Abstract
OBJECTIVES
To evaluate the adequacy of discharge room cleaning and the impact of a cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) on environmental surfaces in intensive care unit (ICU) rooms.
DESIGN
Prospective environmental study.
SETTING AND SAMPLE
Convenience sample of ICU rooms in an academic hospital.
METHODS AND INTERVENTION
The intervention consisted of (1) a change from the use of pour bottles to bucket immersion for applying disinfectant to cleaning cloths, (2) an educational campaign, and (3) feedback regarding adequacy of discharge cleaning. Cleaning of 15 surfaces was evaluated by inspecting for removal of a preapplied mark, visible only with an ultraviolet lamp (“black light”). Six surfaces were cultured for MRSA or VRE contamination. Outcomes of mark removal and culture positivity were evaluated by χ2 testing and generalized linear mixed models, clustering by room.
RESULTS
The black-light mark was removed from 44% of surfaces at baseline, compared with 71% during the intervention (P <.001). The intervention increased the likelihood of removal of black-light marks after discharge cleaning (odds ratio, 4.4; P < .001), controlling for ICU type (medical vs surgical) and type of surface. The intervention reduced the likelihood of an environmental culture positive for MRSA or VRE (proportion of cultures positive, 45% at baseline vs 27% during the intervention; adjusted odds ratio, 0.4; P = .02). Broad, flat surfaces were more likely to be cleaned than were doorknobs and sink or toilet handles.
CONCLUSIONS
Increasing the volume of disinfectant applied to environmental surfaces, providing education for Environmental Services staff, and instituting feedback with a black-light marker improved cleaning and reduced the frequency of MRSA and VRE contamination.
Environmental contamination with pathogens commonly occurs during routine medical care. Many studies have described transmission of pathogenic organisms through contact with contaminated room surfaces.1–3 Of particular concern is the potential for transmission of multidrug-resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE), which are associated with healthcare-associated infections, increased lengths of stay in hospitals, increased healthcare costs, and increased mortality.4–7
Cleaning is essential to reduce environmental reservoirs of known hospital-acquired pathogens. Surfaces in rooms occupied by MRSA-positive or VRE-positive patients can contaminate the hands of healthcare workers who touch these surfaces without touching the colonized or infected patient.8–10 Both MRSA and VRE have been isolated from various fomites, including beds, linen hampers, doorknobs, and window ledges.11–16 Studies have demonstrated that these antimicrobial-resistant pathogens can persist on room surfaces even after discharge cleaning.17,18 Interventions that address the thoroughness of room cleaning have proven successful in reducing the environmental burden of such organisms.13
Although the goal of environmental cleaning and disinfection is not sterilization, adequate cleaning requires sufficient removal of pathogens to minimize patients’ risk of acquiring infections from hospital environments. This is particularly true in areas serving high-risk patients, such as intensive care units (ICUs). The Environmental Services department at Brigham and Women’s Hospital has routine cleaning policies that exceed national performance standards.19 Examples of added measures include exchanging bed curtains after discharging patients who were placed under contact precautions and using pour bottles to dispense disinfectant, which results in quantities of applied agent that are larger than those dispensed by spray bottles. In addition, all Environmental Services staff receive hands-on training in cleaning protocols and twice-monthly quality-control assessments that affect compensation. Nevertheless, we have recently shown that patients admitted to ICU rooms previously occupied by MRSA or VRE carriers are at increased risk for MRSA or VRE acquisition.18 It is possible that high-risk areas occupied by patients who are critically ill, often with wounds, medical devices, and immunocompromised states, have need of more-intensive cleaning protocols to reduce transmission of and subsequent infection due to these and other pathogens.
Recently, a novel and nontoxic tracking marker that is visible only under a UV lamp (“black light”) has been developed to assess the quality of environmental cleaning. It is invisible and can be removed only with sufficient moisture.20–23 Feedback based on this evaluation system has been shown to improve cleaning technique, but it is not known whether it produces reductions in environmental contamination. We assessed whether an intervention involving improved cleaning practices, staff education, and feedback based on the black-light monitoring system would impact the thoroughness of discharge room cleaning and the environmental prevalence of MRSA and VRE in ICU rooms.
METHODS
Setting and Design
We performed an 8-month prospective study evaluating the impact of an intervention on discharge cleaning of hospital ICU rooms from June 15, 2006, through February 13, 2007. The study consisted of a 6-week baseline period and 6-month intervention period and was performed in all 10 ICUs at Brigham and Women’s Hospital, an 800-bed tertiary-care facility and academic medical center in Boston, Massachusetts. The ICUs each had a 10-bed capacity, and they included cardiac, medical (2 units), general surgery, burn/trauma, cardiac surgery (2 units), neurosurgery (2 units), and thoracic surgery ICUs. This study was performed as a joint Infection Control and Environmental Services quality-improvement project and received approval for analysis and publication from the hospital’s institutional review board.
At baseline, routine discharge room cleaning was performed using a hospital-grade quaternary ammonium disinfectant. Staff dusted and wiped all surfaces and equipment, in compliance with national standards, as described elsewhere.18
Baseline Period
To identify “high-touch” (ie, frequently touched) surfaces in a typical ICU room, an informal survey was conducted among a convenience sample of ICU nurses. Based on the results of this survey, 15 environmental surfaces were selected for marking with a black-light indicator, and 6 environmental surfaces were selected for culture after discharge cleaning (Table 1).
TABLE 1.
Surfaces Evaluated After Postdischarge Room Cleaning
| Surfaces marked with black-light substance |
| Main doorknob |
| Bathroom doorknob |
| Main countertop |
| Linen hamper |
| Monitor touch pad |
| Equipment cart top |
| Equipment cart handle |
| Window countertop |
| Intravenous pump |
| Sink handle |
| Bedside table |
| Curtain |
| Light switch |
| Toilet flush handle |
| Bed raila |
| Surfaces cultured |
| Linen hamper and trash binb |
| Doorknobsc |
| Monitor touch pad |
| Countertop |
| Equipment cart (top and handles) |
| Bed raila |
Only in medical intensive care unit (ICU) rooms; beds in all other ICU rooms traveled with patients on transfer from the ICU.
A single swab was used to sample both surfaces for a qualitative culture; in addition, a quantitative culture sample was taken from the linen hamper alone.
A single swab was used to sample both doorknobs for a qualitative culture; in addition, a quantitative culture sample was taken from the main doorknob alone.
We identified rooms of ICU patients who were known to harbor either MRSA or VRE and who were expected to be discharged from the ICU within 24 hours. Identification occurred during weekday daytime and evening hours for a 6-week period (June 15, 2006 to July 28, 2006). Pending discharges were identified by using an automated Environmental Services database in which imminent discharges were posted and by periodic communication with ICU nursing staff and unit coordinators. Rooms of patients under airborne, transplant, neutropenic, or droplet precautions were excluded, to prevent unmeasured effects of additional precautions on the thoroughness of room cleaning.
Evaluation and culturing during the baseline period were performed with the knowledge of the Environmental Services director but without the knowledge of Environmental Services staff, supervisors, or managers. Before discharge room cleaning, the 15 preselected surfaces were marked with a black-light–sensitive substance, which is nontoxic, invisible, and removable only with a moist cloth applied with moderate pressure. Marks were approximately 1 cm2 in area and were placed in a consistent location on each surface.
After postdischarge room cleaning and before the admission of a new patient, a black light was used to evaluate whether the marks had been removed. Marks were classified as “clean” (completely or partially removed) or “dirty” (not removed). In addition, the preselected 6 surfaces were cultured for MRSA and VRE from samples collected on sterile rayon swabs (Bacteriology Culture Collection and Transport System; Fisher Scientific). For each surface, a sample for quantitative culture was collected from a 1-cm2 sterile template applied to the surface, and a sample for qualitative culture was collected by using a separate swab and liberally sampling a large area of the surface. All specimens were processed within 24 hours of collection.
Quantitative culture swabs were vortexed briefly in 1 mL of sterile phosphate-buffered saline, and 100 µL of this solution was plated on vancomycin-impregnated bile esculin azide agar and oxacillin-impregnated mannitol salt agar. Plates were incubated at 37°C for 24 hours. Confirmation of the presence of MRSA and VRE was performed in accordance with Clinical and Laboratory Standards Institute guidelines.24
Qualitative culture swabs were vortexed in 5 mL of tryptic soy broth and incubated at 37°C for 24 hours. Samples exhibiting turbidity were plated on screening plates as described above. Culture tubes exhibiting no growth after 24 hours were reincubated for an additional 24 hours and rescreened for turbidity.
Intervention Period
The 6-month intervention period lasted from July 30, 2006, through February 13, 2007. At the start of the intervention period, all Environmental Services staff, managers, and supervisors were fully informed of the study and baseline period results. The intervention involved 3 components: (1) a change in the application of disinfectant from the use of pour bottles to immersion of the cleaning cloth in buckets, (2) Environmental Services staff education on transmission of healthcare-associated pathogens and resultant infection, and (3) feedback regarding the thoroughness of room cleaning, using the black-light indicator. Education further emphasized proper dilution of concentrated disinfectant, repeated immersion of cloths in disinfectant before cleaning of each room surface, and discarding of disinfection solution after each room cleaning.
Black-light mark application and screening were continued throughout the intervention period for 2 days per week by Infection Control staff. ICU rooms from which a MRSA-positive or VRE-positive patient was expected to be discharged or transferred within 24 hours were chosen for black-light marking, evaluation of mark removal, and culturing of environmental surfaces, as described above.
In addition, Environmental Services supervisors were trained to integrate black-light screening into their routine evaluation of approximately 50% of all ICU rooms undergoing discharge cleaning. Supervisors were trained to mark 5 surfaces before discharge cleaning and to screen for removal of the marks after cleaning was performed. Room selection was at the discretion of the supervisors. Every 2 months, the 5 surfaces rotated, such that all 15 of the surfaces chosen for evaluation were monitored within 6 months. Supervisors were instructed to provide immediate feedback to their staff after screening a room. Although the placement and assessment of black-light marks by supervisors was part of the intervention protocol, only data collected by infection control staff contributed to the analysis of the intervention impact, to maintain standardization in room selection.
During the intervention period, results of all black-light evaluations were reported weekly to the Environmental Services director and managers for dissemination to their supervisors and staff. These reports tabulated the results by ICU, surface, and Environmental Services staff member, to identify targets for improvement.
Analysis
We calculated the percentage of black-light marks removed after discharge cleaning, stratified by study period. We assessed potential predictors of mark removal in bivariate tests (χ2) and generalized linear mixed models (GLIMMIX; SAS version 9.1; SAS Institute), which accounted for clustering by room. Assessed variables included surface type, study period (baseline or intervention), ICU type (medical or surgical), and prior occupant status (MRSA positive or VRE positive).
In addition, we calculated the percentage of surface cultures yielding MRSA or VRE. Results were evaluated by (1) study period (baseline or intervention), (2) black-light mark status of the surface (clean or dirty), and (3) prior occupant status (MRSA positive or VRE positive). The association between culture positivity and black-light mark status, study period, and prior occupant status were assessed using 2-tailed χ2 tests and generalized linear mixed models, accounting for clustering by room. In a separate model, we further assessed whether the proportion of cleaned marks in each room was predictive of whether any surface yielded a culture positive for MRSA or VRE. Assessed variables in this latter model included surface type, percentage of removed black-light marks, study period (baseline or intervention), and ICU type (medical or surgical). Lastly, we performed 2 separate multivariate models evaluating whether MRSA-positive prior occupant status or VRE-positive prior occupant status predicted a surface culture positive for the same organism. In all models, effect modification was assessed using interaction terms, and predictors were retained at P < .05.
RESULTS
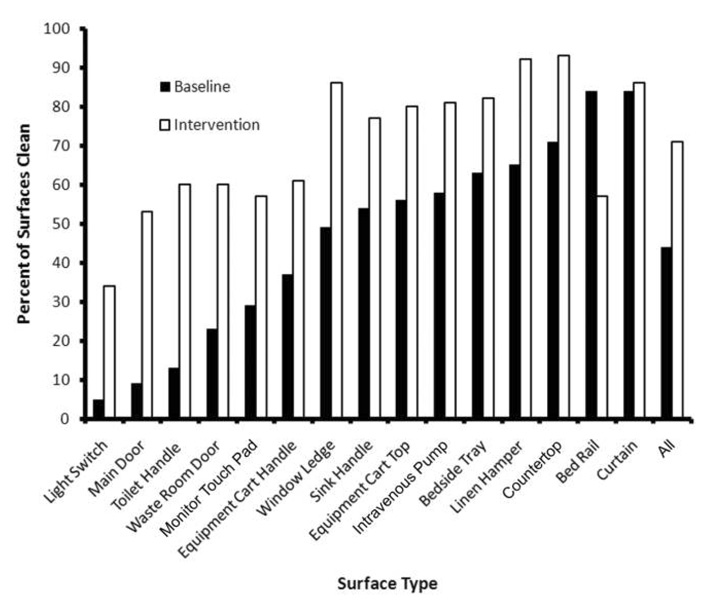
The black light was used to evaluate a total of 41 ICU rooms (545 surfaces marked) during the baseline period and 44 rooms (576 surfaces marked) during the intervention period. The percentages of surfaces that were clean during both study periods are shown in Figure 1. Not all surfaces were evaluated in every room, because mobile room items, such as linen hampers and bed trays, were occasionally removed during discharge cleaning. Bed rails were evaluated only in the medical ICU, because beds in all other ICUs transferred with the discharged patient. All surfaces showed an improvement in the percentage of clean surfaces in the intervention period, with the exception of bed rails, which had half the number of black-light evaluations compared with the other surfaces. Overall, the percentage of total ICU precaution rooms sampled was 25% in the baseline period and 5% in the intervention period.
FIGURE 1.
Impact of the intervention on the removal of a black-light mark on 15 high-touch surfaces. A total of 41 rooms (545 surfaces) were evaluated during the baseline period, and a total of 44 rooms (576 surfaces) were evaluated during the intervention. The bed rail was evaluated only in medical intensive care units, with a <50% sample size in both periods. No. of bed rails marked: baseline, n = 19; intervention, n = 14.
In bivariate testing, mark removal was more frequent during the intervention period than during the baseline period (71% vs 44%; P < .001). Additional predictors of mark removal included type of ICU (medical vs surgical, 62% vs 54%; P =.005) and type of surface (P < .001). The results of the multivariate analysis were similar (Table 2), with the intervention period remaining a strong predictor of mark removal from room surfaces. There was no difference in the effect of the intervention between surgical and medical ICUs. Surfaces with the highest likelihood of mark removal either were removed at the time of discharge cleaning for laundering (eg, curtains) or involved a flat surface (eg, countertops or linen hampers). Doorknobs and handles were the least likely to be cleaned.
TABLE 2.
Multivariate Model Predicting Likelihood of Black-Light Mark Removal, Clustering by Room
| Variable | Odds ratio (95% CLs) | P |
|---|---|---|
| ICU type: medical | 2.1 (1.4, 3.2) | <.001 |
| Surface type | ||
| Light switch | 1.0 | |
| Main door | 1.8 (0.8, 4.0) | .12 |
| Toilet handle | 2.7 (1.3, 5.6) | .007 |
| Waste room door | 3.6 (1.8, 7.4) | <.001 |
| Monitor touch pad | 3.7 (1.8, 7.5) | <.001 |
| Equipment cart handle | 4.8 (2.4, 9.8) | <.001 |
| Sink handle | 10.1 (4.9, 20.9) | <.001 |
| Equipment cart top | 11.4 (5.5, 23.7) | <.001 |
| Bed rail | 11.9 (4.5, 31.8) | <.001 |
| Window ledge | 12.1 (5.8, 25.3) | <.001 |
| Intravenous pump | 13.3 (5.8, 30.8) | <.001 |
| Bedside tray | 15.7 (7.1, 34.5) | <.001 |
| Linen hamper | 22.0 (9.7, 49.8) | <.001 |
| Countertop | 29.0 (13.0, 64.9) | <.001 |
| Curtain | 33.0 (13.6, 80.2) | <.001 |
| Intervention period | 4.4 (3.2, 6.2) | <.001 |
NOTE. CLs, confidence limits; ICU, intensive care unit.
Samples for qualitative and quantitative MRSA and VRE culture were collected from 6 surfaces in each of 37 rooms during the baseline period and 44 rooms during the intervention period, for a total of 199 quantitative and 199 qualitative cultures during the baseline period and 234 quantitative and 234 qualitative cultures during the intervention for each organism. Bed rails were cultured only in the medical ICU. One set of cultures (from a countertop) was lost.
Culture results stratified by surface type are shown in Table 3, and results stratified by prior occupant MRSA and VRE carriage status are shown in Table 4. No quantitative cultures yielded any MRSA or VRE growth in either study period. Among qualitative cultures, 16 (45%) of 37 rooms had at least 1 surface with a culture positive for MRSA or VRE at baseline, compared with 12 (27%) of 44 rooms after the intervention, although the difference was not statistically significant in bivariate testing. Type of ICU was predictive of positive surface-culture results (surgical vs medical, 11% vs 5% of rooms; P = .02), as was type of surface (P < .001).
TABLE 3.
Methicillin-Resistant Staphylococcus aureus (MRSA) and Vancomycin-Resistant Enterococci (VRE) Culture Data, by Study Period and Surface Type
| No. (%) of cultures with positive results | ||||
|---|---|---|---|---|
| MRSA | VRE | |||
| Surface | Baseline | Intervention | Baseline | Intervention |
| Doorknobs | 4 (11) | 2 (5) | 2 (5) | 1 (2) |
| Monitor touch pad |
1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Equipment carts |
0 (0) | 3 (7) | 0 (0) | 0 (0) |
| Linen hamper and trash bin |
6 (16) | 2 (5) | 4 (11) | 6 (14) |
| Countertop | 0 (0) | 3 (7) | 2 (5) | 0 (0) |
| Bed rail | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| All surfaces | 11 (6) | 10 (4) | 9 (5) | 7 (3) |
TABLE 4.
Dectection of Methicillin-Resistant Staphylococcus aureus (MRSA) and Vancomycin-Resistant Enterococci (VRE) in Any Surface Culture by Prior Occupant Status
| No. (%) of rooms with a positive culture result | ||||
|---|---|---|---|---|
| Prior occupant carriage status |
MRSA | VRE | ||
| Baseline | Intervention | Baseline | Intervention | |
| MRSA positive | 9 (24) | 7 (16) | 4 (11) | 2 (5) |
| MRSA negative | 1 (3) | 0 (0) | 4 (11) | 4 (9) |
| VRE positive | 5 (14) | 5 (11) | 8 (22) | 6 (14) |
| VRE negative | 5 (14) | 2 (5) | 0 (0) | 0 (0) |
Results of multivariate analysis assessing surface culture positivity for either MRSA or VRE, clustering by room, are shown in Table 5. In contrast to bivariate tests, which used rooms as the unit of analysis, multivariate models showed a significant intervention effect, with reduced environmental MRSA and VRE contamination when cultures were used as the unit of analysis and data were clustered by room. Notably, there was no direct association between the removal of the black-light mark from a specific surface and the likelihood that the surface culture would yield MRSA or VRE. However, the proportion of removed marks in a given room was significantly predictive of fewer MRSA-positive or VRE-positive environmental cultures and was interchangeable (collinear) with the intervention effect in all models. Multivariate models assessing the proportion of marks removed showed that there were 30% fewer positive cultures for every 10% increase in the proportion of removed marks (odds ratio, 0.7; P = .05). Prior occupant VRE carriage status was not significantly predictive in models limited to VRE-positive cultures, but MRSA-positive prior occupant status was significantly predictive in models limited to MRSA-positive cultures (data not shown).
TABLE 5.
Multivariate Model Predicting the Likelihood of an Environmental Culture Positive for Methicillin-Resistant Staphylococcus aureus or Vancomycin-Resistant Enterococci, Clustering by Room
| Variable | Odds ratio (95% CLs) | P |
|---|---|---|
| ICU type: surgical | 3.0 (1, 9.2) | .05 |
| Surface type | ||
| Monitor touch pad | 1.0 | |
| Equipment cart | 3.4 (0.7, 17.2) | .15 |
| Countertop | 6.2 (1.3, 29.2) | .02 |
| Bed rail | 7.1 (0.9, 55.9) | .06 |
| Doorknobs | 11.7 (2.6, 53.3) | .002 |
| Linen hamper and trash bin | 19.7 (4.4, 89.0) | <.001 |
| Intervention period | 0.4 (0.2, 0.9) | .02 |
NOTE. CLs, confidence limits; ICU, intensive care unit.
DISCUSSION
An intervention consisting of bucket application of disinfectant, Environmental Services staff education, and feedback using a black-light monitoring system improved the thoroughness of discharge room cleaning and reduced the likelihood of isolating either MRSA or VRE from the ICU environment. Adequate disinfection is particularly important in ICUs, because the higher acuity of care contributes to contamination of environmental surfaces with potential pathogens. Furthermore, contamination may present a substantial risk to ICU patients, who are critically ill and especially vulnerable to infection because of wounds, comorbidities, and use of medical devices. Our previous work18 has shown that patients admitted to ICU rooms previously occupied by MRSA or VRE carriers are at higher risk for MRSA or VRE acquisition and that 33% of patients who acquire MRSA develop invasive disease within 1 year.25 These risks make high-quality environmental cleaning especially crucial in ICUs.
Although we are unable to determine the attributable impact of each component of our intervention, the literature supports the individual effectiveness of each component in reducing the environmental burden of nosocomial pathogens. Recent studies using the black-light marker have demonstrated improved cleaning compliance.20–23 Other studies have shown that immersion of cleaning cloths in disinfectant is superior to application of disinfectant via spray bottles for eradicating environmental VRE and reducing VRE transmission.12,13 Finally, studies have shown that environmental cleaning education and surveillance reduces levels of environmental contamination with pathogens.12,26 In this work, we combined a return to bucket immersion (standard practice in our hospital 20 years ago) with cleaning surveillance and feedback based on the use of a black-light marker to show that improved black-light mark removal was associated with decreased environmental contamination with MRSA and VRE.
Despite the fact that our institution’s cleaning practices exceeded national standards, only approximately 50% of black-light marks were removed during the baseline period. This finding is consistent with other studies.20–23 In general, we found that flat, horizontal surfaces (countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than were small, vertical surfaces (doorknobs, toilet handles, light switches, and electronics). Use of the black-light marker not only allowed us to identify and target specific surfaces for improvement but also allowed us to improve their cleaning using a visually observable feedback tool that was well received by patients, cleaning staff, and Environmental Services supervisors.
The intervention revealed key insights into barriers to effective environmental cleaning and opportunities for improvement. Although visual inspection was not a formal part of the protocol, anecdotal observations suggested that failure of black-light mark removal was often a result of inadequate saturation of cleaning cloths with disinfectant rather than a failure to wipe surfaces. Inadequate saturation may have been intentional on vertical surfaces, such as door knobs and handles, since soaked cloths could produce noticeable dripping. Additionally, educational discussions with cleaning staff revealed substantial reluctance to clean light switches and electronic equipment with saturated cloths, for fear of electric shock or damage to the equipment. Environmental services staff often mentioned other constraints, such as pressure to expedite cleaning to accommodate an incoming patient. Often, staff did not feel comfortable reporting that inadequate time was allowed for cleaning in response to such pressures. Additionally, there was substantial confusion regarding whether Environmental Services staff were responsible for cleaning mobile objects, such as equipment carts and intravenous pumps. This intervention was helpful in identifying these concerns and implementing appropriate responses, although additional improvement in assigning responsibility for cleaning of all mobile objects is still needed.
Overall, placement of the 15 marks took less than 5 minutes. Since the end of this study, assessment of the 15 surfaces has continued in the routine compliance assays of Environmental Services supervisors through 2-month cycles of 5 marks each. Our finding that surfaces in surgical ICUs were more likely to be contaminated with MRSA or VRE suggests that particular attention may be needed to improve cleaning on surgical units. Although we did not control for the prevalence of these organisms in this study, we have found in our prior work that rates of nosocomial MRSA and VRE transmission are higher in surgical ICUs than in medical ICUs, despite lower colonization pressure.27,28
Our study has a number of limitations. First, the study design does not allow us to individually evaluate the effect of each component of the intervention. Second, because of financial constraints, we were able to culture only a fraction of marked surfaces. Furthermore, the sensitivity of our cultures may have been limited by the small surface area sampled for quantitative culture or by the nonstandardized liberal swabbing for qualitative culture. Nevertheless, we were able to show an impact of the intervention on both surface cleaning and environmental culture results when multivariate models were applied. Lastly, our study does not address the clinical significance of the positive cultures in this study, in terms of the effect of bacterial burden on the patient-specific risk of MRSA or VRE acquisition. Prior work has suggested that nosocomial transmission depends on a number of factors other than environmental bacterial burden. One study, for example, has suggested that the duration of contact with VRE-contaminated surfaces may have more of an impact on VRE transmission than does bacterial burden.29 Other work has suggested that MRSA carriage at different body sites contaminates the environment with varying frequency.8 Further work is needed to understand the thresholds at which environmental contamination leads to pathogen acquisition.
In conclusion, this study demonstrates that an intervention consisting of increased application of disinfectant, Environmental Services staff education, and the use of a black-light monitoring system improved cleaning and decreased the likelihood of cultures positive for either MRSA or VRE. Further work is needed to evaluate the long-term effect of this intervention on MRSA and VRE acquisition rates.
ACKNOWLEDGMENTS
We extend our gratitude to Philip C. Carling, MD (Boston Medical Center and Boston University School of Medicine), for supplying the black-light marking solution and for providing us with guidance in the use of this monitoring system. We also extend our thanks to Andrea M. DuBois, BS, and Mary L. Delaney for their assistance with the microbiological assays. Lastly, we thank the Environmental Services managers Luis Alberto Soto, CHSP (Assistant Director, Environmental Services, Brigham and Women’s Hospital), Loay Kitmitto, Mostafa Boudal, and their staff, for their support in this study.
Footnotes
Potential conflicts of interest. R.P. has received research grants from Sanofi-Aventis, GlaxoSmithKline, Pfizer, and TAP Pharmaceuticals in the past 2 years. D.S.Y. has received research support from Sage Products. All other authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Bures S, Fishbain JT, Uyehara CF, Parker JM, Berg BW. Computer keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. Am J Infect Control. 2000;28:465–471. doi: 10.1067/mic.2000.107267. [DOI] [PubMed] [Google Scholar]
- 2.Noskin GA, Bednarz P, Suriano T, Reiner S, Peterson LR. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control. 2000;28:311–313. doi: 10.1067/mic.2000.108129. [DOI] [PubMed] [Google Scholar]
- 3.Zachary KC, Bayne PS, Morrison VJ, Ford DS, Silver LC, Hooper DC. Contamination of gowns, gloves, and stethoscopes with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2001;22:560–564. doi: 10.1086/501952. [DOI] [PubMed] [Google Scholar]
- 4.Healthcare Infection Control Practices Advisory Committee. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Atlanta: Centers for Disease Control and Prevention; Management of multidrug-resistant organisms in healthcare settings, 2006. 2006 doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed]
- 5.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 6.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 7.Patel R. Clinical impact of vancomycin-resistant enterococci. J Antimicrob Chemother. 2003;51 Suppl 3:iii1321. doi: 10.1093/jac/dkg272. [DOI] [PubMed] [Google Scholar]
- 8.Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18:622–627. [PubMed] [Google Scholar]
- 9.Ray AJ, Hoyen CK, Taub TF, Eckstein EC, Donskey CJ. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA. 2002;287:1400–1401. doi: 10.1001/jama.287.11.1400. [DOI] [PubMed] [Google Scholar]
- 10.Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004;25:164–167. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 11.Sexton T, Clarke P, O’Neill E, Dillane T, Humphreys H. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect. 2006;62:187–194. doi: 10.1016/j.jhin.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Hayden MK, Bonten MJ, Blom DW, Lyle EA, van de Vijver DA, Weinstein RA. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis. 2006;42:1552–1560. doi: 10.1086/503845. [DOI] [PubMed] [Google Scholar]
- 13.Byers KE, Durbin LJ, Simonton BM, Anglim AM, Adal KA, Farr BM. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol. 1998;19:261–264. doi: 10.1086/647806. [DOI] [PubMed] [Google Scholar]
- 14.Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–1619. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 15.Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2001;49:109–116. doi: 10.1053/jhin.2001.1013. [DOI] [PubMed] [Google Scholar]
- 16.Oie S, Hosokawa I, Kamiya A. Contamination of room door handles by methicillin-sensitive/methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2002;51:140–143. doi: 10.1053/jhin.2002.1221. [DOI] [PubMed] [Google Scholar]
- 17.French GL, Otter JA, Shannon KP, Adams NM, Watling D, Parks MJ. Tackling contamination of the hospital environment by methicillinresistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hosp Infect. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166:1945–1951. doi: 10.1001/archinte.166.18.1945. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. [Accessed May 13, 2008];Guideline for Environmental Infection Control in Health-Care Facilities, 2003. Available at http://www.cdc.gov/ncidod/dhqp/gl_environinfection.html.
- 20.Carling PC, Briggs J, Hylander D, Perkins J. An evaluation of patient area cleaning in 3 hospitals using a novel targeting methodology. Am J Infect Control. 2006;34:513–519. doi: 10.1016/j.ajic.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Carling PC, Briggs JL, Perkins J, Highlander D. Improved cleaning of patient rooms using a new targeting method. Clin Infect Dis. 2006;42:385–388. doi: 10.1086/499361. [DOI] [PubMed] [Google Scholar]
- 22.Carling PC, Parry MF, Von Beheren SM. Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:1–7. doi: 10.1086/524329. [DOI] [PubMed] [Google Scholar]
- 23.for The Healthcare Environmental Hygiene Study Group. Carling PC, Von Beheren S, Kim P, Woods C. Intensive care unit environmental cleaning: an evaluation in sixteen hospitals using a novel assessment tool. J Hosp Infect. 2008;68:39–44. doi: 10.1016/j.jhin.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing: sixteenth informational supplement. M100-S16. Wayne, Pennsylvania: CLSI; 2004. [Google Scholar]
- 25.Huang SS, Hinrichsen VL, Stulgis L, et al. Methicillin-resistant Staphylococcus aureus infection in the year following detection of carriage; Program and abstracts of the 16th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; March 18–21, 2006; Chicago, IL. Abstract 157. [Google Scholar]
- 26.Eckstein BC, Adams DA, Eckstein EC, et al. Reduction of Clostridium difficile and vancomycin-resistant enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis. 2007;7:61. doi: 10.1186/1471-2334-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis. 2007;195:330–338. doi: 10.1086/510622. [DOI] [PubMed] [Google Scholar]
- 28.Huang SS, Rifas-Shiman SL, Pottinger JM, et al. Improving the assessment of vancomycin-resistant enterococci by routine screening. J Infect Dis. 2007;195:339–346. doi: 10.1086/510624. [DOI] [PubMed] [Google Scholar]
- 29.Duckro AN, Blom DW, Lyle EA, et al. Transfer of vancomycin-resistant enterococci via health care worker hands. Arch Intern Med. 2005;165:302–307. doi: 10.1001/archinte.165.3.302. [DOI] [PubMed] [Google Scholar]