Abstract
Study Objectives:
To evaluate the relation among several symptoms that occur commonly in cancer patients: trouble sleeping, fatigue/sleepiness, depressed mood, and pain in a large cohort of cancer patients undergoing treatment in a community oncology practice.
Methods:
Demographic, clinical, and patient reported outcomes data from 11,445 cancer patients undergoing treatment in a large community oncology practice were analyzed using structural equation modeling. The data were split so that a model was constructed using half of the patients; this model was then cross-validated on the remaining patients.
Results:
Fatigue was best represented as a latent variable, and significant direct effects were found for trouble sleeping, depressed mood, and pain. Also, there were significant indirect effects of these variables on fatigue. The effect of depressed mood on fatigue and pain was mediated by trouble sleeping, and the effect of trouble sleeping on fatigue was mediated by pain.
Conclusions:
These results predict that interventions aimed at treatment of trouble sleeping, depressed mood, and pain will improve fatigue in patients with cancer. Further, these data predict that treatment of trouble sleeping will improve pain management in this population.
Citation:
Stepanski EJ; Walker MS; Schwartzberg LS; Blakely LJ; Ong JC; Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med 2009;5(2):132-136.
Keywords: Fatigue, insomnia, pain, depression, cancer
Patients undergoing treatment for cancer experience a myriad of disease- and treatment-related symptoms. Fatigue, excessive daytime sleepiness, pain, depressed mood, and disturbed sleep are among the most common symptoms self-reported by patients undergoing treatment for cancer.1–4 The rate of insomnia in cancer patients exceeds that of the general population and varies according to the definition used to make this diagnosis. The percentage of patients meeting formal criteria for an insomnia disorder has been reported at 19%,5 and patients self-reporting disturbed sleep is as high as 63%.6 Nocturnal sleep duration, as measured objectively by ambulatory polysomnography, has been shown to be shorter in patients with cancer.7 In addition to decreased total sleep time at night, patients demonstrate increased sleep fragmentation and increased sleep time during the day.
Understanding the causal direction among these symptoms will lead to improved symptom management strategies. This is especially important in the context of cancer, in which symptom management is needed to assure delivery of optimal anti-neoplastic treatment. Fatigue can be a dose-limiting toxicity, leading to dose reduction or treatment discontinuation, thereby reducing treatment efficacy. Several recent studies have shown a systematic relation between sleep, depression, pain, and fatigue. For example, insomnia has been linked with increased rates of depression, decreased quality of life, and increased fatigue in other patient populations.8–10 Recent work has also shown decreased pain thresholds in research subjects following sleep deprivation.11 Clinical management of cancer is complicated by these symptoms, and supportive care interventions may be needed to facilitate the successful delivery of planned treatment regimens.
It is difficult to understand the significance of a single symptom in isolation since there are complex patterns of co-variation expected among these symptoms. In the past it was thought that insomnia occurred as a consequence of depression, such that the term secondary insomnia was used to describe insomnia in the presence of depression. Studies have now shown that individuals with chronic insomnia are at increased risk to develop depression,12 suggesting a bi-directional relation between these conditions. Fatigue and pain have both been shown to commonly co-occur with insomnia. The same pattern has been noted in the relation between pain and disturbed sleep, such that pain is noted to cause insomnia, and short sleep causes increased pain.11,13 Although these relationships have been shown in non-cancer patient populations, they would be expected to extend to cancer patients.
Awareness of the common co-occurrence of cancer-related symptoms has led to emerging research on a set of symptoms, or symptom cluster, rather than individual symptoms in isolation. A symptom cluster has been defined as a group of symptoms that are interrelated but do not necessarily share a common etiology.14 Pain, fatigue, sleep disturbance, and depressed mood have received notable attention as a symptom cluster.3,4,14,15 One study found that a subgroup of outpatients who reported high levels on all symptoms in the cluster reported the lowest quality of life scores.3 Another study found a 3-way interaction between pain, fatigue, and insomnia, with no significant change when covariates of age, comorbidities, and stage of cancer were added.4 Understanding how these common symptoms affect quality of life and treatment outcome in the context of cancer is important but difficult.
While previous research has focused on the identification of a symptom cluster or subgroup of patients with a specific symptom profile, the relationship among these symptoms in the context of cancer remains unclear. Small sample sizes typically limit the statistical analyses that can be conducted to examine the potential effects of one variable on another. However, when adequate sample sizes are available, structural equation modeling (SEM) provides a framework for simultaneously examining the relationship among many variables. This includes modeling of causal relations among variables, examination of direct and indirect effects, modeling of variables as latent or observed, and testing of competing models.16,17 Employing SEM to examine data from a large sample of cancer patients could yield important insights into the causal relationships among variables in a symptom cluster.
To our knowledge, SEM has not been previously used to investigate the relationship among insomnia, fatigue, pain, and depressed mood in cancer, particularly with a cross-section of patients such as reported in this study. Therefore, the aim of this paper was to examine the relationship among these variables in a sample of patients with cancer, heterogeneous with respect to tumor type and stage of treatment, using SEM. This analysis was intended to be exploratory, and specifically to evaluate the interrelationships among these specific symptoms to develop a causal model to inform further research in this area.
METHODS
Participants
Cross-sectional patient-reported outcomes from 11,445 consecutive patients evaluated at the West Clinic in Memphis, Tennessee, were analyzed in the present study. Patients completed the Patient Care Monitor as a standard part of their clinical care.
Measures
The Patient Care Monitor (PCM; Supportive Oncology Services, Memphis, TN) is a validated software package assessing oncology-related patient symptoms, using an 11-point Likert scale (Figure 1).17,18 Each item has anchors displayed with each numeral as follows: 0: Not a problem, 1-3: Mild problem, 4-6: Moderate problem, 7-9: Severe problem, and 10: As bad as possible. This instrument is administered routinely to patients at each office visit through a tablet computer. The instrument assesses 86 individual symptoms and generates 6 indices that describe global function. The specific patient-reported symptoms extracted for analysis were depressed mood, trouble sleeping, physical pain, and 2 items addressing fatigue: tiredness/weakness and daytime sleepiness. The disease and demographic characteristics were age, gender, ethnicity, disease site (breast cancer, lung cancer, or other disease site), and recent chemotherapy administration (within past 30 days).
Figure 1.
Screen shot for Patient Care Monitor item assessing fatigue
Data Analysis
Structural equation modeling (SEM) analysis was employed to examine the relations among patient reported outcomes and individual and disease characteristics. SEM is a general method that allows formulation and testing of a statistical model of the relations among variables. SEM is often described as causal modeling because the method typically examines the cause and effect relations among variables. A regression equation is a simple example of a causal model. SEM extends this simple causal model in 2 ways. First, it allows for simultaneous estimation of many regression equations, each controlling for other variables in the model. Second, it allows us to infer the existence of an unobserved (latent) variable from the pattern of correlations present among observed variables. For example, suppose we observe correlations among variables related to decreased enjoyment, hopelessness, and sadness. We might infer the presence of an underlying shared cause of these symptoms (depression) that accounts for the correlations among them. SEM allows us to model the presence of that underlying variable, and to examine its role as either a cause or an effect of other variables. A further strength of SEM is that, in addition to testing of individual coefficients, the overall model can be evaluated to assess its fit with the data.
In our application of SEM, the study sample of 11,445 patients was randomly split into training and validation halves, with all exploratory and model building analyses conducted on the training sample. Structural equation modeling was limited to complete cases, and this reduced the sample for this stage of the analysis to 9,504 cases. The training sample had 4,713 cases, and the model validation sample had 4,791 cases. Models were estimated using EQS version 6.1. Comparative fit index (CFI) and standardized root mean square residual (SRMR) were employed as the primary criteria of model fit, with cutoff values of CFI > 0.95 and SRMR < 0.08 interpreted to indicate good model fit.20 Chi-square and degrees of freedom for the models are also reported. Disease site was modeled as 3-level, corresponding to breast cancer, lung cancer, or other disease site. Ethnicity was also modeled as 3-level, with patients coded as Caucasian, African American, or Other/unspecified ethnicity. For disease site and ethnicity, specific categories collapsed into other were either of low frequency or were shown in preliminary analysis to not significantly predict the study outcomes.
Results
The demographics and clinical data are presented in Table 1. Approximately three-fourths of the sample was female, and breast cancer was the most common tumor site. Over half the sample reported having trouble sleeping, with 26% reporting moderate or severe trouble sleeping (score ≥ 4 on PCM item).
Table 1.
Demographics and Clinical Characteristics of Sample
| Age (SD) | 61.5 (14.2, range 18-95) |
| Sex | 74.3% female |
| Race | |
| African American | 1691 (14.7%) |
| Caucasian | 7915 (68.8%) |
| Other | 1849 (16.1%) |
| Not specified | 44 (0.4%) |
| Tumor site | |
| Breast | 3,316 |
| Genitourinary | 2,966 |
| Gastrointestinal | 1,634 |
| Hematologic | 1,373 |
| Lung | 1,224 |
| Head and Neck | 501 |
| Skin | 321 |
| Received chemotherapy within 30 days | 24.9% |
| Report of any trouble sleeping | 55% |
| Report of “moderate or severe” trouble sleeping | 26% |
Mean values from patient reported symptoms on the PCM are reported in Table 2; 21.9% of patients without trouble sleeping had recent chemotherapy, compared with 27.5% of patients with trouble sleeping who had recent therapy (p < 0.001). Compared to patients who reported no trouble sleeping, patients with moderate to severe trouble sleeping were significantly younger and reported significantly more fatigue, pain, and depressed mood (all p < 0.001).
Table 2.
Mean Ratings of Patient Reported Symptoms from the Patient Care Monitor
| No Insomnia n = 5,151 | Mod-Severe Insomnia n = 3,031 | t | p-value | |
|---|---|---|---|---|
| Age | 62.1 | 59.7 | 7.17 | < 0.0001 |
| Fatigue or tiredness | 2.38 | 5.23 | −45.53 | < 0.0001 |
| Physical pain | 1.58 | 4.33 | −40.38 | < 0.0001 |
| Sad or depressed | 0.69 | 2.56 | −29.56 | < 0.0001 |
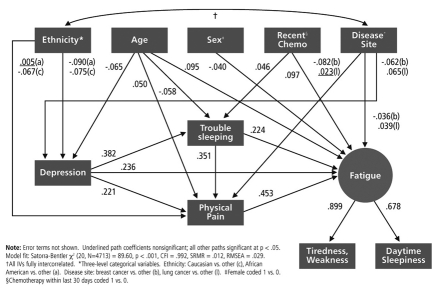
A Path Diagram representing the model development results is shown in Figure 2. As shown in the figure, fatigue was modeled as a latent variable, directly influenced by depressed mood, trouble sleeping, and physical pain, as well as by several demographic and disease characteristics. We examined a number of competing models of the relations among the patient reported outcomes, including models in which pain caused sleep problems rather than vice versa, and in which latent fatigue was modeled as a cause rather than an effect. Although the competing models were not nested (and therefore not statistically comparable), the model presented in Figure 2 appeared to be the best fitting among the models examined as indicated by fit indices. Overall model fit appeared quite good, Satorra-Bentler χ2 (20, N = 4713) = 89.60, p < 0.001, CFI = 0.992, SRMR = 0.012. We also attempted to fit nonrecursive models of the role of pain, given its plausible role as an effect and also a cause of depressed mood, sleep difficulty, and fatigue. However, despite inclusion of instrumental variables, the models appeared under-identified, and the estimation failed to converge.
Figure 2.
Structural equation model of pain, depressed mood, trouble sleeping, and fatigue in cancer patients
In addition to the direct effects among depressed mood, trouble sleeping, physical pain, and fatigue, we also tested for the presence of indirect effects with the Sobel test of mediation within EQS version 6.1.21 Results of these analyses indicated that in addition to the significant direct effects shown in Figure 2, the effect of depressed mood on physical pain is mediated by trouble sleeping, the effect of trouble sleeping on fatigue is mediated by physical pain, and the effect of depressed mood on fatigue is mediated by trouble sleeping and physical pain. Indeed, the analysis indicates that less than half of the effect of depressed mood on fatigue is direct. Over half of the effect is carried by trouble sleeping and by pain. These indirect effects were all significant at p < 0.05.
Cross-validation of this model with the validation sample supported the fit of the model to the data, χ2 (20, N = 4791) = 116.47, p < 0.001, CFI = 0.990, SRMR = 0.013. Individual model parameters varied only slightly from the model shown in Figure 2; the most notable change was that the effect of sex on fatigue—already a very small effect in the training sample—dropped to nonsignificance in the validation sample. The direction, magnitude, and significance of other parameters were essentially unchanged.
Discussion
The present study examined the relationship between trouble sleeping, depressed mood, pain, and fatigue in a large sample of patients with cancer, as measured with a standardized, validated, self-report instrument. As expected, trouble sleeping was found to occur commonly in patients with cancer, and was associated with significantly increased fatigue, pain, and depressed mood. While this is not surprising, systematic measurement of sleep parameters has often been neglected in studies of cancer-related fatigue. A consensus statement recommended routine evaluation of sleep disorders after review of the literature in this area.22
There were moderate to strong associations among depressed mood, pain, trouble sleeping, and fatigue in this sample. In the best fitting model, the SEM analysis showed that trouble sleeping led to increased ratings of pain. The relation between pain and sleep often has been assumed to be reciprocal—pain can lead to disturbed sleep, and vice versa.23 In the present study, a model of reciprocal causation could not be fit to the data, and models in which pain caused trouble sleeping rather than vice versa fit less well than the model as specified in Figure 2. However, an implication of the present data is that attenuation of trouble sleeping will improve pain control in patients with cancer.
The analysis led to an unexpected finding regarding the role of age. In contrast to the general population, younger age was associated with increased trouble sleeping in patients with cancer. This may reflect that the causes of sleep disturbance in these patients are related to disease- and treatment-related factors, as opposed to being an expected consequence of healthy aging. This is supported by the finding in these data that recent administration of chemotherapy was also associated with increased trouble sleeping. Younger patients would be expected to have a better performance status, and therefore are more likely to receive more aggressive chemotherapy than an older patient with a poorer performance status. Therefore, younger patients may be exposed to more treatment-related toxicity. Future research will need to control for performance status, as well as use of specific chemotherapy drugs and regimens.
Fatigue ratings were found to increase with increases in trouble sleeping, depressed mood, and pain. While depressed mood acted directly to increase fatigue, it also acted indirectly through increasing pain and trouble sleeping. Based on this finding, an intervention targeting depressed mood would be expected to improve several downstream symptoms—fatigue, trouble sleeping, and pain. This is not to say that other symptoms should be ignored, but only to state that an aggressive program targeting a single symptom would be predicted to have more widespread benefit if depressed mood were the target. This is potentially very important as pain management is critically important in this population, and a complete understanding of relevant factors driving pain ratings will lead to better management programs. This finding illustrates the increased explanatory power available with SEM, and the importance of considering multiple symptoms simultaneously to more fully appreciate the complexity of the interrelationships of these symptoms.
A remaining question is the extent to which patients with cancer experience fatigue versus daytime sleepiness. Items assessing both domains were included in the present study, but it is well known that patients (and even occasional researchers) interchange these two constructs.24 Most patients will interpret a question about feeling “tired” in the same way was a question about feeling “sleepy.” Both items were included in our analysis, and as expected, these items were very highly inter-correlated. Future work in this area will need more complex measures of sleepiness to distinguish between fatigue and daytime sleepiness in cancer populations.
Sample size was a particular strength of this study. Nevertheless, there are several limitations that should be considered. We limited the analysis to one observation per patient. Inclusion of multiple observations per patient would have allowed for examination of change over time within patient. The analysis also did not control for when assessments were made relative to diagnosis, although some symptoms plausibly would vary over time. An additional consideration is that only two indicators of fatigue were available for analysis. This was sufficient to statistically identify the structural equation model, but additional indicators of fatigue would have been preferable. Finally, it is plausible that bidirectional causal influences exist among depressed mood, trouble sleeping, pain, and fatigue. However, these effects could not be modeled with the available data. As a result, the model presented here should be interpreted as fitting the data very well, but not as ruling out the possibility of reciprocal causation or feedback loops among the variables in question.
In summary, these data demonstrate significant interrelationships between several common symptoms in cancer patients: fatigue, depressed mood, trouble sleeping, and pain. Structural equation modeling in a large cohort of cancer patients found that depressed mood, trouble sleeping, and pain all exert a direct influence on fatigue ratings. Additionally, increases in depressed mood and/or trouble sleeping lead to increased pain. Since quality of life for patients with cancer is very much affected by these symptoms, interventions aimed at depressed mood and trouble sleeping would be expected to improve both pain and fatigue in this patient population.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D.Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Fox SW, Lyon DE. Symptom clusters and quality of life in survivors of lung cancer. Oncol Nurs Forum. 2006;33:931–6. doi: 10.1188/06.ONF.931-936. [DOI] [PubMed] [Google Scholar]
- 3.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman AJ, Given BA, von Eye A, et al. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum. 2007;34:785–92. doi: 10.1188/07.ONF.785-792. [DOI] [PubMed] [Google Scholar]
- 5.Savard J, Simard S, Blanchet J, et al. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–90. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 6.Koopman C, Nouriani B, Erickson V, et al. Sleep disturbances in women with metastatic breast cancer. Breast J. 2002;8:362–70. doi: 10.1046/j.1524-4741.2002.08606.x. [DOI] [PubMed] [Google Scholar]
- 7.Parker KP, Bliwise DL, Ribeiro M, et al. Sleep/wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26:2464–72. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SD, Patel SS, Khetpal P, et al. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:919–25. doi: 10.2215/CJN.00820207. [DOI] [PubMed] [Google Scholar]
- 9.Zammit GK, Weiner J, Damato N, et al. Quality of life in people with insomnia. Sleep. 1999;22(Suppl 2):S379–385. [PubMed] [Google Scholar]
- 10.Piperidou C, Karlovasitou A, Triantafyllou N, et al. Influence of sleep disturbance on quality of life of patients with epilepsy. Seizure. 2008;17:588–94. doi: 10.1016/j.seizure.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Roehrs T, Hyde M, Blaisdell B, et al. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Angst J, Gamma A, et al. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewes AM, Nielsen KD, Arendt-Nielsen L, et al. The effect of cutaneous and deep pain on the electroencephalogram during sleep--an experimental study. Sleep. 1997;20:632–40. doi: 10.1093/sleep/20.8.632. [DOI] [PubMed] [Google Scholar]
- 14.Miaskowski C, Aouizerat BE, Dodd M, et al. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J Natl Cancer Inst Monogr . 2007:39–46. doi: 10.1093/jncimonographs/lgm003. [DOI] [PubMed] [Google Scholar]
- 15.Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum. 2007;34:971–80. doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- 16.Bollen KA. Structural equations with latent variables. New York, NY: Wiley; 1989. [Google Scholar]
- 17.Kline RB. Principles and practice of structural equation modeling. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 18.Fortner B, Okon T, Schwartzberg L, et al. The Cancer Care Monitor: psychometric content evaluation and pilot testing of a computer administered system for symptom screening and quality of life in adult cancer patients. J Pain Symptom Manage. 2003;26:1077–92. doi: 10.1016/j.jpainsymman.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Fortner B, Baldwin S, Schwartzberg L, et al. Validation of the Cancer Care Monitor items for physical symptoms and treatment side effects using expert oncology nurse evaluation. J Pain Symptom Manage. 2006;31:207–14. doi: 10.1016/j.jpainsymman.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struc Equ Modeling. 1999;6:1–55. [Google Scholar]
- 21.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. In: Leinhardt S, editor. Sociological Methodology. Washington DC: American Statistical Association; 1982. pp. 290–312. [Google Scholar]
- 22.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: Pain, depression, and fatigue, July 15-17, 2002. J Natl Inst. 2003;95:1110–17. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 23.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 24.Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus in definition and measurement. Sleep Med Rev. 2006;10:63–76. doi: 10.1016/j.smrv.2005.05.004. [DOI] [PubMed] [Google Scholar]