Abstract
A series of N-substituted 9-azabicyclo[3.3.1]nonan-3α-yl phenylcarbamate analogs were synthesized. Among them, WC-26 and WC-59 were identified as the most potent σ2 receptor ligands (Ki = 2.58 and 0.82 nM, respectively) with high selectivity against σ1 (Ki of σ1/σ2 ratio = 557 and 2,087, respectively). [18F]WC-59 was radiolabeled via a nucleophilic substitution of a mesylate precursor by [18F]fluoride, and in vitro direct binding studies of [18F]WC-59 were conducted using membrane preparations from murine EMT-6 solid breast tumors. The results indicate that [18F]WC-59 binds specifically to σ2 receptors in vitro (Kd = ~2 nM). Biodistribution studies of [18F]WC-59 in EMT-6 tumor-bearing mice indicated that the tracer was a less suitable candidate for clinical imaging studies than existing F-18 labeled σ2 receptor ligands. The ability of WC-26 to enhance the cytotoxic effects of the chemotherapy drug, doxorubicin, was evaluated in cell culture using the mouse breast tumor EMT-6 and the human tumor MDA-MB435. WC-26 greatly increased the ability of doxorubicin to kill these two tumor cell lines in vitro. These results indicate that WC-26 is potentially a useful chemosensitizer for the treatment of cancer when combined with conventional chemotherapeutics.
Keywords: Sigma-2 ligands, PET radiotracers, cancer, chemosensitization
1. Introduction
Sigma receptors are a distinct class of proteins with a widespread distribution in the central nervous system and peripheral tissues.1 It is now widely accepted that there are at least two types of sigma receptors, σ1 and σ2.2 Although the function of these receptors are not clearly defined yet, these receptors are distinguishable functionally, pharmacologically, and by their molecular size. The σ1 receptor has a molecular weight of ~25 kDa and has been cloned; the σ2 receptor has a molecular weight of ~21.5 kDa and has not been cloned.3-5 σ2 receptors are overexpressed in a wide variety of human tumor cells, and the σ2 receptor density is 10-fold higher in proliferative cells compared to quiescent mouse mammary adenocarcinoma cells both in vitro and in vivo.6-10 The high σ2 receptor density in a wide variety of tumor types suggests that σ2 receptors could be a target for the development of new radiotracers for imaging tumors with positron emission tomography (PET) and/or single photon emission computed tomography (SPECT).11-13
Recent studies have shown that σ2 selective ligands induce reactive oxygen species (ROS) and apoptosis in human tumor cell lines.14-16 Although the mechanism of cell death is largely unknown, several studies have revealed that σ2 receptor ligands induce apoptosis by caspase-dependent and/or caspase-independent pathways.14-16 Most antitumor drugs have severe adverse effects at high doses or following chronic use, and these adverse effects limit their clinical utility. One of the strategies to overcome this obstacle is to use a drug with no overlapping toxicity to enhance the ability of another antitumor drug to kill tumor cells. This phenomenon is called chemosensitization and can result in either an increase in tumor cell kill at the same level of toxicity or a decrease in toxicity at the same level of tumor cell kill. Additional studies with some σ2 selective ligands have demonstrated their ability to enhance the cytotoxicity of anticancer drugs.14-16 For example, cytotoxicity is increased when MCF-7 cells are treated with combinations of either doxorubicin or actinomycin and the σ2 selective ligand, CB-184.14 Consequently, there is also a great interest in developing high affinity σ2 receptor ligands as anticancer drugs or chemosensitizing agents.
Although a number of sigma receptor ligands have been reported, most of these ligands have either a high selectivity for the σ1 receptor (i.e. (+)-pentazocine), or bind with similar affinities to both σ1 and σ2 receptors (i.e. haloperidol (1) and DTG (2))1. Only a few sigma ligands, such as siramesine (3), CB-184 (4), and the benzamide analogs represented by RHM-1 (7), have a moderate to high selectivity for the σ2 receptor (Fig. 1).11,12,17-22 BIMU-1 (5), a potent 5-hydroxytryptamine receptor ligand with selectivity for 5-HT3 and 5-HT4, has been shown to possess a moderate affinity and selectivity for σ2 versus σ1 receptors.23 Based on a structural modification of BIMU-1, we previously reported that the σ2 selective ligand, 9-benzyl-9-azabicyclo[3.3.1]nonan-3-yl 2-methoxy-5-methylphenylcarbamate (6) has a higher selectivity for σ2 receptors than for σ1 receptors (σ1/σ2 = 31) with no binding to 5-HT3 and 5-HT4 (Kis: >1,000 nM).24,25 Thus, 6 may serve as an excellent lead compound for the development of novel highly selective σ2 ligands.
Figure 1.
Structures of representative σ receptor ligands.
In the present study, a series of N-substituted-9-azabicyclo[3.3.1] carbamate analogs was prepared, and their affinities for the σ receptors measured. Two promising candidates for further study were identified: WC-26 and WC-59. The highly selective σ2 receptor ligand, WC-59, was radiolabeled with 18F to generate [18F]WC-59, and its direct saturation binding affinity measured using membranes from EMT-6 mouse breast tumor. In addition, the biodistribution of [18F]WC-59 in female BALB/c mice implanted with EMT-6 tumor cells was investigated. The in vivo anti-tumor properties of the highly selective σ2 receptor ligand WC-26 were previously evaluated using Panc-02, a murine pancreatic tumor model implanted in C57BL/6 mice.27 These studies demonstrated a reduction in tumor volume during and immediately following treatment with WC-26, despite continued tumor growth in vehicle-treated mice. WC26 treated mice showed no evidence of systemic toxicity. The WC-26 treated tumors displayed dose-dependent increases in apoptosis when analyzed by flow cytometry methods. Although the tumors resumed growing when treatment was halted, these results indicate that WC-26 is biologically active; therefore additional in vitro studies were conducted to evaluate the potential utility of WC-26 in combination chemotherapy as a chemosensitizer. Using the mouse breast tumor cell line EMT-6 and the human MDA-MB435 cell line, the ability of WC-26 to increase the cytotoxicity of the anticancer drug, doxorubicin (DOX), was explored. The results of these studies indicate that although [18F]WC-59 is not a superior PET radiotracer for detecting solid tumors; WC-26 is potentially a useful chemosensitizer for treating cancer.
2. Results
2.1. Chemistry
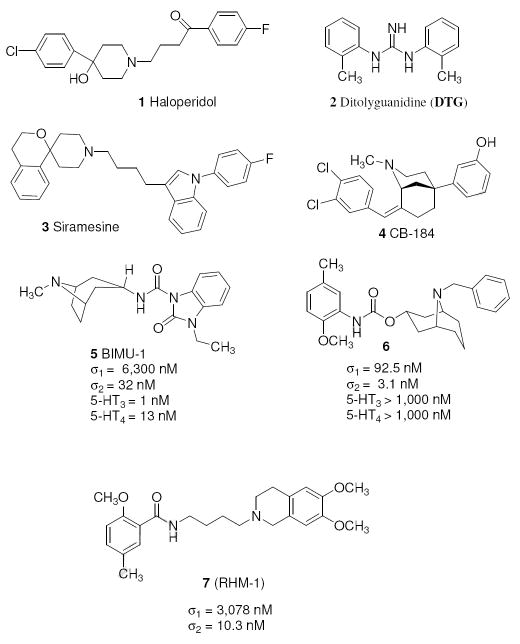
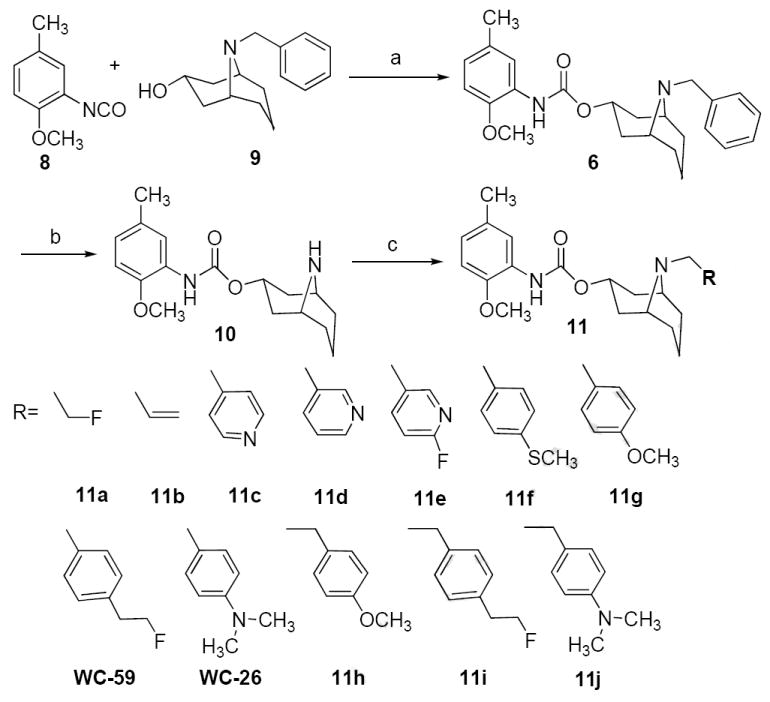
The synthesis of sigma receptor ligands based on the phenylcarbamate lead compound 6 is shown in Scheme 1. Coupling of 2-methoxy-5-methylphenyl isocyanate 8 with the alcohol 9 gave the phenylcarbamate compound 6, after which the benzyl group of 6 was cleaved by transfer hydrogenation to generate the secondary amine 10. The bridgehead nitrogen of 10 was alkylated by treatment of 10 with alkyl halide to give compounds 11a-j, and WC-59, or compound 10 was reacted with 4-(dimethylamino)benzaldehyde under NaBH3CN conditions to give WC-26. The synthesis of the key intermediates, 14, 18, and 22 used to generate WC-59, 11i, and [18F]WC-59, respectively, is shown in Scheme 2.
Scheme 1.
Reagents: (a) CH2Cl2, (CH3COO)2SnBu2; (b) Pd(OH)2/C, HCO2NH4, CH3OH/EtOAc; (c) RCH2X, (X=Br, Cl), K2CO3, CH3CN, or R-CHO, NaBH3CN (WC-26).
Scheme 2.
Reagents: (a) LiAlH4, ether; (b) CBr4, Ph3P; (c) Ph4PBr, NaCN, CH2Cl2/H2O; (d) NaOH, H2O; (e) TBDPSCl, Et3N, CH2Cl2.
2.2. Sigma receptor binding affinity of the compounds generated in Scheme 1
Competitive inhibition experiments were performed to measure the σ1 and σ2 binding affinities (Ki) of 11a-j, WC-59, and WC-26 as previously described.26 The σ1 binding sites were assayed with the σ1 selective radioligand, [3H](+)-pentazocine (~3 nM), using guinea pig brain homogenates. The σ2 binding sites were assayed with the novel σ2-selective radioligand, [3H]RHM-1 (~1 nM), using rat liver homogenates.26 The results of these in vitro binding studies are shown in Table 1. The compounds, 11a-j, had low to moderate selectivity and affinity for σ2 receptors. However, two compounds, WC-59, and WC-26, had both a high selectivity and a high affinity for σ2 receptors. WC-59, and WC-26 also had log P values that make them suitable for in vivo studies either imaging or treating proliferative solid tumors. Consequently, these two compounds were selected for further development respectively as a PET radiotracer and a chemotherapeutic agent.
Table 1.
In vitro σ receptor binding and log P data for the phenylcarbamate analogs.
| Compounds |
Ki (nM) |
σ1/σ2 | Log P | |
|---|---|---|---|---|
| σ1 | σ2 | |||
| 11a | 2,003.3 ± 215.7 | 143.3 ± 4.2 | 14 | 2.85 |
| 11b | 1,256.8 ± 87.9 | 85.9 ± 3.2 | 15 | 2.86 |
| 11c | 1,563.3 ± 415.9 | 521.6 ± 15.1 | 3 | 2.94 |
| 11d | 2,346.7 ± 340.7 | 183.9 ± 13.0 | 13 | 2.70 |
| 11e | 2,510.0 ± 477.6 | 571.5 ± 142.8 | 4 | 3.21 |
| 11f | 2,023.3 ± 344.0 | 8,192.1 ± 25.9 | 11 | 3.92 |
| 11g | 323.3 ± 40.8 | 40.7 ± 4.4 | 8 | 3.3 |
| WC-59 | 1,710.5 ± 84.0 | 0.82 ± 0.13 | 2,087 | 3.68 |
| WC-26 | 1,436.5 ± 166.1 | 2.58 ± 0.59 | 557 | 3.02 |
| 11h | 447.1 ± 66.9 | 125.8 ± 27.2 | 4 | 3.22 |
| 11i | 2,633.3 ± 672.9 | 275.8 ± 78.2 | 10 | 3.69 |
| 11j | 1,870.0 ± 338.3 | 195.2 ± 32.0 | 10 | 3.36 |
| Haloperidol | 1.45 ± 0.33 | 24.2 ± 3.0 | 0.06 | 3.36 |
2.3. Radiolabeling of WC-59
The synthesis of both the mesylate precursor 25 and [18F]WC-59 is shown in Scheme 3. The secondary amine 10 was alkylated with (4-(bromomethyl)phenethyloxy)(tert-butyl)diphenylsilane 22 to generate 23, and the (tert-butyl)diphenylsilane group in 23 was deprotected with n-Bu4NF to give the alcohol 24. The hydroxyl group of 24 was then mesylated to generate the precursor 25. The synthesis of [18F]WC-59 was accomplished by nucleophilic substitution of the mesylate precursor 25 with [18F]fluoride under standard conditions. The total synthesis and purification time was 100 min with an overall decay corrected yield of 15%. The radiochemical purity of the [18F]WC-59 was >99.9%, and the specific activity was 4,200 mCi/μmol.
Scheme 3.
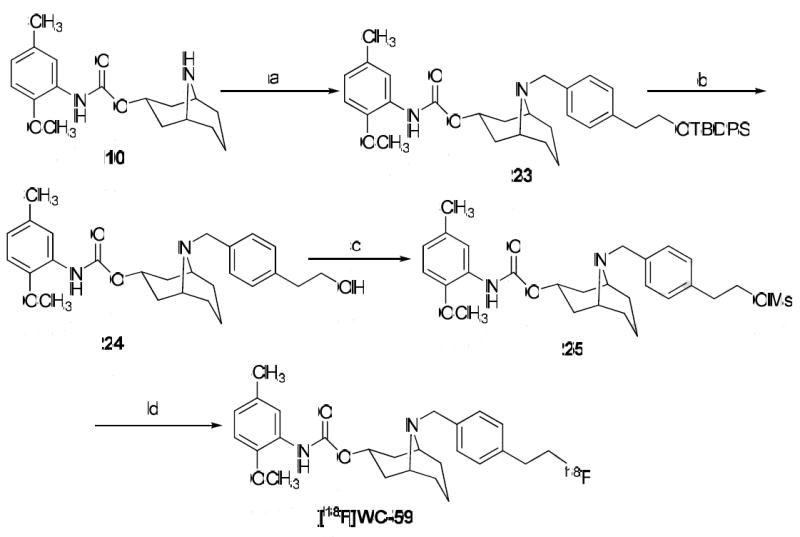
Reagents: (a) (4-(Bromomethyl)phenethyloxy)(t-butyl)diphenylsilane (22), Et3N, CH2Cl2; (b) n-Bu4NF·H2O, THF; (c) MsCl, Et3N; (d) [18F]fluoride, K2CO3, K222, DMSO, 110°C.
2.4. [18F]WC-59 binding study with EMT-6 tumors
To determine if the binding affinity of [18F]WC-59 was suitable for a solid tumor imaging agent, membrane homogenates were prepared from ~800 mg of EMT-6 mouse breast tumors, and a total direct saturation binding study performed using these membranes (Figure 2). The σ2 receptor density was high (~3,700 fmol/mg protein), and the binding affinity of [18F]WC-59 to the σ2 receptors in EMT-6 membranes was ~2 nM; a value consistent with the previous in vitro results (Table 1). The high in vitro direct binding affinity indicated that this radioligand was suitable for further evaluation in imaging solid tumors.
Figure 2.
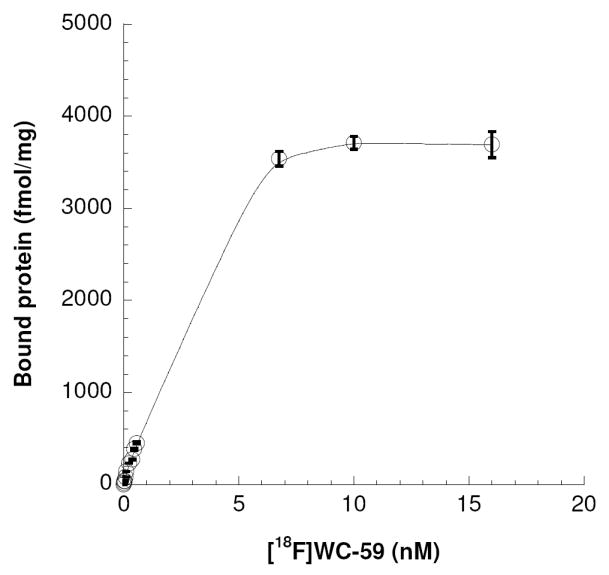
A representative total saturation σ2 binding experiment for [18F]WC-59 using membrane homogenates from EMT-6 mouse breast tumors. Each point is the mean of duplicate samples.
2.5. Biodistribution of [18F]WC-59 in tumor-bearing mice
A biodistribution study was conducted in EMT-6 tumor-bearing female BALB/c mice. There was a high initial uptake of [18F]WC-59 in lung, kidney and heart, but this uptake was reduced rapidly over the 2 h post-injection period (Table 2): the initial activity in lung, kidney, and heart was reduced by 95%, 75%, and 93% at 2 h post-injection, respectively. Liver also had a high initial uptake followed by a minimal washout of the radiotracer over the 2 h post-injection period. However, as previously reported, liver has a high density of σ2 receptors.1 The uptake of the [18F]WC-59 radiotracer in EMT-6 tumors was modest at 5 min post-injection (1.39 ± 0.34 %ID/g), but increased rapidly to ~3.5 %ID/g by 30 min post-injection; this level was stable for least 2 h (Table 2). Uptake in fat 5 min post-injection was also initially modest (2.33 ± 0.83 %ID/g) but increased more than 5.5-fold (12.63 ± 0.61 %ID/g) by 2 hours post-injection while bone activity level showed a modest increase and relatively stable levels from 30 min to 2 h. Despite the rapid washout, lung activity 2 h post injection (4.98 %ID/g) remained higher than the activity seen in tumor (4.08 %ID/g). These data indicate that [18F]WC-59 is a not superior to other 18F-labeled σ2 receptor ligands developed by our group as PET imaging agents for detecting and determining the proliferative status of solid tumors.12
Table 2.
Biodistribution of [18F]WC-59 in EMT-6 tumor-bearing BALB/C mice
| %ID/g |
||||
|---|---|---|---|---|
| 5 min. | 30 min. | 1 hour | 2 hour | |
| Blood | 1.88 ± 0.61 | 0.99 ± 0.10 | 0.93 ± 0.16 | 0.59 ± 0.03 |
| Lung | 111.00 ± 13.52 | 16.32 ± 1.14 | 8.73 ± 2.54 | 4.98 ± 0.27 |
| Liver | 21.14 ± 3.59 | 28.50 ± 1.82 | 23.35 ± 2.00 | 17.11 ± 1.57 |
| Kidney | 22.42 ± 2.91 | 16.29 ± 1.35 | 10.38 ± 1.29 | 5.82 ± 0.33 |
| Muscle | 4.60 ± 0.76 | 2.90 ± 0.68 | 1.92 ± 0.51 | 1.62 ± 0.27 |
| Fat | 2.33 ± 0.83 | 8.58 ± 2.13 | 8.49 ± 1.41 | 12.63 ± 0.61 |
| Heart | 18.49 ± 2.50 | 3.70 ± 0.39 | 2.13 ± 0.45 | 1.22 ± 0.18 |
| Brain | 2.65 ± 0.24 | 2.12 ± 0.21 | 1.55 ± 0.40 | 1.16 ± 0.09 |
| Bone | 2.98 ± 0.53 | 4.76 ± 0.50 | 4.87 ± 1.15 | 5.68 ± 1.02 |
| Tumor | 1.39 ± 0.34 | 3.62 ± 0.55 | 3.47 ± 0.51 | 4.08 ± 0.66 |
| Tumor:Blood | 0.82 | 3.67 | 3.86 | 6.96 |
| Tumor:Muscle | 0.30 | 1.32 | 1.92 | 2.53 |
| Tumor:Fat | 0.65 | 0.45 | 0.41 | 0.32 |
| Tumor:Lung | 0.01 | 0.22 | 0.42 | 0.82 |
2.6. Evaluation of WC-26 as a chemosensitizer
WC-26 has been previously shown to have modest antitumor activity as a single agent in a mouse pancreatic tumor model.27 Studies have shown that σ2 receptor ligands generate ROS in tumor cells grown in tissue culture.15 Thus, we hypothesized that WC-26 when used as in combination therapy, may increase the antitumor activity of chemotherapeutic agents such as doxorubicin (DOX) that are thought to kill tumor cells, at least in part, by generating intracellular ROS.
When exponentially growing mouse EMT-6 (Figure 3) or human MDA-MB435 (Figure 4) tumor cells were treated for 16 h with 2 μM WC-26 alone and then a range of concentrations of DOX was added for an additional 24 h, a large decrease in clonogenic survival relative to control samples was observed compared to the clonogenic survival for cells treated with DOX alone. It should be noted that, for both cell lines, a 40 h treatment with 2 μM WC-26 alone resulted in no measureable cell kill. It is also important to note that a substantial increase in tumor cell kill following sequential administration of the drugs occurred at doses where neither drug killed any cells as a single agent. Therefore, these data suggest that the σ2 receptor ligand, WC-26, is a potent chemosensitizer of drugs that produce their antitumor effects by generating intracellular ROS.
Figure 3.
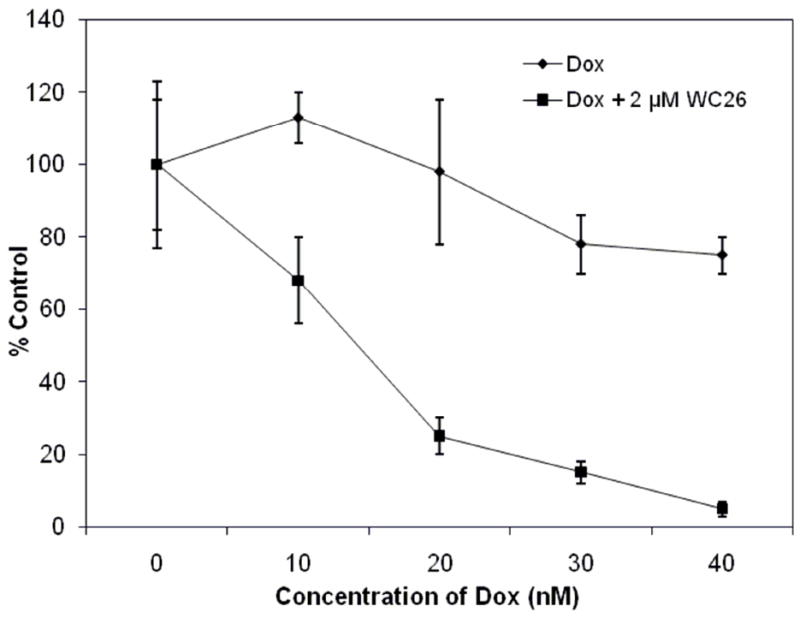
Percent survival of EMT-6 cells treated with WC-26 for 16 h, followed by a combination of WC-26 and doxorubicin (DOX) for an additional 24 h. The data points represent the mean ± 1 SD of triplicate samples in a representative experiment.
Figure 4.
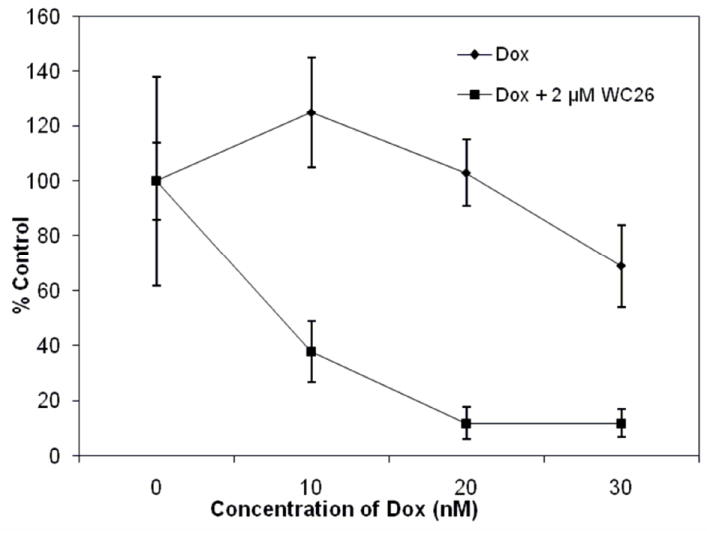
Percent survival of MDA-MB435 cells treated with WC-26 for 16 h, followed by a combination of WC-26 and doxorubicin (DOX) for an additional 24 h. The data points represent the mean ± 1 SD of triplicate samples in a representative experiment.
2. Discussion
The goal of the current study was to develop new sigma receptor ligands possessing a high affinity and selectivity for the σ2 receptor versus the σ1 receptor using 6 as a lead compound. When the benzyl group in 6 was substituted by a fluoroethyl or allyl group, the compounds, 11a and 11b, had lowered affinities for both σ1 and σ2 receptors. Similarly, a pyridinyl ring substituent (11c, 11d, 11e) on the same benzyl ring resulted in lower affinities for σ1 and σ2 receptor compared with 6. However, the results were different when the 4-position on the benzyl ring of compound 6 was modified. Adding a 4-methylthio (11f) or a 4-methoxy group (11g) reduced the binding affinity to σ1 and σ2 receptors, while adding a 4-fluoroethyl (WC-59) or a 4-dimethylamino (WC-26) group increased the binding affinity to σ2 receptors and decreased the binding affinity to σ1 receptors. The σ1/σ2 ratio for WC-59 and WC-26 increased to 2,087 and 557, respectively. Extending the spacer length of the methylene group to an ethylene group between the bridgehead nitrogen and the benzene ring of the N-benzyl moiety (11h, 11i, and 11j) resulted in a much lower σ2 receptor selectivity and affinity compared to the N-benzyl compounds, 11g, WC-59, and WC-26. These data demonstrate that the phenylcarbamate 6 is an excellent lead compound for the development of ligands with a high affinity and selectivity for σ2 receptors.
Of the two compounds that had a high selectivity and affinity for σ2 receptors, WC-59 was selected to be radiolabeled with 18F because its structure already contains a fluorine atom and because [18F]WC-59 could then be compared to our existing library of F-18 labeled σ2 receptor ligands.12 The half life of 18F (110 min.) is preferred for radiosynthesis and PET investigations over 11C (20.4 min half-life). The total synthesis and purification time of ~100 min, the decay corrected radiochemical yield of 15%, the radiochemical purity of >99.9%, and the specific activity of 4,200 mCi/μmol were all acceptable for a PET imaging agent. The [18F]WC-59 direct saturation binding studies on membrane homogenates derived from EMT-6 solid tumors (Figure 2) gave a σ2 receptor density of ~3,700 fmol/mg protein and a σ2 receptor binding affinity ~2 nM; these values are consistent with those determined with EMT-6 cells from tissue culture and liver membrane homogenates. The [18F]WC-59 biodistribution studies in EMT-6 tumor-bearing female BALB/c mice showed that although tumor: blood (6.92) and tumor: muscle (2.52) ratios at 2 h post-injection were acceptable for a PET imaging agent; however, tumor: fat ratios were below unity, and even at 2 h post-injection, the activity retained in the lung (4.98 %ID/g) remained higher than the activity seen in tumor (4.08 %ID/g). Consequently, although [18F]WC-59 was successfully radiolabeled and generated promising initial results in vitro, it is not a superior candidate PET radioligand for clinical imaging studies when compared with existing F-18 labeled σ2 receptor ligands used to detect and determine the proliferative status of solid tumors.12
Previously published studies using WC-26 as an anticancer agent in a mouse model of pancreatic cancer showed good biological activity, but tumor growth inhibition was short-lived.27 Based on that data and other published studies using sigma-receptor ligands in combination with standard chemotherapeutic agents14, the compound was chosen for in vitro evaluation as a chemosensitizer. The ability of the σ2 receptor selective ligand, WC-26, to enhance the cytotoxicity of the antitumor drug, DOX, was studied using a clonogenic survival assay in two different cell lines. Our data demonstrate that WC-26 greatly enhances both the mouse EMT-6 (Figure 3) and the human MDA-MB435 (Figure 4) tumor cell kill obtained with DOX. This chemosensitization occurred at WC-26 (2 μM, 40 h) and DOX (<20 μM, 24 h) exposure: doses where each drug produced no tumor cell kill as a single agent. Both of these drugs are known to produce ROS in cells (data not shown); high levels of ROS are known to kill tumor cells. Thus, our working hypothesis is that WC-26 increases DOX cytotoxicity by increasing the intracellular ROS to a lethal concentration. However, other mechanisms such as the activation of caspase-3 and induction of apoptosis cannot be excluded at this time. Further explorations of the mechanism of cell kill are under investigation at this time.
4. Conclusion
Two highly selective and potent σ2 receptor ligands, WC-59 and WC-26, were synthesized using 9-azabicyclo[3.3.1]nonan-3α-yl phenylcarbamate as the lead compound. WC-59 was radiolabeled with 18F and a series of radiochemical binding and biodistribution studies were performed which demonstrated that although [18F]WC-59 demonstrated good in vitro binding, it was not superior to existing 18F σ2 receptor ligands when evaluated in vivo at a PET imaging agent. Because σ2 receptor ligands have been shown to have antitumor activity14-16 and generate intracellular ROS15, and because of the compound’s prior evaluation as a single agent chemotherapeutic27, the ability of WC-26 to enhance the cytotoxicity of DOX, an antitumor drug used to treat solid tumors, was investigated in vitro. WC-26 greatly increased the ability of DOX to kill both mouse EMT-6 and human MDA-MB435 tumor cells at concentrations where neither drug as a single agent produced any cell kill. These results indicate that WC-26 has the potential to be useful as a chemosensitizer for the treatment of solid tumors.
5. Experimental
5.1. General methods and materials
All chemicals were obtained from standard commercial sources and used without further purification. All reactions were carried out using standard air-free and moisture-free techniques under an inert nitrogen atmosphere with dry solvents unless otherwise stated. Flash column chromatography was conducted using Scientific Adsorbents, Inc. silica gel, 60A, “40 Micron Flash” (32-63 μm). Melting points were determined using a MEL-TEMP 3.0 (Barnstead International, Dubuque, IA) and are uncorrected. 1H NMR spectra were recorded on a Varian Unity-300 (300 MHz) NMR spectrometer. All chemical shifts were reported as parts per million (ppm) downfield from tetramethylsilane (TMS), and coupling constants (J) are given in Hertz (Hz). Splitting patterns are typically described as follows: s, singlet; d, doublet; t, triplet; m, multiplet. The elemental analyses (C, H, N) were determined by Atlantic Microlab, Inc. (Norcross, GA)
5.1.1. (4-(2-Fluoroethyl)phenyl)methanol (13)
A solution of 1228 (0.75 g, 4.1 mmol) in ether (10 mL) was added to a solution of LiAlH4 (311 mg, 8.2 mmol) in ether (10 mL) at ambient temperature over 10 min. The mixture was stirred overnight, after which ice water (3 mL) was added. The white solid of the inorganic salt was filtered out and washed with ether, and then the filtrate of the ether was washed with saturated NaCl solution (30 mL), and finally dried over Na2SO4. After evaporation of the ether, the crude product was purified by flash column chromatography with hexane-ether (1:1) to obtain 13 (473 mg, 75%) as colorless oil. 1H NMR (CDCl3) δ: 7.32 (d, J = 8.1 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 4.65 (s, 2H), 4.63 (dt, J = 47.1, 6.6 Hz, 2H), 3.02 (dt, J = 23.4, 6.6 Hz, 2H), 1.99 (s, 1H).
5.1.2. 1-(Bromomethyl)-4-(2-fluoroethyl)benzene (14)
Ph3P (1.53 g, 5.84 mmol) was added to a solution of 13 (450 mg, 2.92 mmol) and CBr4 (1.45 g, 4.38 mmol) in CH2Cl2 (10 mL) at 0 °C, and the mixture was stirred for 20 min at 0 °C. After evaporation of CH2Cl2, hexane-ether (1:1, 50 mL) was added and the precipitated white solid was removed by filtration. The filtrate was evaporated under vacuum. The crude product was purified by flash column chromatography with hexane-ether (10:1) to obtain 14 (432 mg, 72%) as a white solid. 1H NMR (CDCl3) δ: 7.35 (d, J = 8.1 Hz, 2H), 7.21 (d, J = 8.1 Hz, 2H), 4.62 (dt, J = 47.1, 6.6 Hz, 2H), 4.48 (s, 2H), 3.00 (dt, J = 23.7, 6.6 Hz, 2H).
5.1.3. 2-(4-(2-Fluoroethyl)phenyl)acetonitrile (15)
A solution of 14 (280 mg, 1.29 mmol), tetraphenylphosphonium bromide (541 mg, 1.29 mmol) and KCN (840 mg, 12.9 mmol) in CH2Cl2-H2O (10 mL/10 mL) was refluxed for 3 h. After addition of ether (70 mL), the organic layer was washed first with water (30 mL), then with saturated NaCl solution (30 mL) and finally dried over Na2SO4. The solvent was evaporated, and the crude product was purified by flash column chromatography with hexane-ether (2:1) to obtain 15 (165 mg, 79%) as a colorless oil. 1H NMR (CDCl3) δ: 7.25 (s, 4H), 4.61 (dt, J = 47.1, 6.6 Hz, 2H), 3.69 (s, 2H), 2.99 (dt, J = 24.6, 6.3 Hz, 2H).
5.1.4. 2-(4-(2-Fluoroethyl)phenyl)acetic acid (16)
A solution of 15 (165 mg, 1.0 mmol) and NaOH (200 mg, 5.0 mmol) in EtOH-H2O (3 mL/3 mL) was refluxed for 6 h. Water (5 mL) was added to the reaction mixture, followed by extraction with ether. The aqueous layer was acidified with 6N HCl to pH = 1, then it was extracted with ethyl acetate (2 × 30 mL). The ethyl acetate extraction was dried over Na2SO4. Ethyl acetate was evaporated to obtain 16 (182 mg, 100%) as a white solid, mp 110.1-111.1 °C. 1H NMR (CDCl3) δ: 7.22 (s, 4H), 4.62 (dt, J = 47.1, 6.6 Hz, 2H), 3.63 (s, 2H), 3.00 (dt, J = 23.4, 6.6 Hz, 2H).
5.1.5. 2-(4-(2-Fluoroethyl)phenyl)ethanol (17)
17 was prepared from 16 in a manner similar to that described for 13, The crude product was purified by flash column chromatography with hexane-ether (1:1) to obtain 17 (131 mg, 78%) as a colorless oil. 1H NMR (CDCl3) δ: 7.20 (s, 4H), 4.63 (dt, J = 47.4, 6.6 Hz, 2H), 3.81 (t, J = 6.6 Hz, 2H), 3.00 (dt, J = 23.4, 6.6 Hz, 2H), 2.84 (t, J = 6.6 Hz, 2H), 2.17 (s, 1H).
5.1.6. 1-(2-Bromoethyl)-4-(2-fluoroethyl)benzene (18)
18 was prepared from 17 in a manner similar to that described for 14. The crude product was purified by flash column chromatography with hexane-ether (100:5) to obtain 18 (155 mg, 86%) as a colorless oil. 1H NMR (CDCl3) δ: 7.20 (s, 4H), 4.64 (dt, J = 47.1, 6.6 Hz, 2H), 3.58 (t, J = 7.8 Hz, 2H), 3.16 (t, J = 7.8 Hz, 2H), 3.02 (dt, J = 23.1, 6.6 Hz, 2H).
5.1.7. 9-Benzyl-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (6)
Dibutyltin diacetate (0.5 mL) was added to a solution of 2-methoxy-5-methylphenyl isocyanate (8) (3.34 g, 20.5 mmol) and 9-benzyl-9-aza-bicyclo [3.3.1]nonan-3-ol (9) (4.50 g, 19.5 mmol) in CH2Cl2 (45 mL) at ambient temperature, and the mixture was stirred overnight. After evaporation of CH2Cl2 under vacuum, ether (100 mL) was added, the ether solution was first washed with water (50 mL), then with saturated NaCl solution (50 mL), and finally dried over Na2SO4. After removal of ether, the crude product was purified by flash column chromatography with hexane-ether (2:1) to obtain 6 (7.40 g, 96%) as a pale yellow oil. 1H NMR (CDCl3) δ 7.97 (s, 1H), 7.37-7.22 (m, 5H), 7.15 (s, 1H), 6.76 (m, 2H), 5.26 (m, 1H), 3.83 (s, 3H), 3.80 (s, 2H), 3.06 (m, 2H), 2.47 (m, 2H), 2.30 (s, 3H), 2.14 (m, 1H), 1.97 (m, 2H), 1.51 (m, 3H), 1.17 (m, 2H).
5.1.8. 9-Azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (10)
Pd(OH)2/C (20%, 750 mg) was added to a solution of 6 (3.67 g, 9.3 mmol) and ammonium formate (2.93 g, 4.65 mmol) in methanol (90 mL) and ethyl acetate (90 mL). The mixture was refluxed for 2 h. After evaporation of the solvents under vacuum, ethyl acetate (100 mL) was added. The mixture was washed with saturated Na2CO3 solution (40 mL), water (40 mL), saturated NaCl solution (40 mL), and finally the organic solution was dried over Na2SO4. Ethyl acetate was then removed to obtain 10 (2.82 g, 100%) as a pale yellow solid. 1H NMR (CDCl3) δ: 7.93 (s, 1H), 7.15 (s, 1H), 6.76 (m, 2H), 4.99 (m, 1H), 3.83 (s, 3H), 3.34 (m, 2H), 2.36 (m, 2H), 2.29 (s, 3H), 2.08 (m, 1H), 1.64 (m, 4H), 1.50 (m, 4H).
5.1.9. 9-(2-Fluoroethyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (11a)
K2CO3 (0.5 g) was added to a solution of 10 (203 mg, 0.67 mmol) and 1-bromo-2-fluoroethane (1 mL) in acetonitrile (8 mL). The mixture was stirred overnight at ambient temperature. After the addition of ether (75 mL), the organic solution was first washed with water (30 mL), then with saturated NaCl solution (30 mL), and finally dried over Na2SO4. After removal of solvent under reduced pressure, the crude product was purified by flash column chromatography with hexane-ether (1:2) to obtain 11a (133 mg, 51%) as a colorless oil. The HCl salt of 11a is a white solid, mp 201.4-202.5 °C. 1H NMR (CDCl3) δ: 7.95 (s, 1H), 7.14 (s, 1H), 6.76 (m, 2H), 5.13 (m, 1H), 4.45 (dt, J = 47.7, 6.6 Hz, 2H), 3.84 (s, 3H), 3.08 (m, 2H), 2.91 (dt, J = 18.9, 5.4 Hz, 2H), 2.47 (m, 2H), 2.30 (s, 3H), 2.14 (m, 1H), 1.89 (m, 2H), 1.54 (m, 3H), 1.22 (m, 2H). Anal. Calcd for C19H27FN2O3·HCl: C, 58.98; H, 7.29; N 7.24. Found: C, 58.48; H, 7.26; N, 7.16.
5.1.10. 9-Allyl-9-azabicyclo[3.3.1]nonan-3-yl 2-methoxy-5-methylphenylcarbamate (11b)
11b was prepared from allyl bromide in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with ether to obtain 11b (90 mg, 34%) as a colorless oil. The HCl salt of 11b is a white solid, mp 221.3-222.3 °C. 1H NMR (CDCl3) δ: 7.95 (s, 1H), 7.13 (s, 1H), 6.76 (m, 2H), 5.79 (m, 1H), 5.13 (m, 3H), 3.84 (s, 3H), 3.26 (d, J = 6.0 Hz, 2H), 3.05 (m, 2H), 2.44 (m, 2H), 2.29 (s, 3H), 2.17 (m, 1H), 1.88 (m, 2H), 1.53 (m, 3H), 1.22 (m, 2H). Anal. Calcd for C20H28N2O3·HCl: C, 63.06; H, 7.67; N, 7.35. Found: C, 62.64; H, 7.66; N, 7.31.
5.1.11. 9-((Pyridine-4-yl)methyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (11c)
11c was prepared from 4-bromomethyl-pyridine hydrogen bromide in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with hexane-ether (1:2) to obtain 11c (133 mg, 51%) as a colorless oil. The HCl salt of 11c is a white solid, mp 225.5-227.0 °C. 1H NMR (CDCl3) δ: 8.51 (d, J = 5.4 Hz, 2H), 7.96 (s, 1H), 7.30 (d, J = 5.7 Hz, 2H), 7.17 (s, 1H), 6.76 (m, 2H), 5.25 (m, 1H), 3.85 (s, 3H), 3.82 (s, 2H), 3.01 (m, 2H), 2.48 (m, 2H), 2.30 (s, 3H), 2.15 (m, 1H), 1.93 (m, 2H), 1.54 (m, 3H), 1.23 (m, 2H). Anal. Calcd for C23H29N3O3·2HCl·0.5H2O: C, 57.86; H, 6.76; N, 8.80. Found: C, 58.01; H, 6.95; N, 8.68.
5.1.12. 9-((Pyridine-3-yl)methyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (11d)
11d was prepared from 3-bromomethyl-pyridine hydrogen bromide in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with ether to obtain 11d (90 mg, 34%) as a colorless oil. The HCl salt of 11d is a white solid, mp 242.7-243.9 °C. 1H NMR (CDCl3) δ 8.51 (s, 1H), 8.46 (m, 1H), 7.95 (s, 1H), 7.71 (m, 1H), 7.24 (m, 1H), 7.17 (s, 1H), 6.74 (m, 2H), 5.21 (m, 1H), 3.82 (s, 3H), 3.79 (s, 2H), 3.01 (m, 2H), 2.45 (m, 2H), 2.28 (s, 3H), 2.14 (m, 1H), 1.93 (m, 2H), 1.53 (m, 3H), 1.19 (m, 2H). Anal. Calcd for C23H29N3O3·2HCl: C, 58.97; H, 6.67; N, 8.97. Found: C, 58.69; H, 6.87; N, 8.57.
5.1.13. 9-((6-Fluoropyridine-3-yl)methyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (11e)
11e was prepared from 5-bromomethyl-2-fluoro-pyridine in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with hexane-ether (1:1) to obtain 11e (264 mg, 96%) as a colorless oil. The HCl salt of 11e is a white solid, mp 166.7-167.9 °C. 1H NMR (CDCl3) δ: 8.10 (s, 1H), 7.95 (s, 1H), 7.84 (td, J = 8.25, 2.4 Hz, 1H), 7.16 (s, 1H), 6.89 (dd, J = 8.25, 2.7 Hz, 1H), 6.76 (m, 2H), 5.20 (m, 1H), 3.84 (s, 3H), 3.78 (s, 2H), 3.01 (m, 2H), 2.43 (m, 2H), 2.30 (s, 3H), 2.16 (m, 1H), 1.93 (m, 2H), 1.56 (m, 3H), 1.23 (m, 2H). Anal. Calcd for C23H28FN3O3·HCl·H2O: C, 59.03; H, 6.68; N, 8.98. Found: C, 58.77; H, 6.39; N, 8.61.
5.1.14. 9-(4-(Methylthio)benzyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (11f)
11f was prepared from 1-bromomethyl-4-methylsulanyl-benzene in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with hexane-ether (1:1) to obtain 11f (245 mg, 81%) as a colorless oil. The HCl salt of 11f is a white solid, mp 179.3-180.8 °C. 1H NMR (CDCl3) δ: 8.01 (s, 1H), 7.31 (d, J = 8.1 Hz, 2H), 7.23 (d, J = 8.1 Hz, 2H), 7.21 (s, 1H), 6.76 (m, 2H), 5.27 (m, 1H), 3.83 (s, 3H), 3.78 (s, 2H), 3.07 (m, 2H), 2.48 (s, 3H), 2.46 (m, 2H), 2.32 (s, 3H), 2.17 (m, 1H), 1.96 (m, 2H), 1.58 (m, 3H), 1.21 (m, 2H). Anal. Calcd for C25H32N2O3S·HCl: C, 62.94; H, 6.97; N, 5.87. Found: C, 62.58; H, 7.02; N, 5.42.
5.1.15. 9-(4-Methoxybenzyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (11g)
11g was prepared from 1-chloromethyl-4-methoxybenzene in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with hexane-ether (1:2) to obtain 11g (221 mg, 72%) as a colorless oil. The HCl salt of 11g is a white solid, mp 203.9-204.8 °C. 1H NMR (CDCl3) δ: 7.99 (s, 1H), 7.28 (d, J = 8.7 Hz, 2H), 7.17 (s, 1H), 6.86 (d, J = 8.7 Hz, 2H), 6.76 (m, 2H), 5.26 (m, 1H), 3.85 (s, 3H), 3.81 (s, 3H), 3.75 (s, 2H), 3.05 (m, 2H), 2.47 (m, 2H), 2.31 (s, 3H), 2.16 (m, 1H), 1.95 (m, 2H), 1.53 (m, 3H), 1.19 (m, 2H). Anal. Calcd for C25H32N2O4·HCl·0.25H2O: C, 64.50; H, 7.25; N, 6.02. Found: C, 64.54; H, 7.28; N, 5.65.
5.1.16. 9-(4-Methoxyphenethyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methylphenylcarbamate (11h)
11h was prepared from 1-(2-bromo-ethyl)-4-methoxybenzene in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with ether to obtain 11h (86 mg, 29%) as a colorless oil. The HCl salt of 11h is a white solid, mp 209.8-210.9 °C. 1H NMR (CDCl3) δ: 7.96 (s, 1H), 7.16 (s, 1H), 7.14 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 6.76 (m, 2H), 5.14 (m, 1H), 3.85 (s, 3H), 3.79 (s, 3H), 3.10 (m, 2H), 2.79 (m, 2H), 2.64 (m, 2H), 2.46 (m, 2H), 2.30 (s, 3H), 2.17 (m, 1H), 1.89 (m, 2H), 1.53 (m, 3H), 1.22 (m, 2H). Anal. Calcd for C26H34N2O4·HCl: C, 65.74; H, 7.43; N, 5.90. Found: C, 65.91; H, 7.57; N, 5.64.
5.1.17. 9-(4-(2-Fluoroethyl)phenethyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methyl-phenylcarbamate (11i)
11i was prepared from 18 in a manner similar to that described for 11a. The crude product was purified by flash column chromatography ether to obtain 11i (162 mg, 53%) as a colorless oil. The HCl salt of 11i is a white solid, mp 202.2-203.7 °C. 1H NMR (CDCl3) δ: 7.91 (s, 1H), 7.10 (s, 5H), 6.68 (m, 2H), 5.08 (m, 1H), 4.55 (dt, J = 46.8, 6.6 Hz, 2H), 3.77 (s, 3H), 3.04 (m, 2H), 2.92 (dt, J = 22.8, 6.6 Hz, 2H), 2.74 (m, 2H), 2.64 (m, 2H), 2.40 (m, 2H), 2.24 (s, 3H), 2.09 (m, 1H), 1.83 (m, 2H), 1.46 (m, 3H), 1.18 (m, 2H). Anal. Calcd for C27H36ClFN2O3·HCl: C, 66.04; H, 7.39; N, 5.70. Found: C, 66.18; H, 7.54; N, 5.61.
5.1.18. 9-(4-(Dimethylamino)phenethyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methyl-phenylcarbamate (11j)
11j was prepared from 4-(2-bromoethyl)-N,N-dimethylaniline in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with ether to obtain 11j (131 mg, 44%) as a pale yellow oil. The HCl salt of 11j is a white solid, mp 253.6-255.2 °C. 1H NMR (CDCl3) δ: 7.96 (s, 1H), 7.14 (s, 1H), 7.10 (d, J = 9.0 Hz, 2H), 6.76 (m, 2H), 6.71 (d, J = 8.7 Hz, 2H), 5.15 (m, 1H), 3.85 (s, 3H), 3.13 (m, 2H), 2.92 (s, 6H), 2.79 (m, 2H), 2.64 (m, 2H), 2.45 (m, 2H), 2.30 (s, 3H), 2.17 (m, 1H), 1.91 (m, 2H), 1.52 (m, 3H), 1.22 (m, 2H). Anal. Calcd for C27H37N3O3·2HCl: C, 61.83; H, 7.49; N, 8.01. Found: C, 61.99; H, 7.51; N, 7.84.
5.1.19. 9-(4-(2-Fluoroethyl)benzyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methyl-phenylcarbamate (WC-59)
WC-59 was prepared from 14 in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with hexane-ether (1:2) to obtain WC-59 (212 mg, 87%) as a colorless oil. The HCl salt of WC-59 is a white solid, mp 134.7-135.8 °C. 1H NMR (CDCl3) δ: 8.01 (s, 1H), 7.33 (d, J = 8.1 Hz, 2H), 7.20 (s, 1H), 7.19 (d, J = 8.1 Hz, 2H), 6.76 (m, 2H), 5.28 (m, 1H), 4.64 (dt, J = 47.1, 6.6 Hz, 2H), 3.84 (s, 3H), 3.80 (s, 2H), 3.07 (m, 2H), 3.01 (dt, J = 22.8, 6.6 Hz, 2H), 2.50 (m, 2H), 2.33 (s, 3H), 2.17 (m, 1H), 1.97 (m, 2H), 1.55 (m, 3H), 1.18 (m, 2H). Anal. Calcd for C26H34ClFN2O3·HCl·0.25H2O: C, 64.85; H, 7.22; N, 5.82. Found: C, 65.03; H, 7.45; N, 5.33.
5.1.20. 9-(4-(Dimethylamino)benzyl)-9-azabicyclo[3.3.1]nonan-3-yl-2-methoxy-5-methyl-phenylcarbamate (WC-26)
NaBH3CN (57 mg, 0.91 mmol) was added to a solution of 10 (260 mg, 0.85 mmol) and 4-(dimethylamino)benzaldehyde (127 mg, 0.85 mmol) in methanol (10 mL), and the reaction mixture was stirred for 3 days. After evaporation of methanol and addition of ethyl acetate (50 mL), the mixture was washed with water (30 mL), then with saturated NaCl solution (30 mL), and finally the organic layer was dried over Na2SO4. The crude product was purified by flash column chromatography with hexane-ether (1:2) to obtain WC-26 (185 mg, 50%) as a colorless oil. The HCl salt of WC-26 is a white solid, mp 192.2-193.1 °C. 1H NMR (CDCl3) δ: 8.00 (s, 1H), 7.25 (d, J = 8.7 Hz, 2H), 7.18 (s, 1H), 6.76 (m, 2H), 6.73 (d, J = 8.4 Hz, 2H), 5.27 (m, 1H), 3.85 (s, 3H), 3.74 (s, 2H), 3.08 (m, 2H), 2.95 (s, 6H), 2.49 (m, 2H), 2.32 (s, 3H), 2.17 (m,1H), 1.97 (m, 2H), 1.54 (m, 3H), 1.19 (m, 2H). Anal. Calcd for C26H35N3O3·2HCl·0.5H2O: C, 60.11; H, 7.37; N, 8.09. Found: C, 59.99; H, 7.50; N, 7.74.
5.1.21. 4-[2-(t-Butyl-diphenyl-silyloxy)-ethyl]-benzoic acid methyl ester (20)
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) (2 mL) was added to a solution of 4-(2-hydroxyethyl)benzoic acid methyl ester 19 (721 mg, 4.0 mmol) and t-butyldiphenylsilyl chloride (TBDPSCl) (2.20 g, 8.0 mmol) in CH2Cl2 (15 mL), and the reaction mixture was stirred overnight. After evaporation of CH2Cl2 under vacuum, ether (75 mL) was added and the mixture was washed with water (2 × 50 mL), then with saturated NaCl solution (50 mL), and finally the organic layer was dried over Na2SO4. The crude product was purified by flash column chromatography with hexane-ether (10:1) to obtain 20 (1.64 g, 98%) as a colorless oil. 1H NMR (CDCl3) δ: 7.97 (d, J = 7.2 Hz, 2H), 7.59 (d, J = 6.6 Hz, 4H), 7.47-7.35 (m, 6H), 7.27 (d, J = 7.8 Hz, 2H), 3.95 (s, 3H), 3.89 (t, J = 6.6 Hz, 2H), 2.92 (t, J = 6.6 Hz, 2H), 1.04 (s, 9H).
5.1.22. [4-[2-(t-Butyl-diphenyl-silyloxy)-ethyl]-phenyl]-methanol (21)
21 was prepared from 20 in a manner similar to that described for 13. The crude product was purified by flash column chromatography with hexane-ether (1:1) to obtain 21 (540 mg, 35%) as a colorless oil. 1H NMR (CDCl3) δ: 7.64 (m, 4H), 7.45-7.36 (m, 6H), 7.30 (d, J = 7.8 Hz, 2H), 7.19 (d, J = 8.4 Hz, 2H), 4.69 (s, 2H), 3.88 (t, J = 6.9 Hz, 2H), 2.90 (t, J = 6.9 Hz, 2H), 1.08 (s, 9H).
5.1.23. [2-(4-Bromomethyl-phenyl)-ethoxy]-t-butyl-diphenyl-silane (22)
22 was prepared from 21 in a manner similar to that described for 14. The crude product was purified by flash column chromatography with hexane-ether (10:1) to obtain 22 (148 mg, 65%) as a colorless oil. 1H NMR (CDCl3) δ: 7.60 (m, 4H), 7.45-7.34 (m, 6H), 7.30 (d, J = 7.8 Hz, 2H), 7.14 (d, J = 7.8 Hz, 2H), 4.50 (s, 2H), 3.84 (t, J = 6.9 Hz, 2H), 2.85 (t, J = 6.9 Hz, 2H), 1.06 and 1.04 (s, 9H).
5.1.24. (2-Methoxy-5-methyl-phenyl)-carbamic acid 9-[4-[2-(t-butyl-diphenyl-silyloxy)-ethyl]-9-aza-bicyclo[3.3.1]non-3-yl ester (23)
23 was prepared from 22 in a manner similar to that described for 11a. The crude product was purified by flash column chromatography with hexane-ether (1:1) to obtain 23 (146 mg, 65%) as a colorless oil. 1H NMR (CDCl3) δ: 7.99 (s, 1H), 7.61 (m, 4H), 7.39 (m, 6H), 7.25 (m, 2H), 7.16 (s, 1H), 7.10 (m, 2H), 6.76 (m, 2H), 5.27 (m, 1H), 3.84 (m, 2H), 3.83 (s, 3H), 3.77 (s, 2H), 3.04 (m, 2H), 2.85 (m, 2H), 2.45 (m, 2H), 2.31 (s, 3H), 2.19 (m, 1H), 1.95 (m, 2H), 1.54 (m, 3H), 1.18 (m, 2H), 1.03 (s, 9H).
5.1.25. (2-Methoxy-5-methyl-phenyl)carbamic acid 9-[4-(2-hydroxy-ethyl)-benzyl]-9-aza-bicyclo[3.3.1]non-3-yl ester (24)
Tetrabutylammonium fluoride (53 mg, 0.2 mmol) was added to a solution of 23 (120 mg, 0.18 mmol) in THF (5 mL) at ambient temperature, and the mixture was stirred overnight. After addition of ethyl acetate (50 mL), the mixture was washed with water (30 mL), then with saturated NaCl solution (30 mL), and finally the organic layer was dried over Na2SO4. The solvent was evaporated, and the crude product was purified by flash column chromatography with ether to obtain 24 (56 mg, 72%) as a colorless oil. 1H NMR (CDCl3) δ: 7.96 (s, 1H), 7.34 (d, J = 7.5 Hz, 2H), 7.18 (d, J = 7.8 Hz, 2H), 7.16 (s, 1H), 6.76 (m, 2H), 5.24 (m, 1H), 3.86 (t, J = 6.6 Hz, 2H), 3.84 (s, 3H), 3.81 (s, 2H), 3.08 (m, 2H), 2.86 (t, J = 6.6 Hz, 2H), 2.51 (m, 2H), 2.30 (s, 3H), 2.17 (m, 1H), 1.98 (m, 2H), 1.57 (m, 3H), 1.23 (m, 2H).
5.1.26. Methanesulfonic acid 2-[4-[3-(2-methoxy-5-methyl-phenylcarbamoyloxy)-9-aza-bicyclo[3.3.1]non-9-ylmethyl]-phenyl]ethyl ester (25)
Triethylamine (26 mg, 0.2 mmol) was added at 0 °C to a solution of 24 (44 mg, 0.1 mmol) and methanesulfonyl chloride (29 mg, 0.15 mmol) in CH2Cl2 (5 mL), and the mixture was stirred overnight at ambient temperature. After addition of ethyl acetate (50 mL), the mixture was washed first with water (30 mL), then with saturated NaCl solution (30 mL), and finally the organic layer was dried over Na2SO4. The solvent was evaporated, and the crude product was purified by flash column chromatography with ether-MeOH (10:1) to obtain 25 (42 mg, 71%) as a colorless oil. 1H NMR (CDCl3) δ: 7.96 (s, 1H), 7.32 (d, J = 8.1 Hz, 2H), 7.18 (s, 1H), 7.17 (d, J = 8.1 Hz, 2H), 6.76 (m, 2H), 5.24 (m, 1H), 4.41 (t, J = 7.2 Hz, 2H), 3.84 (s, 3H), 3.78 (s, 2H), 3.06 (m, 2H), 3.04 (t, J = 7.2 Hz, 2H), 2.86 (s, 3H), 2.47 (m, 2H), 2.30 (s, 3H), 2.15 (m, 1H), 1.96 (m, 2H), 1.56 (m, 3H), 1.20 (m, 2H).
5.2. Synthesis of [18F]WC-59
5.2.1. General information
Classic C18 SepPak cartridges and Oasis HLB cartridges were purchased from Waters Corporation (Milford, MA). For the TLC analyses, EM Science Silica Gel 60 F254 TLC plates were purchased from Fisher Scientific (Pittsburgh, PA). Radio-TLC was accomplished using a Bioscan 200 imaging scanner (Bioscan, Inc., Washington, DC). Radioactivity was counted with a Beckman Gamma 8000 counter (Beckman Instruments, Inc., Irvine, CA). [18F]Fluoride was generated by the [18O(p,n)18F] reaction when 95% enriched [18O] water was irradiated with protons from Washington University’s RDS111 cyclotron.
5.2.2. Radiolabeling
[18F]Fluoride (120 mCi) was dried by azeotropic distillation using CH3CN (3 × 1 mL) in the presence of K2CO3 (1 mg) and K222 (5.5 mg) at 110 °C under a flow of N2, and then a solution of precursor 25 (1.0 mg, 1.9 μmol) in DMSO (350 μL) was added. The reaction mixture was heated in an oil bath (110 °C) for 10 min. This resulted in an 18F incorporation of 52% according to a radio-TLC analysis (silica, 20% methanol/80% CH2Cl2). The mixture was diluted in water (20 mL), and free [18F]fluoride was removed by passing the dilution through an Oasis HLB cartridge (500 mg). The trapped radioactivity in the cartridge was eluted with CH3CN (10 mL), and the elute was concentrated to dryness under reduced pressure. The residue was dissolved in CH3CN (1 mL) and water (3.5 mL) for HPLC injection.
[18F]WC-59 was purified by a reversed phased HPLC (Phenomenex Prodigy ODS3 column 250 × 10 mm, 10 μ), being eluted with 42% CH3CN, 58% 0.1 M ammonium formate buffer (pH = 4.5) at a flow rate of 4 mL/min and UV at 239 nm. [18F]WC-59 (~10 mCi) was collected at 18 min. The HPLC purified [18F]WC-59 was isolated from the HPLC solvent by dilution in water (200 mL), extraction in a C18 classic SepPak, and elution with ethanol (1-2 mL). The total synthesis and purification time was 100 min, the decay-corrected radiochemical yield was ~15%, the radiochemical purity was >99.9%, and the specific activity was 4,200 mCi/μmol at the end of the synthesis. This analysis was performed using an analytical HPLC column (Phenomenex Prodigy ODS3 column, 250 × 4.6 mm, 5μ) and comparing the integrated UV absorbance of the product with a calibrated mass/UV absorbance curve for WC-59. The identity of [18F]WC-59 was also confirmed by coeluting the radioactive compound with nonradioactive WC-59 on the analytical HPLC system. After purification, [18F]WC-59 was prepared in 10% ethanol and 90% water for injection into animals.
5.3. Biodistribution of [18F]WC-59
All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals established by Washington University’s Animal Studies Committee. Female BALB/c mice were implanted subcutaneously in the scapular region with ~5 × 105 EMT-6 cells in 100 μL PBS 11 days prior to the study. At the time of the biodistribution study tumors were ~100-150 mg. [18F]WC-59 (~20 μCi per 150 μL) was injected via the tail vein, and the mice were sacrificed at 5 min, 30 min, 1h and 2 h post-injection. Blood was collected then tumor and non-target tissues were removed, weighed, and the radioactivity was measured in a Beckman 8000 automated gamma well counter with a diluted standard of the injected dose. The data were analyzed as the percent injected dose per gram of tissue (%ID/g).
5.4. Colony Formation Assay
EMT-6 or MDA-MB435 cells were seeded at 6,000 cells per well in 96-well plates. After incubating for 24 h, the attached cells were treated for 16 h with WC-26 alone. After another 16 h, various concentrations of DOX were added to the wells, and the cells were incubated for an additional 24 h. Upon completion of treatment with drugs or vehicle controls, the cells were removed from the wells, counted, diluted, and 300 single cells were replated in 60 mm petri dishes for colony formation. After incubating the dishes for 14 days at 37 °C in a humidified CO2 incubator, the colonies were fixed with methanol-acetic acid (3:1) and stained with 0.25% crystal violet. Colonies containing >25 cells were scored, and the percent survival calculated by comparison to untreated controls. The data are shown as the mean ± 1 SD of triplicate samples from a representative experiment.
Acknowledgments
This research was funded by NIH grant CA 102869. The authors gratefully thank Susan Adams, Bill Margenau and Pat Margenau for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker JM, Bowen WD, Walker FO, Matsumoto RR, de Costa BR, Rice KC. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- 2.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Eur J Pharmacol Mol Pharmacol Sect. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 3.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Proc Natl Acad, Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- 5.Seth P, Leibach FH, Ganapathy V. Biochem Biophys Res Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- 6.Bem WT, Thomas GE, Mamone JY, Homan SM, Levy BK, Johnson FE, Coscia C. J Cancer Res. 1991;55:6558–6562. [PubMed] [Google Scholar]
- 7.Vilner BJ, John CS, Bowen WD. Cancer Res. 1995;55:408–413. [PubMed] [Google Scholar]
- 8.Mach RH, Smith CR, al-Nabulsi I, Whirrett BR, Childers SR, Wheeler KT. Cancer Res. 1997;57:156–161. [PubMed] [Google Scholar]
- 9.Wheeler KT, Wang LM, Wallen CA, Childers SR, Cline JM, Keng PC, Mach RH. British J Cancer. 2000;82(6):1223–1232. doi: 10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Nabulsi I, Mach RH, Wang LM, Wallen CA, Keng PC, Sten K, Childers SR, Wheeler KT. British J Cancer. 1999;81:925–933. doi: 10.1038/sj.bjc.6690789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu ZD, Dence CS, Ponde DE, Jones LA, Wheeler KT, Welch MJ, Mach RH. Nucl Med Biol. 2005;32(5):423–430. doi: 10.1016/j.nucmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Tu ZD, Xu JB, Jones LA, Li SH, Dumstorff C, Vangveravong S, Chen DL, Wheeler KT, Welch MJ, Mach RH. J Med, Chem. 2007;50:3194–3204. doi: 10.1021/jm0614883. [DOI] [PubMed] [Google Scholar]
- 13.Kassiou M, Dannals RF, Liu X, Wong DF, Ravert HT, Scheffel UA. Bioorg Med Chem. 2005;13(11):3623–3626. doi: 10.1016/j.bmc.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Crawford KW, Bowen WD. Cancer Res. 2002;62(1):313–322. [PubMed] [Google Scholar]
- 15.Ostenfeld MS, Fehrenbacher N, Hoyer-Hansen M, Thomsen C, Farkas T, Jäättelä M. Cancer Res. 2005;65(19):8975–8983. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- 16.Azzariti A, Colabufo NA, Berardi F, Porcelli L, Niso M, Simone GM, Perrone R, Paradiso A. Mol Cancer Ther. 2006;5(7):1807–1816. doi: 10.1158/1535-7163.MCT-05-0402. [DOI] [PubMed] [Google Scholar]
- 17.Costa BR, He X, Dominguez C, Cutts J, Williams W, Bowen WD. J Med Chem. 1994;37(2):314–21. doi: 10.1021/jm00028a016. [DOI] [PubMed] [Google Scholar]
- 18.Hang Y, Williams W, Bowen WD, Rice KC. J Med Chem. 1996;39:3564–3568. doi: 10.1021/jm9600813. [DOI] [PubMed] [Google Scholar]
- 19.Bowen WD, Bertha CM, Vilner BJ, Rice KC. Eur J Pharmacol. 1995;278(3):257–60. doi: 10.1016/0014-2999(95)00176-l. [DOI] [PubMed] [Google Scholar]
- 20.Maier CA, Wuensch B. J Med Chem. 2002;45:438–448. doi: 10.1021/jm010992z. [DOI] [PubMed] [Google Scholar]
- 21.Costantino L, Gandolfi F, Sorbi C, Franchini S, Prezzavento O, Vittorio F, Ronsisvalle G, Leonardi A, Poggesi E, Brasili L. J Med Chem. 2005;48:266–273. doi: 10.1021/jm049433t. [DOI] [PubMed] [Google Scholar]
- 22.Berardi F, Ferorelli S, Abate C, Colabufo NA, Contino M, Perrone R, Tortorella V. J Med Chem. 2004;47:2308–2317. doi: 10.1021/jm031026e. [DOI] [PubMed] [Google Scholar]
- 23.Bonhaus DW, Loury DN, Jakeman LB, To Z, DeSouza A, Eglen RM, Wong EHF. J Pharmacol Exp Ther. 1993;267:961–970. [PubMed] [Google Scholar]
- 24.Mach RH, Yang B, Wu L, Kuhner RJ, Whirrett BR, West T. Med Chem Res. 2001;10(6):339–355. [Google Scholar]
- 25.Mach RH, Vangveravong S, Huang Y, Yang B, Blair JB, Wu L. Med Chem Res. 2002;11(7):380–398. [Google Scholar]
- 26.Xu J, Tu Z, Jones LA, Vangveravong S, Wheeler KT, Mach RH. Eur J Pharmacol. 2005;525:8–17. doi: 10.1016/j.ejphar.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Kashiwagi H, McDunn JE, Simon PO, Jr, Goedegebuure PS, Xu J, Jones L, Chang K, Johnston F, Trinkaus K, Hotchkiss RS, Mach RH, Hawkins WG. Mol Cancer. 2007;6:48. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Bioorg Med Chem. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]