Abstract
Objective
Quantitative measures of pre-attentional, attentional and frontal lobe processes were compared to evaluate quantitative measures of these deficits in Ex-Preterm vs. Ex-Term adolescents.
Methods
We compared 43 Ex-Preterm with 26 Ex-Term adolescents using the P50 auditory potential, the Psychomotor Vigilance Task (PVT), a reaction time (RT) test, and Near Infrared Spectroscopy (NIRS).
Results
The mean amplitude (±SE) of the P50 amplitude was similar in the Ex-Preterm (1.8±1.4µv) vs. Ex-Term adolescents (1.8±0.6µv, df=68, F=0.05, p=0.8), but the Ex-Preterm group showed a trimodal distribution in amplitude (High, 3.3±0.4 µv,df=42.25, F=19.2, p<0.01; Medium, 1.7±0.1 µv, df=39, F=0.41, p=0.53; Low, 0.7±0.1 µv, df=40, F=49.5, p<0.01) suggested by statistically significant variance between populations (Kolmogorov-Kuiper test, df=42.25, F=5.4, p<0.01). Mean RT was longer in Ex-Preterm (250±8 msec) vs. Ex-Term subjects (200±5 ms, df=68, F=18.8, p<0.001). PVT lapses were increased in Ex-Preterm subjects, and varied inversely with P50 amplitude (Overall Mean 17±5 lapses, df=67, F=5.34, p<0.05; Low P50 amplitude, 25±10, df=40, F=8.8, p<0.01; Medium, 21±11, df=38, F=5.37, p<0.05; High, 6±2, df=39, F=6.78, p<0.01) vs. Ex-Term subjects (2±0.4 lapses, p<0.01). NIRS levels did not differ statistically, but tended to correlate with P50 amplitude in the Ex-Preterm group.
Conclusions
These findings suggest differential pre-attentional, attentional and frontal lobe dysfunction in ex-preterm adolescents.
Significance
These measures could provide a means to objectively assess differential dysregulation of arousal and attention in Ex-Preterm adolescents, allowing optimization of therapeutic designs.
Keywords: Attention, attention deficit hyperactive disorder, infant, premature, P50, near infrared spectroscopy
Introduction
Preterm delivery affects 8% of the total deliveries in the United States. Of the preterm deliveries, 1.5% (60,000) are Very Low Birth Weight (VLBW), weighing less than 1,500 gm. These neonates require prolonged intensive care and 51% of VLBW neonates suffer major disability later in life (Wood et al. 2000). Many of these Ex-Preterm children develop cognitive deficits and abnormal behaviors during childhood (Achenbach et al. 1993; Bhutta et al. 2002), often without ultrasonographic abnormalities (Dammann and Leviton 2004; Olsen et al. 1997). Newer imaging techniques have documented reduced cortical and subcortical gray matter volumes, white matter damage, and abnormal cerebellar development with concomitant increases in cerebrospinal fluid (Inder et al. 2005; Messerschmidt et al. 2005; Peterson et al. 2000). The regional brain volume abnormalities and cognitive deficits in Ex-Preterm children may be partially explained by an increased vulnerability to neuronal cell death in immature neurons, inflammation, and the effects of stress, such as repetitive neonatal pain, maternal separation during intensive care, increased auditory and visual stimulation, and sleep deprivation, all of which are developmentally unexpected (Anand and Scalzo 2000; Hagberg and Mallard 2005; Meaney et al. 1989; Perlman 2001; Porter et al. 1998; Simons et al. 2003). These noxious stimuli may affect programmed and excitotoxic cell death resulting in adverse neurological sequelae (Mitani et al. 1998; Pryce et al. 2002). Thus, it is not surprising that prematurely born children show a number of persistent developmental disturbances including pre-attentional (arousal and sleep-wake abnormalities) and attentional (reaction time and sensory gating) (Dupin et al. 2000; Fletcher et al. 1997; Lawson and Ruff 2004; O’Keefe et al. 2003) deficits. A recent report described selective loss of gamma-amino-butyric acid (GABA) neurons in Preterm children (Robinson et al. 2006), suggesting lowered inhibitory control systems may be responsible for some of the observed deficits.
These findings also suggest that multiple brain structures may be involved in generating the deficits observed. Moreover, some, but not all, Preterm neonates are affected by intraventricular hemorrhage, periventricular leukomalacia, and hypoxia/ischemia, all of which are associated with adverse neurological outcomes, while other Preterm neonates are spared those sequelae. However, virtually all Preterm neonates are affected by various forms of stress. In experimental animals, these stressors have led to changes in arousal and attention (Mason 2001). Alterations in arousal (pre-attentional processes) and attention in human subjects are associated with learning disabilities and cognitive difficulties. Some, but not all, patients suffer from attentional disturbances that may manifest as attention deficit and hyperactivity disorder (ADHD) (Bhutta et al. 2002). We sought to determine which measures of attention and cortical processing might be compromised in Ex-Preterm adolescents compared to Term adolescents, and if different sub-populations manifested differential deficits. We used the P50 midlatency auditory evoked potential, which has been used extensively to measure pre-attentional mechanisms and is mediated by brainstem-thalamus processes (Buchwald et al. 1991; Garcia-Rill and Skinner 2001). This waveform occurs from 45–75 milliseconds after an auditory stimulus and is usually paired with a second auditory stimulus. The decreasing amplitude is thought to represent the potential to “screen” continuous or irrelevant information; thus the amplitude can be used to assess the degree of habituation to repetitive auditory stimuli. The Psychomotor Vigilance Task (PVT) is a simple, not choice reaction time (RT) test to study attentional processes (Hazlett et al. 2001; Sturm and Willmes 2001). It consists of an auditory stimulus followed by the depression of a lever., Near Infrared Spectroscopy (NIRS) was used to measure oxygen extraction of frontal lobe blood flow. It is an indirect measure of frontal cortical processes (Hatakenaka et al 2007; Bortfield et al 2007). The overall goal was to determine if these measures could provide functional profiles indicative of differential deficits, which could then be treated more effectively. Preliminary results have been reported (Wallace et al. 2005).
Methods
Subjects
After approval by the local institutional review board, we enrolled and consented the parents/guardians of 43 Ex-Preterm adolescents who were born at less than 33 weeks gestational age and 26 Ex-Term born adolescents who were born at greater than 36 weeks gestation from local pediatric clinics and the adolescent clinic at Arkansas Children’s Hospital. All subjects were between 13 and 20 years of age inclusive without a history of head injury, unconsciousness or neurological sequelae. Subjects were excluded if they were unable to comply with the directions of the prescribed tests. A detailed history was obtained from the caregiver and subject including a description of the newborn course, subsequent surgical procedures, socioeconomic status including maternal education and income, and a description of possible cognitive or attentional problems that were encountered in school or at home, such as ADHD (Smith et al. 2003, 2004). A diagnosis of ADHD was made if there had been a specific diagnosis of ADHD in the past and/or if medication had been prescribed as ascertained by history, but there was no formal testing. For these measurements and analysis, all investigators were blinded to birth history.
P50 Potential Recording Procedures
Techniques for recording and analyzing the P50 potential have been published previously (Boop et al. 1993; Dornhoffer et al. 2003, 2006; Fann et al. 2005; Garcia-Rill and Skinner 2001; Rasco et al. 2000; Skinner et al. 1999; Teo et al. 1997, 1998). Subjects were seated on a recliner in a well lit, sound attenuating room easily observed by the experimenter. Gold-plated surface electrodes with a water-soluble conducting paste were used, and electrode resistance maintained at <5 Kohm. The P50 potential was recorded at the vertex (Cz) referenced to a frontal electrode (Fz), which yields a clearer waveform than reference to linked mastoids. Eye movements were detected using diagonally placed canthal electrodes, while jaw movements were detected using a lead over the masseter muscle referred to the chin. A subclavicular ground was also used. Each channel was led to a Grass Instruments 5P11 amplifier with a high resistance input stage. The gain and bandpass were as follows: P50 potential x100 K and 3 Hz-1 KHz; EOG x20 K and 3 Hz-1 KHz; and EMG x10 K and 30 Hz-10 KHz, with a 60 Hz notch filter on each amplifier. Fast Fourier Transform analysis has shown that the P50 potential is not degraded by the notch filter. The rack of instruments in the Electrophysiology Core Facility of the Center for Translational Neurosciences was approved for use on human subjects by the Biomedical Engineering Unit.
Prior to the recording, earphones were placed on each subject and the hearing threshold for each ear determined using a Grass Instruments Audiostimulator STM10. The test stimulus was a rarefaction click of 0.1 msec duration set at least 50 dB above threshold, usually 85–103 dB. Testing sessions of 4–5 min in duration consisted of paired click stimuli at 250, 500 or 1000 msec interstimulus intervals (ISIs). Pairs of clicks were delivered once every 5 sec until 64 pairs of evoked potentials were acquired, averaged and stored by the computer. Amplified signals were displayed on an oscilloscope for visual monitoring, digitized using a GW Instruments I/O module, averaged using Superscope software (GW Instruments) and stored on computer disk.
PVT Recording Procedures
The PVT is a test of behavioral alertness and involves a simple (not choice) RT test designed to evaluate the ability to sustain attention and respond in a timely manner to salient signals (Dinges and Powell 1985). It consists of responding to a 90 dB SPL tone of 1,000 Hz frequency by pressing a response button as soon as the stimulus appears, which stops the stimulus counter and displays the RT in milliseconds. The ISI varied from 2–10 sec, and the task duration typically lasted 10 min (which yielded 80 RTs per trial). The subject was instructed to press the button as soon as each stimulus appeared, in order to keep the RT as low as possible, but not to press the button too soon (which yielded a false start -FS warning on the display). Special software was used to extract multiple performance parameters from each PVT trial (Dinges and Kribbs 1991; Jewett et al. 1999), such as mean RT (overall attentional performance), fastest 10% of RTs (optimum response times), slowest 10% of RTs (worst response times), number of lapses (failure to respond in a timely manner), etc.
Near Infrared Spectroscopy (NIRS)
The clinical tissue oxygenation monitor, INVOS 1400, can measure changes in hemoglobin concentration and redox state of cytochrome oxidase, as well as the ratio of oxygenated to total tissue hemoglobin. In NIRS, the slope of light attenuation vs distance is measured at a distant point from the light input, from which the ratio is calculated using light diffusion theory. One light-emitting diode is used as the light source and harmless, low-intensity near-infrared light passes into the patient’s forehead. A high gain, low noise amplifier is used in the two detectors, enabling a large emitter-detector separation of 5 cm. The diode and detectors are contained in a non-reusable self-adhesive pad. Measurement of frontal lobe blood flow using NIRS parallel those made with a blood gas analyzer. These monitors are used clinically in Japan in rehabilitation/orthopedic settings, in neonatal intensive care units (continuous brain blood flow measured over the fontanelle), and emergency medicine applications. They are approved for research in the U.S. and are used, for example, to assess sensory and motor performance (Bortfeld et al. 2007; Hatakenaka et al. 2007; Otsuka et al. 2007). Sampling occurred every 10sec over a 5 min baseline period, then every 10sec during the 10 min PVT test and during recording of each P50 potential ISI. A mean value was obtained during each epoch for each subject.
Statistical methodology
All data was analyzed using Analysis of Variance (ANOVA) using GB-STAT 6.5.6. Data are expressed as the mean ± Standard Deviation (SD) or Standard Error (SE) and a probability of 0.05 was selected a priori as the level of significance. In the analysis of P50 potential amplitude, the distribution of amplitudes was different between the Ex-Preterm and Ex-Term groups, specifically in the multiple modality and variability (Kolmogorov-Kuiper test, p = 0.01). Based on our observed empirical histogram for our Ex-Preterm subjects (Fig. 1A), three distinct peaks emerged, suggesting ‘low,’ ‘medium,’ and ‘high,’ distribution groupings. We conjecture that the distribution of P50 potential amplitude for the Ex-Preterm group is a mixture of three groups. Statistical mixture models are available to give estimates of the mean and variance for several normal distributions that may have been combined to create one distribution. These methods rely on somewhat larger data sets and are not powerful enough for the data at hand. This being the case, we applied an ad hoc approach by calculating the 33rd (1.08) and 67th (2.32) percentiles of the data. These percentile values correspond to points that appear to ‘cut’ between the three peaks consistent with values in which the three peaks begin and end in the empirical distribution.
Figure 1.
Figure 1A. Frequency Histogram demonstrating 3 distinct subpopulations of ex-preterm adolescents. The amplitude of the P50 potential is on the x-axis, and the frequency of occurrence is shown on the Y-axis. The distribution of P50 potential amplitudes in Ex-Term adolescents (dark line) was unimodal, whereas the distribution of P50 potential amplitudes in Ex-Preterm adolescents appeared to have three peaks (gray line). A test of Variance revealed a significant difference between the two distributions (p<0.005). A Mann-Whitney-U test of the two distributions revealed that they were statistically different (P<0.01).
Figure 1B. Mean amplitude of the P50 Potential for all Ex-Term (open bar) and Ex-Preterm (filled bar) adolescents demonstrating no difference in amplitude when all Ex-Preterm subjects were pooled. The multimodal distribution of P50 amplitudes among Ex-Preterm subjects showed the presence of 3 distinct subpopulations differing by more than 1 SD. Comparisons between High (vertical stripes), Medium (horizontal stripes) and Low (diagonal stripes) amplitude subpopulations of Ex-Preterm adolescents showed significant differences between groups at **p<0.01
Figure 1C. Representative P50 potential averages in response to the first stimulus of a pair delivered at the arrows in three subjects, AP62 with High amplitude, AP72 with Medium amplitude and AP37 with Low amplitude P50 potentials. Calibration bars, vertical 1.5 uV, horizontal 25 msec for all three recordings.
Results
The baseline characteristics of the Ex-Preterm and Ex-Term adolescents are shown in Table 1A and Table 1B. There was no significant difference in age or socioeconomic status between the Ex-Preterm and Ex-Term adolescents. Mean birth weight (±SD) for the Ex-Term subjects, both male and female, was 3.3±0.4 kg, which was 47% higher than the Ex-Preterm subjects, both male and female (1.8±0.9 kg, ANOVA, df=68, F=96.1, p<0.01). Mean gestational age was significantly higher in the Ex-Term children (38.9±1.7 weeks) compared to the Ex-Preterm adolescents (30±3 weeks, ANOVA, df=68, F=151, p<0.01). The incidence of ADHD was significantly higher in the Ex-Preterm adolescents compared to the Ex-Term born adolescents (Fisher’s Exact Test, p=0.011) and is shown in Table 1B.
Table 1.
| Table 1A. Baseline Characteristics. All Values expressed as Mean (±SD) | ||||||
|---|---|---|---|---|---|---|
| ExPreterm Total |
ExPreterm Males |
ExPreterm Females |
ExTerm | ExTerm Males |
ExTerm Females |
|
| Number | 43 | 14 | 29 | 26 | 8 | 18 |
| Gestation (wks) | 30 (±3) | 31.5 (±2.2) | 29.2(±3.6) | 38.9(±1.7) | 38.9(±1.4) | 38.9 (±1.8) |
| Age (yrs) | 17.1 (±2.6) | 16.6 (±2.6) | 17.3 (±2.6) | 16 (±2) | 16.3 (±1.4) | 16.1 (±1.8) |
| Birthweight | 1.8 (±0.7) | 2.0 (±0.7) | 1.6 (±0.7) | 3.3 (±0.4) | 3.6 (±0.3) | 3.2 (±0.4) |
| Table 1B. Baseline Characteristics. | ||
|---|---|---|
| Term | Preterm | |
| Education | ||
| <12 years | 1 | 2 |
| High School Graduate | 13 | 18 |
| Some College | 12 | 13 |
| Economic status | ||
| <$25,000 | 16 | 25 |
| $25,000–$50,000 | 6 | 8 |
| >$50,000 | 4 | 5 |
| ADHD | ||
| Yes | 0 | 9 |
| No | 26 | 34 |
Pre-attentional processes
The mean (±SE) P50 potential amplitude for the Ex-Term subjects (1.8±0.6µv) was similar to that of the Ex-Preterm subjects (1.8±1.4µv). Because of the unusual distribution of the amplitudes of that group, the variances of the populations were tested. For P50 potential amplitude, the difference in variance between the Ex-Preterm and Ex-Term groups was large (Kolmogorov-Kuiper test, df=(42,25), F=5.44, p<0.01) suggesting that the Ex-Preterm and Ex-Term groups had different population distributions. A histogram of the Ex-Term and Ex-Preterm groups showed that the latter exhibited a trimodal distribution based on P50 potential amplitude, compared to the unimodal distribution of the Ex-Term subjects (see Figure 1A). When we calculated the 33rd (1.08) and 67th (2.32) percentiles of the mean P50 potential amplitudes (±SE) of the Ex-Preterm subjects, they segregated into High (3.3µv, n=14), Medium (1.7µv, n=14), and Low amplitude (0.7µv, n=15) peaks (See Figure 1B). Representative waveforms for High, Medium and Low amplitude P50 potentials are shown in Figure 1C. Interestingly, the degree of prematurity was not responsible for these differences with comparable gestational ages among the subjects in the High (30.1±3.8 weeks), Medium (30.2±2.5 weeks, ANOVA, df=27, F=0, p=1 compared to High) and Low (29.7±3.5 weeks, ANOVA, df=28, F=0.100, p=0.75 compared to High) amplitude peaks. Moreover, birth weight did not differ significantly across the subjects in different P50 potential amplitude peaks of the Ex-Preterm distribution (High, 1.7±0.7 kg, Medium, 1.8±0.9 kg, ANOVA, df=27, F=0.029, p=0.87 compared to High, and Low, 1.8±0.6 kg, ANOVA, df=28, F=0.80, p=0.80 compared to High. See the statistical methods section for justification).
Habituation to repetitive stimulation can be assessed using a paired stimulus paradigm. The use of different ISIs can generate a recovery curve showing decreasing habituation as ISI increases, and decreased habituation is indicative of a sensory gating deficit. As expected, habituation of the P50 potential measured as the amplitude of the second response as a percent of the first response was progressively lower (i.e. a higher percent) as the ISI increased. Habituation at the different ISIs is shown in Table 2. Habituation was not significantly different between the Ex-Term controls and the Ex-Preterm groups. There were no significant changes in the habituation to repetitive inputs in subjects in the High, Medium or Low P50 potential amplitude peaks of the Ex-Preterm distribution, indicating no major disturbances in habituation/sensory gating processes.
Table 2.
P50 potential habituation of the study population
| Ex-Term | Ex-Preterm (Total) |
Ex-Preterm (High P50) |
Ex-Preterm (Medium P50) |
Ex-Preterm (Low P50) |
|
|---|---|---|---|---|---|
| 250 ISI (%) | 23(±28) | 22(±29) | 20(±31) | 25(±27) | 42(±45) |
| 500 ISI (%) | 39(±32) | 46(±36) | 41(±24) | 34(±38) | 52(±46) |
| 1000 ISI (%) | 58(±32) | 67(±29) | 56(±36) | 73(±24) | 76(±68) |
Attentional processes
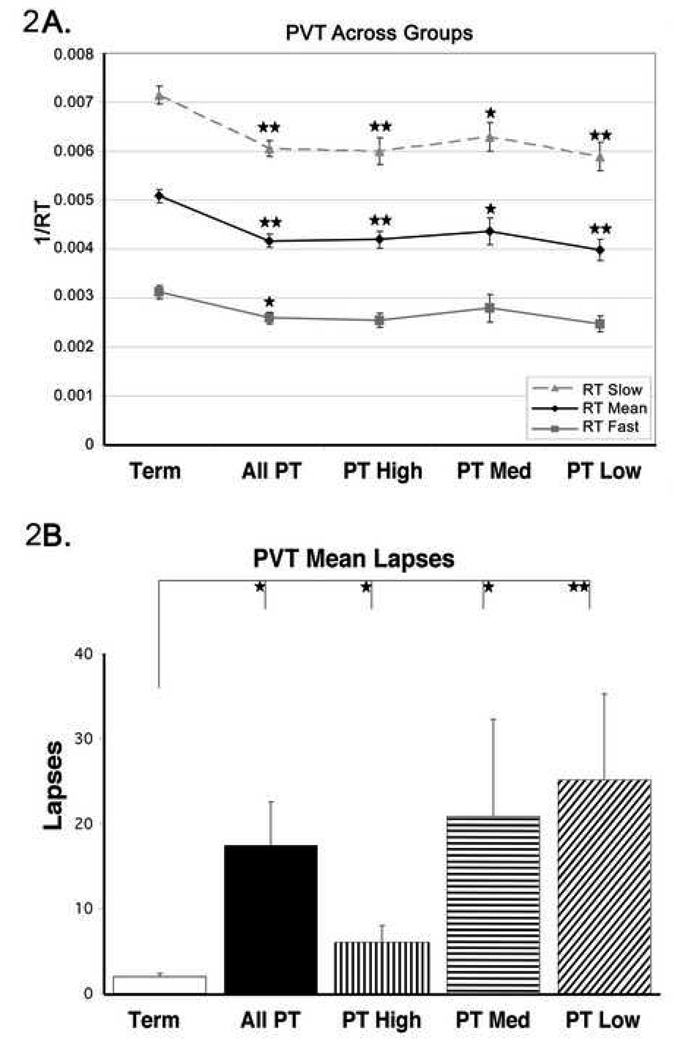
The mean RT (±SE) for the Ex-Preterm group (250±8.4 msec) was significantly slower (ANOVA, df=68, F=35.5, p<0.01) than that of the Ex-Term group (200±5.3 msec). When the Ex-Preterm group was divided in terms of the trimodal P50 potential amplitude distribution the mean RT was similar across Ex-Preterm peaks, which were all significantly slower than the mean RT in the Ex-Term subjects (High 245±12 msec, ANOVA, df=39, F=116.5, p<0.05; Medium 241±16.6 msec, ANOVA df=39, F=388.5, p<0.01; Low 263±15.1 msec, ANOVA, df=40, F=636, p<0.01), suggesting that P50 potential amplitude was unrelated to Mean RT (see Figure 2A). Because the mean RT performance can increase linearly as a function of increasing standard deviation, gross differences in variance could skew the results. Therefore, raw RT data was transformed into its reciprocal, 1/RT, to remove the proportionality and to maximize the likelihood of observing subtle shifts in responses (Dinges 1992). Figure 2A shows that 1/RT of the slowest response times (worst performance) was significantly different between Ex-Term subjects and all Ex-Preterm subjects (ANOVA, df=4, F=18.12, p<0.01), and between Ex-Term and those in P50 amplitude peaks (High, F=11.12, p<0.01; Medium, F=5.98, p<0.05; and Low, F=14.19, p<0.01). A similar difference was evident in 1/RT mean between Ex-Term and all Ex-Preterm subjects, and subjects in each P50 potential amplitude peak (ANOVA, df=4, All F=20.53, p<0.01; High F=10.98, p<0.01; Medium F=6.85, p<0.05; Low F=17.85, p<0.01). In terms of the fastest response times (optimal performance), only when all the Ex-Preterm subjects were compared to the Ex-Term group was there a significant difference (ANOVA, df=4, All F=8.16, p<0.05).
Figure 2.
Figure 2A. PVT results showing mean reaction time (RT) comparing all Ex-Term adolescents, Ex-Preterm adolescents, and subgroups of Ex-Preterm adolescents classified by their P50 potential mean amplitude. These data demonstrate a lack of relationship between the P50 potential (Pre-attentional processes) and mean reaction time (Attentional processes). **p<0.01
Figure 2B. Frequency of lapses comparing all Ex-Term adolescents and subgroups of Ex-Preterm adolescents classified by their P50 potential mean amplitude, demonstrating inverse relationship between the P50 potential amplitude (Pre-attentional processes) and PVT reaction time lapses (Attentional errors). Note that all Ex-Preterm sub-groups committed more lapses than Ex-Term subjects, which were related to their P50 potential amplitude. Ex-preterm subjects showed an increasing number of Lapses as P50 potential amplitude decreased. * p<0.05; **p<0.01
Frequency of lapses, or failure to respond in a timely manner, yielded the results shown in Figure 2B. The mean number of Lapses (±SE) in Ex-Term subjects was 2±0.4, which was significantly lower than in the Ex-Preterm group as a whole (17±5.1, ANOVA, df=68, F=9.49, p<0.01), who overall exhibited a higher lapse frequency. When the Ex-Preterm group was divided in terms of the trimodal P50 potential amplitude distribution, the number of Lapses was significantly different across peaks (High 6±2.0; Medium 21±11.4; Low 25±10.2). The mean number of Lapses of the subjects in the High amplitude peak showed significantly more Lapses than those in the Ex-Term group (ANOVA, df=39, F=55.6, p<0.05). Those in the Medium amplitude peak, which was similar to that of the Ex-Term group in most other measures, nevertheless committed a significantly higher number of Lapses than those in the Ex-Term group (ANOVA, df=39, F=31.2, p<0.05). The subjects in the Low amplitude peak committed the most Lapses and was significantly different from those in the Ex-Term group (ANOVA, df=40, F=42.6, p<0.01).
Weeks of gestation and birth weight
Figure 3A shows that the birth weight of the Ex-Preterm adolescents was significantly lower than that of Term adolescents, as described above. When subjects were divided according to P50 potential amplitude peaks, there was no difference between subjects in these peaks, so that P50 potential amplitude seems unrelated to birth weight, as long as it is significantly lower than term. Weeks gestation, which was lower in the Ex-Preterm population compared to the Term population, was also unrelated to P50 potential amplitude levels, since subjects falling within the three amplitude peaks had similar gestational ages.
Figure 3.
The trend of PVT decreases as P50 increases in the Ex-Preterm group. Linear regression methods for data are non standard because the variance of PVT is greatest for lesser P50 values. Two approaches suggested by Neter (Neter J, 1996) were employed to estimate the slope and its standard error. Both approaches suggest a strong relationship with slightly different levels of significance. The first, using the White (White H, 1980) robust consistent variance estimator for regression coefficients and the second a bias corrected bootstrap (Efron B, 1993).
The slope using the White estimator was borderline significant, (slope = −8.93, se = 4.66, p = 0.055; 95% CI(−18.07, 0.20), while the corresponding bias corrected bootstrap estimate was significant (slope = −8.74, se = 4.96, empirical p = 0.032, 95% CI (−21.22, −1.22).
Frontal lobe processes
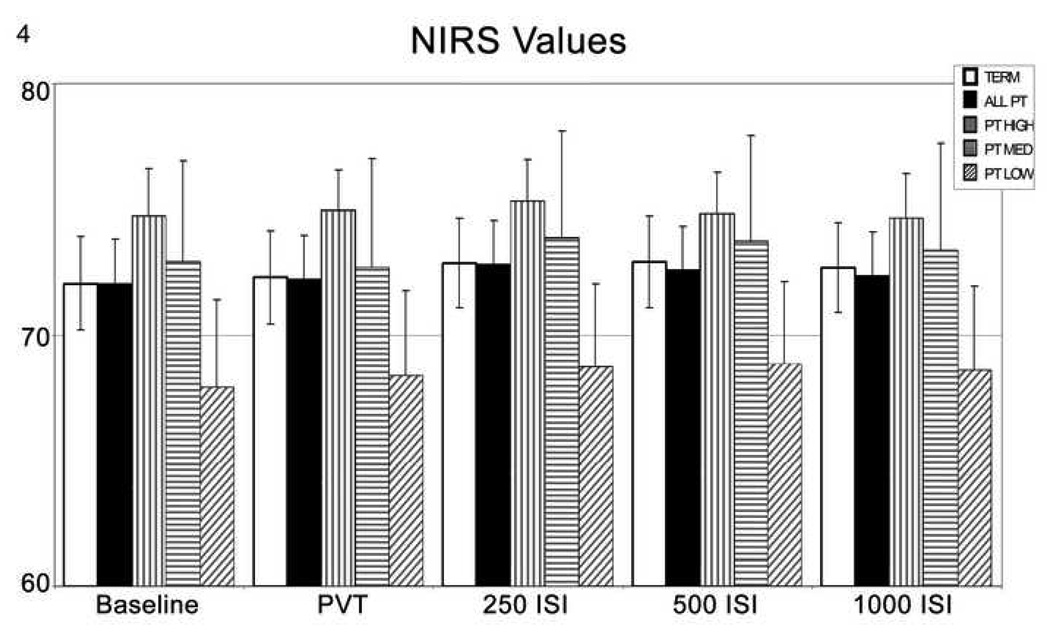
The results of NIRS recordings are shown in Table 3 and Figure 4. Briefly, the mean ±SE of the NIRS value over the 10 min Baseline (resting condition) recording was compared to those taken during performance of the PVT, and then during each of the randomly administered P50 potential recordings across the 250, 500 and 1,000msec ISIs. Because of the considerable variance, there was no difference between Ex-Term (n=22) and Ex-Preterm (n=25) populations (72±2 vs 72±2, ANOVA, p=0.34), or between the Ex-Term and subjects in each of the Ex-Preterm peaks according to P50 potential amplitude (High n=10, 75±2, Medium n=7, 73±4, Low n=8, 68±3) (ANOVA, p=0.62, 0.39 and 0.51, respectively). However, there was a numerical difference such that higher NIRS values were observed in Ex-Preterm subjects with High P50 potential amplitude and the lowest NIRS values in subjects with Low P50 potential amplitude. These trends were present during each of the recording epochs, whether during baseline, PVT, 250, 500 or 1,000 msec ISIs (Table 3).
Table 3.
NIRS Values. Shown as mean ±SD during baseline testing (no stimulus), during the PVT, and during different ISI’s
| Baseline | PVT | 250 ISI | 500 ISI | 1000 ISI | |
|---|---|---|---|---|---|
| High P50, n=10 | 74.7±5.9 | 75.0±5.2 | 73.9±11.3 | 74.9±11.4 | 74.7±5.1 |
| Medium P50, n=7 | 72.9±10.7 | 72.7±11.3 | 73.9±11.1 | 73.7±11.3 | 73.4±11.5 |
| Low P50, n=8 | 67.9±9.8 | 68.4±9.6 | 68.7±9.3 | 68.8±9.4 | 68.6±9.2 |
Figure 4.
The trend of NIRS increases as P50 increases in the Ex-Preterm group. Standard linear regression methods were appropriate for this data. A significant positive relationship was observed between NIRS and P50. (slope = 3.63, se = 1.54, p = 0.018), 95% CI (0.60, 6.66).
ADHD
The population of Ex-Term subjects did not include anyone with ADHD. In the Ex-Preterm subjects, there were 9/43 (21%) with ADHD, 2/14 (14%) males and 7/29 (24%) females. When the Ex-Preterm subjects were divided into subjects in High, Medium and Low P50 potential amplitude peaks, there were 3/14 (21%) ADHD subjects in the High amplitude peak of which 2/6, (33%) were males and 1/8 (13%) were females. There was 1/14 (7%) with ADHD in the Medium amplitude peak with 0/5 males and 1/9 (11%) females. There were 5/15 (33%) with ADHD in the Low amplitude peak with 0/3 males and 5/12 (42%) females. Females showed a somewhat higher prevalence of ADHD than males in all three peaks.
Discussion
We are the first to test Ex-Preterm with Ex-Term born controls using a battery of quantitative measures including the P50 potential, PVT and NIRS to compare pre-attentional, attentional and frontal lobe processes. A single recent effort reported decreased P50 potential amplitude in a small group of 13 preterm children at 5 years of age, but no other processes were investigated (Mikkola et al. 2007). Our results represent an important first step to a deeper understanding of the pre-attentional, attentional and cognitive problems commonly found in Ex-Preterm adolescents. We suggest these tests may represent an objective means of assessment for these deficits in the population of Ex-Preterm adolescents that is composed of a trimodal distribution, probably based on the degree of structural damage and responses to multiple stressors early in life. Briefly, we detected three peaks based on P50 potential amplitude, which showed differential performance on certain parameters of reaction time measured by the PVT, and suggested a trend towards differences in frontal lobe blood flow levels according to NIRS recordings. The potential next steps are discussed below.
The P50 potential
Early electroencephalographic (EEG) work based on topographical studies of human auditory evoked potentials concluded that auditory evoked potentials in the 20–60 msec latency range were generated in the posterior temporal lobe (Vaughan and Ritter 1970); however, other workers found the P50 potential still to be present after bilateral lesions of the temporal lobe (Woods et al. 1984). Depth recording electrodes passing vertically and medially to the primary auditory cortex on the superior temporal gyrus failed to find a reversal across the Sylvian fissure, as did electrodes passing along the long axis of the temporal lobe from the occipital cortex to the tip of the temporal lobe (Goff et al. 1980). A recent study on epilepsy patients with electrodes implanted in the hippocampus and parahippocampal gyrus found “no P50-like activity within the hippocampus in any of our subjects” (Grunwald et al. 2003). Using subdural electrodes for cortical recordings, these authors observed responses at ∼50 msec latency that habituated to repetitive stimulation in only 6/24 patients, and these were confined to prefrontal cortex and temporo-parietal regions near, but not in, the primary auditory cortex (Grunwald et al. 2003). A more recent magnetoencephalographic study concluded that the magnetic equivalent of the P50 potential, the M50, was not localized to the auditory cortex. That study showed distributed fronto-parietal foci indicative of a non-auditory, arousal-related, waveform (Garcia-Rill et al. 2007). The P50 potential is characterized by sleep state-dependence (present during waking and paradoxical sleep but not slow wave sleep, i.e. during states of cortical desynchronizaton) (Erwin and Buchwald 1986a; Kevanishvili and von Specht 1979), habituation at stimulation rates >2Hz (indicative of a non-primary pathway), and is blocked by the cholinergic antagonist scopolamine (suggesting this waveform is generated by the cholinergic arm of the reticular activating system (RAS)) (Erwin and Buchwald 1986a, b). Work on animal equivalents of the P50 potential (Garcia-Rill and Skinner 2002), lends further support to the probable involvement of brainstem-thalamus sources reflecting arousal or pre-attentional processes. Previous studies in narcoleptic patients (Boop et al. 1994) and recent studies on spatial neglect (Woods et al. 2006) and other conditions (Garcia-Rill and Skinner 2002) suggest that P50 potential amplitude reflects level of arousal. The present results showed that a trimodal distribution of Ex-Preterm adolescents manifested different peaks of P50 potential amplitudes, tentatively suggesting that subjects with Low amplitude may suffer from lowered arousal levels, while others were in the control or normal range indicative of no deficits in arousal or pre-attentional processes, and yet others showed High amplitude P50 potentials that may indicate increased arousal levels.
A key element in P50 potential manifestation is its habituation to repetitive stimulation. Many studies have used the P50 potential to study habituation, a component of the process of sensory gating, the ability to filter incoming information in order to attend to salient stimuli. Normal subjects show a markedly lower response to the second of closely spaced stimuli (Erwin and Buchwald 1986a, Boutros and Belger 1999); however, decreased habituation of the P50 potential has been reported in schizophrenia (Adler et al. 1982), autism (Buchwald et al. 1992), narcolepsy (Boop et al. 1994), post traumatic stress disorder (Skinner et al. 1999), Parkinson’s Disease (Teo et al. 1997, 1998), Huntington’s Disease (Uc et al. 2002) and depression (Garcia-Rill et al. 2002). We observed no decrement in habituation in the Ex-Preterm population as a whole compared to the Ex-Term population. However, when considering the trimodal distribution based on P50 potential amplitude, neither the Ex-Preterm population as a whole nor each of the peaks of their trimodal distribution showed statistically significant changes in habituation, although the Low P50 potential amplitude peak showed a trend towards significance. We would expect that the incidence of ADHD would decrease habituation of the P50 potential, but only a minority of the Ex-Preterm population exhibited ADHD (see below), and these were distributed evenly throughout the multimodal distribution of P50 potential amplitude values. That is, subjects expected to show decrements in P50 potential habituation, were a minority of the population in each P50 potential amplitude range, so that the mean habituation for the groups was not statistically different from each other. A larger sample may reveal if an effect is evident in habituation to repetitive inputs, especially in the low P50 amplitude population.
The PVT
In terms of attentional processes, Ex-Preterm adolescents showed increased mean RT compared to the Ex-Term controls, pointing to disturbances in sustained attention, regardless of P50 potential amplitude (Dinges and Kribbs 1991). These differences likely represent delayed thalamo-cortical processing. Our work agrees with the suggestion that thalamo-cortical mechanisms form the basis of anticipatory attention (Bastiaansen and Brunia 2001). Interestingly, when Ex-Preterm adolescents were analyzed according to the trimodal distribution of P50 potential amplitudes, those with High, Medium and Low amplitude P50 potentials committed increasing numbers of Lapses. Thus, depressed arousal, as suggested by decreased P50 potential amplitude (and perhaps a decrement in habituation to repetitive stimuli), may be associated with the greatest deficit in sustained attention, reflected in increased failure to respond in a timely manner. Subjects in this peak may suffer from more extensive thalamo-cortical dysregulation. The number of Lapses was lower in the Medium P50 amplitude group, and closest to normal, although still statistically increased, in the High P50 amplitude group.
NIRS
As far as frontal lobe blood flow is concerned, there were no statistically significant differences between peaks, although there was a consistent tendency to manifest increased blood flow in subjects with High amplitude P50 potentials, and decreased blood flow in those with Low amplitude P50 potentials. Additional studies are required to determine if, in a larger sample, such differences become significant and thus indicate a real decrement in flow. Until such data are available, the results described should be regarded with caution.
ADHD
The frequency of occurrence of ADHD in our Ex-Preterm population (21%) was generally higher than in the total population (6%), in keeping with previous results indicating higher frequency of ADHD in low birth weight children that demonstrate a relative risk of 2.62 for Ex-Preterm children (Bhutta et al. 2002). Our results are worrisome because abnormalities in arousal and attention are related to the development of clinical ADHD (Aleksandrov et al. 2005; Huang-Pollock et al. 2006; Koschack et al. 2003). Children with ADHD have numerous co-existing morbidities, including oppositional/defiant disorder in up to 75% (Barkley 1998; Connor et al. 2003), substance abuse (Fischer et al. 2003), antisocial behavior (Rasmussen and Gillberg 2000), cognitive, and academic problems because of their inability to “stay on task” (Fischer et al. 2003; Rasmussen and Gillberg 2000). Other areas of adolescent life such as driving are also adversely affected by ADHD, leading to an increased risk of accidents associated with injuries (Barkley 2004). Of particular concern is a 12 fold increase in dysthymia and sleep disorders, which are found in adults with arousal dysfunction (Corkum et al. 1999). In general, the incidence of ADHD did not seem related to P50 potential amplitude. Interestingly, our study sample had a higher representation of females with ADHD (24% in females and 14% in males), consistent with others who have found a similar male to female ratio for ADHD in Ex-Preterm children compared to male predominance of ADHD in the general population (Milich et al. 2001).
Clinical implications
We believe these data have important implications for Ex-Preterm adolescents. An objective test for arousal/pre-attentional and attentional problems would be an invaluable asset in the care of these young adults. Since these problems are well known to coexist with ADHD, the extreme morbidity and life style changes associated with ADHD could at least be anticipated by patients, teachers, families and caregivers. We also believe treatment of arousal disturbances could be beneficial and deserves further study. For example, the results described suggest that there is a trimodal distribution of Ex-Preterm adolescents such that the extremes of the P50 potential amplitude spectrum may require differential treatment, one for “hyperarousal”, the other for “hypoarousal” in combination with potential sensory gating deficits. Only electrophysiological screening may detect such differences since birth weight and weeks of gestation did not provide clues regarding potential future arousal levels.
Conclusion
These results suggest that, regardless of birth weight or weeks of gestation, premature birth induces differential and persistent deleterious effects on pre-attentional, attentional and cortical mechanisms in a significant portion of the population. P50 potential, PVT and NIRS could also be useful in monitoring therapeutic efficacy.
Acknowledgements
The authors wish to acknowledge the patients and families who gave generously of their time for this project, grant USPHS RR020146 and the Center for Translational Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest.
References
- Achenbach TM, Howell CT, Aoki MF, Rauh VA. Nine-year outcome of the Vermont intervention program for low birth weight infants. Pediatrics. 1993;91:45–55. [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neuropsychological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiat. 1982;17:639–654. [PubMed] [Google Scholar]
- Aleksandrov AA, Polyakova NV, Stankevich LN. Evoked brain potentials in adolescents in normal conditions and in attention deficit during solution of tasks requiring recognition of short-duration acoustic stimuli. Neurosci Behav Physiol. 2005;35:153–157. doi: 10.1007/s11055-005-0058-5. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biology of the Neonate. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- Barkley R. Attention Deficit Disorder: A Handbook for Diagnosis and Treatment. New York, NY: Guilford Press; 1998. [Google Scholar]
- Barkley RA. Driving impairments in teens and adults with attention-deficit/hyperactivity disorder. Psychiat Clin North Amer. 2004;27:233–260. doi: 10.1016/S0193-953X(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Brunia CH. Anticipatory attention: an event-related desynchronization approach. Int J Psychophysiol. 2001;43:91–107. doi: 10.1016/s0167-8760(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Boop FA, Garcia-Rill E, Dykman R, Skinner RD. The P1: insights into attention and arousal. Pediat Neurosurg. 1994;20:57–62. doi: 10.1159/000120765. [DOI] [PubMed] [Google Scholar]
- Bortfeld H, Wruck E, Boas DA. Assessing infants’ cortical response to speech using near-infrared spectroscopy. NeuroImage. 2007;34:407–415. doi: 10.1016/j.neuroimage.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Belger A. Midlatency evoked potentials attenuation and augmentation reflect different aspects of sensory gating. Biol Psychiat. 1999;45:917–922. doi: 10.1016/s0006-3223(98)00253-4. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Erwin R, Van Lancker D, Read s, Cummings JL. Midlatency auditory evoked responses: different abnormality of P1 in Alzheimer’s disease. Electroenceph clin Neurophysiol. 1989;74:378–384. doi: 10.1016/0168-5597(89)90005-1. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Erwin R, Van Lancker D, Guthrie D, Schwafel J, Tanguay P. Midlatency auditory evoked responses: P1 abnormalities in adult autistic subjects. Electroenceph clin Neurophysiol. 1992;84:164–171. doi: 10.1016/0168-5597(92)90021-3. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Rubinstein EH, Schwafel J, Strandburg J. Midlatency auditory evoked responses: Differential effects of a cholinergic agonist and antagonist. Electroenceph clin Neurophysiol. 1991;80:303–309. doi: 10.1016/0168-5597(91)90114-d. [DOI] [PubMed] [Google Scholar]
- Connor DF, Edwards G, Fletcher KE, Baird J, Barkley RA, Steingard RJ. Correlates of comorbid psychopathology in children with ADHD. J Amer Acad Child Adoles Psychiat. 2003;42:193–200. doi: 10.1097/00004583-200302000-00013. [DOI] [PubMed] [Google Scholar]
- Corkum P, Moldofsky H, Hogg-Johnson S, Humphries T, Tannock R. Sleep problems in children with attention-deficit/hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J Amer Acad Child Adolesc Psychiat. 1999;38:1285–1293. doi: 10.1097/00004583-199910000-00018. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Biomarker epidemiology of cerebral palsy. Ann Neurol. 2004;55:158–161. doi: 10.1002/ana.20014. [DOI] [PubMed] [Google Scholar]
- Dinges DF. Probing the limits of functional capability: the effects of sleep loss on short-duration tasks. In: Broughton RJ, Ogilvie RD, editors. Sleep, arousal and performance. Boston: Birkhauser; 1992. pp. 177–188. [Google Scholar]
- Dinges D, Powell Microcomouter analysis of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth. 1985;17:652–655. [Google Scholar]
- Dinges D, Kribbs . Performing while sleepy: effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley; 1991. pp. 97–128. [Google Scholar]
- Dornhoffer J, Mamiya N, Bray P, Skinner RD, Garcia-Rill E. Effects of rotation on the sleep state-dependent midlatency auditory evoked potential in the human. J Vestib Res. 2003;12:205–209. [PubMed] [Google Scholar]
- Dornhoffer J, Danner C, Mennemeier M, Blake D, Garcia-Rill E. Arousal and attention deficits in patients with tinnitus. Int Tinnitus J. 2006;12:9–16. [PubMed] [Google Scholar]
- Dupin R, Laurent JP, Stauder JE, Saliba E. Auditory attention processing in 5-year-old children born preterm: evidence from event-related potentials. Devel Med Child Neurol. 2000;42:476–480. doi: 10.1017/s0012162200000888. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: differential effects of sleep in the human. Electroenceph clin Neurophysiol. 1986a;65:383–392. doi: 10.1016/0168-5597(86)90017-1. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: differential recovery cycle characteristics. Effects of sleep in the human. Electroenceph clin Neurophysiol. 1986b;64:417–423. doi: 10.1016/0013-4694(86)90075-1. [DOI] [PubMed] [Google Scholar]
- Fann AV, Preston MA, Bray P, Mamiya M, Williams DK, Skinner RD. The P50 midlatency auditory evoked potential in patients with chronic low back pain (CLBP) Clin Neurophysiol. 2005;116:681–689. doi: 10.1016/j.clinph.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: self-reported psychiatric disorders, comorbidity, and the role of childhood conduct problems and teen CD. J Abnorm Child Psychol. 2002;20:463–475. doi: 10.1023/a:1019864813776. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Landry SH, Bohan TP, Davidson KC, Brookshire BL, Lachar D, et al. Effects of intraventricular hemorrhage and hydrocephalus on the long-term neurobehavioral development of preterm very-low-birthweight infants. Devel Med Child Neurol. 1997;39:596–606. doi: 10.1111/j.1469-8749.1997.tb07495.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The sleep state-dependent P50 midlatency auditory evoked potential. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley & Belfus, Inc; 2002. pp. 697–704. [Google Scholar]
- Garcia-Rill E, Skinner RD, Clothier J, Dornhoffer J, Uc E, Fann AV, et al. The sleep state-dependent midlatency auditory evoked P50 potential in various disorders. Thal Rel Syst. 2002;2:9–19. [Google Scholar]
- Garcia-Rill E, Moran K, Garcia J, Findley W, Walton K, Strotman B, et al. Magnetic sources of the M50 response are localized to frontal cortex. Clin. Neurophysiol. 2008;119:388–398. doi: 10.1016/j.clinph.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff WR, Williamson PD, Van Gilder JC, Allison T, Fisher TC. Neural origins of long latency evoked potentials recorded from the depth and from the cortical surface of the brain in man. Prog Clin Neurophysiol. 1980;7:126–145. [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, et al. Neuronal substrates of sensory gating within the human brain. Biol Psychiat. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005;18:117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- Hatakenaka M, Miyai I, Mihara M, Sakoda S, Kubota K. Frontal regions involved in learning of motor skill- a functional NIRS study. NeuroImage. 2007;34:109–116. doi: 10.1016/j.neuroimage.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Tang CY, Fleischman MD, Wei TC, Byne W. Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol Psychiat. 2001;504:281–291. doi: 10.1016/s0006-3223(01)01094-0. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT, Halperin JM. Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychol. 2006;20:420–429. doi: 10.1037/0894-4105.20.4.420. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Dijk DJ, Kronauer RE, Dinges DF. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. 1999;22:171–179. doi: 10.1093/sleep/22.2.171. [DOI] [PubMed] [Google Scholar]
- Koschack J, Kunert HJ, Derichs G, Weniger G, Irle E. Impaired and enhanced attentional function in children with attention deficit/hyperactivity disorder. Psychol Med. 2003;33:481–489. doi: 10.1017/s0033291702007067. [DOI] [PubMed] [Google Scholar]
- Lawson KR, Ruff HA. Early focused attention predicts outcome for children born prematurely. J Devel Behav Pediatrics. 2004;204:399–406. doi: 10.1097/00004703-200412000-00003. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Ann Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinol. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A, Brugger PC, Boltshauser E, Zoder G, Sterniste W, Birnbacher R, et al. Disruption of cerebellar development: potential complication of extreme prematurity. Amer J Neuroradiol. 2005;26:1659–1667. [PMC free article] [PubMed] [Google Scholar]
- Mikkola K, Kushnerenko E, Partanen E, Serenius-Sirve S, Leipala J, Houtilainen M, et al. Auditory event-related potentials and cognitive function of preterm children at five years of age. Clin Neurophysiol. 2007;118:1494–1502. doi: 10.1016/j.clinph.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentine A, Lynam D. ADHD combined type and inattentive type are distinct and unrelated disorders. Clin Psychol Sci Pract. 2001;8:463–488. [Google Scholar]
- Mitani A, Watanabe M, Kataoka K. Functional change of NMDA receptors related to enhancement of susceptibility to neurotoxicity in the developing pontine nucleus. J Neurosci. 1998;18:7941–7952. doi: 10.1523/JNEUROSCI.18-19-07941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. In: Applied Linear Statistical Models. 4th Edition. Richard D, editor. Burr Ridge, Illinois: Irwin, Inc.; 1996. [Google Scholar]
- O'Keeffe MJ, O'Callaghan M, Williams GM, Najman JM, Bor W. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Pediatrics. 2003;112:301–307. doi: 10.1542/peds.112.2.301. [DOI] [PubMed] [Google Scholar]
- Olsen P, Paakko E, Vainionpaa L, Pyhtinen J, Jarvelin MR. Magnetic resonance imaging of periventricular leukomalacia and its clinical correlation in children. Ann Neurol. 1997;41:754–761. doi: 10.1002/ana.410410611. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Nakato E, Kanazawa S, Yamaguchi MK, Watanabe S, Kakigi P. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. NeuroImage. 2007;34:399–406. doi: 10.1016/j.neuroimage.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Neurobehavioral deficits in premature graduates of intensive care-potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339–1348. doi: 10.1542/peds.108.6.1339. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Porter FL, Wolf CM, Miller JP. The effect of handling and immobilization on the response to acute pain in newborn infants. Pediatrics. 1998;102:1383–1389. doi: 10.1542/peds.102.6.1383. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Felton J. Early life stress: long-term physiological impact in rodents and primates. News Physiol Sci. 2002;17:150–155. doi: 10.1152/nips.01367.2001. [DOI] [PubMed] [Google Scholar]
- Rasco LM, Skinner RD, Garcia-Rill E. Effects of age on sensory gating of the sleep state-dependent P1/P50 midlatency auditory evoked potential. Sleep Res Online. 2000;3:97–105. HTTP://www.SRO.ORG/2000/Rasco/97/. [PubMed] [Google Scholar]
- Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Amer Acad Child Adolesc Psychiat. 2000;39:1424–1431. doi: 10.1097/00004583-200011000-00017. [DOI] [PubMed] [Google Scholar]
- Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatrics Adolesc Med. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Rasco LM, Fitzgerald J, Karson CN, Matthew M, Williams DK, et al. Reduced sensory gating of the P1 potential in rape victims and combat veterans with post traumatic stress disorder. Dep Anx. 1999;9:122–130. doi: 10.1002/(sici)1520-6394(1999)9:3<122::aid-da4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Aiding diagnosis of attention-deficit/hyperactivity disorder and its subtypes: discriminant function analysis of event-related potential data. J Child Psychol Psychiat Allied Disc. 2003;44:1067–1075. doi: 10.1111/1469-7610.00191. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Inhibitory processing during the Go/NoGo task: an ERP analysis of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2004;115:1320–1331. doi: 10.1016/j.clinph.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:S76–S84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco LM, Al-Mefty K, Skinner RD, Boop FA, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson's disease. Movement Dis. 1997;12:655–664. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco LM, Skinner RD, Garcia-Rill E. Disinhibition of the sleep-state dependent P1 potential in Parkinson's disease-improvement after pallidotomy. Sleep Res Online. 1998;1:62–70. HTTP://www.SRO.ORG/1998/Teo/62/. [PubMed] [Google Scholar]
- Uc E, Skinner RD, Rodnitzky L, Garcia-Rill E. The midlatency auditory evoked potential P50 is abnormal in Huntington’s Disease. J Neurol Sci. 2003;212:1–5. doi: 10.1016/s0022-510x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Vaughan HG, Ritter W. The sources of the auditory evoked responses recorded from the human scalp. Electroenceph clin Neurophysiol. 1970;28:360–367. doi: 10.1016/0013-4694(70)90228-2. [DOI] [PubMed] [Google Scholar]
- Wallace TG, Hall RW, Anand JKS, Garcia-Rill E. Preattentional and attentional dysregulation in ex-preterm adolescents. Neurosci Abst. 2005;31:512.6. [Google Scholar]
- White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. 2000:378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Mennemeier M, Garcia-Rill E, Meythaler J, Mark VW, Jewel GR, et al. Bias in magnitude estimation following left hemisphere injury. Neuropsychologia. 2006;44:1406–1412. doi: 10.1016/j.neuropsychologia.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DL, Knight RT, Neville HJ. Bitemporal lesions dissociate auditory evoked potentials and perception. Electroenceph clin Neurophysiol. 1984;57:208–220. doi: 10.1016/0013-4694(84)90122-6. [DOI] [PubMed] [Google Scholar]