Abstract
Presbycusis – age-related hearing loss, is the number one communication disorder, and one of the top three chronic medical conditions of our aged population. Aquaporins, particularly aquaporin 4 (Aqp4), are membrane proteins with important roles in water and ion flux across cell membranes, including cells of the inner ear and pathways of the brain used for hearing. To more fully understand the biological bases of presbycusis, 39 CBA mice, a well-studied animal model of presbycusis, underwent non-invasive hearing testing as a function of sound frequency (auditory brainstem response – ABR thresholds, and distortion-product otoacoustic emission – DPOAE magnitudes), and were clustered into four groups based on age and hearing ability. Aqp4 gene expression, as determined by genechip microarray analysis and quantitative real-time PCR, was compared to the young adult control group in the three older groups: middle aged with good hearing, old age with mild presbycusis, and old age with severe presbycusis. Linear regression and ANOVA showed statistically significant changes in Aqp4 gene expression and ABR and DPOAE hearing status in the cochlea and auditory midbrain – inferior colliculus. Down-regulation in the cochlea was seen, and an initial down-, then up-regulation was discovered for the inferior colliculus Aqp4 expression. It is theorized that these changes in Aqp4 gene expression represent an age-related disruption of ion flux in the fluids of the cochlea that are responsible for ionic gradients underlying sound transduction in cochlear hair cells necessary for hearing. In regard to central auditory processing at the level of the auditory midbrain, aquaporin gene expression changes may affect neurotransmitter cycling involving supporting cells, thus impairing complex sound neural processing with age.
Keywords: Gene discovery, Gene expression, Presbycusis, Hearing, Hearing Loss, Auditory System
1. Introduction
Age-related hearing loss (presbycusis) is the number one communication disorder, and is among the top three chronic medical conditions of the elderly. Given the prevalence of this sensory deficit, identifying the underlying neural and molecular mechanisms is of high value, from both basic science and healthcare vantage points. The CBA mouse has proven to be a useful animal model for characterizing and investigating the neural and molecular bases of presbycusis (Willott 1991; Willott et al. 1991; Frisina and Walton 2001; Frisina and Rajan 2005). Moreover, studying gene expression changes related to age and hearing loss can possibly elucidate some of the etiologies for this common sensory disorder.
Aquaporins are integral tetrameric membrane proteins that are responsible for rapid transmembrane transport of water molecules, and they also participate in diffusion of other functionally-relevant biomolecules (Beitz et al. 1999). Given the relatively poor permeability to water of lipid bilayer cell membranes, aquaporins play a ubiquitous and crucial role in directing water transport at specific locations in an energy efficient manner, and for optimal cell osmolality (Huang et al. 2002). Aquaporins are found in different tissues throughout the body, but are highly expressed in cells where rapid fluid transport is vital, including kidney, lung, salivary gland and brain. To date, 11 different members of the aquaporin protein family have been identified, and Aquaporin 4 (Aqp4), which has the highest water permeability of the family, is of particular importance. It has been found to be highly expressed in glial cell membranes and ependymal cells of brain ventricles (Beitz et al. 2003).
Aqp4 has also been discovered in the mouse cochlea where it was localized to the supporting cells of the organ of Corti. Specifically, Aqp4 was localized to the basolateral plasma membrane of Hensen's cells, the inner sulcus cells, and the basal plasma membrane of the Claudius cells (Li and Verkman 2001). An Aqp4 knockout mouse demonstrated not only a urinary concentrating defect, but also a significant hearing loss as measured by auditory brainstem responses (ABRs) (Li and Verkman 2001; Mhatre et al. 2002). It has been hypothesized that Aqp4-mediated water transport is vital to the normal functioning of the potassium ion transport system of the inner ear, and that impaired water/ion transport due to Aqp4 gene depletion is consistent with severe cochlear hearing loss (Li and Verkman 2001; Beitz et al. 2003).
The auditory midbrain, the watershed of ascending sound information, is also a location of functionally important changes in gene expression with regard to presbycusis. Changes in the expression of genes associated with glutamate, the primary excitatory neurotransmitter of the auditory system (Tadros et al. 2007a), and serotonin (Tadros et al. 2007b) have been demonstrated with regard to age and/or hearing loss in the mouse inferior colliculus (IC, auditory portion of the midbrain). For example, it is postulated that the demonstration of gene-regulated changes of glutamate receptors and the serotonin 2B receptor are related to age-linked changes in complex sound processing. Specifically, serotonin serves as an important neuromodulator for information processing of complex sounds in the IC (Hurley and Pollak 1999, 2001) Age-related changes have been detected in other neurotransmitters, including gamma amino butyric acid (GABA, Caspary et al. 1995a, 1999, 2005), and it is thought that the dysfunction and altered regulation of the balance of the excitatory/inhibitory neurotransmitter pathways plays a key role in the complex sound processing deficits characteristic of presbycusis.
In the present study, by use of gene microarray and quantitative real-time polymerase chain reaction (qPCR) analysis, changes in aquaporin gene expression in the cochlea and IC were examined relative to presbycusis in the CBA mouse model of age-related hearing loss. Correlations with hearing measures revealed possible functional implications of these gene expression changes in the peripheral (ear) and central (brain) auditory system with age.
2. Results
2.1 Group selection and hearing results
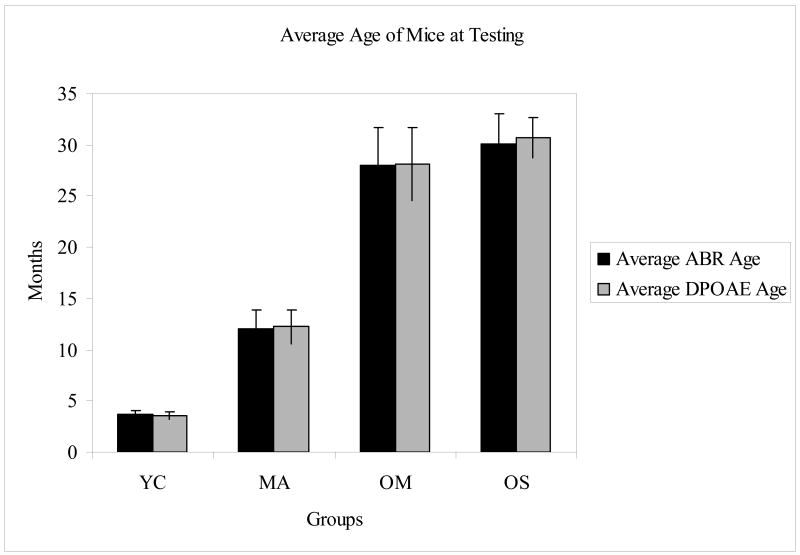
The mice segregated into four groups, based on age and hearing ability: young controls with good hearing (YC), middle aged with good hearing (MA), old aged with mild presbycusis (OM), and old aged with severe presbycusis (OS) (Tadros et al. 2007a, 2007b). Table 1 provides the averaged ABR threshold and distortion-product otoacoustic emission (DPOAE) level values for each group at representative frequencies. The age of the mice at the time of the testing is detailed in Figure 1. The number of animals and percent of female mice for each group was as follows: 8 YC – 50% female, 17 MA – 59% female, 9 OM – 44% female, and 5 OS – 80% female.
Table 1.
Hearing capabilities of the four CBA mouse subject groups of the present investigation measured in dB SPL for ABR thresholds and DPOAE levels, adapted from Tadros et al. (2007a). H=hearing, L=loss, N=normal.
| Young NH-YC | Middle Age NH-MA | Old Mild HL-OM | Old Severe HL-OS | |
|---|---|---|---|---|
| Low Frequency DPOAE (5-14 kHz) | 17.40 | 18.76 | 7.69 | -13.61 |
| Mid Frequency DPOAE (15-29 kHz) | 17.92 | 18.08 | 4.12 | -19.43 |
| High Frequency DPOAE (31-45 kHz) | 11.26 | 8.52 | -1.47 | -21.95 |
| 3 kHz ABR | 53.33 | 64.41 | 78.89 | 85.00 |
| 6 kHz ABR | 26.11 | 33.24 | 58.33 | 73.33 |
| 12 kHz ABR | 8.33 | 15.29 | 37.78 | 48.33 |
| 24 kHz ABR | 14.44 | 21.47 | 45.00 | 66.67 |
| 32 kHz ABR | 21.11 | 30.59 | 59.44 | 71.67 |
| 48 kHz ABR | 25.00 | 35.29 | 67.78 | 73.33 |
Figure 1.
Average age in months of each group (YC=young controls, MA=middle aged, OM=old aged with mild presbycusis, OS=old aged with severe presbycusis) at the time of testing for ABRs and DPOAEs. Error bars denote SD.
2.2 Microarray results
Two probe set locations on the microarray assessed the expression of the Aqp4 gene: 1425382_a_at, and 1434449_at. Correlations between the log signal ratio data for these two probe set IDs were analyzed with regard to the ABR and DPOAE data. Table 2 lists the r and p values for the linear regression analysis of the different groups for the functional hearing assessments. There were statistically significant differences seen at all frequencies studied for the 1425382_a_at probe set in the IC. In addition, significant correlations were obtained at 6, 24, and 32 kHz for the ABR for the 1434449_at probe set in the IC. Figure 2 shows an example of a significant correlation in the IC between the log signal ratio values for the probe set ID 1425382_a_at and the ABR measurements at 48 KHz (r2=0.2570, p=0.0036, F=10.03). There were no statistically significant correlations with the hearing measures for down-regulation of the cochlear gene expression values for either probe set ID.
Table 2.
Linear regression analyses of log signal ratios for two Aqp4 genechip probes and individual mouse ABR and DPOAE data. p values and r are given, and statistically significant correlations are in bold.
| Cochlea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Probe Set ID | ABR Frequency (kHz) | DPOAE Frequency Range (kHz) | ||||||||
| 3 | 6 | 12 | 24 | 32 | 48 | Mid-Low | Mid-Middle | Mid-High | ||
| 1425382_a_at | p value | 0.709 | 0.433 | 0.178 | 0.442 | 0.660 | 0.916 | 0.948 | 0.920 | 0.970 |
| r | 0.191 | 0.249 | 0.366 | 0.271 | 0.230 | 0.132 | 0.160 | 0.146 | 0.116 | |
| 1434449_at | p value | 0.889 | 0.236 | 0.337 | 0.158 | 0.413 | 0.907 | 0.136 | 0.096 | 0.064 |
| r | 0.031 | 0.190 | 0.118 | 0.162 | 0.049 | 0.088 | 0.117 | 0.156 | 0.183 | |
| Inferior Colliculus | ||||||||||
| Probe Set ID | ABR Frequency (kHz) | DPOAE Frequency Range (kHz) | ||||||||
| 3 | 6 | 12 | 24 | 32 | 48 | Mid-Low | Mid-Middle | Mid-High | ||
| 1425382_a_at | p value | 0.044 | 0.004 | 0.031 | 0.035 | 0.007 | 0.004 | 0.009 | 0.010 | 0.006 |
| r | 0.364 | 0.505 | 0.387 | 0.381 | 0.478 | 0.507 | 0.460 | 0.454 | 0.485 | |
| 1434449_at | p value | 0.344 | 0.040 | 0.051 | 0.047 | 0.027 | 0.136 | 0.002 | 0.005 | 0.011 |
| r | 0.176 | 0.371 | 0.353 | 0.359 | 0.396 | 0.274 | 0.541 | 0.489 | 0.451 | |
Figure 2.
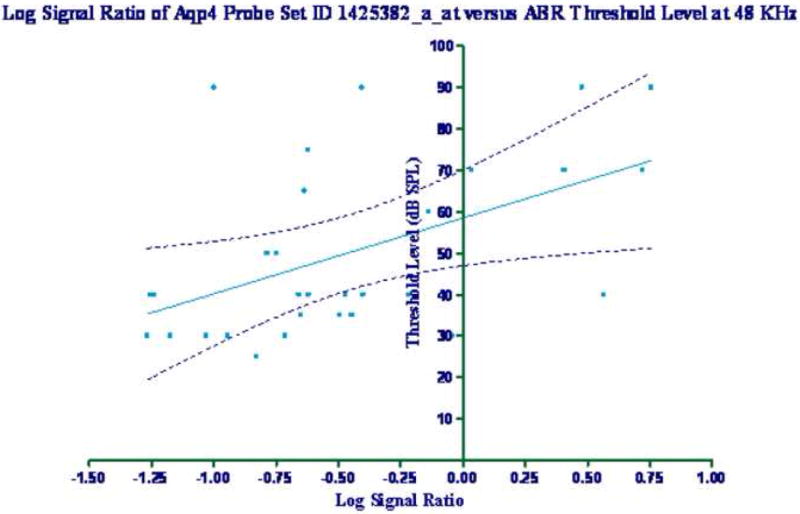
Log signal ratio of the genechip microarray probe set ID 1425382_a_at versus the measured ABRs threshold values at 48 kHz. Linear regression analysis showed r=0.507, p=0.004, and F=10.03. The 99% confidence limit is denoted on the graph.
A Kruskal-Wallis nonparametric ANOVA of the log signal ratios of the two probe set IDs was also performed. Table 3 lists the Kruskal-Wallis p values, and the difference in rank sum for each group. A statistically significant difference was found between the MA and OS groups for each probe set ID in the IC samples.
Table 3.
Kruskal-Wallis ANOVA analyses of the log signal ratios of two Aqp4 probe set IDs. Statistically significant values are in bold.
| Cochlea | ||||
|---|---|---|---|---|
| Probe Set ID | ANOVA | |||
| Kruskal-Wallis Subject Group Main Effect | MA vs. OM | MA vs. OS | OM vs. OS | |
| 1425382_a_at | p=0.814 Kruskal-Wallis Statistic=0.518 |
0.51 | 2.84 | 2.33 |
| 1434449_at | p=0.772 Kruskal-Wallis Statistic=0.411 |
1.36 | -2.2 | -3.56 |
| Inferior Colliculus | ||||
| Probe Set ID | ANOVA | |||
| Kruskal-Wallis Subject Group Main Effect | MA vs. OM | MA vs. OS | OM vs. OS | |
| 1425382_a_at |
p=0.028 Kruskal-Wallis Statistic=6.607 |
-3.28 | -12.34 | -9.07 |
| 1434449_at |
p=0.037 Kruskal-Wallis Statistic=7.135 |
-4.23 | -11.72 | -7.49 |
2.3 qPCR results
Quantitative real-time PCR using a TaqMan® Gene Expression probe for Aqp4 was performed on each of the 39 individual animals for both the cochlea and the IC. The resulting fold changes were calculated using the methods described above, as provided in Table 4. Fold changes greater than 1.2 (positive or negative) were seen in all age groups in both locations. Note that down-regulation of Aqp4 was seen with age and hearing loss in the cochlea. For the IC, the MA and OM groups showed prominent down-regulations, and the OS mice showed an up-regulation.
Table 4.
Fold changes Aqp4 expression in cochlea and inferior colliculus for each method and hearing group. Fold changes greater than 1.2 are in bold.
| Cochlea | |||
|---|---|---|---|
| Probe Set ID | Fold Change | ||
| MA | OM | OS | |
| qPCR | -1.71 | -3.29 | -1.35 |
| 1425382_a_at | -1.04 | -1.04 | -1.01 |
| 1434449_at | -1.18 | -1.33 | -1.41 |
| Inferior Colliculus | |||
| Probe Set ID | Fold Change | ||
| MA | OM | OS | |
| qPCR | -1.28 | -1.31 | 1.58 |
| 1425382_a_at | -1.57 | -1.28 | 1.04 |
| 1434449_at | -1.02 | 1.04 | 1.42 |
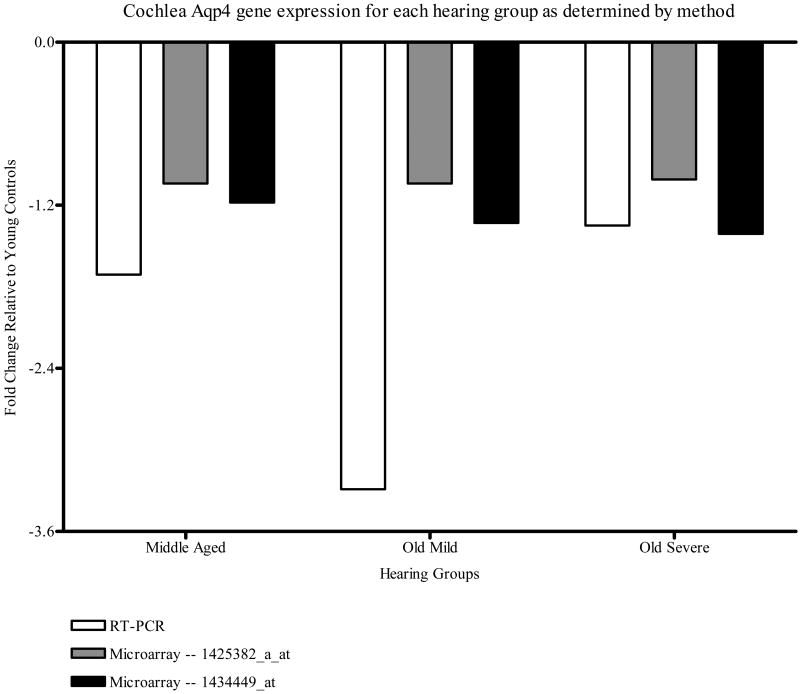
Figure 3 displays the fold changes seen in the cochlea by both methods (microarray analysis for both probe sets and qPCR). The gene expression was down-regulated in all hearing groups relative to the YC group, for both methods studied. Fold changes greater than 1.2 were seen in all hearing groups as measured by the qPCR and in the OM and OS groups as measured by microarray analysis for the 1434449_at probe set.
Figure 3.
CBA mouse cochlea Aqp4 gene expression fold change relative to the YC group for each method measured, i.e., qPCR and genechip microarray analysis for the two Aqp4 probe sets, 1425382_a_at and 1434449_at.
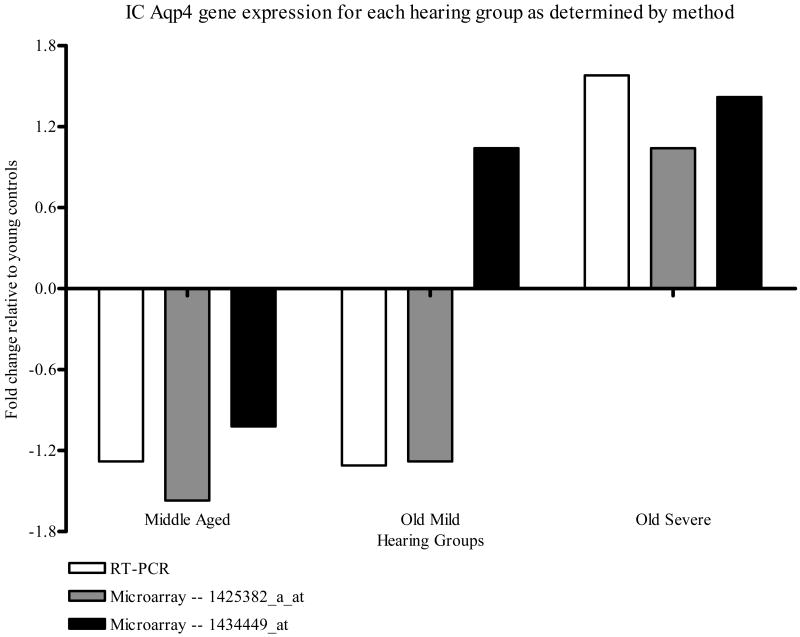
Figure 4 gives the fold changes observed in the IC by both methods. The Aqp4 gene expression was down regulated with age alone, but up-regulated with age and severe hearing loss, relative to the YC group. There was strong concurrence with the direction of regulation across all hearing groups and methods with the exception of the results of the microarray probe set ID 1434449_at OM group, which was up-regulated non-significantly, whereas the other two methods were significantly down regulated. Fold changes greater than 1.2 were seen in all hearing groups as measured by qPCR, in the MA and OM groups as measured by the microarray probe set ID 1425382_a_at method, and in the OS group as measured by the microarray probe set ID 1434449_at and qPCR techniques.
Figure 4.
CBA mouse IC Aqp4 gene expression fold change relative to the YC group for each method measured, i.e., qPCR and genechip microarray analysis for the two Aqp4 probe sets, 1425382_a_at and 1434449_at.
3. Discussion
Aquaporins play a fundamental role in water and ion transport across cell membranes, including sensory cells and neurons, and have been shown to play important roles in underlying mechanisms of cerebral edema, renal function, vision, and hearing (Verkman 2002; Papadapalous and Verkman 2007). In the present study, a gene expression analysis was carried out to determine if there are significant changes in the expression of Aqp4 in the cochlea and auditory midbrain in mice with age-related hearing loss. Based on the microarray analysis with qPCR confirmation, we discovered that the expression of Aqp4 is down-regulated in the cochlea in mice as they age and acquire hearing loss. The expression of Aqp4 in the IC is also down-regulated with age, but upregulated with aging and severe hearing loss, with the latter possibly a compensatory up-regulation when peripheral inputs originating in the cochlea show severe losses with age.
As summarized above, the presence of Aqp4 expression has been demonstrated in supporting cells of the mouse organ of Corti (Takumi et al. 1998; Huang et al. 2002; Mhatre et al. 2002). In addition, the absence of this channel protein in Aqp4 knockout mice results in significant hearing loss (Li and Verkman 2001), emphasizing the necessity of Aqp4 for normal cochlear functioning. The exact roles of the aquaporin channel protein family in the inner ear have not been fully elucidated. It has been theorized that aquaporins play a central role in facilitating the flow of K+ ions in the organ of Corti and lateral wall supporting cells by allowing swift osmolality adjustments in supporting epithelial cells via rapid water flux (Li and Verkman 2001; Mhatre et al. 2002). Gene microarray analysis and qPCR of the present report demonstrate that the expression of the Aqp4 gene in the cochlea decreases as the mouse ages and acquires hearing loss. This could lead to disruption of the normally tightly-controlled ion homeostasis of the cochlear K+ recycling network, a finely tuned hydro-chemical-electrical system. The normal endocochlear potential maintains a steep electrochemical gradient, keeping the organ of Corti in a state of readiness that allows for rapid inner hair cell depolarization and neural signal transduction necessary for complex sound coding. Failure of the ability of the surrounding epithelial cells to support the rapid ion flux via Aqp4 mediated water flow for osmotic adjustment could lead to age-related hearing loss via premature hair cell dysfunction, and eventually cochlear cell death.
As discussed above, rapid K+ ion fluxes seen in the hair cells and supporting cells of the cochlea result in osmotic changes that are thought to be offset by the flux of water through aquaporin channels. The importance of the aquaporin-mediated water flux is thought to be a general paradigm of excitable neural tissues (Beitz et al. 2003). Therefore, changes in Aqp4 expression, such as those seen in the auditory midbrain in this study could result in impaired neuro-excitability and disrupted auditory coding of complex sounds, including animal vocalizations and speech. In the current study, it was demonstrated that concurrent with the down regulation of Aqp4 expression in the cochlea, there is an initial down regulation, then a significant up regulation of Aqp4 expression in the mouse auditory midbrain when hearing loss becomes most severe. This could represent an age-related down regulation seen in the MA and OM groups, followed by a compensatory up-regulation seen in the old-aged severe presbycusis group. The gene expression change pattern in the old mice could represent the transition occurring as the hearing loss intensifies in old age. These Aqp4 gene expression changes seen in the auditory midbrain could lead to a deficit in central auditory processing, contributing to the development of central presbycusis, and possibly the difficulty aged listeners have with comprehending complex sounds in background noise.
Summary and Conclusions
Aquaporins—and specifically Aqp4—play an important role in the maintenance of normal hearing function. By gene microarray analysis and qPCR, Aqp4 was discovered to be down regulated in the mouse auditory midbrain as the mouse ages, with a subsequent up-regulation with the development of severe presbycusis. In the ear, there was a consistent trend towards down-regulation of Aqp4 expression in the mouse cochlea with the progression of presbycusis from middle age through old age. Much work remains to be done to understand the cell cycle pathways involved in the pathogenesis of presbycusis. Thus, the changes seen here in Aqp4 expression likely play an important role in K+ recycling and neurotransmitter cycling deficits in the course of presbycusis in a mouse model of the aging auditory system.
4. Experimental Procedure
Subject groups, gene microarray procedures and qPCR protocols have been described in detail in our previous reports (Tadros et al. 2007a,b; D'Souza et al. 2008). All animal procedures were approved by the Univ. of Rochester Committee on Animal Resources and NIH.
4.1 Animal model
CBA/CaJ mice were bred in-house and housed according to institutional protocol, with original breeding pairs obtained from Jackson Laboratories. All animals had similar environmental and non-ototoxic history. The young adult (YC) group was used as the baseline control group for gene expression data analyses (e.g., calculation of fold changes).
4.2 Functional hearing assessment
The non-invasive hearing tests were conducted in a sound-proof room, utilizing procedures similar to our previous investigations (Jacobson et al. 2003; Guimaraes et al. 2004; Varghese et al. 2005; Frisina et al. 2007). Normal body temperature was maintained at 38° C with a servo heating pad.
4.2.1 Distortion product otoacoustic emissions (DPOAEs)
Ipsilateral acoustic stimulation and simultaneous measurement of DPOAEs were accomplished with the Tucker-Davis Tech. (TDT, Alachua, FL) BioSig III hardware/software system. Stimuli were digitally synthesized at 200 kHz using (TDT SigGen software) with the ratio of frequency 2 (f2) to frequency 1 (f1) constant at 1.25; L1 was equal to 65 dB and L2 was equal to 50 dB SPL, as calibrated in a 0.1 mL coupler simulating the mouse ear canal. After synthesis, f1, f2, and the wideband noise were each passed through an RP2.1 D/A converter to PA5 programmable attenuators. Following attenuation, the signals went to ED1 speaker drivers which fed into the EC1 electrostatic loudspeakers coupled to the ear canal through a short, flexible tube with rigid plastic tapering tip. For DPOAE measurements, resultant ear canal sound pressure was recorded with a low noise microphone and probe (Etymotic, Elk Grove Village, IL, ER10B+) housed in the same coupler as the f1 and f2 speakers. The output of the ER10B+ amplifier went into an MA3 microphone amplifier, whose output went to an RP2.1 A/D converter for sampling at 200 kHz. A fast Fourier transform (FFT) was performed with the BioSig software on the resultant waveform. The magnitude of f1, f2, the 2f1–f2 distortion product, and the noise floor of the frequency bins surrounding the 2f1–f2 components were measured from the FFT. The procedure was repeated for geometric mean frequencies ranging from 5.6 to 44.8 kHz (eight frequencies/octave) to adequately assess the neuroethologically functional range of mouse hearing. Before data acquisition, individual mice were microscopically examined for evidence of external ear canal and middle ear obstruction. Mice with clearly visualized, healthy tympanic membranes were included. Mice were anesthetized with a mixture of ketamine and xylazine (120 and 10 mg/kg body weight, respectively) by intraperitoneal injection before all experimental sessions. The coupler was placed close to the tympanic membrane. The recording session duration was limited by depth of anesthesia, and lasted approximately one hour per animal.
4.2.2 Auditory brainstem responses (ABRs)
Auditory brainstem responses were measured in response to tone pips of 3, 6, 12, 24, 32, and 48 kHz presented at a rate of 11 bursts/s. Auditory brainstem responses were recorded with subcutaneous platinum needle electrodes placed at the vertex (non-inverting input), right-side mastoid prominence (inverted input), and tail (indifferent site). Electroencephalographic (EEG) activity was differentially amplified (50 or 100×) (Grass Tech., West Warwick, RI, Model P511 EEG amplifier), then put into an A/D converter (AD1, TDT) and digitized at 50 kHz. Each averaged response was based on 300–500 stimulus repetitions recorded over 10 ms epochs. Contamination by muscle and cardiac activities was prevented by rejecting data epochs in which the single trace electroencephalogram contained peak-to-peak amplitudes exceeding 50 μV. During this procedure, 5 mg/10 g body weight general anesthetic, Avertin (Tribromoethanol, delivered IP), was used to anesthetize the mice.
4.3 Sample isolation
Several days later, upon completion of the physiological recording sessions, the mice were sacrificed by decapitation. The cochleae and brains were immediately dissected using a Zeiss stereomicroscope and placed in ice-cold saline. The IC was dissected from each brain. Forty pairs of cochleae and 39 brain (IC) samples were collected. The soft tissue of the cochlear duct from the two cochleae of each animal was combined as one gene expression sample. All samples were placed in cold Trizol (Invitrogen, Carlsbad, CA) and stored at −80 ° C for gene microarray and qPCR processing.
4.4 Microarray gene expression processing
4.4.1 GeneChip
One Affymetrix (Santa Clara, CA) M430A high-density oligonucleotide array set (A) was used for each IC or cochlea sample. Each array contains 22,600 probe sets analyzing the expression of over 14,000 mouse genes. Eleven pairs of 25mer oligonucleotides that span the coding region of the genes represent each gene. Each probe pair consisted of a perfect match sequence that is complementary to the mRNA target and a mismatch sequence that has a single base pair mutation in a region critical for target hybridization; this sequence serves as a control for non-specific hybridization. Sequences used in the design of the array were selected from GenBank, dbEST, and RefSeq.
4.4.2 Samples preparation
4.4.2.1 RNA extraction
Each sample was homogenized in 1 mL of Trizol reagent per 50–100 mg of tissue using a polytron power homogenizer. The total RNA was isolated from the tissue homogenates of each sample using a modified Trizol protocol (Invitrogen, Gibco BRL). Each sample was centrifuged at 12,000×g for 10 min at 4° and the clear supernatant was transferred to a new tube and incubated for 5 min at 15–30 ° C to permit the complete dissociation of nucleoprotein complexes. A 0.2 mL of chloroform per each milliliter of trizol reagent was added and the tube was shaken vigorously by hand for 15 s, then incubated at 15–30° C for 2 min and centrifuged at 12,000×g for 15 min at 4° C. The aqueous phase was transferred to a new tube, 0.5 mL of isopropyl alcohol per 1 mL Trizol reagent, then incubated at 15–30° C for 10 min and centrifuged at 12,000×g for 10 min at 4° C. The supernatant was separated and the RNA pellet was washed once with 1mL 75% ethanol (EtOH) for each 1 mL trizol reagent. The sample was mixed by vortex and then centrifuged at 7500×g for 5 min at 4° C. The new RNA pellet was air-dried, dissolved in 10–20 μL of RNase-free water and incubated at 42° C for 5 min. The RNA quality was assessed by Agilent (Santa Clara, CA) Bioanalyzer 2100 and absorbance measurements at A260/A280 using the nanodrop.
4.4.2.2 cDNA synthesis
For gene array analysis, cDNA synthesis was performed with 20 μg of total RNA using the Superscript Choice cDNA Synthesis Kit (Invitrogen). For qPCR, nuGen cDNA reagents kit was used to generate a high fidelity cDNA, which was modified at the 3′ end to contain an initiation site for T7 RNA polymerase. The detailed protocol is found in www.nugeninc.com.
4.4.2.3 In vitro transcription (IVT) and fragmentation
Clean up of double-stranded cDNA was done according to the Affymetrix GeneChip Expression analysis protocol. Synthesis of Biotin-labeled cRNA was performed by adding 1 μg of cDNA to 10×IVT labeling buffer, IVT labeling NTP mix, IVT labeling enzyme mix, and RNase-free water, then incubated at 37 ° C for 16 h. The Biotin-labeled cRNA was cleaned up according to the Affymetrix GeneChip expression analysis protocol and a 20 g of full-length cRNA from each sample was fragmented by adding 5× fragmentation buffer and RNase-free water, followed by incubation at 94° C for 35 min. The standard fragmentation procedure produces a distribution of RNA fragment sizes from approximately 35–200 bases. After the fragmentation, cDNA, full-length cRNA and fragmented cRNA were analyzed by electrophoresis using the Agilent Bioanalyzer 2100 to assess the appropriate size distribution prior to microarray hybridization.
4.4.2.4 Target hybridization, washing, staining, and scanning
GeneChip M430A probe arrays (Affymetrix) were hybridized, washed, and stained according to the manufacturer's instructions in a fluidics station. The arrays were scanned using a Hewlett Packard confocal laser scanner and visualized using GeneChip 5.1 software. Three data files were created, namely image data (.dat), cell intensity data (.cel), and expression probe analysis data (.chp) files. Detailed protocols for sample preparation and target labeling assays for expression analysis can be found at www.Affymetrix.com
4.5 Real-time PCR (qPCR)
The primer/probe used in quantification of gene expression was acquired from TaqMan® GeneExpression assays on demand products (AOD) from Applied Biosystems (Foster City, CA). The primer/probe consisted of a 20× mix of unlabeled PCR primers and TaqMan MGB probe (FAM dye-labeled). The Applied Biosystems 7500 real-time PCR system was used to perform the real-time experiments using a 20 μL volume reaction in a 96-well plate. Each well contained 9 μL template (cDNA) and/or RNase-free water (control) with total cDNA concentration of 88 ng, 1 μL 20× assays on demand GeneExpression assay mix (primer/probe) for the studied gene, 10 μL TaqMan 2× universal PCR master mix. A parallel PCR reaction was performed with glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-specific primer/probe, as an endogenous control, in triplicates in order to control technical replicates. The reactions' thermal cycle conditions were adjusted as 10 min initial set-up at 95°C, followed by 50 cycles, each of which consisted of 15 s denaturing at 95°C and 1 min annealing/extend at 60°C. The results of each plate were analyzed using PRISM (Applied Biosystems) software to calculate the CT value of each well and compare these values in studied gene wells with control and endogenous control wells. In this investigation, qPCR was done to confirm and quantify the Aqp4 receptor gene expression in both the cochlea and IC of different age groups of CBA mice as revealed by the microarrays. Protocols can be found at www.appliedbiosystems.com.
4.6 Statistical analyses
4.6.1 GeneChip expression analysis
After assessing chip quality, the Affymetrix GeneChip Operating Software (GCOS) automatically generates the (.cel) image file from the (.dat) data file. The signal log ratio specified the difference in expression of the studied gene in a particular sample relative to the mean expression of that gene in all samples from the young adult mice. A signal log ratio of 1.0 indicates an increase of the transcript level by 2-fold and −1.0 indicates a decrease by 2-fold. A signal log ratio of zero indicates no change. Fold changes of all samples were calculated from signal log ratios using the following equations:
Kruskal-Wallis one way analysis of variance (ANOVA) was used to compare between the signal log ratio values of the different subject groups, with significance set at the p<0.5 level. In addition, linear regression analyses were employed to find correlations between the signal log ratio values and the functional hearing measurements. These measurements were the distortion product otoacoustic emission (DPOAE) amplitudes at low frequencies (5.6–14.5 kHz), mid frequencies=(15.8–29.0 kHz) and high frequencies (31.6–44.8 kHz), in addition to auditory brainstem response thresholds (ABR) at 3, 6, 12, 24, 32, and 48 kHz.
4.6.2 Real-time PCR analysis
The threshold cycle (CT) values, defined as the number of qPCR cycles taken to reach the greatest amplification level, were measured to detect the threshold of Aqp4 and GAPDH genes in all cochlear and IC samples. The CT value of each well was determined and the average of the three wells of each sample was calculated. Delta CT (ΔCT) for the Aqp4 gene of each sample was calculated using the equation:
The resulting ΔCT values were then averaged within each hearing-defined test group. Delta-ΔCT (ΔΔCT) was calculated for the Aqp4 gene of each group using the following equation:
Where the test groups included: MA, OM, and OS. The control group consisted of YC mice. The fold change for the gene expression of Aqp4 was calculated from the formula:
GraphPad Prism 4 software was used to perform a Kruskal-Wallis ANOVA, followed by Dunn's post-hoc test corrected for multiple comparisons, and the linear regression statistics.
Acknowledgments
We thank John Housel for collecting the ABR data, Martha Lynch-Erhardt for cochlear dissections, Martha Zettel for inferior colliculus dissections and Enza Daugherty for project assistance. The research was funded by NIH Grants P01 AG09524, from the National Institute on Aging, and P30 DC05409 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beitz E, Kumagami H, Krippeit-Drew P, Ruppersberg JP, Schultz JE. Expression pattern of aquaporin water channels in the inner ear of the rat: the molecular basis for a water regulation system in the endolymphatic sac. Hear Res. 1999;132:76–84. doi: 10.1016/s0378-5955(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Beitz E, Zenner HP, Schultz JE. Aquaporin-mediated fluid regulation in the inner ear. Cell Mol Neurobiol. 2003;23:315–329. doi: 10.1023/A:1023636620721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neurosci. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol. 1995;30:349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Armour BAL, Pippin J, Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci. 1990;10:2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci. 2005;47:10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza M, Zhu X, Frisina RD. Novel approach for selecting genes from RMA normalized microarray data using functional hearing tests in aging mice. J Neurosci Met. 2008;171:279–287. doi: 10.1016/j.jneumeth.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD, Newman SR, Zhu X. Auditory efferent activation in CBA mice exceeds that of C57s for varying levels of noise. J Acoust Soc Am. 2007;121:EL-29–34. doi: 10.1121/1.2401226. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Rajan R. Inferior colliculus: Aging and plasticity. In: Winer J, Schreiner C, editors. The Inferior Colliculus. Springer; New York: 2005. pp. 559–584. [Google Scholar]
- Frisina RD, Walton JP. Aging of the mouse central auditory system. In: Willott JP, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. CRC Press; New York: 2001. pp. 339–379. [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim SH, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Huang D, Chen P, Chen S, Nagura M, Lim DJ, Lin X. Expression patterns of aquaporins in the inner ear: evidence for concerted actions of multiple types of aquaporins to facilitate water transport in the cochlea. Hear Res. 2002;165:85–95. doi: 10.1016/s0378-5955(02)00288-5. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. J Neurophysiol. 2001;85:828–842. doi: 10.1152/jn.2001.85.2.828. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci. 1999;19:8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M, Kim SH, Romney J, Zhu X, Frisina RD. Contralateral suppression of distortion-product otoacoustic emissions declines with age: A comparison of findings in CBA mice with human listeners. Laryngoscope. 2003;113:1707–1713. doi: 10.1097/00005537-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Li J, Verkman AS. Impaired hearing in mice lacking aquaporin-4 water channels. J Biol Chem. 2001;276:31233–31237. doi: 10.1074/jbc.M104368200. [DOI] [PubMed] [Google Scholar]
- Mhatre AN, Stern RE, Li J, Lalwani AK. Aquaporin 4 expression in the mammalian inner ear and its role in hearing. Biochem Biophys Res Comm. 2002;297:987–996. doi: 10.1016/s0006-291x(02)02296-9. [DOI] [PubMed] [Google Scholar]
- Papadopolous MC, Verkman AS. Aquaporin-4 and brain edema. Ped Nephrol. 2007;22:778–784. doi: 10.1007/s00467-006-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, D'Souza M, Zettel ML, Zhu X, Waxmonsky NC, Frisina RD. Glutamate-related gene expression changes with age in the mouse auditory midbrain. Brain Res. 2007a;1127:1–9. doi: 10.1016/j.brainres.2006.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, D'Souza M, Zettel ML, Zhu X, Lynch-Erhardt M, Frisina RD. Serotonin 2B receptor: upregulated with age and hearing loss in mouse auditory system. Neurobiol Aging. 2007b;28:1112–1123. doi: 10.1016/j.neurobiolaging.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Takumi Y, Nagelhus EA, Eidet J, Matsubara A, Usami S, Shinkawa H, Nielsen S, Ottersen OP. Select types of supporting cell in the inner ear express aquaporin-4 channel protein. Eur J Neurosci. 1998;10:3548–3595. doi: 10.1046/j.1460-9568.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- Varghese GI, Zhu X, Frisina RD. Age-related declines in contralateral suppression of distortion product otoacoustic emissions utilizing pure tones in CBA/CaJ mice. Hear Res. 2005;209:60–67. doi: 10.1016/j.heares.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Physiological importance of aquaporin water channels. Ann Med. 2002;34:192–200. [PubMed] [Google Scholar]
- Willott JF. Aging and the Auditory System: Anatomy, Physiology, and Psychophysics. Singular Pub. Group; San Diego: 1991. pp. 56–131. [Google Scholar]
- Willott JF, Parham K, Paris Hunter K. Comparison of the auditory sensitivity of neurons in the cochlear nucleus and inferior colliculus of young and aging C57BL/6J and CBA/J mice. Hear Res. 1991;53:78–94. doi: 10.1016/0378-5955(91)90215-u. [DOI] [PubMed] [Google Scholar]