Abstract
OBJECTIVE
Although a short cervix is known to be associated with preterm birth, the patterns of three-dimensional, anatomic changes leading to a short cervix are unknown. Our objective was to 1) construct three-dimensional anatomic models during normal pregnancy and 2) use the models to compare cervical anatomy in the second and third trimester.
STUDY DESIGN
A cross sectional study was performed in a population of patients referred to magnetic resonance imaging (MRI) for a fetal indication. Using magnetic resonance images for guidance, three-dimensional solid models of the following anatomic structures were constructed: amniotic cavity, uterine wall, cervical stroma, cervical mucosa and anterior vaginal wall. To compare cervical anatomy in the second and third trimester, models were matched according the size of the bony pelvis.
RESULTS
Fourteen patients were imaged and divided into two groups according to gestational age: 20 – 24 weeks (n=7)) and 31 – 36 weeks (n=7). Compared to the second trimester, the third trimester was associated with significant descent of the amniotic sac. (p=.02). Descent of the amniotic sac was associated with modified anatomy of the uterocervical junction. These 3-dimensional changes were associated with a cervix that appeared shorter in the third trimester.
CONCLUSION
We report a technique for constructing MRI-based, three-dimensional anatomic models during pregnancy. Compared to the second trimester, the third trimester is associated with three-dimensional changes in the cervix and lower uterine segment.
Keywords: Anatomy, Cervix, Magnetic Resonance Imaging, Pregnancy
Introduction
Preterm birth is an important obstetrical complication that causes substantial childhood morbidity.[1,2] The rate of preterm birth is increasing[3] and the cost of caring for preterm newborns is high.[4] Spontaneous preterm birth refers to those preterm births that occur without apparent medical illness and accounts for the majority of all preterm births.[5] Although the etiology of spontaneous preterm birth is multifactorial, a short cervix is known to be an important antepartum risk factor.[6] Measurement of cervical length using two-dimensional (2D) ultrasound is commonly used to assess the risk of spontaneous preterm birth and target therapy to prevent preterm birth.[7]
Cervical length is a simple measurement of complex, three-dimensional (3D) anatomy. The cervix forms a continuous anatomic connection between the anterior surface of the vagina and the lower uterine segment. The cervix is known to be shorter in the third trimester compared to the second trimester.[8,9] Presumably, shortening of the cervix is accompanied by 3D changes in cervical geometry and surrounding anatomy. Yet, the 3D anatomic changes that result in the observed decrease of cervical length are unknown.
A short cervix in the midtrimester is suspected to indicate that its biomechanical function is impaired.[10,11] Investigation of biomechanical function during pregnancy is limited by a paucity of 3D anatomic data. Imaging-based 3D models of the cervix and surrounding structures in pregnancy are needed to formulate accurate hypotheses on the mechanism through which mechanical loading (gravity, tension in the uterine walls, intrauterine pressure, etc.) can lead to the observed decrease in cervical length.
The purpose of this study was to develop a technique to evaluate 3-dimensional anatomy of the cervix and surrounding structures during pregnancy. For this purpose, we constructed magnetic resonance (MR)-based, 3D solid models of cervical anatomy in the second and third trimester. MR imaging was chosen because it demonstrates superior soft tissue contrast and wider field of view compared to ultrasound. A better understanding of the 3D cervical anatomy associated with normal pregnancy may help elucidate the anatomic and biomechanical changes that cause a cervix to become short in cases of spontaneous preterm birth.
Material and Methods
Patient Selection
Cervical magnetic resonance imaging (MRI) was performed in a population of patients referred for fetal MRI. A cross sectional study design was used: all cervical imaging was performed on the same day as the clinically indicated fetal MRI. The study was performed at a single tertiary care center from January 2005 to September 2007. The study protocol was approved by the IRB. Informed consent was obtained prior to MR imaging.
Magnetic Resonance Imaging
Scans were performed on a Siemens Symphony 1.5 Tesla system (Malvern, PA) with a phased array surface coil. The pulse sequence used in the present study was modified from a previously published pulse sequence.[12] To increase the depth of the imaging volume, the slice thickness was increased to 2.5 mm. Fifty-two slices were obtained, which resulted in an imaging volume thickness of 130 mm. To shorten the acquisition time, the matrix size was decreased. The final pulse sequence was a fast-spin echo, proton density-weighted sequence (repetition time 9900 milliseconds, echo time 9.1 milliseconds) with 2 averages and flip angle of 150 degrees. A 192 × 192 matrix was interpolated to a 384 × 384 matrix with 15 % phase oversampling in the phase-encoding direction. The field of view was 250 mm × 250 mm. The acquisition time was 4 minutes 20 seconds. In most cases, the images were obtained in the coronal plane. The posterior aspect of the imaging volume was positioned at the apex of the vagina.
Solid Model Construction
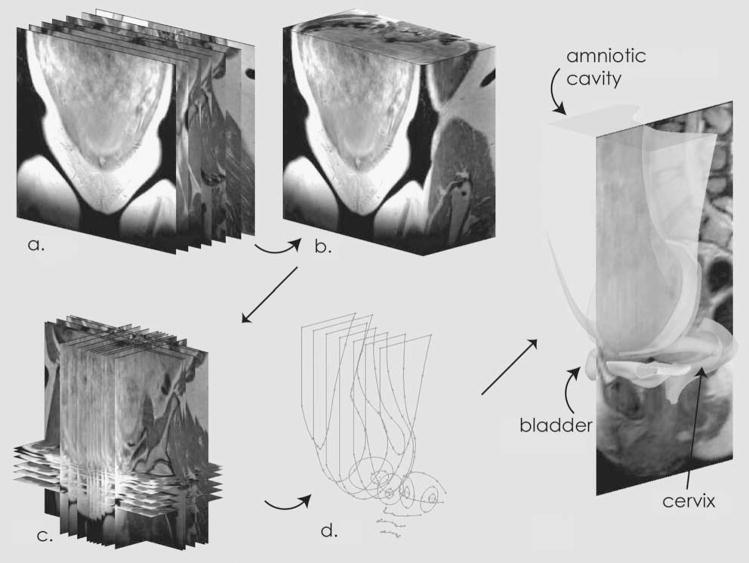
MR-guided solid models were constructed using solid modeling software (Solidworks, Concord, MA).[13,14] Figure 1 demonstrates the procedure. First, 2D MR images were converted to a 3D volume (Analyze 7.5, AnalyzeDirect, Lenexa KS) and reformatted so that voxel dimensions were 1.0 × 1.0 × 1.0 cubic millimeters. A coordinate system was defined within the modeling workspace such that there was a one to one correspondence between points in the 3-dimensional MRI volume and points in the modeling workspace. Model generation followed a specific strategy: images were selected from the 3D volume and placed in the modeling workspace in the correct anatomic location. Choice of the specific images depended on the anatomy of interest. The strategy was to choose a minimum number of images to adequately capture the shape of the anatomic object. As experience accrued with the technique, it was found that 20 – 25 images were sufficient to generate an accurate anatomic model. Model construction took approximately six hours to complete.
Figure 1.
Construction of Solid Models. Two-dimensional images were collected in the coronal plane (a) and converted to a three-dimensional volume (b). Individual images were selected from the three-dimensional volume and placed in the modeling workspace (c). Using these images as guides, two-dimensional tracings were made (d), which were combined (lofted) into three-dimensional solids.
Models were limited to anatomy below the pelvic inlet as defined by the diagonal conjugate of the pelvis. Six structures were defined in all models: amniotic cavity, uterine wall, cervical stroma, cervical mucosa, anterior vaginal wall and bladder. The cervix displayed three zones of signal intensity on MR images.[15] The innermost zone of high signal intensity was used to represent the cervical mucosa. The middle zone (low signal intensity) and outer zone (medium signal intensity) were used to represent the cervical stroma.[15] The anterior vaginal wall was limited to DeLancey levels I, II and part of level III.[16] In selected models, the left uterine artery was also defined.
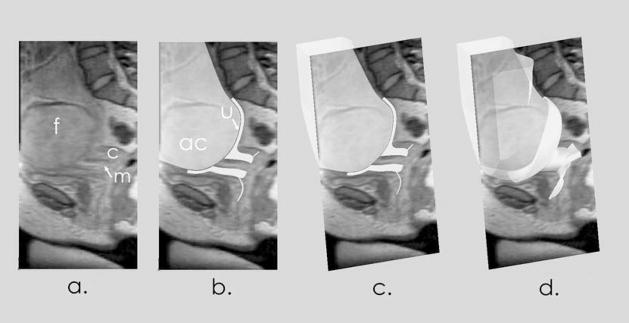
The six anatomic structures were defined in the following manner. Using image guidance, the anatomy of interest was traced and the tracings were combined into a solid. The uterine wall was defined by its relation to the amniotic cavity. To define the cervix, cervical profiles were traced on planes that were perpendicular to the path of the cervical canal. The uterine wall, cervix and vagina were combined into a single solid. The uterine artery was defined using the 3D line creation tool. To compare models at the same magnification and orientation, models were imported into a single assembly as individual parts. Model accuracy was verified by comparing the model to the appropriate MR image (figure 2).
Figure 2.
Accuracy of Solid Models. The first panel (a) shows a midline, sagittal MR image with the fetal head (f), posterior cervix (c) and mucosa (m). The second panel (b) shows the anatomic model superimposed on the image. The amniotic cavity (ac) and uterus (u) are indicated. In the third panel (c), the model and image are viewed at an oblique angle. The 3D model is cut at the level of the image. The fourth panel (d) shows the full 3D anatomic model.
Results
A total of 23 patients were approached to participate. In 9 patients, movement artifact was present, leaving 14 patients (61%) in the study group. Indications for MR imaging included suspected central nervous system anomaly (N=12) and suspected abdominal anomaly (N=2). The age range of the patients was 19 to 39 years. The median age was 28 years. Of the 14 patients, there were 3 primiparas and 11 multiparas - 3 with previous cesarean delivery and 8 with previous vaginal delivery. There were no marked anatomic differences between primiparas and multiparas, though the sample size was too small to make firm conclusions. Also, the cesarean section scar was not seen on the MR images.
To compare 3-dimensional anatomic changes in the third trimester compared to the second trimester, the fourteen models were grouped into a second trimester group (20 – 24 weeks (n=7)) and a third trimester group (31 – 36 weeks (n=7)). The mean cervical lengths of the second trimester and third trimester groups were 3.3 ± 1.2 cm and 2.6 ± 1.0 cm respectively. A side-by-side comparison of second and third trimester groups was performed by matching one model from each group using the diagonal conjugate of the bony pelvis. The diagonal conjugate was chosen as the matching variable to control for differences in the size of the pelvis. Models were limited to anatomy below the diagonal conjugate because the uterine size was larger than the MR volume.
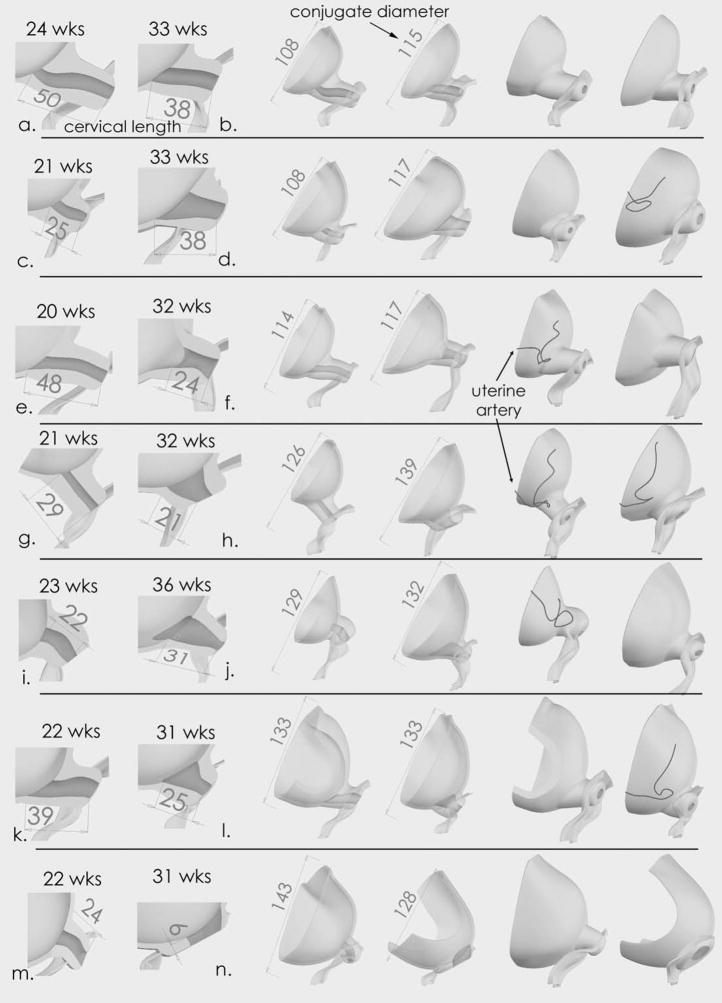
Figure 3 demonstrates fourteen anatomic models: seven models from the second trimester group and seven from the third trimester group. Each row presents a side-by-side comparison of a second and third trimester model. For example, the first row of figure 3 shows two models – one model from patient (a) and one model from patient (b). Three views from the two patients are shown for a total of six views. The first two views demonstrate the cervical lengths of these patients. In the next two views, the two models are viewed from an anterior angle. The cervix is transparent. The diagonal conjugate diameters for patient (a) and (b) are 108 mm and 115 mm respectively. In the next two views, the two models are viewed from a posterior angle. The second row of figure 3 shows model views from patient (c) and (d) in a similar fashion.
Figure 3.
Fourteen Solid Models. Each row shows two, matched models - one model from the second trimester and one from the third trimester. The models are matched by the distance of the conjugate diameter of the pelvic bone. The first two columns demonstrate cervical length. The middle two columns show an anterior view and the cervix is transparent. The last two columns show a posterior view. In all models, the volume of the amniotic cavity below the diagonal conjugate is larger in the third trimester compared to the second trimester. In addition, the transition from the cervix to the uterus is wider in the third trimester compared to the second trimester (compare (c) vs. (d), (g) vs. (h), (i) vs. (j), (k) vs. (m)). Model (n) shows a cervix that is completely effaced at 31 weeks. This patient delivered at 33 weeks. Dimensions are in millimeters.
When 3D cervical anatomy in the second trimester is compared to the third trimester, several findings are apparent. In all seven matched pairs, the volume of amniotic cavity below the diagonal conjugate is increased in the third trimester compared to the second trimester (p =.016, sign test). This finding is consistent with increasing size and descent of the amniotic sac. Amniotic sac descent is associated with qualitative changes in the uterocervical junction. The transition from the cervix to the uterus is more gradual in the third trimester compared to the second trimester (compare (c) vs. (d), (g) vs. (h), (i) vs. (j), (k) vs. (m). Also, the diameter of the internal os appeared wider in the third trimester. These 3D anatomic changes are associated with a shorter cervical length in the third trimester.
Comment
In this first study of 3-dimensional, MRI-based anatomy of the cervix and surrounding structures during pregnancy, increasing gestational age is associated with 3-dimensional changes in the cervical stroma, cervical mucosa and lower uterine segment. The third trimester is associated with significant descent of the amniotic sac. Descent of the amniotic sac is associated with modified anatomy of the uterocervical junction. Taken together, these 3D changes are associated with a shorter cervix in the third trimester.
It is important to understand 3-dimensional anatomic changes that cause a short cervix because different anatomic changes suggest unique pathways to preterm birth. For example, in cases of multiple gestation, it is likely that the cervix becomes short because physiological effacement is accelerated. – the upper part of the cervix is pulled into the lower uterine segment as the uterus is stretched by the expanding amniotic cavity. However, in cases of cervical funneling, it is possible that the cervical length appears to decrease not because the cervix is pulled up but because the amniotic membrane stretches and protrudes inside the cervical canal. Alternative mechanisms to explain the decrease in cervical length in the third trimester can also be proposed. For example, the increased weight of the uterus and amniotic sac might induce longitudinal loads compressing the cervix against the pelvic floor. These differences in the driving forces for cervical shortening are reflected in differences in the patterns of anatomical evolution. To distinguish between competing hypotheses, it may be possible to track 3-dimensional cervical changes over time using an anatomic marker. For example, figure 3 shows the position of the left uterine artery in selected models. The left uterine artery appears higher in the third trimester compared to the second trimester, which suggests the upper aspect of the cervix is pulled into the uterus as the fetal head grows and descends. By performing longitudinal studies of individual patients according to the methodologies introduced in this preliminary work, these hypotheses can be tested by matching the observed patterns of deformation with the predictions of computational (finite element) models.
In this study, 3D models were constructed with the aid of commercially available, solid modeling software. Essentially, MR images were manually segmented in 3D space. This method was chosen for several reasons. First, automatic segmentation algorithms were ineffective for the anatomy of interest. Second, solid modeling software produced smooth models, which is important for future studies involving biomechanical simulation.[11,17] Third, the anatomic shape was captured with minimal information. For example, the shape of the amniotic cavity was often captured with only eight 2-dimensional images. A disadvantage of using solid modeling software is the significant time investment that is required.
Our study has several strengths and weaknesses. In contrast to prior sonographic studies of the 3D cervical anatomy,[8] this study used MR imaging, which was able to demonstrate improved cervical – uterine anatomy and a wider field of view. The primary weakness is the cross-sectional study design. The patients were referred to MRI for a fetal indication. Hence, there was no data on longitudinal changes in individual patients. Also, there was marked anatomic variability among different patients, which limited the ability to draw quantitative conclusions from the data. In addition, relatively few patients were studied. We regard the results of this study as primarily hypothesis-generating. In a subsequent study, we expect to image patients at two different time points to reduce variability and allow more quantitative conclusions.
In conclusion, increasing gestational age is associated with 3-dimensional cervical changes in the cervical stroma, cervical mucosa and lower uterine segment. It is important to understand 3-dimensional anatomic changes that cause a short cervix because different anatomic changes suggest unique pathways to preterm birth.
Acknowledgments
Grant Support: Society for Maternal Fetal Medicine/American Association of Obstetricians and Gynecologists Foundation
Footnotes
Reprints not available
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 2.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343:378–84. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Kirmeyer S, Munson ML. Births: Final data for 2005. National vital statistics reports. 6. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 4.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington D.C: National Academies Press; 2006. [Google Scholar]
- 5.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 6.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 8.Rovas L, Sladkevicius P, Strobel E, Valentin L. Reference data representative of normal findings at two-dimensional and three-dimensional gray-scale ultrasound examination of the cervix from 17 to 41 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;27:392–402. doi: 10.1002/uog.2658. [DOI] [PubMed] [Google Scholar]
- 9.Gramellini D, Fieni S, Molina E, Berretta R, Vadora E. Transvaginal sonographic cervical length changes during normal pregnancy. J Ultrasound Med. 2002;21:227–32. doi: 10.7863/jum.2002.21.3.227. quiz 234–5. [DOI] [PubMed] [Google Scholar]
- 10.House M, Socrate S. The cervix as a biomechanical structure. Ultrasound Obstet Gynecol. 2006;28:745–9. doi: 10.1002/uog.3850. [DOI] [PubMed] [Google Scholar]
- 11.Myers KM, Paskaleva AP, House M, Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008;4:104–16. doi: 10.1016/j.actbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 12.House M, O’Callaghan M, Bahrami S, Chelmow D, Kini J, Wu D, et al. Magnetic resonance imaging of the cervix during pregnancy: effect of gestational age and prior vaginal birth. Am J Obstet Gynecol. 2005;193:1554–60. doi: 10.1016/j.ajog.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Chen WP, Tai CL, Tan CF, Shih CH, Hou SH, Lee MS. The degrees to which transtrochanteric rotational osteotomy moves the region of osteonecrotic femoral head out of the weight-bearing area as evaluated by computer simulation. Clin Biomech (Bristol, Avon) 2005;20:63–9. doi: 10.1016/j.clinbiomech.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Chang KH, Magdum S, Khera SC, Goel VK. An advanced approach for computer modeling and prototyping of the human tooth. Ann Biomed Eng. 2003;31:621–31. doi: 10.1114/1.1568117. [DOI] [PubMed] [Google Scholar]
- 15.Scoutt LM, McCauley TR, Flynn SD, Luthringer DJ, McCarthy SM. Zonal anatomy of the cervix: correlation of MR imaging and histologic examination of hysterectomy specimens. Radiology. 93(186):159–162. doi: 10.1148/radiology.186.1.8416558. [DOI] [PubMed] [Google Scholar]
- 16.DeLancey JO. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36:897–909. doi: 10.1097/00003081-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Paskaleva AP. PhD Thesis. Massachusetts Institute of Technology; 2007. Biomechanics of Cervical Function in Pregnancy - Case of Cervical Insufficiency. [Google Scholar]