Abstract
We investigated whether pannexin-1, a carbenoxolone-sensitive hemichannel activated in erythrocytes by swelling, could contribute to swelling-activated chloride currents (ICl,swell) in HEK-293 cells. We used ethidium bromide uptake as an index of pannexin-1 activation and IC,swell activation as an index of plasma membrane stretching. ICl swell activated by a hypotonic solution was reversible inhibited by carbenoxolone (IC50 98 ± 5 μM), a blocker of pannexin-1. However, the hypotonic solution that activated ICl,swell did not induce ethidium bromide uptake indicating that pannexin-1 was not activated by cell swelling. The mimetic peptide 10panx1, a pannexin-1 antagonist, did not affect ICl,swell activation but completely inhibited the ATP-induced ethidium bromide uptake coupled to P2X7 receptors activation. We conclude that carbenoxolone directly inhibited ICl,swell independent of pannexin-1 and that pannexin-1 hemichannels are not activated by swelling in HEK-293 cells.
Keywords: cell swelling, swelling-activated chloride current, carbenoxolone, pannexin-1, dye uptake, ATP release
INTRODUCTION
Pannexins 1, 2 and 3 comprise a family of membrane proteins that share homology to innexins, the invertebrate counterparts of connexins [1]. Pannexin-1 (panx-1) assembles into hexameric hemichannels [2,3] permeable to relatively large organic molecules [4,5] like ATP, Yopro 1, ethidium bromide [3,6] and ions like Ca2+ [7].
Panx-1 is opened by activation of P2X7 receptors (P2X7R) but cell swelling seems to activate it too. In agreement with this idea, swelling human erythrocytes with a hypotonic media induces ATP release that can be prevented by carbenoxolone (CBX), a blocker of panx-1[8]. In addition, mechanical stress of membrane patches activates panx-1 [3] and hypotonic stimulation can induces a CBX-sensitive uptake of 5,6-carboxyfluorescein (REF). In the retina CBX and 5-nitro-2-(3-phenylpropylamino) benzoic acid—a blocker of panx-1 and chloride channels—prevent ATP release induced by hydrostatic pressure [9]. The mechano-sensitivity of panx-1 could explain its activation by cell swelling and thus panx-1 may function as pathway for ATP release.
ATP release is an important feature of many cells [10] and can be activated by a variety of signals including hypotonic stress. Yet the mechanism of ATP release induced by hypotonic solutions in different cell types is unsettled [10]. Even when panx-1 could operate as a pathway for ATP release under hypotonic conditions, membrane proteins activated by mechanical stress such as maxi-anion channels could also function as a pathway for ATP release [11]. It is interesting to note that expression of panx-1 in Xenopus oocytes induces a current similar to the ubiquitous swelling-activated chloride current (ICl,swell) [12, 13]. In the present study, we investigated whether endogenous panx-1 was activated and contributed to membrane current in osmotically swollen HEK-293 cells.
MATERIALS AND METHODS
Cell culture and transfection
HEK-293 cells (Invitrogen; Carlsbad, CA) were maintained in DMEM medium (Gibco, BRL) at 37°C in a 95% O2/ 5% CO2 atmosphere. Cells were grown to 50–60% confluence on 30 mm Petri dishes and transfected with pIRES2-EGFP-P2X7R (2 μl/dish from a 1 μg/μl DNA stock) using the Polyfect Transfection Reagent (Qiagen; Valencia, CA). Green fluorescence was used to identify transfected cells [14]. Cells were detached with trypsin (0.1 %), re-plated onto 5 mm glass coverslips and allowed to attach for at least 5 h before use.
Electrophysiological recordings
A coverslip containing HEK cells was placed in the recording chamber mounted on the stage of an inverted microscope (Nikon) equipped with ultraviolet illumination. Whole-cell currents were recorded using a PC-One Patch-Clamp amplifier (Dagan Corp., Minneapolis, MN) and pClamp8 software (Molecular Devices, Sunnyvale, CA). Pipettes (Corning 8161, Warner Instruments Inc.; Hamden, CT) had resistances between 3-4 MΩ when filled with internal solution. Bath solutions were gravity-perfused at a flow rate of ~4 ml/min. The composition of the various external and pipette solutions used is given in mM. Low Tonicity TEACl 190: TEACl 84, CaCl2 0.5 (Osm = 190 mOsm/kg). Hypotonic TEACl: TEACl 140, CaCl2 0.5 (Osm = 270 mOsm/kg). Hypertonic TEACl: same as hypotonic plus 100 mM D-Mannitol (Osm = 370 mOsm/kg). Pipette TEACl: TEACl 140, EGTA 20 (Osm = 340 mOsm/kg). Hypotonic Sodium-Potassium-Glucose (SPG): NaCl 117, KCl 2, glucose 13, CaCl2 2, MgCl2 1 (Osm = 250 mOsm/kg). Hypertonic SPG: same as SPG plus 115 mM D-Mannitol (Osm = 370 mOsm/kg). Pipette NaCl: NaCl 140, EGTA 20, D-Mannitol 30 (Osm = 370 mOsm/kg). The pH was adjusted with 20 (or 10 in SPG solutions) mM HEPES. TEA+ was used to abolish Na+ and K+ currents. The mimetic peptide 10panx1 was tested in SPG solution since it inhibited dye uptake better than in TEACl (data not shown). Osmolality was measured using a vapor pressure osmometer (Wescor, Logan, UT). Voltage commands were delivered from a holding potential of 0 mV. Tandem square voltage pulses (100 ms) to -80 and +120 mV (in this order) were applied every 2.5 s and current amplitude was measured 50 ms before ending the pulse. Raw data were filtered at 5 kHz and sampled at 10 kHz. Experiments were carried out at room temperature (20-22 °C).
Ethidium Bromide uptake
Ethidium Bromide (EtBr) uptake was used as an indication of panx-1 activity [4]. Cells were observed using a Nikon Pan Fluor 60X objective and 528-553 nm green light was used to excite them. Images were digitized with a Hamamatsu camera, acquired and analyzed using the Imaging Workbench 6.0 software (Indec BioSystems, Santa Clara, CA). Cellular fluorescence was measured in regions of interest selected around single cells. Background fluorescence (average of at least three similar signals from nearby cell-free regions) was subtracted from mean intensity during offline analysis. Frames were acquired every three seconds. Cells were incubated for at least five minutes in EtBr (0.6 μM) to make sure that there was no significant uptake under basal conditions (otherwise cells were excluded from the analysis). Fluorescence is given in arbitrary units (au). Experiments were carried out at room temperature.
Analysis and presentation of data
Dose-response curve to CBX was fit using the Hill equation:
| (Equation 1) |
where I is the percentage of inhibition, Imax is the maximum percent of inhibition (=100 %), IC50 is the [CBX] at which 50 % inhibition is achieved, and h is the Hill slope. Data shown are mean ± SEM and n indicates the number of experiments. White bars in figures correspond to hypertonic solution.
Reagents
10panx1 mimetic peptide was form GenScript Corporation Piscataway, NJ. Other chemicals were from Sigma.
RESULTS AND DISCUSSION
A recent report suggests that panx-1 in erythrocytes is activated by cell swelling [8]. In this work, we assessed panx-1 activity expressed engenously in HEK-293 cells [4,7] by measuring EtBr uptake after swelling the cells with hypotonic solutions. Figure 1A shows a typical fluorescence time course (n=5) from a HEK 293 cell transfected with P2X7R. Exposing the cells to Low Tonicity TEACl (190 mOsm/kg) for as long as ten minutes did not induce EtBr uptake. In contrast, brief stimulation of P2X7R with 500 μM ATP did induce large EtBr uptake indicating panx-1 activation (Fig. 1A). Longer exposures to hypotonic medium also failed to induce EtBr uptake in non-transfected cells (data not shown). Lack of panx-1 activation was not due to insufficient cell swelling since the cells were notoriously swollen by the hypotonic challenge (see pictures in Fig. 1A, right panel). The area covered by the cells after ten minutes exposure to the hypotonic solution (point b in Fig. 1A) increased 37 ± 6 % (n=5) above the initial value (point a).
Figure 1. Panx-1 hemichannels are not activated by cell swelling.
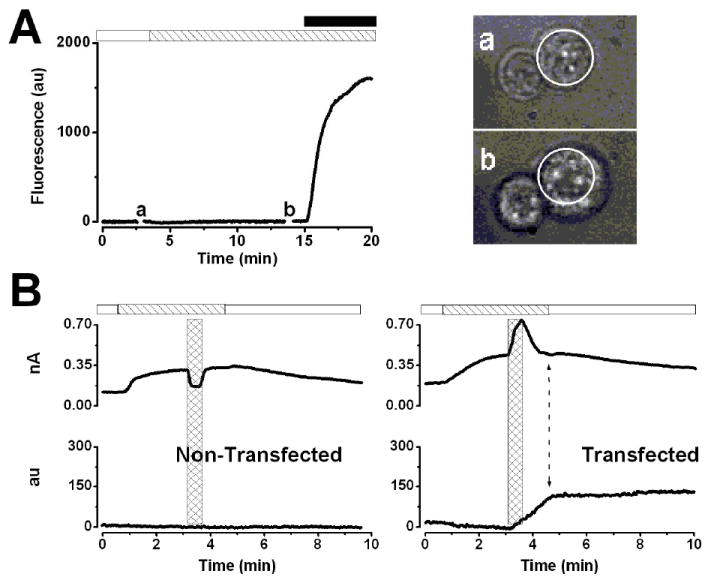
A.- Time course of fluorescence intensity of a representative P2X7-transfected HEK-293 cell (n=5) exposed to Low Tonicity TEACl 190 solution containing EtBr (striped bar). Stimulation with 0.5 mM ATP (black bar) induced EtBr-uptake (end of the trace). Cell images (right) were taken at the time indicated by a and b in the time course. Dotted circles indicate the cell perimeter before the hypotonic challenge. B.- Simultaneous recording of IClswell at +80 mV (upper traces) and fluorescence intensity (lower traces) in non-transfected (n=4) and P2X7-transfected cells (n=4). Cells were exposed to hypotonic TEACl solution containing EtBr (striped bar) and then to 5 mM ATP (hash bars) in a hypotonic solution during 30 s. ATP was washed out using a hypotonic solution, and then ICl,swell was turned off by applying a hypertonic solution (white bar). The double head arrow in the lower time course of the right panel indicates the end of the fluorescence increase.
To further assay the mechanosensitivity of dye uptake we performed simultaneous recordings of IClswell and fluorescence intensity in non-transfected and P2X7-transfected HEK 293 cells. IClswell was used as an indicator of membrane stress imposed by the hypotonic solution. Figure 1B shows the time course of both IClswell amplitude (upper) and fluorescence (lower) at +80 mV from non-transfected (left) and P2X7-transfected (right) cells. Exposure to hypotonic medium activated ICl,swell without dye uptake in both groups of cells. Application of ATP (5 mM) in hypotonic medium (hash bars) induced a drop in ICl,swell amplitude in non-transfected cells (Fig. 1B, left panel) because ATP inhibits ICl,swell [15]. Yet, no EtBr uptake was induced by ATP in non-transfected cells, denoting the need for functional coupling between P2X7R and panx-1 [4,5] and the fact that cell swelling is insufficient to trigger dye uptake in HEK-293 cells. In contrast, in P2X7-transfected cells ATP evoked a current on top of IClswell (Fig. 1B, right panel). This increase in current can be attributed to P2X7R and panx-1 activation since it was accompanied by an increase in dye uptake (Fig 1B lower trace right). Moreover, uptake continued until the ATP-activated current was turned off (indicated by the double arrow head). In agreement with previous reports [4,5] our results indicate that P2X7R activation promotes dye uptake presumably through panx-1.
It has been suggested that panx-1 could serve as the ATP release pathway [8]. However, in HEK cells the dye uptake mechanism mediated by panx-1 is not activated by hypotonic stress therefore the ATP release induced by hypotonic stress cannot be mediated by panx-1. Alternatively, anion channels activated by hypotonic media could be implicated in this process [11]. Thus, we tested whether or not CBX—a licorice root derivative that inhibits gap junctions and panx-1 [13]—blocks IClswell in non-transfected cells dialyzed and bathed in TEA+ solutions. Figure 2A shows a typical time course of ICl,swell (recorded at +120 and -80 mV) activated by hypotonic TEACl (striped bar) before, during and after application of 600 μM CBX (gray bar). ICl,swell was rapidly inhibited by CBX in a reversible and voltage-independent manner (Fig. 2B). Figure 2C shows a dose-response curve for CBX obtained using data like that shown in Fig. 2A. The dose-response curve was fitted with equation 1 and yielded an IC50 value of 98 ± 5 μM and a Hill coefficient of 1.9 ± 0.2. This IC50 value is higher than the IC50 value (2-4 μM) reported for inhibition of panx-1-dependent dye uptake and panx-1 current [4]. In addition, preliminary experiments (n=4) were carried out to analyze the voltage-dependent blockade of ICl,swell by 50 mM external ATP. In agreement with the idea that ATP enters the permeation pathway of ICl,swell we estimated that ATP sits on a binding site located 32% within the electrical field and from the reversal potential shift we estimated a PATP/PCl ratio of 0.07. Our estimates are similar to values previously reported in mouse C127 cells [11].
Figure 2. ICl,swell is inhibited by CBX.
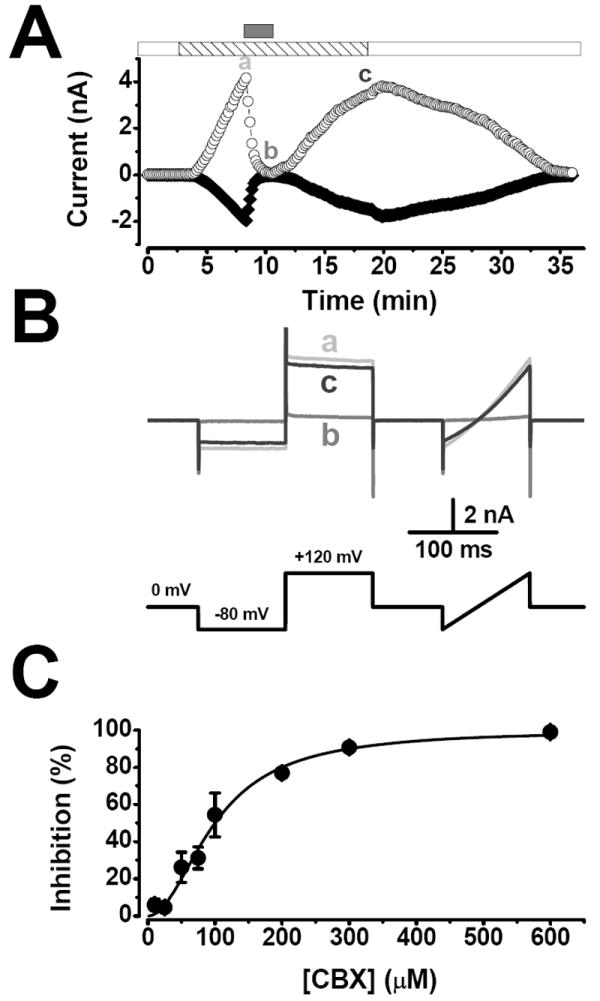
A.- Time course of ICl,swell activation at +120 (○) and −80 mV (◆) by exposure to hypotonic TEACl solution (striped bar). CBX (600 μM; gray bar) reversibly inhibited ICl,swell. After washing CBX with a hypotonic solution IClswell was turned off by a hypertonic TEACl solution (white bar). B.- Representative current traces at the time indicated: a (hypotonic solution alone), b (CBX in a hypotonic solution) and c (hypotonic solution alone). The voltage protocol used is shown below the traces. C.- Dose-response curve for inhibition of ICl,swell by CBX. Percentage of inhibition at +120 mV was calculated relative to inhibition observed with 600 μM CBX. Continuous line is the fit of equation 1 with IC50 of 98 μM. n=3-8.
To test whether the effect of CBX on ICl,swell has a component mediated by panx-1, we used the mimetic peptide 10panx1 which inhibits a variety of panx-1-dependent phenomena, including ion conduction, dye uptake and interleukin-1β processing and release [4]. If the inhibitory effect of CBX on ICl,swell involves panx-1, then a similar inhibitory effect of 10panx1 would be expected. Cells were preincubated during 30 minutes with 500 μM 10panx1 in hypertonic SPG. Figure 3A shows that 10panx1 (cross-hatched bar) completely inhibited EtBr uptake in P2X7-transfected cells stimulated with 2.5 mM ATP (black bar). Partial recovery of dye uptake was observed after a 7.5 minutes washout period. Recovery was evident since a second ATP application induced dye-uptake (Fig. 3A). In contrast, under the same conditions 10panx1 did not inhibit ICl,swell. Figure 3B (lower time course labelled as “peptide”) shows a typical IClswell time course recorded at +80 mV from a cell pre-incubated during 30 minutes with 500 μM 10panx1 in hypertonic solution. A hypotonic challenge (striped bar) was applied in the presence of 10panx1 (cross-hatched bar) which resulted in activation of ICl,swell. After 5 minutes wash with 10panx1-free hypertonic solution, a second hypotonic challenge was applied resulting in faster ICl,swell activation. Control experiments (Fig. 3B upper time course labelled as “control”) to evaluate an effect of 10panx1 on ICl,swell activation show no differences between control and peptide-treated cells. ICl,swell amplitude during the first hypotonic challenge was quite similar in both groups of cells, as well as the slope ratio for the current activation time course during the second and first hypotonic challenges (see summary in Fig. 3B bar graphs). This result strongly suggests that panx-1 has no influence on ICl,swell activation. Hence, it is unlikely that the inhibitory effect of CBX on ICl,swell could be mediated by panx-1.
Figure 3. The mimetic peptide 10panx1 inhibits ethidium bromide uptake but no ICl,swell.

A.- P2X7-transfected HEK-293 cells were pre-incubated during 30 minutes with 500 μM 10panx1 in hypertonic SPG solution. Fluorescence time course of a representative cell (n=4) after the pre-incubation period is shown. In the presence of 10panx1 (cross-hatched bar) cell was stimulated with 2.5 mM ATP (black bar). ATP and 10panx1 peptide were then washed out and a second ATP stimulation was applied. B.- Representative time courses of IClswell at +80 mV of non-transfected HEK-293 cell. Cell was exposed to hypertonic SPG solution and then two consecutive challenges with hypotonic SPG solution were applied (striped bars). Control cell (upper trace) was not exposed to 10panx1, while test cell (lower trace) were pre-incubated during 30 minutes with 500 μM 10panx1 in hypertonic SPG solution; the cell remained in the presence of the peptide (cross-hatched bar) until the end of the first hypotonic challenge. Bar graphs.- Left: IClswell amplitude in control and peptide treated cells evoked by the first hypotonic challenge. Right: slope ratio; slopes were calculated from the activation phase of the time courses evoked by the second (slope 2) and first hypotonic challenges (slope 1). C=Control; P=peptide-treated cells; n=5 for both experimental groups.
Since cell swelling did not induce dye uptake and 10panx1 abolished dye uptake but did not inhibit IClswell, then our data argue against the idea that panx-1 activation by cell swelling could be a universal mechanism. More likely, particular conditions or mechanisms not present in HEK-293 cells work to engage panx-1 activation by swelling in erythrocytes [8]. Furthermore, ICl,swell is inhibited by CBX by a mechanism independent of panx-1 and ICl,swell and panx-1 constitute two independent pathways that are differentially activated in HEK-293 cells.
Of physiological interest is the ATP release induced by mechanical stress in different cell types, including HEK-293, astrocytes, epithelial cells, erythrocytes and fibroblasts [16,17]. Based on the inhibitory effect that CBX has on swelling-induced ATP release from erythrocytes it has been suggested that panx-1 could function as a pathway for ATP release [8]. However, the sole inhibitory effect of CBX on ATP release does not necessarily imply that panx-1 is involved in this process. CBX also inhibits the enzyme 11β-hydroxysteroid dehydrogenase [18], calcium channels [19] and ICl,swell as we show here. Hence, we would suggest that inhibition of ATP release by CBX could be due to blockade of ATP-permeable swelling-activated anion channels. In support of this idea recent evidence shows that maxi-anion channels but not panx-1 [20] are responsible for ATP release in astrocytes [20], cardiomyocytes [21] and macula densa [22].
Acknowledgments
We thank Dr. Pablo Pelegrin for valuable comments. This work was supported by grants from CONACyT (59889 to JA, 79897 to JA, 45895 to PPC) and NIH (PO1-HL18208 to R. Waugh and JA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 2.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin-1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 3.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, Prevarskaya N. Functional implications of calcium permeability of the channel formed by pannexin-1. J Cell Biol. 2006;174:535–546. doi: 10.1083/jcb.200601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. J Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.08.036. 10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 11.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1:311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 13.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 14.Reyes JP, Pérez-Cornejo P, Hernández-Carballo CY, Srivastava A, Romanenko VG, Gonzalez-Begne M, Melvin JE, Arreola J. Na+ modulates anion permeation and block of P2X7 receptors from mouse parotid glands. J Membr Biol. 2008;223:73–85. doi: 10.1007/s00232-008-9115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson PS, Strange K. Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. J Gen Physiol. 1995;105:661–676. doi: 10.1085/jgp.105.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyot AM, Evagelidis A, Gibson C, Xavier GD, Rutter GA, Hanrahan JW. Luciferase mutant expressed in HEK cells reveals transmembrane ATP efflux during mechanical stimulation. FASEB J. 2005;19:A659–A659. [Google Scholar]
- 17.Franco R, Panayiotidis MI, de la Paz LD. Autocrine signaling involved in cell volume regulation: the role of released transmitters and plasma membrane receptors. J Cell Physiol. 2008;216:14–28. doi: 10.1002/jcp.21406. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh D, Wawrzak Z, Pletnev V, Erman M, Duax WL, Pangborn W, Zhu DW, Labrie F, Lin SX. Molecular mechanism of inhibition of steroid dehydrogenases by licorice-derived steroid analogs in modulation of steroid receptor function. Ann NY Acad Sci. 1995;761:341–343. doi: 10.1111/j.1749-6632.1995.tb31388.x. [DOI] [PubMed] [Google Scholar]
- 19.Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca2+ channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- 20.Liu HT, Sabirov RZ, Okada Y. Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal. 2008;4:147–154. doi: 10.1007/s11302-007-9077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutta AK, Sabirov RZ, Uramoto H, Okada Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol. 2004;559:799–812. doi: 10.1113/jphysiol.2004.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell PD, Lapointe JY, Sabirov RZ, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]


