for element mapping (left). Element mapping images (right).
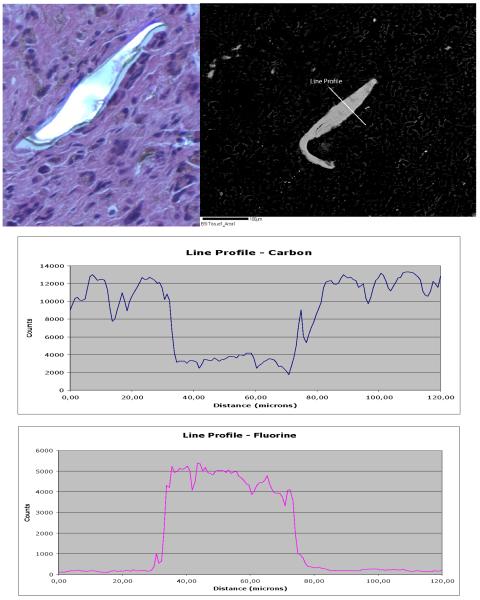
Since a giant cell reaction surrounding the implant material was found in the histological studies, an EMA was performed on the histological section represented in Figure 6. There are scattered bright features and most of the larger bright ones suggest particles of Teflon. (Fig. 6) The size of these particles varied between 20 and 230 μm. Line profile across the brighter particles showed that these are very F-rich materials; therefore, the assumption is that it is Teflon. (Fig. 6). Element maps for C, P and Cl were completely blank, indicating no significant concentrations of those chemical elements are present. Small inclusions of other phases were also present in the implant attached tissue: Al, NaCl, NaKCl, KAl silicate and CaO (or carbonate). These inclusions were not displayed here.
Figure 6.
Upper left corner: Selected H&% histological section for EMA showing a multinucleated giant cell reaction to PTE implant fragment. (H & E. Magnification: 200X). Upper right corner: BSI with a labeled Line profile. Bottom: Line profile graphs with Carbon (upper) and Fluorine (bottom) x-ray counts.
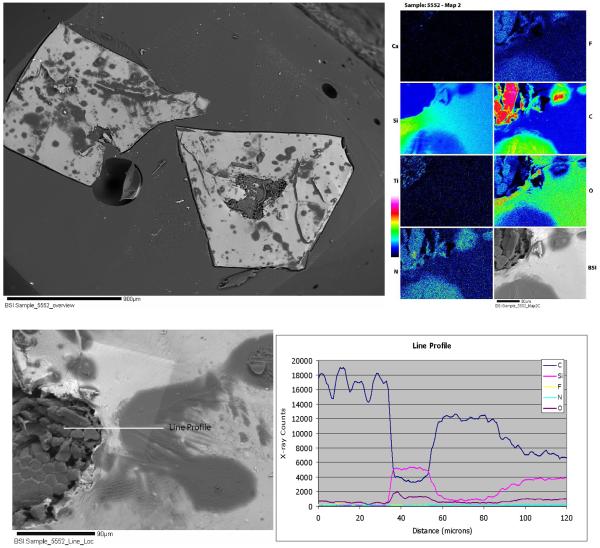
Silastic® implant sections suggest a silicon gel. (Fig. 7) However, inside the gel were small patches of fibrous material in the element maps (Fig. 7). The fibrous materials were rich in F and contained an abundance of Ti inclusions (Fig. 7). The gel was a heterogeneous material well visualized in the element maps. The line profile showed a mix of two phases, one rich in Si and oxygen; and the other phase was rich in F, C and N (Fig. 7).
Figures 7.
Silastic implant sample #8. Top: BSI of the Silastic implant (left). Element mapping images (right). Bottom: BSI of same Silastic implant with a labeled line profile (left), and its corresponding line profile graph with C, Si, F, N, and O x-ray counts (right).
CLINICAL AND STATISTICAL DATA
SSI data is seen in Table 1. The reported temporomandibular/facial pain complaints varied in location, with the temple pain being the most prevalent (60%), and jaw and jaw joint pain (30% each). JFQ values are seen in Table 1. Failure measures for each implant were recorded in Table 1.
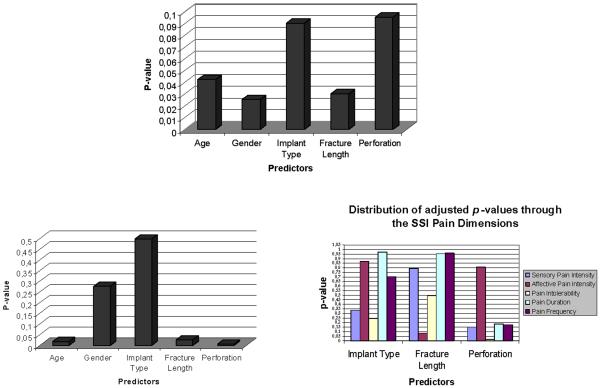
Mean age and standard deviations were similar between the implant groups. Considerable difference in the outcome mean values is observed between the implant groups for SSI but not for the JFQ. (Table 2) When testing the possible association of implant failure measures and other predictors with the SSI primary outcome, statistical significance (p<0.05) was found for the presence of perforation (p=0.0279). The other predictors did not approach significance (p>0.1). In regards to the JFQ primary outcome, the results showed no association with all the specified predictors, including implant type. Following adjustment of the statistical models for age and gender, significance (p<0.05) was again observed for the association between the clinical outcome SSI and presence of perforation in the implant (p=0.0326). (Table 3)
Table 3.
Adjusted p-values (for age and gender) to confirm if 3 Implant Predictors are statistically related to the SSI and JFQ. Adjustments (Proc GLM procedure, SAS Institute) were made for age and gender.
| Independent Variable/ Predictor | Symptom Severity Index p-value | Jaw Function Questionnaire p-value |
|---|---|---|
| Implant type | 0.5212 (p>0.05) | 0.4151 (p>0.05) |
| Presence of Perforation | 0.0326 (p<0.05) | 0.4991 (p>0.05) |
| Fracture Length | 0.8496 (p>0.05) | 0.3191 (p>0.05) |
In the full multivariate linear regression model for the SSI with all predictors entered, a statistically significant association was found between the SSI and the presence of perforation, SSI and the length of fracture, and SSI with age, thus indicating that age is an important covariate. (Fig.8) The model for the JFQ revealed statistically significant results for length of fracture, but age and gender were also important covariates. (Fig.8) Interestingly, while searching for SSI symptom domains implicated in the association between the SSI and presence of perforation, the Pain endurance component (which evaluates how disturbing the temple/ jaw or jaw joint pain symptom is for the patient) was statistically related (p<0.05) to the above implant failure measure (p=0.0148). (Fig.8)
Figures 8.
Top. p-values for JFQ to test what Predictors are related to this outcome measure. Bottom Left. p-values for SSI to test what predictors (including age and gender variables) are possibly related to this outcome measure. Bottom Right. Adjusted p-values (for age and gender) for all SSI symptom domains to test what Predictors are related to these outcome domains.
DISCUSSION
Proplast/Teflon® IP showed specific patterns of polymeric breakdown such as perforation, layer tearing and cleavage, and implant fiber extrusion. The presence of perforation and fracture lines in these implants have also been reported in the literature.25,39,40 In contrast, the stereo zoom observations for the Silastic® implants revealed structural breakdown features, such as extensive fracture lines and less fiber extrusion. These characteristics for Silastic® IP have been poorly described in the literature. Only one of the implants, a Proplast/Teflon® IP, had remained intact with no signs of fracture, although a perforation was observed in its center. The other 9 implants were fractured into more than one piece. These observations indicate that these implants may not withstand the high TMJ vector forces. In regards to fracture length, no obvious difference could be seen between the Proplast/Teflon® and Silastic® IPs. No Silastic® IP showed a verifiable perforation. Although only three Proplast/Teflon® IPs had this failure characteristic, suggesting that such presence of wear occurs mainly in this type of implant. Some breakdown features could have been just a result from the implant surgical removal procedures. Indeed, for certain implant specimens, the implant device was not totally complete, possibly due to wear breakup and/or surgical procedure. The damage at the periphery of all implants from the method of device fixation was rarely found. A large retrospective multicenter study conducted by Spagnoli et al.25 reported that 44% of 96 removed Proplast/Teflon® IP had gross observable wear signs and 14.6% were fractured. Additionally, 10.4% of the 96 Proplast/Teflon® IP were found to be displaced in the TMJ, 9.4% were loose and 3.1% buckled; suggesting lack of implant osteointegration, poor implant surgical fixation, use of aggressive postoperative physical therapy, or high TMJ loading forces.25 The literature suggests that preoperative extrinsic oral parafunctional activity, occlusal factors and intrinsic joint factors, not be able to be controlled postoperatively, can lead to high TMJ loading and consequently implant breakdown.25
Histomorphometric analysis showed osteointegration in the Proplast® porous matrix of the PTE implants. Histological investigation complemented with clearly confirmed that fluorine rich (Teflon) implant wear debris particles play apart in the multinucleated giant cell reaction in the Proplast/Teflon® tissue samples. The size of these particles appeared to vary between 20 and 230 μm. The Trumpy et al.39 study showed aluminum particles in the tissues surrounding the PTE implants, suggesting that these particles could be associated with the FBGCR, but on our EMA no aluminum phase was found to be associated with FBGCR. Particulate Teflon interaction studies by Zardeneta et al.67 suggest that the degree of biological autoimmune response is proportionally related to the amount of serum protein adsorbed by Teflon particles. Typically, a particle size of 50 μm or less in the Zardeneta study67 appeared to dramatically increase the biological signals to local cell populations and activate cellular cascades leading to inflammation. Surprisingly, this FBR was not observed by Zardeneta67 in the Silastic® selected peri-implant soft tissues; only implant wear debris and non-associated, scattered inflammatory cells were present. Moreover, Hartman et al.57 confirmed that particulate silicon debris migrate to peri-implant TMJ fibrous pseudocapsular and lymph nodal tissues, causing foreign body reaction, synovitis, dystrophic calcification, fibrocartilaginous metaplasia, hyalinization, and scarring. Other studies by Shanbhag68 on the interaction macrophage/implant particulate wear debris revealed a macrophage response dependent on the particle size, composition and dose.
Electron Microprobe element mapping and line profile revealed the existence of two main different chemical phases in the Proplast/Teflon® implants: calcium and phosphorus-rich. The F and Al-rich material is most probably the Teflon layer, and the Ca-phosphate would be the Proplast ceramic layer, confirming that the retrieved implant samples were Proplast/Teflon® category II. Energy dispersive spectrometry revealed inclusions of several types of chemical elements in these implant materials, which include: Fe, Fe-Cr, Bi-Al, NaCl, NaKCl, KAl silicate, CaO phases. The presence of these potential toxic materials might be related with the leftovers of the implant manufacturing process, and it confronts the prosthetic principles of biocompatibility. In relation to the Silastic® implants, the element mapping and line profile showed a silicon gel matrix with a fibrous material rich in F and contained an abundance of Ti inclusions.
Statistical analysis showed a statistically significant correlation between the presence of perforation and the SSI outcome. Furthermore, the presence of perforation was found to highly predict the pain tolerability dimension of the SSI (p=0.0148). No apparent association was seen between this implant failure predictor and the JFQ. These points to the fact that patients with an implant perforation report increased pain symptoms and increased difficulty with enduring the pain, but they do not have increased mandibular function impairment. Indeed these implant patients tended to have very low JFQ scores for impairment. It should be emphasized that SSI values varied greatly among the implant subjects. Given a sample of only 10 implants/patients, this should be considered a pilot study. Nevertheless, the sample size needs to be increased in future studies. After adjusting the linear models for age and gender, significance (p<.05) was also observed for the association between one clinical outcome (SSI) and presence of perforation in the implant (p=0.0326). The linear regression model for the SSI with all predictors entered also showed that age could be an important predictor, depending on the model.
SUMMARY AND CONCLUSIONS
This pilot study demonstrated that pain was a significant correlate to perforation of the alloplastic TMJ IP implants. This finding has been speculated elsewhere12,78 but it is scientifically demonstrated here for the first time. Patients with these TMJ implants devices report difficulties in tolerating their pain symptoms. Moreover, Teflon materials present in these implants appeared to structurally fail and cause a FBGCR in the TMJ. The cause of this breakdown may be multifactorial.
Stereo zoom microscopy may constitute a good quality technique to investigate biomechanical failure patterns on the surface of implant devices. Electron Microprobe analysis (with line profile and element mapping) can be an appropriate procedure to evaluate the surface of implant devices, analyze the quality and quantity of different chemical phases present.
This pilot research also provided new information on the problems associated with past implant materials, and these findings may assist in developing other appropriate types of TMJ implant materials more resistant to perforation and breakage. These new TMJ materials should be biocompatible and able to resist to TMJ biomechanical forces, without producing wear debris.
ACKNOWLEDGEMENTS
The author would like to express his deep gratitude to Ana Velly, Abeer Yakout, Delci Sotillo, Jennifer Springsteen, Jim Hodges, John Look, Jorge Perdigão, Mary Warrings, Patricia Carlson, Shanti Kaimal, Yajing Li, Wei Ouyang for their active contribution to this research study.
FINANCIAL INTERESTS: This study was supported by a grant from the National Institute of Dental and Craniofacial Research's Temporomandibular Joint Implant Registry and Repository (NIDCR's TIRR, grant number N01-DE-22635).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Okeson JP. Orofacial pain: guidelines for assessment, diagnosis, and management, The American Academy of Orofacial Pain. Quintessence Publishing Co Inc.; Carol Stream, IL: 1996. pp. 113–158. [Google Scholar]
- 2.Okeson JP. Management of Temporomandibular disorders and Occlusion. 5th edition Mosby Inc.; 2003. pp. 3–27. [Google Scholar]
- 3.Okeson JP. Bell's Orofacial Pains: The Clinical Management of Orofacial Pain. 6th edition Quintessence Publishing Co Inc.; Carol Stream, IL: 2005. pp. 287–373. [Google Scholar]
- 4.Wright EF. Manual of Temporomandibular disorders. Blackwell Munksgaard; Ames, IO: 2005. pp. 181–256. [Google Scholar]
- 5.Dimitroulis G. The role of surgery in the management of disorders of the Temporomandibular Joint: a critical review of the literature. Int J Oral Maxillofac Surg. 2005;34(Part 1):107–113. doi: 10.1016/j.ijom.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Dimitroulis G. The role of surgery in the management of disorders of the Temporomandibular Joint: a critical review of the literature. Int J Oral Maxillofac Surg. 2005;34(Part 2):231–237. doi: 10.1016/j.ijom.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Milam S. Failed Implants and Multiple Operations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:156–62. doi: 10.1016/s1079-2104(97)90107-4. [DOI] [PubMed] [Google Scholar]
- 8.Annandale T. On displacement of the inter-articular cartilage of the lower jaw and its treatment by operation. Lancet. 1887;1:411. [Google Scholar]
- 9.Lanz AB. Discitis mandibularis. Zentralbl Chir. 1909;36:289–291. [Google Scholar]
- 10.Boman K. Temporomandibular joint arthrosis and its treatment by extirpation of the disc. Acta Chir Scand Suppl. 1947;118:95. [Google Scholar]
- 11.Kiehn CL. Menisectomy for internal derangement of the temporomandibular joint. Am J Surg. 1952;83:364–373. doi: 10.1016/0002-9610(52)90271-7. [DOI] [PubMed] [Google Scholar]
- 12.Laskin DM. Etiology of the pain dysfunction syndrome. J Am Dent Assoc. 1969;79:147. doi: 10.14219/jada.archive.1969.0234. [DOI] [PubMed] [Google Scholar]
- 13.McCarty WL, Farrar WB. Surgery for internal derangements of the temporomandibular joint. J Prosthet Dent. 1979;42:191. doi: 10.1016/0022-3913(79)90174-4. [DOI] [PubMed] [Google Scholar]
- 14.Katzberg RW, Keith DA, et al. Internal derangement and osteoarthritis of the temporomandibular joint. Radiology. 1983;146:107–112. doi: 10.1148/radiology.146.1.6849030. [DOI] [PubMed] [Google Scholar]
- 15.Westesson P-L. Arthrography of the temporomandibular joint. J Prosthet Dent. 1984;51:535. doi: 10.1016/0022-3913(84)90310-x. [DOI] [PubMed] [Google Scholar]
- 16.Dolwick MF. Disc preservation surgery for the treatment of internal derangements of the temporomandibular joints. J Oral Maxillofac Surg. 2001;59:1047–1050. doi: 10.1053/joms.2001.26681. [DOI] [PubMed] [Google Scholar]
- 17.Dolwick MF, Katzberg RW, et al. Internal derangement of the temporomandibular joint: fact or fiction? J Prosthet Dent. 1983;49:415–418. doi: 10.1016/0022-3913(83)90287-1. [DOI] [PubMed] [Google Scholar]
- 18.Dolwick MF, Riggs RR. Diagnosis and treatment of internal derangements of the temporomandibular joint. Dent Clin North Am. 1983;27:561–572. [PubMed] [Google Scholar]
- 19.Hall HD, Nickerson JW. Is it time to pay more attention to disc position? J Orofacial Pain. 1994;8:90–95. [PubMed] [Google Scholar]
- 20.Ryan DE. Alloplastic Implants in the Temporomandibular Joint. Oral Maxillofac Surg Clin North Am. 1989;1(2):427–441. [Google Scholar]
- 21.Ryan DE. Alloplastic Disc Replacement. Oral Maxillofac Surg Clin North Am. 1994 May;6(2):307–321. [Google Scholar]
- 22.Estabrooks LN, Fairbanks CE, et al. A retrospective evaluation of 301 TMJ Proplast-Teflon implants. Oral Surg Oral Med Oral Pathol. 1990;70:381–386. doi: 10.1016/0030-4220(90)90164-n. [DOI] [PubMed] [Google Scholar]
- 23.Van Loon J, De Bont LGM, et al. Evaluation of temporomandibular Joint Prostheses: review of the literature from 1946 to 1994 and implications for future prosthesis designs. J Oral Maxillofac Surg. 1995;53:984–996. doi: 10.1016/0278-2391(95)90110-8. [DOI] [PubMed] [Google Scholar]
- 24.Martukas VJ, Lachner J. The use of autogenous auricular cartilage for temporomandibular joint disc surgery. J Oral Maxillofac Surg. 1990;48:438. doi: 10.1016/0278-2391(90)90429-6. [DOI] [PubMed] [Google Scholar]
- 25.Spagnoli D, Kent JN, et al. Multicenter evaluation of temporomandibular joint Proplast-Teflon disk implant. Oral Surg Oral Med Oral Pathol. 1992;74:411–21. doi: 10.1016/0030-4220(92)90285-x. [DOI] [PubMed] [Google Scholar]
- 26.Dusek J, Kent J, et al. Proplast-Teflon implants for treatment of TMJ degenerative disease [Abstract]. Presented at the 60th Annual AAOMS meeting; Chicago. September.1978. [Google Scholar]
- 27.Malloy R, Kent J, et al. Retrospective study of Proplast-Teflon implants for TMJ arthritis [Abstract]. Presented at the 63rd annual AAOMS meeting; Washington. September.1981. [Google Scholar]
- 28.Homsy CF. Biocompatibility of perfluorinated polymers and composites of these polymers. In: Williams DF, editor. Biocompatibility of clinical implant materials. vol. 2. CRC Press Inc.; Boca Raton: 1982. pp. 59–77. [Google Scholar]
- 29.Kiersch TA. The use of Proplast-Teflon implants for menisectomy and disk repair in the temporomandibular joint [Abstract]. Presented at AAOMS Clinical Congress on Reconstructive Biomaterials: Current Assessment and Temporomandibular Joint: Surgical Update; San Diego, CA. January.1984. [Google Scholar]
- 30.Eriksson L, Westesson PL. Long term evaluation of meniscectomy of the temporomandibular joint. J Oral Maxillofac Surg. 1985;43:263–9. doi: 10.1016/0278-2391(85)90284-8. [DOI] [PubMed] [Google Scholar]
- 31.American Academy of Oral and Maxillofacial Surgery: Recommendations for Management of Patients with Temporomandibular Joint Implants. J Oral Maxillofac Surg. 1993;51:1164–1172. doi: 10.1016/s0278-2391(10)80464-1. [DOI] [PubMed] [Google Scholar]
- 32.Goss AN. Towards an international consensus on temporomandibular joint surgery. Int J Oral Maxillofac Surg; Report on the Second International Consensus Meeting; Buenos Aires, Argentina. 1993. pp. 78–81. [DOI] [PubMed] [Google Scholar]
- 33.Fricton JR, Look JO, Schiffman E, Swift J. Long-term study of temporomandibular joint surgery with alloplastic implants compared with nonimplant surgery and nonsurgical rehabilitation for painful temporomandibular joint disc displacement. J Oral Maxillofac Surg. 2002;60:1400–11. doi: 10.1053/joms.2002.36091. discussion 1411-2. [DOI] [PubMed] [Google Scholar]
- 34.Hansen WC, Deshazo BW. Silastic reconstruction of the temporomandibular joint meniscus. Plast Reconstruct Surg. 1969;43:388–91. doi: 10.1097/00006534-196904000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Rubin JP, Yaremchuck MJ. Complications and Toxicities of Implantable Biomaterials used in Facial Reconstructive and Aesthetic Surgery: A Comprehensive Review of the Literature. Plast Reconstr Surg. 1997;100(5):1336–1353. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 36.Wolford LM, Henry CH, et al. Temporomandibular Disorders and Related Pain Conditions, Progress in Pain Research and Management. vol. 4. IASP Press; Seattle: 1995. The Temporomandibular Joint Alloplastic Implant Problem. [Google Scholar]
- 37.Charnley J. An artificial bearing in the hip joint: implications in biological lubrication. Fed Proc. 1966;25:1079–81. [PubMed] [Google Scholar]
- 38.Gallagher DM, Wolford LM. Comparison of Silastic and Proplast implants in the Temporomandibular Joint after Condylectomy for Osteoarthritis. J Oral Maxillofac Surg. 1982:627–630. doi: 10.1016/0278-2391(82)90110-0. [DOI] [PubMed] [Google Scholar]
- 39.Trumpy IG, Lyberg T. In vivo deterioration of proplast-teflon temporomandibular joint interpositional implants: a scanning electron microscopic and energy-dispersive X-ray analysis. J Oral Maxillofac Surg. 1993;51:624–9. doi: 10.1016/s0278-2391(10)80259-9. [DOI] [PubMed] [Google Scholar]
- 40.Valentine JD, Jr., Reiman BE, Beuttenmuller EA, Donovan MG. Light and electron microscopic evaluation of Proplast II TMJ disc implants. J Oral Maxillofac Surg. 1989;47:689–96. doi: 10.1016/s0278-2391(89)80007-2. [DOI] [PubMed] [Google Scholar]
- 41.Yih WY, Merrill RG. Pathology of Alloplastic Interpositional Implants in the Temporomandibular Joint. Oral Maxillofac Surg Clin North Am. 1989;1(2):415–426. [Google Scholar]
- 42.Mercuri LG, Giobbie-Hurder A. Long-term outcomes after total alloplastic temporomandibular joint reconstruction following exposure to failed materials. J Oral Maxillofac Surg. 2004;62:1088–1096. doi: 10.1016/j.joms.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Kulber DA, et al. Severe Cutaneous Foreign Body Giant Cell Reaction After Temporomandibular Joint Reconstruction with Proplast-Teflon (article and discussion) J Oral Maxillofac Surg. 1995;53:719–723. doi: 10.1016/0278-2391(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 44.Chuong R, Piper MA. Cerebrospinal fluid leak associated with proplast implant removal from the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1992;74:422–5. doi: 10.1016/0030-4220(92)90286-y. [DOI] [PubMed] [Google Scholar]
- 45.Berarducci JP, Thompson DA, Scheffer RB. Perforation into middle cranial fossa as a sequel to use of a Proplast-Teflon implant for temporomandibular joint reconstruction. J. Oral Maxillofac. Surg. 1990;48:496. doi: 10.1016/0278-2391(90)90239-x. [DOI] [PubMed] [Google Scholar]
- 46.Smith RM, Goldwasser MS, Sabol SR. Erosion of a Teflon-Proplast implant into the middle cranial fossa. J. Oral Maxillofac. Surg. 1993;51:1268. doi: 10.1016/s0278-2391(10)80300-3. [DOI] [PubMed] [Google Scholar]
- 47.Ta LE, Pillemer SR, et al. Clinical Evaluation of Patients with Temporomandibular Joint Implants. J Oral Maxillofac Surg. 2002;60:1389–1399. doi: 10.1053/joms.2002.36089. [DOI] [PubMed] [Google Scholar]
- 48.Raphael KG, Marbach JJ, et al. Self-reported Systemic, Immune-mediated disorders in patients with and without Proplast-Teflon implants of the Temporomandibular joint. J Oral Maxillofac Surg. 1999;57:364–370. doi: 10.1016/s0278-2391(99)90268-9. [DOI] [PubMed] [Google Scholar]
- 49.Dorsay TA, Youngberg RA, et al. Case Report: Cine MRI in the Evaluation of the Proplast-Teflon TMJ Interpositional Implant. J Computer Assisted Tomography. 1995;19(5):800–803. doi: 10.1097/00004728-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Heffez L, Mafee MF, et al. CT evaluation of TMJ Disc Replacement with a Proplast-Teflon Laminate. J Oral Maxillofac Surg. 1987;45:657–665. doi: 10.1016/0278-2391(87)90303-x. [DOI] [PubMed] [Google Scholar]
- 51.Trumpy IG, et al. Soft Tissue Response to Polytetrafluoroethylene and Silicone Rubber in Humans: morphological and immunohistochemical observations. Scand J Plast Reconstr Hand Surg. 1997;31:295–301. doi: 10.3109/02844319709008975. [DOI] [PubMed] [Google Scholar]
- 52.Nalbandian RM, Swanson AB, et al. Long-term silicone implant arthroplasty. Implications of animal and human autopsy findings. JAMA. 1983;250:1195–1198. [PubMed] [Google Scholar]
- 53.Herndon JH. Long-term results of silicone arthroplasty. Surgical Rounds. 1983;6:94–105. [Google Scholar]
- 54.Charnley J. Tissue reactions to polytetrafluoroethylene. Lancet. 1963;2:1379. [Google Scholar]
- 55.Moriconi ES, Popowich LD, et al. Alloplastic Reconstruction of the Temporomandibular Joint. Dent Clin North Am. 1986;30(2):307–325. [PubMed] [Google Scholar]
- 56.Westesson P, Eriksson L, et al. Destructive lesions of the mandibular condyle following diskectomy with temporary silicone implant. Oral Surg Oral Med Oral Pathol. 1987;63:143–150. doi: 10.1016/0030-4220(87)90302-1. [DOI] [PubMed] [Google Scholar]
- 57.Hartman LC, Bessette RW, et al. Silicone rubber temporomandibular joint (TMJ) meniscal replacements: postimplant histopathologic and material evaluation. J Biomedical Materials Research. 1988;22:475–484. doi: 10.1002/jbm.820220604. [DOI] [PubMed] [Google Scholar]
- 58.Dolwick MF, Aufdemorte TB. Silicone-induced foreign body reaction and lymphadenopathy after temporomandibular joint arthroplasty. Oral Surg Oral Med Oral Pathol. 1985;59:449–452. doi: 10.1016/0030-4220(85)90079-9. [DOI] [PubMed] [Google Scholar]
- 59.Taylor DE, Penhallow JE. Comparative biotolerance of polyacylamide-agarose gel, silicone rubber and microporous PTFE as soft tissue implants. Biomaterials. 1986;7:277–282. doi: 10.1016/0142-9612(86)90050-5. [DOI] [PubMed] [Google Scholar]
- 60.Mirra JM, Marder RA, et al. The Pathology of Failed Total Joint Arthroplasty. Clin Orthopaedics Rel Res. 1982;179:175–183. [PubMed] [Google Scholar]
- 61.NIH NIH Technology Assessment 19th Conference Summary: Improving Medical Implant Performance Through Retrieval Information: Challenges and Opportunities. [Google Scholar]
- 62.Takahashi H, Takakura M. EPMA Analysis of Insulating Materials Using the Thin Film Method. JEOL News 2001. 2001;36E(1):40–44. [Google Scholar]
- 63.Fricton JR, Hathaway KM, et al. Interdisciplinary management of patients with TMJ and craniofacial pain: characteristics and outcome. J Craniomandibular Disorders. 1987;1:115. [PubMed] [Google Scholar]
- 64.Granger CV, et al. Physical Medicine and Rehabilitation. R.L. Braddom, W.B. Saunders; Philadelphia: 1996. Quality and outcome measures for medical rehabilitation; pp. 239–253. [Google Scholar]
- 65.Horrell BM, et al. Validity of a self-report scale for TMD-related disability. Journal of Dental Research. 1999;78:292. [Google Scholar]
- 66.Ohrbach R, Dworkin SF, Granger CV. Cross-validation of a disability scale for chronic temporomandibular disorder pain. International Association for the Study of Pain; 9th World Congress on Pain; Vienna, Austria. 1999. [Google Scholar]
- 67.Zardeneta G, Mukai H. Protein Interactions with particulate Teflon: Implications for the Foreign Body Response. J Oral Maxillofac Surg. 1996;54:873–878. doi: 10.1016/s0278-2391(96)90540-6. [DOI] [PubMed] [Google Scholar]
- 68.Shanbhag AS, Jacobs JJ, et al. Macrophage/particle interactions: Effect of size, composition and surface area. J Biomedical Materials Research. 1994;28:81–90. doi: 10.1002/jbm.820280111. [DOI] [PubMed] [Google Scholar]