Abstract
The extent to which immunizing school children reduces the burden of influenza in adults is controversial. We enrolled a systematic sample of adults ≥50 years hospitalized with respiratory symptoms in two counties, one with and one without a school-based immunization program. We tested all subjects for influenza by polymerase chain reaction. Hospitalizations per 1000 adults aged ≥50 years were 1.28 (95% CI 0.59, 2.04) in the intervention county and 1.53 (95% CI 0.71, 2.34) in the control county. These rates did not differ significantly except in the subgroup aged 50-64 years where rates in the intervention county were significantly lower.
Keywords: influenza, older adults, herd immunity
1. Introduction
Although trivalent inactivated influenza vaccination (TIV) is recommended for all persons aged ≥50 years, recent reports have questioned its effectiveness for the prevention of influenza-associated morbidity and mortality in older adults.[1-3] This concern has increased interest in evaluating indirect effects of vaccinating children upon disease burden in older adults. To date, support for indirect or “herd” effects from immunization of school children is based on a few provocative reports, none of which included laboratory-confirmed influenza as an endpoint.[4-6] In a study conducted during the 1968 influenza pandemic, outpatient visits for acute respiratory illness (ARI) were reduced in all age groups in the community where school children received influenza vaccine as compared to another community where children remained unvaccinated.[4] Universal influenza vaccination of school children in Japan was also associated with a striking reduction in seasonal pneumonia and influenza mortality in the older population and young children.[5, 6]
To further investigate the role of indirect protection of the older population through immunization of school children, we performed active, prospective influenza surveillance in adults ≥50 years hospitalized with respiratory symptoms or non-localizing fever in Knox County, where a school-based influenza immunization campaign was conducted, and in Davidson County, where there was no such program. The goal was to compare the burden of influenza-associated hospitalizations in adults aged ≥50 years in the two counties, one with and one without a school-based influenza vaccination program.
2. Methods
2a. Study Design
To compare adult hospitalization rates for laboratory-confirmed influenza in two geographically distinct Tennessee counties, we enrolled adults ≥50 years hospitalized with respiratory symptoms or non-localizing fever at two hospitals in each county during the influenza season. Recruitment occurred from November 2006 through April 2007 beginning two days per week. Once two cases of influenza were identified for two consecutive weeks in the hospital laboratories in each county, surveillance increased to 4-7 days per week in the county. IRB approval was obtained from all participating hospitals.
2b. Pediatric School-Based Program
In the fall of 2006, Knox County Department of Health implemented a school-based immunization program in which live intranasal attenuated influenza vaccine was administered (LAIV, MedImmune, Gaithersburg, MD), similar to the previously reported 2005-2006 campaign.[7] Between September and December 2006, 47% of 54,786 public school students and 61% of 5,998 private school children in Knox County were vaccinated. This represented 61% of elementary students, 45% of middle school students and 26% of high school students (John Lott, personal communication). In children aged <9 years, 53% received the recommended second dose of LAIV. Davidson County did not have such a school-based immunization program.
2c. Study Population
All adults ≥50 years with acute respiratory symptoms or non-localizing fever admitted to one of four hospitals on surveillance days were eligible for enrollment. During that period Davidson County had a population of 575,261 individuals with 51.3% women, 67.8% white, 27.5% black, while Knox County (180 miles east of Davidson County) had a population of 404,972 individuals with 51.4% women, 88.2% white, and 8.7% black. Both counties are urban with surrounding suburban and rural counties. Davidson County surveillance hospitals included one academic medical center, and one community hospital; Knox County hospitals included two community hospitals. Eligible adults, residing in the surveillance county, were those admitted during specific 24-hour surveillance periods for each enrollment day (4 to 7 days per week during influenza season) and had one or more of the following admitting symptoms: cough, fever, nasal congestion/coryza, dyspnea (including cardiac causes), or wheezing. All eligible patients were entered into a screening log that included age, sex, race, insurance status, admission symptoms, and provision of informed consent.
2d. Demographic and Clinical Information
Patient questionnaires and chart review data collection instruments captured Centers for Disease Control and Prevention (CDC)-defined high risk conditions,[8] living conditions, clinical symptoms, past medical history, smoking history, use of specific medications (steroids, immunosuppressants, and home oxygen), and influenza vaccination status for the 2006-2007 season. Results of provider-ordered microbiologic and radiographic tests, hospital course (ICU stay, length of hospitalization, intubation, and oxygen use), disposition at discharge, and discharge diagnoses were captured through chart review.
2e. Verification of Influenza Vaccination of Adults
Enrolled adults were asked if they received an influenza vaccine for the current influenza season. Verification of vaccination status in all adults was attempted by contacting the provider; vaccination records were considered the gold standard. However, many adults received their influenza vaccinations from non-traditional providers such as grocery chains and pharmacies, thus we were unable to verify vaccination from these sites. Three vaccination categories were defined: not vaccinated (included both verified and unverified patient-reported non-vaccination as well as those vaccinated within 14 days of hospitalization); verified, vaccinated and not verified, vaccinated. Influenza negative patients were used to estimate the rate of influenza vaccine uptake among county adults.
2f. Laboratory Methods
After informed consent, nasal and throat swabs were obtained. One swab sampled both nostrils, a second swab sampled the posterior pharynx, and both swabs were placed into Hank’s transport media. Specimens were held at 4°C, divided into multiple aliquots within 24 hours, and stored at -80°C. Aliquots stored in lysis buffer (Roche Applied Science, Indianapolis, IN) were used for influenza A and B reverse transcriptase-polymerase chain reaction (RT-PCR). Specimens that were influenza positive by RT-PCR were cultured for influenza typing.
Using standard molecular biologic protocols (Roche Applied Science), RNA was extracted from the frozen aliquots and real-time RT-PCR assays were performed using primers and probes developed and provided by Steve Lindstrom, Influenza Branch, CDC, Atlanta, GA.[9, 10] All samples were tested for β-actin (Applied Biosystems) to insure specimen quality. If β-actin was absent in three consecutive tests on a sample, the RT-PCR results were categorized as indeterminate. Influenza was confirmed by positive RT-PCR on two separate tests.
Viral cultures were performed on influenza RT-PCR positive specimens using Rhesus monkey kidney cell culture tubes (Diagnostic Hybrids). Hemadsorption with guinea pig red blood cells was used to screen isolates and influenza A or B was confirmed using specific monoclonal antibodies (Viromed). Influenza isolates from study subjects were then sent to the CDC World Health Organization Collaborating Center for Surveillance, Epidemiology and Control of Influenza, Atlanta, GA for typing.
2g. Statistical Analysis
The primary study objective was to compare population-based rates of influenza-associated hospitalizations in adults aged ≥50 years living in two Tennessee counties, one with a school-based immunization program (Knox) and the other without such a program (Davidson). Rates of influenza-associated hospitalizations were defined as the seasonal weighted number of hospitalizations attributable to influenza-related acute respiratory tract infections or non-localizing fever divided by the county population according to 2006 U.S. census estimates, multiplied by 1000. The observed number of enrolled hospitalizations was weighed to account for sampling days, number of eligible patients, and hospital market share (defined below). Adults with indeterminate PCR results were considered eligible but non-enrolled. Rates were calculated for adults 50-64 years and ≥65 years of age for each county and 95% confidence intervals and rate differences were determined using 1000 bootstrap samples. P values of rate comparisons were determined by the widest confidence interval excluding zero using the 1000 bootstrap samples.
Since we assumed that school-based immunization would have a major effect on county disease rates, this study was powered to detect a 50% lower rate in the intervention than the control county. In low and moderate influenza seasons with projected influenza hospitalization rates of 1.29 and 2.57 per 1000 population, respectively, we projected >80% power to detect relative rates of 0.39 and 0.55 in the intervention county.
2h. Market Share
The Tennessee Hospital Discharge Database, an electronic database that reports all hospitalizations and emergency department discharges in the state, was used to establish where Davidson and Knox County residents with ARI or non-localizing fever were admitted, and what proportion of those admissions occurred in the surveillance hospitals in each county. Specific International Classification of Diseases version 9 (ICD-9) codes used to define ARI or non-localizing fever included: 036.2, 038, 079.9, 320, 321, 323, 381-383, 410, 411, 413, 428, 460-466, 480-487, 490-494, 496, 507, 510-514, 518.81, 780.6, 771.8, 786, 790.7, 799.02,995.91 and 995.92.
3. Results
Surveillance in both counties began the first full week in November 2006 and continued through April 30, 2007. However, all influenza-positive samples were identified during the 18 weeks from December 10, 2006 through April 14, 2007, defined as the influenza season. The timing of the season was similar in both counties. All analyses included persons admitted during the 18-week influenza season.
3a. Study population
Of 738 eligible adults hospitalized during the influenza season, 190 were enrolled in Davidson County and 367 in Knox County for a total enrollment of 557 (75.5%) adults aged ≥50 years hospitalized with ARI or non-localizing fever (Figure 1). Of 181 adults not enrolled, 52% declined, 31% had a surrogate decline, 17% had no guardian, 13% were missed and/or discharged prior to enrollment, and 2% had no available interpreter. Demographic characteristics of subjects enrolled in each county were similar (Table 1), with the exception of more African-Americans enrolled in Davidson County, consistent with local demographics. Using the market share data the surveillance hospitals admitted an estimated 34% and 29% of adults aged ≥50 years with respiratory illness in Knox and Davidson County, respectively.
Figure 1.
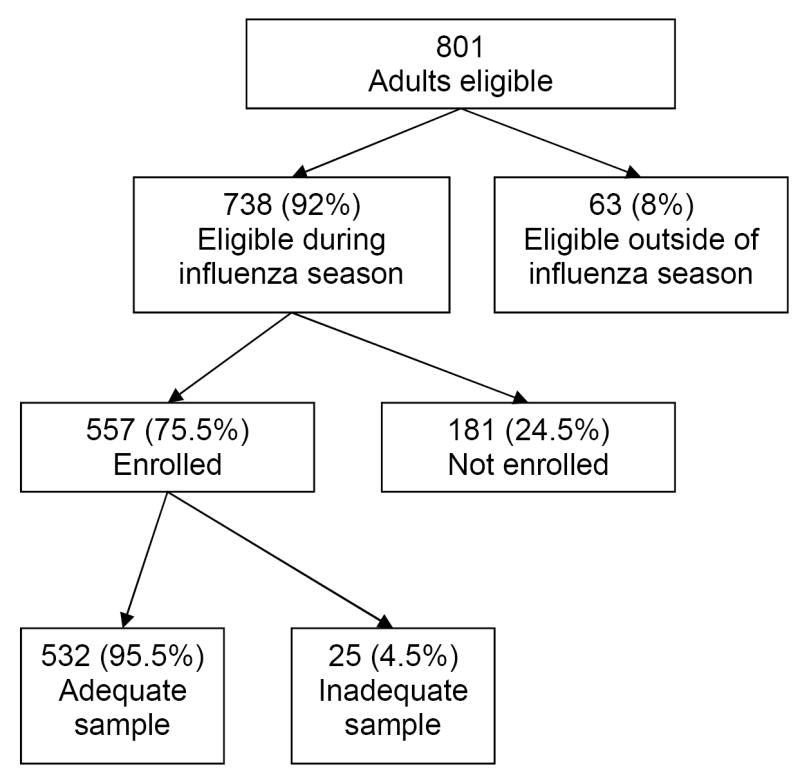
Flowchart of eligibility and enrollment
Table 1.
Demographic characteristics by county and results of PCR testing for influenza
| Knox County (N=345) |
Davidson County (N=187) |
|||
|---|---|---|---|---|
| Influenza Status (PCR) | + (n=16) |
- (n=329) |
+ (n=14) |
- (n=173) |
| Race (%)* | ||||
| White | 100 | 91 | 57 | 64 |
| Black | 0 | 8 | 43 | 36 |
| Mean Age (years) | 74 | 70 | 59 | 69** |
| Age group (%) | ||||
| 50-64 | 25 | 36 | 71 | 37† |
| 65+ | 75 | 64 | 29 | 63 |
| Gender (Male - %) | 38 | 45 | 21 | 37 |
| Insured (%) | 100 | 98 | 93 | 99 |
| Vaccine Status‡ (%) | ||||
| Not vaccinated/unknown | 31 | 31 | 64 | 40 |
| Vaccinated, verified | 31 | 41 | 21 | 39 |
| Vaccinated, non-verified | 38 | 29 | 14 | 21 |
| CDC defined High Risk Condition (%) | ||||
| Any high risk condition | 100 | 96 | 100 | 95 |
| Immunosuppression | 25 | 26 | 36 | 35 |
| Diabetes Mellitus | 38 | 25 | 29 | 25 |
| Pulmonary/Cardiovascular Disease | 38 | 43 | 36 | 34 |
| Living Situation (%) | ||||
| Lives alone at home | 25 | 25 | 14 | 24 |
| With Family | 69 | 62 | 71 | 67 |
| Assisted Living, Nursing Home, Rehabilitation | 6 | 13 | 14 | 9 |
| History of smoking (%) | 25 | 22 | 43 | 23 |
p < 0.001 Race for Davidson County compared to Knox County irrespective of PCR result.
p=0.019 for mean age in Knox county for PCR positive compared to PCR negative
p=0.011 for comparison of the age groups in Knox county for the influenza positive enrolled patients compared to the PCR negative patients.
p=0.004 for those vaccinated in Davidson County compared to Knox County
3b. Influenza Vaccination
More adults ≥50 years were vaccinated in Knox County than in Davidson County (p=0.004). For persons 50-64 years of age, 57.7% were vaccinated (verified and non-verified) in Knox County while 44.6% were vaccinated in Davidson County (p=0.07). For those ≥65 years of age, 76.1% were vaccinated in Knox County while 67.2% were vaccinated in Davidson County (p=0.08, Table 1).
3c. Diagnosis of Influenza
Thirty adults had RT-PCR confirmed influenza; 16 in Knox County and 14 in Davidson County. Of those, 20 were influenza A, and 10 were influenza B, with a similar distribution in both counties.
3d. Circulating and Vaccine Strain Matching
A total of 36 RT-PCR positive samples from both children and adults living in either county were cultured and sent to the CDC for characterization to evaluate the degree to which the circulating strains matched antigens in the 2006-2007 vaccine. The results were similar in the two counties with 11 (50%) good matches, 10 (45%) minor antigenic variants and one (5%) poor match in Knox County and 9 (64%) good matches, 3 minor antigenic variants (21%) and 2 poor matches (14%) in Davidson County (p=0.27).
3e. Rates of Illness
Hospitalization rates for laboratory-confirmed influenza in adults ≥50 years were 1.28 (95% CI 0.59, 2.04) per 1000 in Knox County and 1.53 (95% CI 0.71, 2.34) per 1000 adults in Davidson County (Table 2). Although these rates were not significantly different in adults ≥65 years or in all individuals aged ≥50 years in the two counties (p=0.2 and 0.7 respectively), rates were significantly lower in Knox than Davidson County residents in the 50-64 year group (0.40 vs 1.74 per 1000, p=0.01).
Table 2.
Burden of Influenza-Associated Hospitalizations per 1000 adults ≥50 years of age
| Age Group (years) |
Knox County | Davidson County | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Influenza | Weighted Influenza | Population | Rate (95% CI) |
Number of Influenza | Weighted Influenza | Population | Rate (95% CI) |
||
| 50-64 | 4 | 30.8 | 76962 | 0.40 (0.07,0.81) |
10 | 174.6 | 100170 | 1.74 (0.69,2.85) |
0.01 |
| ≥65 | 12 | 134.9 | 52591 | 2.56 (0.96,4.33) |
4 | 76.7 | 64429 | 1.19 (0.18,2.44) |
0.2 |
| Overall | 16 | 165.7 | 129553 | 1.28 (0.59,2.04) |
14 | 251.3 | 164599 | 1.53 (0.71,2.34) |
0.7 |
4. Discussion
Due to the disproportionate morbidity and mortality of influenza for persons ≥65 years,[11-17] United States public health officials have recommended routine annual influenza vaccination to these individuals for many years. Recently all adults ≥50 years were also recommended to receive yearly influenza vaccination.[18] However, in the past several years there has been increasing concern over suboptimal efficacy in older adults. Therefore, alternative vaccination approaches have been suggested. Universal immunization of all children, particularly school-aged children, has been proposed as a mechanism to achieve indirect protection, or herd effects.[19] Mathematical models have supported this approach with estimates that vaccination of 20% of children aged 6 months to 18 years would decrease the total number of influenza cases by 46%.[20]
To expand the studies on the evaluation of herd effects, we took advantage of a public health experiment (the Knox County school-based immunization program), and based on the previously mentioned modeling data, [20] postulated that it would be possible to detect a substantial effect of the intervention. The objective of our study was to determine if a school-based influenza immunization campaign in Knox county using live, intranasal influenza vaccine, would result in substantially lower influenza hospitalizations of adults ≥50 years in that county when compared to similar adults in Davidson County without such a campaign. The study was designed to evaluate the incremental effects of vaccination of children in addition to the direct effects of vaccination in adults. We found no difference in estimated influenza-associated hospitalization rates in the two counties for all adults aged ≥50 years, our primary study outcome during the mild influenza season. However, we did find significantly lower rates in the 50-64 year subgroup in Knox County when compared with Davidson County. Differences seen in this age group could be due to direct effect of increased vaccination rates in Knox County (57.7% vs. 44.6%), herd effect from vaccinating approximately 45% of school-age children, or both.
Studies done in Texas [21] have shown a reduction of medically-attended acute respiratory infections in adults ≥35 years of age when approximately 20-25% of children in the intervention county received live attenuated influenza vaccine. However these studies were done before the recommendation of yearly influenza vaccination for healthy children. Therefore only 1.5-2.5% of children received any influenza vaccination in the non-intervention counties. During our study period, we estimated that 36% and 33% of children aged <5 years were immunized in Knox and Davidson Counties, respectively. In addition, an estimated 12% of children 5-12 years were immunized in Davidson County despite the absence of a school-based program.[22] With the universal recommendation for immunization of all children in the US, the window of opportunity for studying herd effects in the US has likely closed. The use of cluster randomized trials in vaccine-naïve populations using laboratory-confirmed influenza could definitively answer this question, but will need to be done in countries without universal immunization programs.
Despite the inability to demonstrate an impact of school-based immunization program on all adults, the result in the subgroup of adults aged 50-64 is intriguing and could represent herd effects. In addition, this study has several important findings. First, through prospective surveillance for laboratory-confirmed influenza in two Tennessee counties, we found 1.28-1.53 influenza associated hospitalizations per 1000 adults aged ≥50 years. Previous estimates were based on mathematical models evaluating excess rates of pneumonia and influenza hospitalizations in adults. One study showed the average rate of pneumonia and influenza hospitalizations to be 1.74/1000 with a range of 0 to 4.11/1000 adults ≥65 years of age.[23] This rate is remarkably similar to the prospective population-based rates we observed in adults ≥65 years of 2.56 and 1.19 in Knox County and Davidson County, respectively. Another study evaluated pneumonia and influenza hospitalizations in “epidemic” years and “non-epidemic” years and found 3.99 to 5.18 excess hospitalizations per 1000 population for adults ≥65 years of age during 2 years of high influenza activity when compared to hospitalization rates during a year in which there was no or very low influenza activity.[13] The study year of 2006-2007 would be characterized as a mild influenza season, with no weeks of pneumonia and influenza deaths exceeding the epidemic threshold.[24]
In conclusion, we were unable to demonstrate herd effects from a school-based immunization program in the overall target population aged 50 years and older during a mild influenza season. However, the strata that included younger adults (aged 50-64 years) had significantly lower influenza-associated hospitalization rates. These results are intriguing and support the need for further research. The burden of influenza in both counties was substantial despite a mild influenza season, widespread vaccination programs in older adults, and considerable vaccination of young children.
Acknowledgments
We would like to thank Dr. William Schaffner for his review and recommendations regarding this manuscript.
Financial support: Funding for this study was through multiple sources: an investigator initiated grant from MedImmune (Marie R Griffin, PI) supported the Knox County surveillance activities and the data analysis and the VTEU (N01 AI25462) (Kathryn M. Edwards site PI) supported the adult influenza surveillance activities in Davidson County. Dr. Talbot received salary support and career development from the NIH/NCRR (5 K12 RR017697-05, Dr. Nancy Brown, PI Vanderbilt Mentored Clinical Research Scholar Program) and from the NIAID (1K23AI074863-01). Dr. Poehling received salary support and career development from the NIAID (K23AI065805).
We acknowledge Paul Harris PhD and Carlos Orozco, who designed and implemented the data entry and management system; Vanderbilt study nurses and coordinators: Ann Clay RN, Diane Kent RN, and Dayna Wyatt RN; Covenant Health Systems co-investigator, John Adams MD, study coordinator, Margie Cramer RN, and study nurses Pam McNamee RN, Doris Sklad RN; Vanderbilt laboratory personnel Amy Podsiad and Jody Peters MS, who performed the cultures and polymerase chain reactions; and the Knox County Health Department who performed the intervention and provided data on immunization levels in Knox County children including John Lott RN MS and Martha Buchanan MD. We thank all the adults who generously participated in this study.
Footnotes
Manuscript preparation: The funders had no role in the design and conduct of study, collection, management, analysis, and interpretation, preparation review or approval of manuscript
Potential Conflicts of Interest:
HKBT has received research funds from Protein Sciences and Wyeth. JVW has served as a consultant for MedImmune and Novartis. KME has received funding from the NIH and the CDC to evaluate the impact of influenza vaccines and study new influenza vaccines and has received funding from PATH through the Gates Foundation to evaluate potential new influenza vaccines. KP, YZ, PM, and QC have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165(3):265–72. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 2.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366(9492):1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo C, Viboud C, Montomoli E, Simonsen L, Miller MA. Influenza-related mortality in the Italian elderly: No decline associated with increasing vaccination coverage. Vaccine. 2006 doi: 10.1016/j.vaccine.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 4.Monto AS, Davenport FM, Napier JA, Francis T., Jr Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis. 1970;122(1):16–25. doi: 10.1093/infdis/122.1-2.16. [DOI] [PubMed] [Google Scholar]
- 5.Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344(12):889–96. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 6.Sugaya N, Takeuchi Y. Mass vaccination of schoolchildren against influenza and its impact on the influenza-associated mortality rate among children in Japan. Clin Infect Dis. 2005;41(7):939–47. doi: 10.1086/432938. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter LR, Lott J, Lawson BM, Hall S, Craig AS, Schaffner W, et al. Mass Distribution of Free, Intranasally Administered Influenza Vaccine in a Public School System. Pediatrics. 2007:peds.2006–603. doi: 10.1542/peds.2006-2603. [DOI] [PubMed] [Google Scholar]
- 8.Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR6):1–54. [PubMed] [Google Scholar]
- 9.Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen W, Hadisoedarsuno W, Purba W, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355(21):2186–94. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- 10.Mehlmann M, Bonner AB, Williams JV, Dankbar DM, Moore CL, Kuchta RD, et al. Comparison of the MChip to viral culture, reverse transcription-PCR, and the QuickVue influenza A+B test for rapid diagnosis of influenza. J Clin Microbiol. 2007;45(4):1234–7. doi: 10.1128/JCM.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assaad FA, Reid D. Some factors influencing mortality from influenza. Bull World Health Organ. 1971;45(1):113–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77(6):712–6. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112(6):798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- 14.Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967-1989. Int J Epidemiol. 1993;22(2):334–40. doi: 10.1093/ije/22.2.334. [DOI] [PubMed] [Google Scholar]
- 15.Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med. 1982;142(1):85–9. [PubMed] [Google Scholar]
- 16.Eickhoff TC, Sherman IL, Serfling RE. Observations on excess mortality associated with epidemic influenza. Jama. 1961;176:776–82. doi: 10.1001/jama.1961.03040220024005. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR10):1–42. [PubMed] [Google Scholar]
- 19.Schwartz B, Hinman A, Abramson J, Strikas RA, Allred N, Uyeki T, et al. Universal influenza vaccination in the United States: are we ready? Report of a meeting. J Infect Dis. 2006;194 Suppl 2:S147–54. doi: 10.1086/507556. [DOI] [PubMed] [Google Scholar]
- 20.Weycker D, Edelsberg J, Halloran ME, Longini IM, Jr, Nizam A, Ciuryla V, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23(10):1284–93. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Piedra PA, Gaglani MJ, Kozinetz CA, Herschler G, Riggs M, Griffith M, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005;23(13):1540–8. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Poehling K, Talbot HK, Williams JV, Zhu Y, Lott J, Patterson L, Edwards KM, Griffin MR. Impact of a School-based Influenza Immunization Program on Disease Burden: Comparison of Two Tennessee Counties. doi: 10.1016/j.vaccine.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181(3):831–7. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 24.Update: Influenza activity--United States and worldwide, 2006-07 season, and composition of the 2007-08 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2007;56(31):789–94. [PubMed] [Google Scholar]


