Abstract
Background
Normal growth and development of organisms requires maintenance of a dynamic balance between systems that promote cell survival and those that induce apoptosis. The molecular mechanisms that regulate these processes remain poorly understood, and thus further in vivo study is required. Survivin is a member of the inhibitor of apoptosis protein (IAP) family, that uniquely also promotes mitosis and cell proliferation. Postnatally, survivin is hardly detected in most tissues, but is upregulated in all cancers, and as such, is a potential therapeutic target. Prenatally, survivin is also highly expressed in several tissues. Fully delineating the properties of survivin in vivo in mice has been confounded by early lethal phenotypes following survivin gene inactivation.
Results
To gain further insights into the properties of survivin, we used the zebrafish model. There are 2 zebrafish survivin genes (Birc5a and Birc5b) with overlapping expression patterns during early development, prominently in neural and vascular structures. Morpholino-induced depletion of Birc5a causes profound neuro-developmental, hematopoietic, cardiogenic, vasculogenic and angiogenic defects. Similar abnormalities, all less severe except for hematopoiesis, were evident with suppression of Birc5b. The phenotypes induced by morpholino knockdown of one survivin gene, were rescued by overexpression of the other, indicating that the Birc5 paralogs may compensate for each. The potent vascular endothelial growth factor (VEGF) also entirely rescues the phenotypes induced by depletion of either Birc5a and Birc5b, highlighting its multi-functional properties, as well as the power of the model in characterizing the activities of growth factors.
Conclusion
Overall, with the zebrafish model, we identify survivin as a key regulator of neurogenesis, vasculo-angiogenesis, hematopoiesis and cardiogenesis. These properties of survivin, which are consistent with those identified in mice, indicate that its functions are highly conserved across species, and point to the value of the zebrafish model in understanding the role of this IAP in the pathogenesis of human disease, and for exploring its potential as a therapeutic target.
Background
For normal homeostasis in multicellular organisms, there exists a delicate balance between cell proliferation and cell death, maintenance of which is required to prevent pathological outcomes including developmental abnormalities, cancer, autoimmune diseases, degenerative disorders and poor outcome following wounds and ischemic injury. The major physiologic means by which cell death is achieved in an organism is via apoptosis, a tightly regulated and highly conserved process. In spite of major gains in characterizing the apoptotic pathways in vitro, a better understanding of the precise cellular mechanisms of apoptosis in different tissues and developmental time points in vivo is still required.
The inhibitor of apoptosis proteins (IAPs) are a family of conserved caspase inhibitors originally identified in baculoviruses as proteins capable of preventing virus-mediated cell death in insect cells. Survivin is the smallest of the human inhibitor of apoptosis proteins (IAPs), and has several unique features (reviewed in [1]). It possesses one baculovirus IAP repeat (BIR) domain that is essential for caspase interference, and contains a C-terminal alpha-helical coiled-coil domain [2] that is important for mitosis. Survivin functions as a chromosome passenger protein, complexing with aurora B, borealin and INCENP [3]. Survivin also promotes cell cycle progression [4], and is highly expressed by proliferating cells, while being barely detectable in quiescent adult tissues [5]. Its uniformly elevated expression in essentially all cancers has rendered survivin a therapeutic target (reviewed in [6,7]), and thus a critical understanding of the properties of survivin is key for its successful introduction into the clinic.
In both humans and mice, there is a single survivin gene that generates several different protein products due to alternative pre-mRNA splicing (reviewed in [8]). Transgenic mouse studies revealed that the major, full-length form of survivin is crucial for normal leukocyte [9] and hepatocyte function [10], hematopoiesis [11], and optimal angiogenic response to injury [2,8,12]. Elucidating its developmental role has been limited by the fact that survivin gene inactivation in mice causes early embryonic lethality [3,10]. Nonetheless, conditional gene inactivation studies indicate that survivin is essential for brain development [13], angiogenesis and cardiogenesis [14]. In Xenopus laevis, there are 2 survivin genes, overexpression of one which induces endothelial proliferation [15], while augmented expression of the other had inexplicably lethal effects. Danio rerio (zebrafish) also have 2 survivin genes. Ma et al [16] reported that survivin is important in angiogenesis during zebrafish development, but these studies were limited, as the authors did not examine early developmental time points, they restricted their analyses to only one of the genes, and they did not evaluate the importance of survivin in other organ systems.
To gain further insights into the developmental role of survivin, we used the zebrafish model and detailed the spatio-temporal expression patterns of the 2 survivin genes and characterized their functions. Zebrafish survivin 1 (Birc5a) and survivin 2 (Birc5b) both prevent apoptosis and promote cell proliferation. While they have overlapping patterns of distribution and function, Birc5a predominates in most systems. Consistent with its role in the mouse, both zebrafish survivin genes play critical roles in regulating developmental vasculogenesis, angiogenesis, neurogenesis, cardiogenesis, valvulogenesis and hematopoiesis. The model highlights the conservation of survivin's functions across species, and points to the relevance of utilizing the zebrafish model to evaluate its mechanisms of action, findings that may be extrapolated to human disease.
Results
Orthologues of Survivin in zebrafish
A BLAST search for orthologues of human survivin in zebrafish revealed 2 survivin genes (Birc5a and Birc5b) located on chromosomes 12 and 23, respectively. Using the CLUSTAL W program, the putative predicted proteins were aligned with the orthologous proteins found in human, mouse, Xenopus laevis and Xenopus tropicalis (Additional file 1A). Human survivin is 142 amino acids long, comprised of a 15 amino acid N-terminal domain, a BIR domain (amino acids 16–87), and a C-terminal coiled-coil domain. The proteins encoded by Birc5a and Birc5b exhibit 45–55% overall similarity with those from other species and with each other (Additional file 1B). The greatest sequence conservation between Birc5a and Birc5b resides in the BIR domain (79% similarity). From computer analysis (COILS: http://www.ch.EMBnet.org/software/COILS_form.html), the C-terminal domains of the zebrafish survivin proteins do not form coiled-coil alpha-helical structures, a region that in murine survivin, interacts with the mitotic spindle.
Expression patterns of Birc5a and Birc5b
Ma et al [16] previously described expression of Birc5a in 26 hpf zebrafish embryos restricted to the developing brain, neural tube, and in cells surrounding the axial vessels. We performed detailed studies of the expression patterns of both Birc5a and Birc5b by in situ hybridization using gene-specific probes (Additional file 2). The 2 genes entirely overlap in their spatio-temporal patterns of expression. Since there are no prior reports on the expression of Birc5b in the zebrafish embryo, we therefore only provide images for Birc5b (Figure 1). Birc5a and Birc5b were detected as maternal messages throughout the embryo 1 hour post fertilization (hpf) (not shown). Similar to the report by Ma et al [16] for Birc5a, by 20–24 hpf (20–30 somites), expression of both genes was prominent in neural tissues, including the entire brain, neural tube, the floor plate, and the midbrain-hindbrain barrier (Figure 1A, B). In contrast to the report of Ma et al, at 1 dpf, we also found Birc5 expression in the ventral somites, at the somite boundaries, and in the caudal vein plexus, and in the eye (Figure 1B–E). Although evident from 20 hpf, at 2 dpf, there was more prominent expression of both Birc5 transcripts in the lens and retina of the eyes, in the major axial vessels, and in the branchial arch primordia (Figure 1F–I). Notably, by 2 dpf, expression in the somites was almost absent (Figure 1I). By 3 dpf, both Birc5 transcripts were detected in all the branchial arches, in brain, at the midbrain-hindbrain boundary, in the eyes, but was almost entirely absent in the axial vessels and somites (Figure 1J–L). Overall, the expression patterns of the two zebrafish survivin genes are indistinguishable during development, and are predominantly localized to neural, vascular and ocular structures, with transient expression in the somites and axial vessels during their formation.
Figure 1.
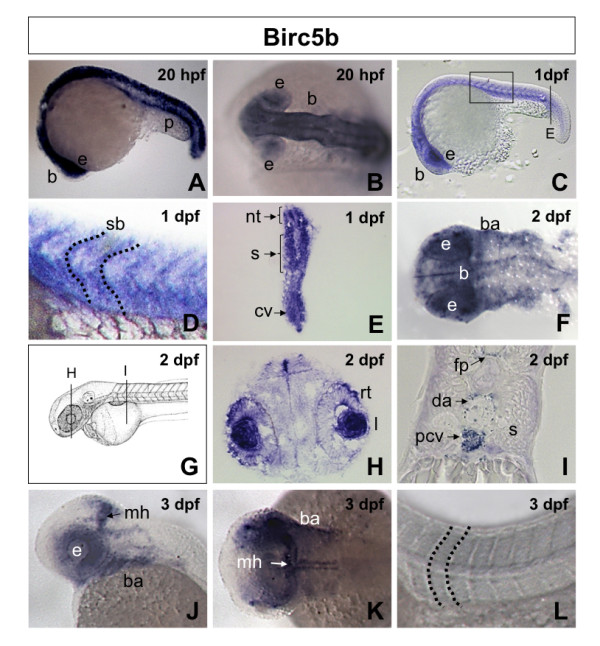
Birc5b spatiotemporal expression in zebrafish embryos. A, B. Lateral (A) and dorsal (B) views of 20 hpf (20 somites) embryos revealing expression of Birc5b in the neural tube, brain, pronephric duct and eyes. C. Lateral view 1 dpf (30 somites) embryo, showing Birc5b expression in brain, eye, neural tube, somite and intersomite boundaries, with higher magnification in D. E. Transverse section through 1 dpf embryo (from C), revealing expression of Birc5b in neural tube, somites and caudal vein plexus. F. Dorsal view of head region of 2 dpf embryo. Birc5a detected in brain, floor plate and branchial arches. G. Diagram of 2 dpf embryo with transverse sections in panels H and I. Transverse sections through 2 dpf embryo reveals expression in retina and iris (H), floor plate, dorsal aorta, posterior cardinal vein; not in somite (I). J-L. Lateral (J) and dorsal (K, L) views of 3 dpf embryo; expression of Birc5b at midbrain-hindbrain barrier, branchial arches and eyes; not in region of axial vessels, somites or intersomite boundaries (L). nt: neural tube, p: pronephric duct, b: brain, e: eye, rt: retina, I: iris, mh: midbrain-hindbrain barrier, fp: floor plate, ba: branchial arches, s: somite, sb: intersomite boundary, da: dorsal aorta, pcv: posterior cardinal vein, cv: caudal vein plexus.
Zebrafish Birc5 morpholino knockdowns
To assess the functions of Birc5a and Birc5b, we used morpholinos to knock down each of the genes. Morpholinos were selected to target sites within each orthologue with the most sequence divergence, including the start codon (ATG morpholinos). Specificity of ATG morpholinos was confirmed by also using morpholinos that target pre-mRNA splice sites or 5' UTR regions (Additional file 3). Controls were performed by injecting a mismatch morpholino (GeneTools, Philomath, Oregon, USA). Each morpholino dose was tested on at least 100 embryos. All morpholinos (ATG, splice site and UTR) for one gene gave identical phenotypes; the results reported reflect those with ATG-morpholinos. We additionally confirmed gene-specificity by in vitro transcription-translation studies. Even at high doses, Birc5a morpholinos had no effect on Birc5b expression, and similarly, Birc5b morpholinos did not affect Birc5a expression (Additional file 4[17,18]). Finally, in all studies, we excluded p53-mediated off-target effects on apoptosis and the observed survivin morpholino-induced phenotypes, by co-injecting p53 morpholinos, as described [19] (data not shown).
Birc5 in neural development
Birc5a and Birc5b morpholino-injected wild-type AB embryos were assessed by brightfield microscopy (Figure 2). After injection of 0.5 ng Birc5a morpholino, morphant embryos at 1 dpf (Fig 2A, B) and 2 dpf (Fig 2D, E) displayed microcephaly (42%, n = 162 at 1 dpf; 57%, n = 157 at 2 dpf) with fluid accumulation in the 4th ventricle (Fig 2E). The effects of Birc5b depletion on brain development using the highest morpholino dose were less dramatic than with the Birc5a knockdown, with 10–15% of Birc5b morphants exhibiting microcephaly at 1 dpf (n = 143) and 2 dpf (n = 105) (Figure 2C, F).
Figure 2.
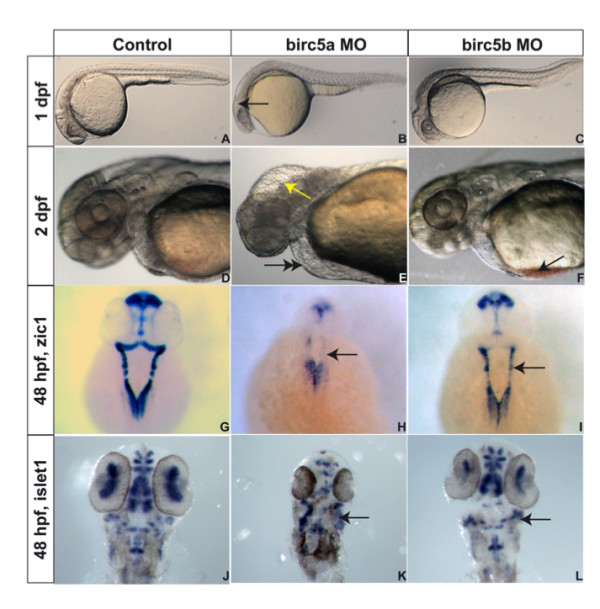
Birc5 in neurodevelopment. Brightfield microscopy of AB zebrafish embryos. A-F: Embryos oriented with head to left. Lateral views at 1 dpf (A, B, C) and 2 dpf (D, E, F), the latter being higher power views of head region. Depletion of Birc5a results in lack of brain development, revealed at 1 dpf (B, arrow) and 2 dpf (E, arrow), with fluid in 4th ventricle, compared to controls (A, D). At 2 dpf, Birc5a knockdown causes cardiogenic defects and pericardial edema (E, double arrow), not observed in controls (D). Majority of Birc5b depleted embryos do not exhibit phenotypic abnormalities under brightfield microscopy at 1 dpf (C) compared to controls (A). At 2 dpf, Birc5b knockdown embryos have smaller head and brain, and accumulate blood in the sinus venosus (F, arrow). Zic1 expression to detect neural tissue, is decreased by Birc5a depletion (H, arrow), compared to controls (G). A similar but less dramatic diminution of Zic1 expression is observed with morpholino knockdown of Birc5b (I, arrow). Depletion of Birc5a also induces disorganization of motor neurons, detected by expression of islet1 (K, arrow), compared to controls (J). Birc5b morphants exhibit less severe but still evident, suppression of islet1 expression (L, arrow).
Disturbances in neuronal development were, however, more readily detectable in both Birc5a and Birc5b mutants by in situ hybridization with probes to detect Zic1, a pan-neural marker [20] (Figure 2G–I), and Islet1, a marker of primary motor neurons [21] (Figure 2J–L). Zic1 staining of the brain was decreased in the 2 dpf Birc5a morphants as compared to controls, reflecting the almost total absence of neural cells. The reduction was also evident in the Birc5b morphants, to a lesser extent when compared to the Birc5a morphant. Similarly, islet1 staining of the Birc5a morphants revealed disorganized or absent motor neurons, while the Birc5b morphants were also affected, but again, less severely.
Overall, both Birc5 genes are critical for normal neural development, with Birc5a predominating.
Birc5 in vasculogenesis and angiogenesis
Recently, Ma et al [16] reported that Birc5a knockdowns primarily induce angiogenic abnormalities, but without affecting vasculogenesis. We extended on their work by examining vasculo-angiogenesis in Birc5a and Birc5b knockdown embryos at different developmental time points, using Tg(Fli:eGFP) or Tg(Flk1:GFP) embryos (Zebrafish International Resource Center [22]) which express green fluorescent protein (GFP) in endothelial cells (Figure 3).
Figure 3.
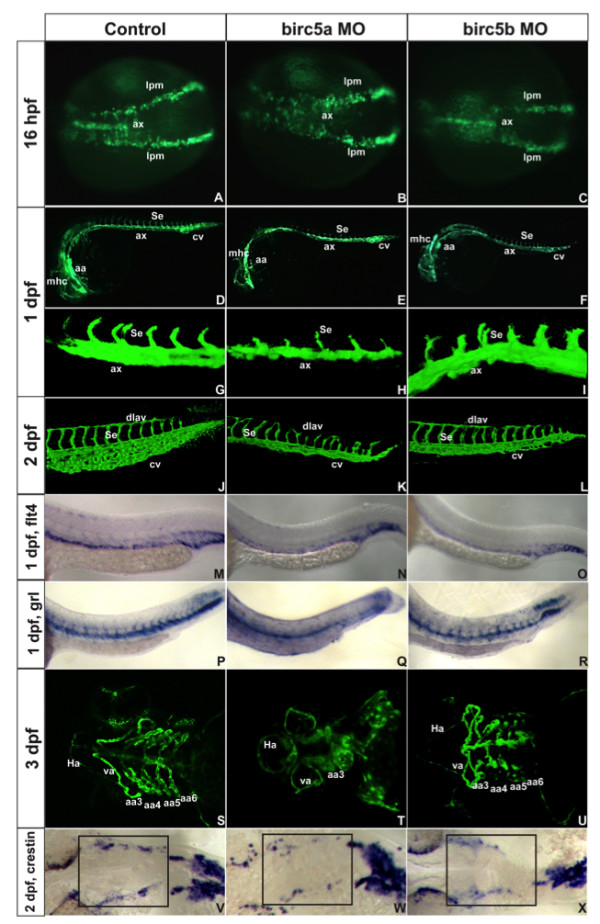
Birc5 in vasculogenesis and angiogenesis. Tg(Fli:eGFP) (A-L) and Tg(Flk1:GFP) (S-U) embryos. A-C: 16 hpf (14 somites), Birc5a morphants with angioblast migration defects from lateral plate mesoderm (B) which are minor in Birc5b morphants (C). D-F: 1 dpf, Birc5a (E, H) and Birc5b (F, I) morphants have thinner axial vessels and poor caudal vein plexus development. G-I: 1 dpf Birc5a depletion (H) delays intersomitic vessels; not with Birc5b depletion (I). J-L: 2dpf Birc5a morphants with abnormal dorsal longitudinal anastomic and intersomitic vessels. Both morphants have poorly developed caudal vein plexus. M-O: flt4 at 1 dpf is reduced in posterior cardinal vein in both morphants (N, O). P-R: gridlock (grl) at 1 dpf is reduced with Birc5a depletion (Q), but not with Birc5b (R). S-U: 3 dpf Birc5a morphants have hypoplastic aortic arches (T). Birc5b depletion at 3 dpf causes hypoplasia of aortic arches 5–6 (U). V-X: Birc5a depletion decreases neural crest cells that migrate to branchial arches, detected with crestin probe. Birc5b depletion (L) reduces neural crest cells. ax: axial vessels, mhc: midbrain-hindbrain channel, Se: intersomitic vessels, aa: aortic arch, cv: caudal vein plexus, pcv: posterior cardinal vein, da: dorsal aorta, dlav, dorsal longitudinal anastomic vessel, Ha: hypobranchial artery, va: ventral aorta, lpm: lateral plate mesoderm.
Precursor angioblasts in zebrafish arise at 12 hpf (6 somites) in the lateral plate mesoderm and migrate from 14 hpf (10 somites) toward the midline, where they coalesce to form primary axial vessels [22]. We first established that depletion of Birc5a or Birc5b does not induce a defect in mesodermal development, by determining that expression of the mesodermal marker, no tail (ntl) [23] in both morphants at 6 hpf, as compared to control zebrafish embryos, was not different (not shown).
We then studied the effect of depleting Birc5a and Birc5b with the highest morpholino doses (2 ng and 4 ng, respectively) on vasculogenesis and angiogenesis. At 16 hpf (14 somites), Birc5a morphant angioblasts migrated in a disorganized fashion, at different rates, with some remaining at the lateral plate mesoderm (Figure 3A, B). Angioblasts in Birc5b morphants migrated normally to the midline at 16 hpf (Figure 3C), but there was reduced signal intensity and thickness of the coalescing axial vessels (22% of embryos, n = 147). With depletion of Birc5a, the dorsal aorta and posterior cardinal vein at 1 dpf and 2 dpf were thinner (45–52%), with a smaller caudal vein plexus (45–52%) (Figure 3D, E, G, H, J, K). A similar effect, although not as prominent and not involving the posterior cardinal vein, was also evident in Birc5b morphants (Figure 3F, I, L). The findings were better visualized following in situ hybridization of 1 dpf embryos with probes flt4 and gridlock (grl) that detect the posterior cardinal vein and the dorsal aorta/intersomitic vessels, respectively [24] (Figure 3M–R). The Birc5a morphants also exhibited delayed sprouting of intersomitic vessels (ISVs), which were occasionally absent, but otherwise were often thin, misdirected, lacking connections, and associated with interruption of the dorsal longitudinal anastomotic vessels. These findings, in concert with the fact that Birc5 expression was transiently detected at the somite boundaries, suggests a role for survivin in ISV patterning and vessel guidance (reviewed in [25]). Interestingly and in contrast, formation of the intersomitic vessels and dorsal longitudinal anastomotic vessels remained intact in the Birc5b morphants.
By 3 dpf, there was underdevelopment and irregular patterning of cranial blood vessels of both the Birc5a and Birc5b morphants (see Additional Files 5, 6, 7) as compared with controls. Only 1 of the branchial arches was seen in the majority of the Birc5a morphants (61%) (Figure 3S, T), while the 5th and 6th branchial arches in the Birc5b morphants were either absent or hypoplastic (65%) (n = 160) (Figure 3U). In attempting to explain the branchial arch defects, we performed in situ hybridization studies at 2 dpf with crestin, a post migratory neural crest cell marker [26]. In both the Birc5a and the Birc5b morphants, but more prominent with depletion of Birc5a, there were fewer crestin-positive neural crest cells in the region of the neural crest, corresponding to that which is critical for development of the branchial arches [27] (Figure 3V–X).
In summary, both Birc5 genes are important for normal vasculogenesis, angiogenesis, and vascular patterning. Although the 2 genes functionally overlap, again, Birc5a appears to play a more prominent role.
Birc5 in hematopoiesis
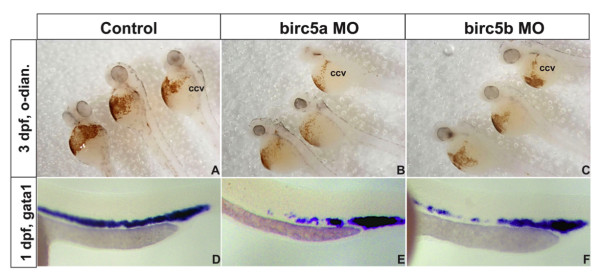
Since, in the zebrafish, at least a subset of hematopoietic and endothelial lineages arise from a common hemangioblast [28], we also examined Birc5a and Birc5b morphants for defective hematopoiesis. Erythrocytes in 3 dpf embryos were identified by staining for hemoglobin with o-dianisidine. Depletion of Birc5a or Birc5b resulted in a reduced number of erythrocytes in the ducts of Cuvier (Figure 4A–C). Moreover, gata1 expression, reflecting hematopoiesis at 1 dpf, was reduced in 35% of Birc5a morphants (n = 112), and 28% of Birc5b morphants (n = 115), as compared to controls (n = 70) (Figure 4D–F). Since defects in circulation may impact on the preceding findings, we examined hematopoiesis at 14 hpf, prior to development of a functional circulation. Flow cytometry of cellular suspensions of dechorianated Tg(gata1:GFP) embryos was used to quantify the number of Gata1+ cells relative to the total number of cells [29]. Depletion of either Birc5a or Birc5b, as compared to control injections, resulted in a decrease in the absolute number of Gata1+ cells from 3.37% for controls to 0.72% for Birc5a, and to 0.42% for Birc5b. In line with these findings, expression of hematopoietic genes gata1, scl and Imo2 [29], quantified by real-time PCR at 14 hpf and 18 hpf in AB zebrafish embryos, was significantly reduced (p < 0.01) by depletion of either Birc5a or Birc5b. Overall, the data support a role for both Birc5 genes in promoting hematopoiesis in the developing zebrafish embryo.
Figure 4.
Birc5 in hematopoiesis. A-C: Depletion of Birc5a (B) or Birc5b (D) causes a reduction in erythropoiesis, shown by staining of erthrocytes with o-dianisidine (control, A). D-F: The preceding is consistent with decreased expression of gata1 by in situ hybridization in both gene knockdowns (E, F) as compared to control (D). ccv: common cardinal vein or duct of Cuvier, o-dian.: o-dianisidine.
Birc5 in cardiogenesis
Depletion of either Birc5a or Birc5b resulted in cardiovascular defects. At the highest morpholino dose, one third of Birc5a morphants displayed pericardial edema, while blood flow in the dorsal aorta and posterior cardinal vein was slowed or absent in at least half the embryos. The heart rate in both Birc5a and Birc5b morphants was significantly reduced (60 ± 8, 56 ± 8, and 86 ± 7 beats/minute in Birc5a morphants, Birc5b morphants, and controls, respectively; p < 0.001 versus controls). In situ hybridization at 30 hpf with the ventricle and cardiac-specific markers, ventricle myosin heavy chain (vmhc) and cardiac myosin light chain (cmlc2) [30], respectively, demonstrated that Birc5a morphant ventricles and hearts were smaller than controls (41%, n = 24 for vmhc; 42%, n = 76 for cmlc2) (Figure 5A, B, D, E). Although not as striking, Birc5b morphants also exhibited smaller ventricles and hearts at 30 hpf (Figure 5C, F) (26%, n = 31 for vmhc; 32%, n = 89 for cmlc2). At 48 hpf when atrio-ventricular (a-v) valve formation is underway, bone morphogenic protein 4 (bmp4) [31] transcripts progressively localize from the atrium and ventricle to myocardial cells at the valve-forming region [32]. With Birc5a depletion, redistribution of bmp4 did not occur in 38% (n = 62) of embryos (Figure 5G, H), predicting defects in valvulogenesis. This was evident in hematoxylin and eosin (H&E) stained sections of 4 dpf embryos, where there was little evidence of an a-v valve in the Birc5a morphants, at a time when the myocardial layer of the morphant ventricle was also thinner, as compared to control embryos (Figure 5J, K). In the Birc5b morphants, bmp4 became localized to the a-v valve, similar to controls, although the opening was smaller in 21% (n = 44) (Figure 5I). At 4 dpf, H&E stained sections also revealed smaller cardiac chambers (20%) (Figure 5L).
Figure 5.
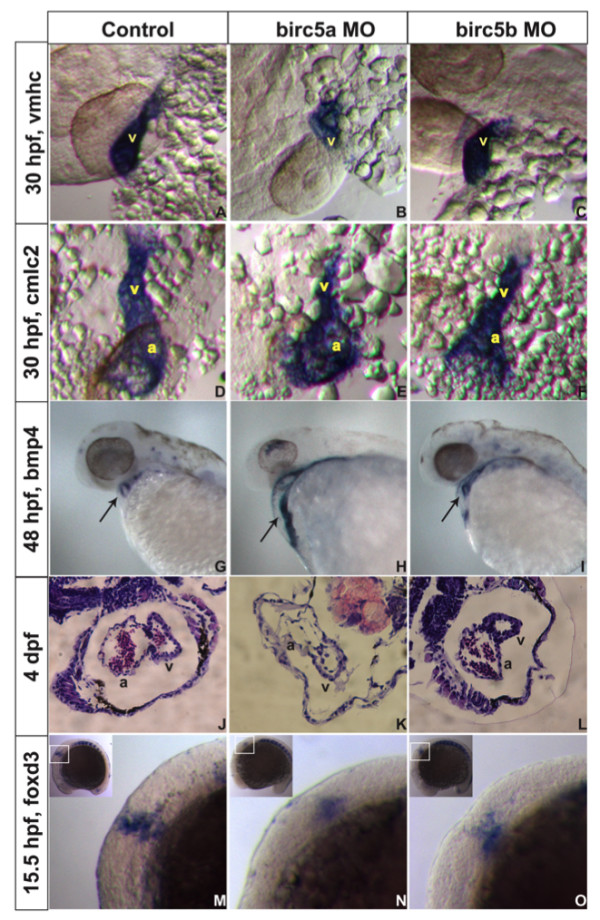
Birc5 in cardiogenesis. In situ hybridizations (A-I, M-O) and histologic sections (J-L) on AB embryos. A-C: At 30 hpf, expression of cardiac ventricle marker vmhc is reduced with Birc5a depletion (A, B), and to lesser extent in Birc5b morphants (C). D-F: cmlc2 staining shows Birc5a (E) or Birc5b (F) morphants with impaired development of atrium and ventricle, compared to controls (D). G-I: At 48 hpf, bmp4 normally localizes in heart to reveal a ring-like structure, representing endocardial cushions of the atrio-ventricular valve (G, arrow). With depletion of Birc5a, bmp4 staining remains diffuse and ring structure is absent (H, arrow). In Birc5b-morphants, bmp4 localizes normally (I, arrow), but the ring is smaller. J-L: H&E stained histologic sections of hearts of normal embryos (J), and those depleted of Birc5a (K) and Birc5b (L) at 4 dpf. Birc5a morphants have thin-walled heart chambers, and little evidence of a-v valve formation. Birc5b-depleted embryos have smaller ventricles. M-O: Premigratory cardiac neural crest cells contributing to heart development, were detected by staining embryos at 15.5 hpf (13 somites) with foxd3. Compared to controls (M), premigratory neural crest cells were barely detectable in embryos depleted of Birc5a (N), and reduced in Birc5b morphants (O). a:atrium, v:ventricle.
Although diminished vascular perfusion may contribute to cardiogenic defects [33], we hypothesized that an additional factor in the Birc5 morphants might be altered formation and/or migration of cardiac neural crest cells, a site of origin of cardiomyocytes in zebrafish [34]. Embryos at 15.5 hpf (13 somites) were therefore hybridized with the foxd3 probe to stain premigratory neural crest cells [35]. Birc5a depletion caused cardiac neural crest cell loss along the rostrocaudal axis (Figure 5M, N), particularly in the region critical for formation of the ventricle, a-v junction, and atrium (region referred to as "Group B" by Sato et al [36]). Birc5b depletion also resulted in loss of cardiac neural crest cells – again, not as dramatically as with Birc5a depletion (Figure 5O). Overall, the findings indicate that Birc5a- and Birc5b-dependent signals are important in maintaining the integrity of cardiac neural crest cells that in turn, contribute to normal cardiogenesis and valvulogenesis.
Birc5 knockdowns result in increased apoptosis and decreased cell proliferation
Ma et al [16] reported that depletion of Birc5a caused increased apoptosis primarily in the neural tube and brain. Given the diverse pro-survival properties of survivin, we assessed the mechanisms of action of each zebrafish survivin gene in the neural and vascular systems.
TUNEL staining of normal Tg(Fli:eGFP) embryos at 1 dpf (Figure 6, left column) and 2 dpf (not shown) revealed minimal evidence of apoptosis. In contrast, at a morpholino dose of 2 ng, Birc5a morphants exhibited significant apoptosis, mostly in the brain and spinal cord (Figure 6A, B, D, E), with lesser amounts in the region of the axial vessels and caudal vein plexus (Figure 6E, H). TUNEL staining in the Birc5b morphants at 1 dpf was, by comparison, less in the neural tissues, but more prominent in the region of the caudal vein plexus and axial vessels (Figure 6C, F). With both gene knockdowns, apoptosis remained spatially unchanged, but was increased at 2 dpf (not shown).
Figure 6.
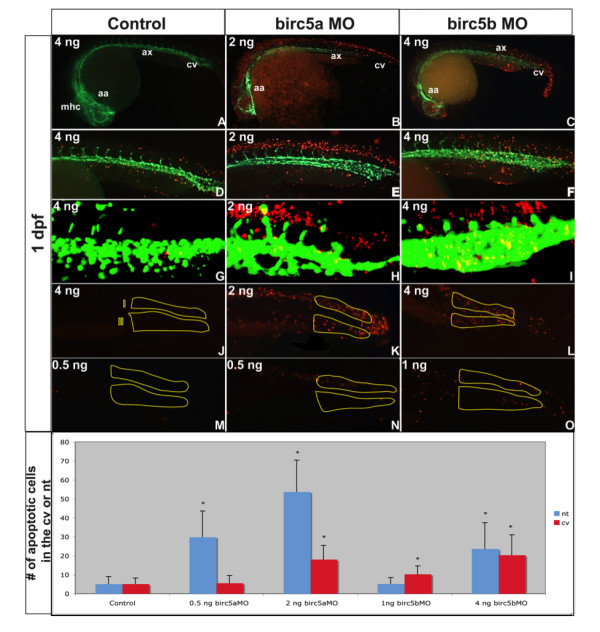
Apoptosis in Birc5-depleted embryos. Tg(Fli:eGFP) embryos reveal apoptosis (red) in relation to blood vessels (green) (A-O). A-I: Morphants at 1 dpf exhibit increased apoptosis, particularly in the brain (B) and along neural tube (E) in Birc5a morphants (morpholino dose 2 ng). Birc5b depletion with 4 ng of morpholino caused apoptosis in axial vessel region, caudal vein plexus, and neural structures (C, F). G-I: Confocal microscopy of 1 μm sagittal "slice" in region of caudal vein plexus and corresponding neural tube (excludes somites): Dose-dependent changes in apoptosis in caudal vein plexus region (J, region II) and corresponding neural tissue (J, region I) after Birc5 knockdowns was quantified at 1 dpf. High dose Birc5a morpholino (2 ng) or Birc5b morpholino (4 ng) causes significant increase in apoptosis in caudal vein plexus and neural tube (K, L). With lower Birc5a morpholino dose 0.5 ng), neural tube apoptosis remains significantly increased, but is almost absent in caudal vein plexus (N). Low dose Birc5b morpholino (1.0 ng) causes significant apoptosis in caudal vein plexus, but not in neural tube (O). Data presented in bottom panel. n = 30 × 3 independent experiments. * p < 0.05 relative to corresponding control. mhc: midbrain-hindbrain channel, aa: aortic arch, ax: axial vessels, cv: caudal vein plexus.
Quantification of TUNEL positive cells in the caudal vein plexus and the corresponding dorsal neural tube region confirmed that depletion of either Birc5a or Birc5b at the higher dose, caused a significant increase in the number of apoptotic cells in both regions (p < 0.05) (Figure 6J–L). However, when the Birc5a morpholino dose was decreased to 0.5 ng, apoptosis in the caudal vein plexus entirely resolved, while TUNEL staining in the neural tube persisted (Figure 6M, N), indicating a greater sensitivity of the neural structures to loss of Birc5a. Conversely, as the Birc5b morpholino dose was decreased, the effect on the neural tube diminished, while apoptosis in the region of the caudal vein plexus persisted (Figure 6M, O).
We also quantified the effects of the two genes on cell proliferation (Figure 7). At 1 dpf, proliferating cells were present in the region of the caudal vein plexus and neural tube (Figure 7A, B). Birc5a depletion using 2 ng of morpholino interfered with cell proliferation in both regions, while the 0.5 ng dose (Figure 7D, E) only suppressed proliferation in the neural tube. Depletion of Birc5b also interfered with cell proliferation in the caudal vein plexus and neural tube, and this anti-proliferative effect in the neural tube disappeared at the lower morpholino dose (Figure 7C, F). Overall, the findings support the notion that both Birc5 genes interfere with apoptosis and promote cell proliferation during early zebrafish development, but that there are dose-dependent and site-specific distinguishing features.
Figure 7.
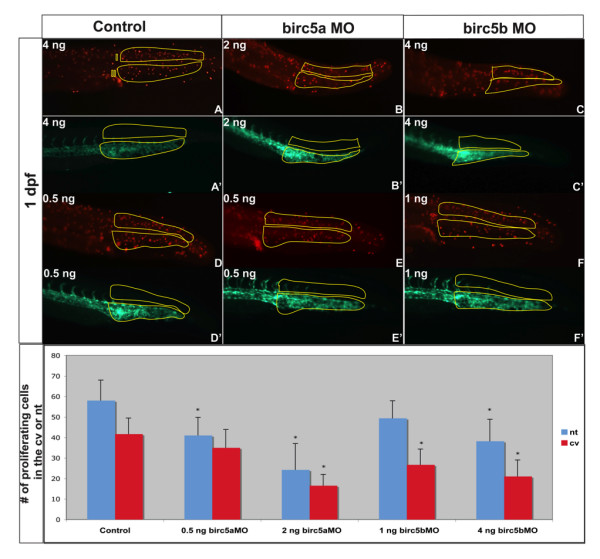
Cell proliferation in Brc5-depleted embryos. Tg(Fli:eGFP) embryos, were used to localize proliferating cells (red, A-F) in relation to blood vessels (green, A'-F') after Birc5 morpholino knockdowns. Proliferating cells immuno-detected by whole-mount staining of 2 dpf embryos with anti-phospho-Histone H3 antibodies (red). The number of proliferating cells in the caudal vein plexus and neural tube (see Figure 6J for regions) was quantified in embryos after high dose (B, C) or low dose (E, F) morpholino knockdowns. With high dose Birc5a or Birc5b morpholino (2 ng or 4 ng, respectively), there is a significant decrease in cell proliferation in the caudal vein plexus and the neural tube (B, C) as compared to the control (A). When the Birc5a morpholino knockdown dose is lowered (0.5 ng) (E), there is still a significant decrease in cell proliferation in the neural tube, but not in the caudal vein plexus, compared to control (D). Conversely, low dose Birc5b morpholino knockdown (1.0 ng) results in a significant diminution of cell proliferation in the caudal vein plexus, but not in the neural tube (F). Quantitative data are presented in the bottom panel. n = 30 × 3 independent experiments. * p < 0.05 relative to corresponding control.
Phenotype rescues of Birc5a and Birc5b morphants
We determined whether we could rescue the Birc5 morphants by co-injecting synthetic mRNAs encoding the open reading frame of either Birc5a or Birc5b. In a dose-dependent manner, co-injection of the respective Birc5 mRNA almost entirely rescued both the vascular and neural phenotypes induced by the highest morpholino dose (Table 1). Thus, at 2 dpf, the Birc5a morphants were rescued by co-injection of 1 ng of Birc5a mRNA, and the Birc5b morphants were rescued by 1 ng of Birc5b mRNA. Furthermore, co-injection of 1 ng of Birc5b mRNA could also partially rescue the Birc5a morphants, while Birc5b morphants could be completely rescued by Birc5a mRNA. The findings indicate that the 2 survivin genes may, under different conditions, compensate for each other.
Table 1.
Rescue of neuro-vascular phenotypes with Birc5 mRNAs
| Buffer or Specific mRNA Co-injected | ||||||
| Phenotype | Buffer | Birc5a mRNA(n = 162) | Birc5b mRNA(n = 180) | Birc5a mRNA(n = 134) | Birc5b mRNA(n = 173) | |
| Birc5a kd | Neuro | 61% (of 276) |
8% | 21% | ||
| Vascular | 40% (of 276) |
10% | 22% | |||
| Birc5b kd | Neuro | 26% (of 263) |
5% | 6% | ||
| Vascular | 38% (of 263) |
4% | 8% | |||
Birc5 morpholinos were injected into Tg(Fli:eGFP) zebrafish embryos simultaneously with injections of specific mRNAs as shown, and phenotypic analyses were performed at 2 dpf. The incidence of neural or vascular abnormalities is illustrated.
Role of VEGF in regulating survivin
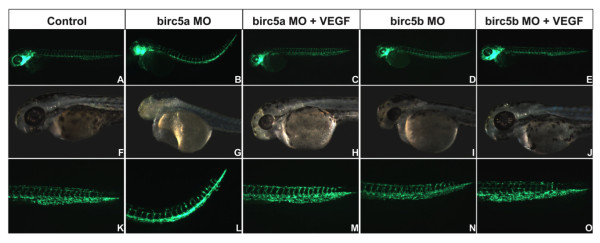
Vascular endothelial growth factor (VEGF) has vasculo-angiogenic, neurogenic, cardiogenic and hematopoietic properties, the effects on endothelial cells mediated in part by upregulating survivin [37]. Ma et al [16] demonstrated that VEGF protein upregulates expression of Birc5a in zebrafish at 96 hpf. We evaluated whether VEGF could rescue the phenotypes induced by depletion of the 2 zebrafish survivin genes. Human VEGF mRNA (500 pg) or vehicle was injected into Tg(Fli:EGFP) embryos alone, or with a control morpholino, or with maximum dose morpholinos to deplete either Birc5a or Birc5b (Figure 8). Embryos were evaluated at 2 dpf. In conjunction with Birc5a morpholino knockdowns (n = 86), VEGF mRNA reduced the incidence of neural and vascular defects to 43% and 37%, respectively, of that observed with Birc5a morpholino alone. VEGF also rescued the phenotype induced by Birc5b depletion. Thus, administration of VEGF mRNA (n = 85) with Birc5b morpholino decreased the incidence of vascular and neural defects in Birc5b morphants (n = 110), to 20% and 24%, respectively, of that found with Birc5b morpholino alone. The results demonstrate that VEGF may protect the integrity of the neural and vascular systems from single birc5 gene depletion, possibly via a compensatory increase in expression of the second birc5 gene.
Figure 8.
Rescue of Birc5 knockdown phenotypes with VEGF mRNA. Birc5a or Birc5b was depleted with 2 ng or 4 ng, respectively, of corresponding morpholino into Tg(Fli:eGFP) embryos, alone or with human VEGF mRNA. Embryos were evaluated at 2 dpf. Morpholino-induced angiogenic, cardiac and neurodevelopmental defects were reversed by VEGF.
Discussion and conclusion
In attempting to elucidate the physiologic relevance of the inhibitor of apoptosis protein, survivin, we have utilized the zebrafish model and characterized the expression patterns and functions of its two genes in early development. Our studies extend those of Ma et al [16], who first reported that Birc5a has angiogenic, but not vasculogenic, properties, a discrepancy that may be partly explained by the fact that their studies were restricted to developmental time points no earlier than 22 hpf. Several novel insights are provided by our work. Both Birc5a and Birc5b are expressed predominantly by neural, vascular, and ocular structures, and in the somites/somite boundaries; both inhibit apoptosis and promote cell proliferation; and both contribute to normal vasculogenesis, angiogenesis, cardiogenesis, neurogenesis and hematopoiesis. No other member of the IAP family has been shown to have similarly profound developmental effects on multiple organ systems following gene inactivation or knockdown in small animal models [38].
Our findings are consistent with immunohistochemical analyses and survivin gene inactivation studies in the mouse embryo, where the single survivin gene is essential for survival, and also plays a key role in angiogenesis, neurogenesis, cardiogenesis and hematopoiesis [5,11,13,14]. Our studies therefore support the concept that the zebrafish paralogs, Birc5a and Birc5b, largely recapitulate the properties of the ancestral gene, represented in the mouse, thereby rationalizing the use of this model to elucidate the role of survivin in health and disease in higher animals.
While the two zebrafish survivin genes have indistinguishable patterns of expression during development, in all of our assays, except those for hematopoiesis, depletion of Birc5a resulted in more severe, and sometimes distinct phenotypes. For example, only Birc5a depletion caused marked alterations in ISV patterning. This phenotype would be most readily attributed to expression of Birc5a at the somite boundaries, where VEGF [39] and other guidance molecules play a crucial role in vessel patterning (reviewed in [25]). However, Birc5b is also expressed in the somites/somite boundaries, and its depletion had no effect on the ISVs. Thus, further studies, including analyses of the expression profiles of relevant guidance molecules, will be required to further elucidate the specific properties of each Birc5 gene in the somites.
Functional differences between the two Birc5 genes were additionally uncovered by titering the respective morpholino doses to evaluate effects of each paralog on apoptosis and cell proliferation in the neural tube and vascular structures. By this approach, Birc5a was found to be more effective at protecting the neural structures, while Birc5b was more effective at protecting the caudal vein plexus. The latter is interesting, because the caudal vein plexus is a major site for primitive hematopoiesis, a process that was more prominently disturbed by Birc5b depletion. Nonetheless, administration of either mRNA could effectively rescue the phenotypes induced by depletion of the other. Thus, the physiologic relevance of the functional differences between the two survivin paralogs is not fully delineated, and it appears that each may act to compensate for deficiencies of the other.
A common feature of both Birc5 genes, not previously recognized, is that their expression in the somites and axial vessels is transient and restricted to an early developmental time period, both essentially gone by 3 dpf. Up until that time, the axial vessels and the ISVs are generated, and based on the morpholino knockdowns, survivin is critical for formation of these structures, after which survivin is no longer necessary at those sites. Interestingly, the IAP Birc2 is expressed in the vasculature of zebrafish beginning at 54 hpf, whereupon it is required to maintain endothelial cell integrity [40]. One could speculate that there exists an intrinsic molecular switch that is "flipped" at 2–3 dpf, when Birc5 expression turns off, and Birc2 turns on, the latter which is required to form a more complex vascular network. Characterization of such a switch mechanism could enhance our understanding of the regulation of angiogenesis.
In mice depleted of endothelial survivin, embryonic heart development was abnormal, and the mutant endocardial lineage cells could not support epithelial-mesenchymal transformation (EMT) [14]. In both survivin gene knockdowns in the zebrafish, we also observed abnormalities in cardiogenesis – more prominent with Birc5a depletion. Fate-mapping studies in zebrafish have revealed that formation of the atrium, ventricle and a-v valves depends on the integrity of the cardiac neural crest cells [36], while intracardiac fluid forces also contribute to normal heart development [33]. Thus, the etiology of the abnormalities in cardiogenesis in the Birc5 morphants may be multifactorial, i.e. secondary to the circulation defect and/or due to loss of the cardiac neural crest cells. Further study is required to elucidate the relevant Birc5-dependent pro-survival pathways for these neural crest cells. Nonetheless, the zebrafish and mouse models highlight the importance of survivin in heart development. Just as single-nucleotide polymorphisms of the VEGF gene have been linked to congenital valvuloseptal defects [41], the possibility that functional alterations in survivin expression might underlie congenital heart defects is worthy of consideration.
The prominent role that survivin plays in regulating vasculo-angiogenesis, neurogenesis, cardiogenesis and hematopoiesis supports the widely accepted notion of co-ordinately regulated development of these systems (reviewed in [42]). For example, the Eph/ephrins regulate fasciculation and guidance of axons, direct neural crest cell fate and migration, modulate neural progenitor cell survival, while also being important for cardiovascular development [43] and erythropoiesis [44]. Vascular endothelial growth factor (VEGF), although best characterized as a critical mediator of vasculogenesis and angiogenesis, also has direct effects on the nervous system (reviewed in [45]). VEGF protects neural cells from hypoxia, facilitates axonal outgrowth, promotes endothelial release of neurogenic factors [46], and induces neural stem cell proliferation [47]. VEGF also promotes hematopoiesis [48], and altered regulation of VEGF results in profound defects in heart development [49]. These properties of VEGF are confirmed in our studies, where the neural and cardiovascular phenotypes induced by depletion of Birc5a or Birc5b, were rescued by VEGF. Although not tested, it is likely that upon depletion of one Birc5 gene, the exogenous VEGF upregulated expression of the other, which in turn compensated for the phenotypic defects. In that respect, Ma et al [37] indeed, demonstrated that VEGF protein can increase accumulation of Birc5a mRNA in zebrafish embryos. Beyond the zebrafish model system, upregulation of survivin in endothelial cells has been well-documented [37]. The finding that survivin is also a downstream effector of VEGF in the neurologic system, and that survivin transcripts are highly expressed in neural progenitor cells [50], implies that the survivin gene in humans may, similar to VEGF [51], be a modifier in the progression of neural diseases, such as amyotrophic lateral sclerosis, and thus have the potential as a therapeutic target.
By regulating cell proliferation and apoptosis, the two zebrafish survivin genes, Birc5a and Birc5b, play important roles in multiple biologic systems. Although we have not examined all organs during development; nor have we yet evaluated its role in the eye; the prominent effects of Birc5 gene depletion on the cardiac, neurovascular and hematopoietic systems in the zebrafish embryo suggest that survivin has temporal and tissue-specific properties. In view of the apparent functional overlap with the murine and human survivin orthologues, the zebrafish model provides an exceptional opportunity to examine the physiologic relevance of the complex molecular and biochemical pathways that govern cell survival and apoptosis. The insights gained will lead to the development of safer, targeted therapeutics for a spectrum of cardio-vascular, hematopoeitic and neurologic diseases, and a better understanding of the etiology and genetics of an array of congenital diseases.
Methods
Zebrafish strains and maintenance
Wild-type AB zebrafish and Tg(Flk1:EGFP), Tg(Fli:EGFP)y1, and Tg(gata1:GFP) zebrafish were maintained under standard laboratory conditions [22]. Embryos were grown in 0.003% 1-phenyl-2-thiourea (PTU, Sigma, Bornem, Belgium) in 0.3× Danieau (17 mM NaCl, 0.21 mM KCl, 0.12 mM MgSO4.7H2O, 0.18 mMCa(NO3)2, 1.5 mM Hepes (Fluka, Bornem, Belgium) pH 7.6) starting from 24 hpf, were kept at 28.5°C, and staged according to standard criteria [52]. After 24 hours, the chorion was removed with trypsin 1.5 mg/ml (Sigma). All animal studies were approved by the University of Leuven Animal Studies Ethics Commission.
Digoxigenin-labeled RNA probes for in situ hybridization
cDNAs were cloned into the pGEM-T Easy vector (Promega, Leiden, the Netherlands). For detection of Birc5a, primer pairs: Birc5a-F1/R1, Birc5a-F2/R2 and Birc5a-F3/R3 (see Additional file 2) were used to generate amplicons, respectively, of 902 bp (3' coding region + UTR), 344 bp (only 3' UTR) and 429 bp (coding region). For detection of Birc5b, probes were made with the following primer pairs: Birc5b-F1/R1 and Birc5b-F2/R2, generating amplicons, respectively, of 491 bp (coding region + 3' UTR) and 387 bp (coding region). After linearization with Spe1 and Nco1, antisense riboprobes were prepared with the DIG RNA labeling kit (Roche, Vilvoorde, Belgium) according to the manufacturer's instructions and purified with the RNeasy mini kit (Qiagen, Venlo, the Netherlands).
Flow cytometry
Tg(Gata1:GFP) zebrafish embryos were injected at the 1-cell stage, and at 14 hpf, they were dechorionated and digested with 0.05% trypsin/EDTA for 15 minutes at 28°C. A single cell suspension was obtained by pipetting up and down, after which 100% fetal calf serum (FCS) was added. Cells were filtered through a 40 μM cell strainer, washed with 2% FCS/PBS, and the percentage of GFP-positive cells was measured by flow cytometry (FacsCalibur, BD Biosciences).
mRNA preparation
cDNAs in plasmid vectors were linearized with the appropriate restriction enzyme, and the digested DNA was ethanol precipitated. mRNA for each survivin gene transcript or vegf was then prepared according to the manufacturers instructions (Ambion, Lennik, Belgium).
Real-time PCR
Quantitative real-time PCR was performed to examine the expression of different hematopoietic genes using SYBR green (Applied Biosystems, Belgium) according to manufacturers instructions. The sequence of the oligonucleotide primers used are as reported by Ma et al [29]. The β-actin mRNA levels were used as an internal reference.
Morpholino and mRNA injection
Morpholinos (Gene Tools LLC, Oregon, USA) were targeted against the ATG or 5'UTR of Birc5a and Birc5b. Morpholinos and synthetic mRNAs were diluted in 1.5% phenol red (Sigma) in 0.2 M KC. Fertilized zebrafish eggs at the 1–4 cell stage were positioned into agar slots, and using a Femtojet (Eppendorf, Haasrode, Belgium), a micromanipulator (Narishige, New York, USA) and a Zeiss-stemi 2000-C light microscope (Zeiss, Zaventum, Belgium), eggs were injected with 1 nl of morpholino using back-filled fine borosilicate, glass capillary needles.
Detection of apoptosis and cell proliferation
Embryos were fixed in 4% paraformaldehyde overnight at 4°C and stored in methanol at -20°C until further processing. In situ hybridization was performed as described [17]. For apoptosis and cell proliferation studies, embryos were first rehydrated with decreasing concentrations of methanol, washed with PBST (PBS, 0.1% Tween 20), treated with proteinase K (10 mg/ml) at 37°C for 30 to 60 minutes, refixed with 4% paraformaldehyde, washed in PBST, and lastly incubated with 0.1 M citrate solution (0.1% citrate/PBS/Triton). TUNEL staining was performed with the ApopTag kit (Chemicon, Heule, Belgium). Cell proliferation was visualized using antibodies against phosphorylated phospho-histone H3 (Upstate, Huissen, the Netherlands). After mounting in 3% methylcellulose (Sigma) in PBS, embryos were visualized by light microscopy or with a Zeiss Lumar fluorescence stereomicroscope, and pictures were taken under green fluorescence Tg(Fli:eGFP) and red fluorescence (apoptotic cells or proliferating cells) with a Zeiss AxioCam MRc digital camera. For each embryo, the entire caudal vein plexus and corresponding overlying neural tube was delineated, and apoptotic cells or proliferating cells were manually counted. 30 embryos were analyzed per condition and 3 experiments were performed. Data were compared with a standard Student t-test.
Authors' contributions
MD and FZ carried out in situ hybridizations and microscopy, and designed experiments, while MD also drafted the manuscript. AD, IB and MM prepared all riboprobes, did cDNA cloning and sequencing, and helped with in situ hybridizations, acquisition of data and analyses. MA provided continuous intellectual input, evaluation and interpretation of data, and writing of the manuscript. EC conceived, designed and co-ordinated the project, and drafted the manuscript. All authors read and approved the final manuscript.
Supplementary Material
A. Using the CLUSTAL W program, protein sequences of human, mouse, Xenopus and zebrafish survivin were aligned.B. The overall similarity (in %) at the level of amino acid sequence between survivins of human, mouse, Xenopus and zebrafish were compared. Homo, Homo sapiens; Mus, Mus musculus; XT, Xenopus tropicalis; Birc5, danio rerio; XL, Xenopus laevis; * identical amino acid; conserved change; highly conserved change.
Primers used to generate in situ hybridization probes.
Morpholinos used for the knockdown of Birc5a and Birc5b.
An oligonucleotide for each zebrafish survivingene containing the binding sites of the different morpholinos directed against the 5' UTR and the ATG of each gene, was cloned into the pCAG-T7-luciferase plasmid, resulting in the pCAG-T7-luciferase-Birc5a and pCAG-T7-luciferase-Birc5b plasmids. These plasmids, together with varying doses of Birc5 morpholinos, were used in an in vitrotranscription/translation assay, as described [18]. Experiments were performed in triplicate. Luciferase activity was measured in arbitrary light units that are noted in the Y-axis. Birc5a is only depleted by the Birc5a morpholino (B-F) and not by the Birc5b morpholino (G-K). Similarly, Birc5b is only depleted by the Birc5b morpholino (R-V) and not by the Birc5a morpholino (M-Q). Birc5a plasmid: pCAG-T7-luciferase-Birc5a; Birc5b plasmid: pCAG-T7-luciferase-Birc5b; MO1: Birc5a morpholino; MO2: Birc5b morpholino.
Confocal microscopy was used to visualize a cross sectional view from the of top of the head region of a 3 dpf zebrafish embryo, revealing the different vascular structures.
When compared to the control (Movie S1), depletion of Birc5a with 2 ng of morpholino results in striking underdevelopment and irregular patterning of several blood vessels in the head of the embryo.
When compared to the control (Movie S1), knockdown of Birc5b with 4 ng of morpholino leads to underdevelopment of vascular structures in the head, although to a lesser extent than in the Birc5a morphants (Movie S2).
Acknowledgments
Acknowledgements
This work was supported in part by the Fonds voor Wetenschappelijk Onderzoek (FWO), Belgium. We thank Lieve Moons and Filip Claes for their advice.
Contributor Information
Mieke Delvaeye, Email: Mieke.Delvaeye@vib-kuleuven.be.
Astrid De Vriese, Email: Astrid.DeVriese@vib-kuleuven.be.
Femke Zwerts, Email: FemkeZwerts@yahoo.com.
Inge Betz, Email: Inge_Betz@yahoo.com.
Michael Moons, Email: Michael.Moons@vib-kuleuven.be.
Monica Autiero, Email: fb238607@skynet.be.
Edward M Conway, Email: Ed.Conway@vib-kuleuven.be.
References
- Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–90. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri D. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Medicine. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–28. doi: 10.1016/S0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ito T, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M, Shiraki K. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene. 2000;19:1346–53. doi: 10.1038/sj.onc.1203429. [DOI] [PubMed] [Google Scholar]
- Adida C, Crotty P, McGrath J, Berrebi D, Diebold J, Altieri D. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Path. 1998;152:43–49. [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–98. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- Altieri DC. New wirings in the survivin networks. Oncogene. 2008;27:6276–84. doi: 10.1038/onc.2008.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ling X. Survivin study: an update of "what is the next wave"? J Cell Physiol. 2006;208:476–86. doi: 10.1002/jcp.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altznauer F, Martinelli S, Yousefi S, Thurig C, Schmid I, Conway E, Schoni M, Vogt P, Mueller C, Fey M, et al. Inflammation-associated cell cycle-independent block of apoptosis by survivin in terminally differentiated neutrophils. J Exp Med. 2004;199:1343–54. doi: 10.1084/jem.20032033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Pollefeyt S, Steiner-Mosonyi M, Luo W, DeVriese A, Lupu F, Bono F, Leducq N, Dol F, Schaeffer P, et al. Deficiency of Survivin in Transgenic Mice Exacerbates Fas-Induced Apoptosis via Mitochondrial Pathways. Gastroenterology. 2002;123:619–631. doi: 10.1053/gast.2002.34753. [DOI] [PubMed] [Google Scholar]
- Leung CG, Xu Y, Mularski B, Liu H, Gurbuxani S, Crispino JD. Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J Exp Med. 2007;204:1603–11. doi: 10.1084/jem.20062395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Zwerts F, Van Eygen V, DeVries A, Nagai N, Luo W, Collen D. Survivin-dependent angiogenesis in ischemic brain: Molecular mechanisms of hypoxia-induced upregulation. Am J Pathol. 2003;163:935–946. doi: 10.1016/S0002-9440(10)63453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, de Bruin A, Caldas H, Fangusaro J, Hayes J, Conway EM, Robinson M, Altura RA. Essential role for survivin in early brain development. J Neurosci. 2005;25:6962–70. doi: 10.1523/JNEUROSCI.1446-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerts F, Lupu F, De Vriese A, Pollefeyt S, Moons L, Altura RA, Jiang Y, Maxwell PH, Hill P, Oh H, et al. Lack of endothelial cell survivin causes embryonic defects in angiogenesis, cardiogenesis, and neural tube closure. Blood. 2007;109:4742–52. doi: 10.1182/blood-2006-06-028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier D, Phung AC, Ymlahi-Ouazzani Q, Sinzelle L, Ballagny C, Bronchain O, Du Pasquier L, Mazabraud A. Survivin increased vascular development during Xenopus ontogenesis. Differentiation. 2006;74:244–53. doi: 10.1111/j.1432-0436.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- Ma A, Lin R, Chan PK, Leung JC, Chan LY, Meng A, Verfaillie CM, Liang R, Leung AY. The role of survivin in angiogenesis during zebrafish embryonic development. BMC Dev Biol. 2007;7:50. doi: 10.1186/1471-213X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-T. [DOI] [PubMed] [Google Scholar]
- Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster RW, Fraser SE. FGF signaling mediates regeneration of the differentiating cerebellum through repatterning of the anterior hindbrain and reinitiation of neuronal migration. J Neurosci. 2006;26:7293–304. doi: 10.1523/JNEUROSCI.0095-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–25. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–18. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Fouquet B, Weinstein BM, Serluca FC, Fishman MC. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev Biol. 1997;183:37–48. doi: 10.1006/dbio.1996.8495. [DOI] [PubMed] [Google Scholar]
- Zhong TP. Zebrafish genetics and formation of embryonic vasculature. Curr Top Dev Biol. 2005;71:53–81. doi: 10.1016/S0070-2153(05)71002-4. [DOI] [PubMed] [Google Scholar]
- Baldessari D, Mione M. How to create the vascular tree? (Latest) help from the zebrafish. Pharmacol Ther. 2008;118:206–30. doi: 10.1016/j.pharmthera.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Rubinstein AL, Lee D, Luo R, Henion PD, Halpern ME. Genes dependent on zebrafish cyclops function identified by AFLP differential gene expression screen. Genesis. 2000;26:86–97. doi: 10.1002/(SICI)1526-968X(200001)26:1<86::AID-GENE11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Snider P, Olaopa M, Firulli AB, Conway SJ. Cardiovascular development and the colonizing cardiac neural crest lineage. ScientificWorldJournal. 2007;7:1090–113. doi: 10.1100/tsw.2007.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–9. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- Ma AC, Liang R, Leung AY. The role of phospholipase C gamma 1 in primitive hematopoiesis during zebrafish development. Exp Hematol. 2007;35:368–73. doi: 10.1016/j.exphem.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–82. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- Glickman NS, Yelon D. Cardiac development in zebrafish: coordination of form and function. Semin Cell Dev Biol. 2002;13:507–13. doi: 10.1016/S1084952102001040. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–7. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Li YX, Zdanowicz M, Young L, Kumiski D, Leatherbury L, Kirby ML. Cardiac neural crest in zebrafish embryos contributes to myocardial cell lineage and early heart function. Dev Dyn. 2003;226:540–50. doi: 10.1002/dvdy.10264. [DOI] [PubMed] [Google Scholar]
- Lister JA, Cooper C, Nguyen K, Modrell M, Grant K, Raible DW. Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Dev Biol. 2006;290:92–104. doi: 10.1016/j.ydbio.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Sato M, Yost HJ. Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Dev Biol. 2003;257:127–39. doi: 10.1016/S0012-1606(03)00037-X. [DOI] [PubMed] [Google Scholar]
- Tran J, Rak J, Sheehan C, Saibil SD, LaCasse E, Korneluk RG, Kerbel RS. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun. 1999;264:781–8. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- O'Riordan MX, Bauler LD, Scott FL, Duckett CS. Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev Cell. 2008;15:497–508. doi: 10.1016/j.devcel.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Xu X, Chin AJ, Balasubramaniyan NV, Teo MA, Lam TJ, Weinberg ES, Ge R. Cloning and characterization of vascular endothelial growth factor (VEGF) from zebrafish, Danio rerio. Biochim Biophys Acta. 1998;1397:14–20. doi: 10.1016/s0167-4781(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DY. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet. 2007;39:1397–402. doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- Vannay A, Vasarhelyi B, Kornyei M, Treszl A, Kozma G, Gyorffy B, Tulassay T, Sulyok E. Single-nucleotide polymorphisms of VEGF gene are associated with risk of congenital valvuloseptal heart defects. Am Heart J. 2006;151:878–81. doi: 10.1016/j.ahj.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Raab S, Plate KH. Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol (Berl) 2007;113:607–26. doi: 10.1007/s00401-007-0228-3. [DOI] [PubMed] [Google Scholar]
- Adams RH. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin Cell Dev Biol. 2002;13:55–60. doi: 10.1006/scdb.2001.0289. [DOI] [PubMed] [Google Scholar]
- Suenobu S, Takakura N, Inada T, Yamada Y, Yuasa H, Zhang XQ, Sakano S, Oike Y, Suda T. A role of EphB4 receptor and its ligand, ephrin-B2, in erythropoiesis. Biochem Biophys Res Commun. 2002;293:1124–31. doi: 10.1016/S0006-291X(02)00330-3. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue – beyond blood vessels. Exp Neurol. 2004;187:246–53. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie S, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial Cells Stimulate Self-Renewal and Expand Neurogenesis of Neural Stem Cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–48. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Chang JR, Chin AJ, Smith A, Kelly C, Weinberg ES, Ge R. The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. Mech Dev. 2001;108:29–43. doi: 10.1016/S0925-4773(01)00468-3. [DOI] [PubMed] [Google Scholar]
- Stalmans I. Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome. Verh K Acad Geneeskd Belg. 2005;67:229–76. [PubMed] [Google Scholar]
- Pennartz S, Belvindrah R, Tomiuk S, Zimmer C, Hofmann K, Conradt M, Bosio A, Cremer H. Purification of neuronal precursors from the adult mouse brain: comprehensive gene expression analysis provides new insights into the control of cell migration, differentiation, and homeostasis. Mol Cell Neurosci. 2004;25:692–706. doi: 10.1016/j.mcn.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, Wyns S, Thijs V, Andersson J, van Marion I, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–94. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Using the CLUSTAL W program, protein sequences of human, mouse, Xenopus and zebrafish survivin were aligned.B. The overall similarity (in %) at the level of amino acid sequence between survivins of human, mouse, Xenopus and zebrafish were compared. Homo, Homo sapiens; Mus, Mus musculus; XT, Xenopus tropicalis; Birc5, danio rerio; XL, Xenopus laevis; * identical amino acid; conserved change; highly conserved change.
Primers used to generate in situ hybridization probes.
Morpholinos used for the knockdown of Birc5a and Birc5b.
An oligonucleotide for each zebrafish survivingene containing the binding sites of the different morpholinos directed against the 5' UTR and the ATG of each gene, was cloned into the pCAG-T7-luciferase plasmid, resulting in the pCAG-T7-luciferase-Birc5a and pCAG-T7-luciferase-Birc5b plasmids. These plasmids, together with varying doses of Birc5 morpholinos, were used in an in vitrotranscription/translation assay, as described [18]. Experiments were performed in triplicate. Luciferase activity was measured in arbitrary light units that are noted in the Y-axis. Birc5a is only depleted by the Birc5a morpholino (B-F) and not by the Birc5b morpholino (G-K). Similarly, Birc5b is only depleted by the Birc5b morpholino (R-V) and not by the Birc5a morpholino (M-Q). Birc5a plasmid: pCAG-T7-luciferase-Birc5a; Birc5b plasmid: pCAG-T7-luciferase-Birc5b; MO1: Birc5a morpholino; MO2: Birc5b morpholino.
Confocal microscopy was used to visualize a cross sectional view from the of top of the head region of a 3 dpf zebrafish embryo, revealing the different vascular structures.
When compared to the control (Movie S1), depletion of Birc5a with 2 ng of morpholino results in striking underdevelopment and irregular patterning of several blood vessels in the head of the embryo.
When compared to the control (Movie S1), knockdown of Birc5b with 4 ng of morpholino leads to underdevelopment of vascular structures in the head, although to a lesser extent than in the Birc5a morphants (Movie S2).