Abstract
Study Objective:
To examine the long-term effects of continuous positive airway pressure (CPAP) therapy on blood pressure (BP) in patients with obstructive sleep apnea and resistant hypertension.
Methods:
Study subjects were 98 patients with obstructive sleep apnea syndrome and hypertension who had 3 or more documented daytime BP measurements taken within 3 months of enrollment and every 3 months after CPAP initiation for 1 year. Resistant hypertension was defined as daytime BP of at least 140 mm Hg systolic or 90 mm Hg diastolic, despite the use of 3 or more antihypertensive medications. Patients in the resistant hypertension group (n = 42) were compared with subjects with controlled hypertension (n = 56).
Results:
Mean difference in mean arterial pressure was −5.6 (95% confidence interval [CI] −2.0 to −8.7 mm Hg; p = 0.03) in the resistant group and −0.8 mm Hg (95% CI −2.9 to 3.3 mm Hg; p = 0.53) in patients with controlled BP at the end of follow up period. CPAP permitted de-escalation of antihypertensive treatment in 71% of subjects with resistant hypertension but did not significantly alter the antihypertensive regimen in the controlled group. Multivariate regression analysis showed that baseline BP (odds ratio 5.4, 95% CI 2.3 to 8.9; p = 0.01) and diuretic therapy (odds ratio = 3.2, 95% CI 1.8 to 6.1; p = 0.02), but not apnea-hypopnea index or hours of CPAP use, were independently associated with a decrease in mean arterial pressure after 12 months of CPAP therapy.
Conclusion:
In this observational study, CPAP was associated with different effects on blood pressure control in hypertensive patients with sleep apnea. A beneficial response to CPAP therapy was found mainly in subjects with the most severe hypertensive disease.
Citation:
Dernaika TA; Kinasewitz GT; Tawk MM. Effects of nocturnal continuous positive airway pressure therapy in patients with resistant hypertension and obstructive sleep apnea. J Clin Sleep Med 2009;5(2):103–107.
Keywords: Sleep apnea, CPAP, hypertension, resistant
Several studies have confirmed the relationship between obstructive sleep apnea syndrome (OSAS) and hypertension.1,5 This relationship has long been recognized and has been attributed to the effects of hypoxemia,6,8 hemodynamic effects of intrathoracic pressure changes,9,10 interruptions in respiration,11,12 or to disruption of sleep.13,14,15
Clinical trials of continuous positive airway pressure (CPAP) represent another line of investigation into the causal role of sleep apnea in hypertension.16 Several studies have shown that CPAP reduces sympathetic activity,17,18 decreases production of free-oxygen radicals and circulating levels of some markers of inflammation,19,20 and reverses the endothelial dysfunction associated with OSAS.21 Therefore, hypothetically, CPAP should improve blood pressure (BP) control in patients with OSAS. In recent years, conflicting results from several clinical studies22,23,24,25 have shown either a clear improvement or only minor but insignificant reductions in BP measurements. However, most of these studies enrolled a relatively small number of participants or did not examine the long-term effects of CPAP therapy on BP in patients with OSAS. To date, there is still no clear agreement whether long-term CPAP therapy improves BP control in patients with OSAS and hypertension, and there is little evidence that the use of nocturnal positive pressure therapy can affect BP in patients suffering from OSA and resistant hypertension.
In this study, we examined whether treating OSA can lower BP in patients who remain hypertensive despite medical therapy. We also investigated whether CPAP therapy affects antihypertensive treatment among patients who are using CPAP for sleep disordered breathing.
METHODS
Study Design
This was a retrospective chart review study. We reviewed the medical records of all patients who had a polysomnography with CPAP study at the Veteran Affairs Medical Center in Oklahoma City and the University of Oklahoma Health Sciences Center.
Inclusion criteria were age at least 18 years of age, a diagnosis of OSAS, a history of hypertension on drug therapy, and 3 or more documented daytime BP measurements obtained within 3 months of enrollment and every 3 months for 6 months and then 1 year from CPAP initiation. Resistant hypertension was defined as a daytime blood pressure of at least 140 mm Hg systolic or at least 90 mm Hg diastolic, despite stable use of a combination of 3 or more antihypertensive medications. BP measurements were obtained from noninvasive measurements using a sphygmomanometer performed during routine examinations and monitoring of screened subjects.
Exclusion criteria were a documented history of noncompliance with drug treatment or CPAP therapy; use of medications that can raise BP; presence of any secondary form of hypertension, including renal insufficiency (defined as a serum creatinine level of at least 1.50 mg/dL); presence of renal artery stenosis; or positive results from a hormone work-up for secondary hypertension (defined as abnormal urine or serum catecholamine levels, plasma cortisol, rennin, aldosterone, or thyroid function tests). Other exclusion criteria were sustained excessive alcohol use and significant modification in drug regimen within 3 months of the study.
Sleep Studies
The diagnosis of OSAS was based on standard polysomnography performed using standard techniques and scoring criteria. Obstructive sleep apnea was defined as an apnea-hypopnea index (AHI) of at least 5 events per hour. Arousals were defined according to the recommendations of the American Sleep Disorders Association Atlas task force.26 Sleep data were staged according to the system of Rechtschaffen and Kales.27
Data Analysis
Demographic characteristics, polysomnography data, comorbidities, and medications were recorded. Two groups of patients were identified at baseline. The first group consisted of patients with controlled hypertension on 2 or fewer drugs, whereas the second group included patients with resistant hypertension, as defined above. The primary outcome variable was change in mean arterial pressure at the end of the follow-up period. Secondary outcomes measured were change in systolic and diastolic BP as well as antihypertensive treatment among different patient groups at the end of follow-up period.
Statistical software (version 4.0; JMP; Cary, NC) was used for data processing and statistical analysis. Continuous variables were expressed as mean ± SD, and categorical variables were expressed as a percentage. A 2-tailed t test for independent samples or a χ2 test was used to compare baseline variables in the 2 groups. Changes in BP within groups were assessed using the paired t test.
Finally we constructed a multivariate logistic regression model to predict variables associated with change in BP in hypertensive patients. The dependent categorical variable was a decrement of 10% or more of the mean arterial pressure at 12 months, compared with baseline, whereas independent variables included in the model were age, comorbidities, weight change, medication use, baseline mean arterial blood pressure, baseline AHI, and hours of CPAP use.
RESULTS
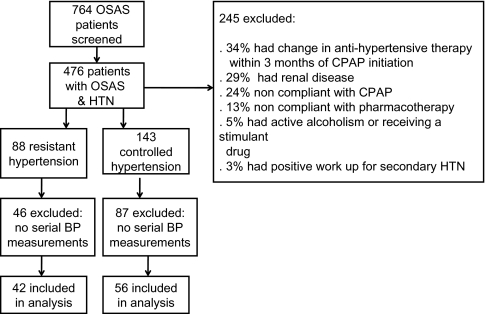
Seven hundred sixty-four patients were screened for entry into the study. Ninety-eight subjects were found to be eligible after meeting the inclusion and exclusion criteria (Figure 1). Of those, 42 patients had resistant hypertension, as defined in the methods. Demographic characteristics and polysomnography data were similar in the two groups of patients. Patients with resistant hypertension (n = 42) had higher arterial pressure measurements and were receiving more antihypertensive agents, as was expected (Table 1).
Figure 1.
Enrollment chart. Patients may have had more than 1 reason for exclusion. OSAS refers to obstructive sleep apnea; BP, blood pressure; CPAP, continuous positive airway pressure; HTN, hypertension.
Table 1.
Patient Characteristics at Baseline
| Controlled hypertension (n = 56) |
Resistant hypertension (n = 42) |
p Value | |
|---|---|---|---|
| Age, y | 56.1 ± 1.4 | 61.8 ± 8.9 | NS |
| BMI, kg/m2 | 37.7 ± 7.1 | 38.5 ± 10.2 | NS |
| Men/women, no. | 54/2 | 41/1 | NS |
| Smoker, no. (%) | 12 (23) | 10 (21) | NS |
| Co-occurring medical condition, no. (%) |
|||
| Diabetes | 14 (25) | 9 (21) | NS |
| Dyslipidemia | 19 (34) | 15 (38) | NS |
| COPD | 8 (14) | 5 (12) | NS |
| CAD | 13 (23) | 13 (31) | NS |
| ESS, score | 12.7 ± 6.0 | 13.0 ± 6.5 | NS |
| Creatinine level, mg/dL |
1.02 ± 0.4 | 1.04 ± 0.35 | NS |
| AHI, no./h | 58.8 ± 32.2 | 60.1 ± 36.2 | NS |
| CPAP use, h | 6.1 ± 0.9 | 6.5 ± 1.2 | NS |
| Blood pressure, mm Hg | |||
| Systolic | 128 ± 8.3 | 146 ± 8.4 | 0.03 |
| Diastolic | 79.8 ± 6.4 | 84.8 ± 7.2 | 0.06 |
| Mean arterial | 96.2 ± 5.7 | 105 ± 5.7 | 0.02 |
| Antihypertensive use, no. (%) |
|||
| ACE inhibitor | 25 (44) | 37 (88) | 0.01 |
| Diuretic | 30 (53) | 33 (78) | 0.02 |
| β blocker | 29 (52) | 27 (64) | 0.01 |
| Calcium-channel blocker |
10 (18) | 18 (43) | 0.02 |
| Other agent | 3 (5) | 9 (21) | 0.03 |
| Antihypertensive drugsa |
1.5 ± 0.5 | 3.4 ± 0.8 | 0.01 |
| Follow-up, days | 368 ± 25 | 344 ± 32 | NS |
Data are presented as mean ± SD, unless otherwise indicated. BMI refers to body mass index; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; ACE, angiotensin converting enzyme inhibitor.
Antihypertensive drugs refers to the number of drugs used per person.
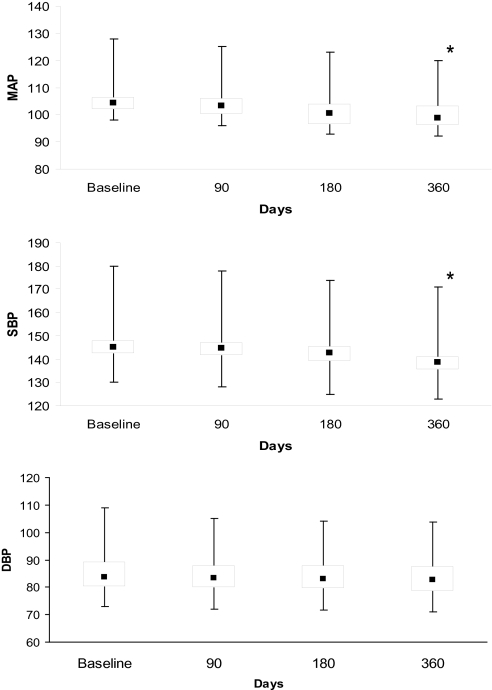
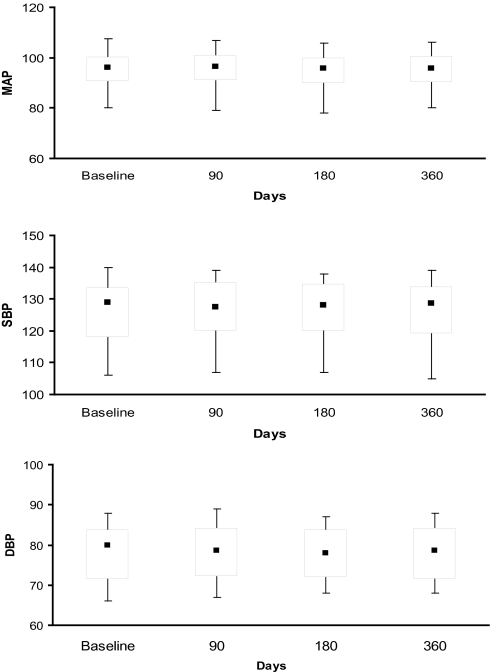
The box and whisker plots in Figure 2 illustrate that, in subjects with resistant hypertension, median mean arterial pressure and systolic BP moved toward lower values at 180 days, with a significant trend continuing at 12 months, whereas patients with controlled hypertension (n = 56) demonstrated no significant change over the same time period (Figure 3). No significant difference in diastolic measurements was seen in either group of patients. Mean difference in mean arterial pressure was −5.6 (95% confidence interval [CI] −2.0 to −8.7 mm Hg; p = .03) in the resistant hypertension group and −0.8 mm Hg (95% CI −2.9 to 3.3 mm Hg; p = .53) in patients with well-controlled BP at baseline. A trend toward a difference in the use of antihypertensive drugs was seen in the 2 groups of patients at end of the follow-up period (Table 2). In subjects with resistant hypertensive disease (n = 42), the antihypertensive drug regimen was unaltered in 12 patients (29%) but modified in 30 subjects (71%) by either dose reduction (n = 12) or by discontinuing 1 or more drugs (n = 18). Patients with controlled hypertension showed an overall stability in their treatment regimen. Only 6 patients (10%) required either escalation (n = 2) or change in their treatment (n = 4). Interestingly enough, 5 out of 14 subjects who were treated with a single agent at baseline were not taking any antihypertensive drugs at 12 months.
Figure 2.
Box whisker plots showing a trend in blood pressure measurements in patients with resistant hypertension. MAP refers to mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure. *p < 0.05 compared with baseline.
Figure 3.
Box whisker plots showing a trend in blood pressure measurements in patients with controlled hypertension. MAP refers to mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 2.
Antihypertensive Therapy and Body Mass Index at Baseline and 1 Year After Initiation of CPAP
| Antihypertensive agent use, no (%) | Controlled Hypertension |
Resistant Hypertension |
||
|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | |
| ACE inhibitor | 25 (44) | 24 (43) | 37 (88) | 34 (81) |
| Diuretic | 30 (53) | 28 (50) | 33 (78) | 30 (71) |
| β blocker | 29 (52) | 30 (53) | 27 (64) | 25 (59) |
| Calcium-channel blocker | 10 (18) | 8 (14) | 18 (43) | 13 (31)a |
| Other | 3 (5) | 2 (3) | 9 (21) | 4 (9)a |
| Antihypertensive drugsb | 1.5 ± 0.5 | 1.4 ± 0.6 | 3.4 ± 0.8 | 2.8 ± 0.05 |
| BMI, kg/m2 | 37.7 ± 7.1 | 36.2 ± 9.1 | 38.5 ± 10.2 | 37.8 ± 9.9 |
Data are presented as mean ± SD, unless otherwise indicated. CPAP refers to continuous positive airway pressure; ACE, angiotensin converting enzyme inhibitor; BMI, body mass index.
p < 0.05;
Antihypertensive drugs refers to the number of drugs used per person.
The results of multivariate regression analysis showed that predictors of a decrease in mean arterial pressure by 10% at 12 months were hypertension severity at baseline (odds ratio [OR] 5.4, 95% CI 2.3 to 8.9; p = 0.01) and diuretic therapy (OR = 3.2, 95% CI 1.8 to 6.1; p = 0.02) but not baseline AHI (OR = 1.4, 95% CI 0.60 to 3.8; p = 0.35) or hours of CPAP use (OR = 0.4, 95% CI 0.9 to 2.2; p = 0.42)
Discussion
Several trials have investigated the effect of CPAP on BP in patients with OSAS. CPAP has been reported to lower nighttime and daytime BP in hypertensive individuals with OSAS.28 The use of CPAP therapy was also been associated with reduction in systolic and, to a lesser extent, diastolic BP in patients with resistant hypertension after 8 weeks of therapy.29 Conversely, Campos-Rodriguez and colleagues reported no significant changes in systolic, diastolic, daytime, or nighttime BP in patients with well-controlled hypertension.30 More recently, investigators from the same group followed 55 hypertensive patients with sleep apnea for 24 months after CPAP initiation.31 A modest but statistically significant decrease in mean arterial pressure (−4.4; 95% CI, −7.9 to −0.9 mm Hg) at the end of follow-up period was observed on an intention-to-treat analysis. Noting that hypertension was relatively well controlled with drug therapy in this cohort of patients, the researchers found that the subjects with higher baseline BP and better compliance with CPAP showed the most significant improvement with treatment.
In this retrospective study, we provide several new observations on the effect of CPAP therapy in patients with OSAS and hypertension. First, the use of CPAP therapy was accompanied by a reduction in daytime BP in patients with resistant hypertension; this reduction was first apparent at 6 months after treatment initiation and was sustained after 12 months of therapy. Second, CPAP therapy permitted deescalation of antihypertensive drug therapy in a significant proportion of patients with resistant hypertension. Third, the effects of CPAP therapy on BP regulation appear to be less noticeable in hypertensive patients controlled on drug regimen.
This study differs from the previous investigations because we examined specifically the long-term effect of CPAP therapy in a particular group of patients with hypertension and found a different effect of CPAP on BP control in patients with resistant versus well-controlled hypertension. We did not observe a measurable significant change in arterial pressure after 3 months of CPAP had elapsed in patients with resistant hypertension. These results are consistent with the findings by Campos-Rodriguez and colleagues in their 2 studies because achieving a significant reduction in BP may take longer than a few months. This could be explained by the effect of CPAP on the mechanisms underlying hypertension. Although, the reversal of sustained sympathetic activation may be seen shortly after CPAP application, control of other neuronal, humoral, and vascular factors may take a longer time to be brought under control.32
The trend in antihypertensive therapy examined in this study further supports the beneficial effect of CPAP in this patient population. There is general agreement derived from meta-analyses of the benefits of antihypertensive treatment. A reduction of mean arterial BP by 5% to 10% in hypertensive patients has the potential to reduce stroke incidence by at least 40%.33,34 Although CPAP alone is unlikely to achieve such reduction in BP, our study showed a positive impact of CPAP on arterial BP. particularly in patients with uncontrolled hypertensive disease.
A few limitations of this study should be noted. Given the retrospective nature of our study, compliance with pharmacotherapy and CPAP were assessed by clinical data obtained from review of patients' charts. We also recognize that BP measurements were recorded during clinic visits and did not follow the American Heart Association guidelines for definition of resistant hypertension because no ambulatory recordings were available to include in this analysis. We did exclude secondary causes for hypertension, with the exception of obesity; however, patients kept a relatively constant body mass index during the entire observation period, and, as assess using multivariate analysis, weight change was not associated with a decrement in BP measurements at the end of the follow-up period.
In summary, we conclude that the long-term use of CPAP therapy is associated with improved BP control in patients with OSAS and resistant hypertension, as reflected by a reduction in mean arterial pressure and a decreased trend in the use of antihypertensive therapy. Randomized larger trials in this particular group of patients are needed to confirm these results.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–65. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard P, Palta M. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 3.Peppard P, Young T, Palta M. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Hla KM, Young TB, Bidwell T. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120:382–8. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Grote L, Hedner J, Peter JH. Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J Hypertens. 2000;18:679–85. doi: 10.1097/00004872-200018060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shepard JW., Jr. Gas exchange and hemodynamics during sleep. Med Clin North Am. 1985;69:1243–64. doi: 10.1016/s0025-7125(16)30985-3. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell CP, Ayuse T, King ED. Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol. 1996;80:773–81. doi: 10.1152/jappl.1996.80.3.773. [DOI] [PubMed] [Google Scholar]
- 8.Cooper VL, Pearson SB, Bowker CM. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia—a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol. 2005;568:677–87. doi: 10.1113/jphysiol.2005.094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buda AJ, Pinsky MR, Ingels NB., Jr. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301:453–9. doi: 10.1056/NEJM197908303010901. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd TC., Jr. Effect of inspiration on inferior vena caval blood flow in dogs. J Appl Physiol. 1983;55:1701–8. doi: 10.1152/jappl.1983.55.6.1701. [DOI] [PubMed] [Google Scholar]
- 11.Howell JB, Permutt S, Proctor DF. Effect of inflation of the lung on different parts on pulmonary vascular bed. J Appl Physiol. 1961;16:71–6. doi: 10.1152/jappl.1961.16.1.71. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman MP, Iwamoto GA, Ashton JH. Response to inflation of vagal afferents with endings in the lungs of dogs. Circ Res. 1982;51:525–31. doi: 10.1161/01.res.51.4.525. [DOI] [PubMed] [Google Scholar]
- 13.Ringler J, Basner RC, Shannon R. Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. J Appl Physiol. 1990;69:2143–8. doi: 10.1152/jappl.1990.69.6.2143. [DOI] [PubMed] [Google Scholar]
- 14.Ali NJ, Davies RJ, Fleetham JA. The acute effects of continuous positive airway pressure on oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101:1526–32. doi: 10.1378/chest.101.6.1526. [DOI] [PubMed] [Google Scholar]
- 15.Davies RJ, Belt PJ, Roberts SJ. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74:1123–30. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- 16.Nieto FJ, Young TB, Lind BK. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 17.Hedner J, Darpo B, Ejnell H. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J. 1995;8:222–9. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 18.Narkiewicz K, Kato M, Phillips BJ. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–5. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 19.Schulz R, Mahmoudi S, Hattar K. Enhanced release of super oxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 20.Chin K, Nakamura T, Shimizu K. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109:562–7. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 21.Ip MS, Lam B, Chan LY. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 22.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension. 2000;35:144–7. doi: 10.1161/01.hyp.35.1.144. [DOI] [PubMed] [Google Scholar]
- 23.Faccenda JF, Mackay TW, Boon NA. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 24.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomized parallel trial. Lancet. 2001;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 25.Becker HF, Jerrentrup A, Ploch T. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 26.BEEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. US Department of Health, Education, and Welfare Public Health Service - NIH/NIND. 1968 [Google Scholar]
- 28.Somers VK. Management of sleep apnea. In: Izzo JL Jr, Black HR, editors. Hypertension Primer: the Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 512–6. [Google Scholar]
- 29.Logan AG, Tkacova R, Perlikowski SM. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–7. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 30.Campos-Rodriguez F, Grilo-Reina L, Perez-Ronchel J. Effect of continuous positive airway pressure on ambulatory bp in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129:1459–67. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 31.Campos-Rodriguez F, Perez-Ronchel J, Grilo-Reina L. Long-term effect of continuous positive airway pressure on bp in patients with hypertension and sleep apnea. Chest. 2007;132:1847–52. doi: 10.1378/chest.07-1478. [DOI] [PubMed] [Google Scholar]
- 32.Iellamo F, Montano N. Continuous positive airway pressure treatment: good for obstructive sleep apnea syndrome, maybe not for hypertension? Chest. 2006;129:1403–5. doi: 10.1378/chest.129.6.1403. [DOI] [PubMed] [Google Scholar]
- 33.Law MR, Wald NJ, Morris JK. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Psaty BM, Smith NL, Siscovick DS. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277:739–45. [PubMed] [Google Scholar]