Abstract
Study Objectives:
Determine whether treatment of sleep disorders identified in brain injured adults would result in resolution of those sleep disorders and improvement of symptoms and daytime function.
Methods:
Prospective evaluation of unselected traumatic brain injury patients with nocturnal polysomnography (NPSG), multiple sleep latency test (MSLT), Epworth Sleepiness Scale (ESS), and neuropsychological testing including Psychomotor Vigilance Test (PVT), Profile of Mood States (POMS), and Functional Outcome of Sleep Questionnaire (FOSQ) before and after treatment with continuous positive airway pressure (CPAP) for obstructive sleep apnea (OSA), modafinil (200 mg) for narcolepsy and posttraumatic hypersomnia (PTH), or pramipexole (0.375 mg) for periodic limb movements in sleep (PLMS).
Setting:
Three academic medical centers.
Participants:
Fifty-seven (57) adults ≥ 3 months post traumatic brain injury (TBI).
Measurements And Results:
Abnormal sleep studies were found in 22 subjects (39%), of whom 13 (23%) had OSA, 2 (3%) had PTH, 3 (5%) had narcolepsy, 4 (7%) had PLMS, and 12 had objective excessive daytime sleepiness with MSLT score < 10 minutes. Apneas, hypopneas, and snoring were eliminated by CPAP in OSA subjects, but there was no significant change in MSLT scores. Periodic limb movements were eliminated with pramipexole. One of 3 narcolepsy subjects and 1 of 2 PTH subjects had resolution of hypersomnia with modafinil. There was no significant change in FOSQ, POMS, or PVT results after treatment.
Conclusions:
Treatment of sleep disorders after TBI may result in polysomnographic resolution without change in sleepiness or neuropsychological function.
Citation:
Castriotta RJ; Atanasov S; Wilde MC; Masel BE; Lai JM; Kuna ST. Treatment of sleep disorders after traumatic brain injury. J Clin Sleep Med 2009;5(2):137-144.
Keywords: Trauma, brain injury, hypersomnia, sleep apnea, narcolepsy, sleep disorders, MSLT, continuous positive airway pressure.
Traumatic brain injury (TBI) has been increasingly recognized as a major health problem in both the civilian and military population as a consequence of motor vehicle accidents and explosive devices.1 Every year, over 124,000 civilians in the U.S. who sustain a TBI develop a long-term disability. Presently, there are over 3.3 million Americans living with a TBI-related long-term disability. Recent studies have revealed a high prevalence of sleep disorders in the TBI population, including narcolepsy, posttraumatic hypersomnia (PTH), periodic limb movements in sleep (PLMS), and especially obstructive sleep apnea (OSA), with serious consequences.2–8 Very little, however, has been published about treatment of these problems.9 Recent reports of long-term studies of patients with OSA have revealed a significantly higher risk of both cardiovascular and all-cause mortality,10,11 as well as reduced cardiac function12 and hypertension.13,14 OSA has been linked with structural changes in the brain15 as well as neurocognitive deficits.16 Both narcolepsy and OSA are significant risk factors for motor vehicle accidents.17,18 The purpose of this present study was to determine whether treatment of specific sleep disorders identified in brain injured adults would result in resolution of those sleep disorders and improvement of symptoms and daytime function.
METHODS
Subjects
Subjects older than 18 years of age who were ≥ 3 months post TBI were recruited from the rehabilitative services at 3 academic medical centers: Memorial Hermann Hospital–Texas Medical Center (Houston, TX), Transitional Learning Center (Galveston, TX), and Philadelphia Veterans Administration Medical Center (Philadelphia, PA). The study was approved by the Committee for the Protection of Human Subjects/Institutional Review Board of all participating institutions. Exclusion criteria were as follows: (1) presence of circadian rhythm disorder, (2) inability to give informed consent, and (3) use of sedating medications. Each consented subject was scheduled to undergo nocturnal and daytime sleep studies along with neuropsychological testing. TBI severity was classified by considering both emergency room Glasgow Coma Scale (GCS) and CT scan findings according to established criteria.19,20 Subjects were classified as having a severe TBI if their GCS score was < 9, regardless of CT scan findings. Subjects were classified as having had a moderate TBI if they had a GCS of 9-12 regardless of CT findings, or if they had a GCS of 13-15 and a positive CT scan.21,22 Subjects were classified as having a moderate/severe TBI if they had a positive CT scan but there was no GCS available to make a finer characterization. Subjects were classified as having a mild TBI if their GCS score was 13-15 and the CT scan was negative.22
METHODS
Sleep Studies
An Epworth Sleepiness Score (ESS) questionnaire23 was completed by each subject on the night of polysomnography. Nocturnal polysomnograms (NPSG) were performed ≥ 3 months post injury in sleep laboratories in each center. Using standard techniques,24,25 a computer data acquisition and analysis system recorded the following signals: electroencephalogram (C3A2, C4A1, O1A2, and O2A1), bilateral electroculogram, submental and bilateral anterior tibialis electromyogram, thoracic and abdominal excursion by piezocrystals, oral and nasal airflow by thermistor and breath sounds, body position, oxygen saturation by pulse oximeter, and electrocardiogram. Throughout the study, subjects were monitored with an infrared video camera and a one-way intercom, which connects the bedroom with the monitoring room. All studies were attended by polysomnographic technologists who also scored the studies using 30-sec epochs by Rechtschaffen and Kales criteria,26 and each was interpreted by a physician certified by the American Board of Sleep Medicine.
During the day subsequent to the sleep study, a multiple sleep latency test (MSLT) was used to assess objective physiologic sleepiness. The test was performed using standard techniques; sleep onset was defined as the first epoch of sleep, (> 50% of the 30-sec epoch of any sleep stage).27 Each subject took 5 naps of 20-min duration at 2 hour intervals. The following signals were recorded during the naps: EEG (C3A2, C4A1, O1A2 and O2A1), bilateral electroculograms, submental electromyogram, and electrocardiogram. The average sleep latency over these 5 naps was the MSLT score. Those with an MSLT score < 10 min were termed sleepy and those with an MSLT score ≥ 10 min were non-sleepy. A urine sample was collected after the NPSG and during the MSLT with analysis for possible opiates, benzodiazepines, cannabinoids, amphetamines, and adrenergic drugs.
Respiratory events were scored as previously described.28 Obstructive apnea was defined by cessation of breathing > 10 sec with ≥ 4% fall in oxygen saturation and/or EEG arousal accompanied by continuous respiratory effort. Central apnea was defined by a cessation of breathing > 10 sec with ≥ 4% fall in oxygen saturation and/or EEG arousal without respiratory effort. Hypopnea was defined as > 50% reduction in airflow > 10 sec accompanied by ≥ 4% fall in oxygen saturation and/or EEG arousal. The diagnosis of obstructive sleep apnea (OSA) was made with ≥ 5 apneas/hour of sleep and/or ≥ 10 apneas + hypopneas/hour of sleep. Narcolepsy was defined as an MSLT score (average sleep latency) < 5 min with ≥ 2 sleep onset REM periods (SOREMPs) after an unremarkable NPSG with adequate total sleep and REM sleep and negative urine drug screen. Posttraumatic hypersomnia (PTH) was defined as an MSLT score ≤ 10 min with < 2 SOREMPs after an unremarkable NPSG and no history of hypersomnolence prior to TBI. Periodic limb movements in sleep (PLMS) were defined as > 5 periodic limb movements (PLMs)/hour of sleep; PLMs were scored according to the standard criteria prevailing at the time the study was designed and initiated.29,30
Neuropsychological Evaluation
Each subject underwent a brief neuropsychological evaluation and completed several self-report measures. All subjects were evaluated on 2 occasions. To control for diurnal variations, all evaluations took place beginning at 10:30, between the second and third MSLT naps. The measures used are described below.
Psychomotor Vigilance Test (PVT):
Sustained attention was evaluated with the Psychomotor Vigilance Test (PVT). The PVT was chosen because it is sensitive to the effects of sleepiness on cognitive functioning as well as cognitive problems associated with OSA and its treatment.31–33 The PVT is administered via a small hand-held computerized device with a 3-digit millisecond LED counter and display window (PVT-192: Ambulatory Monitoring Inc, Ardsley, NY). Subjects are presented with a 10-min trial in which they press a response button as soon as a number counting up from 0 is seen. Once the response button is pressed, the counter stops and feedback is given on their reaction time. The amount of time between stimulus presentations varies between minimum and maximum interstimulus intervals of 2000 and 10,000 ms. Performances are recorded in the PVT device and downloaded into a database after the testing bout. For the purposes of this study, the average of the fastest 10% of reaction times, the average of the slowest 10% reaction times, and the number of lapses (reaction times > 500 ms) from the PVT were selected for this analysis because these variables have been shown in prior research to be sensitive to sustained attention under conditions of sleep deprivation and in sleep disorders.31–33 Normally, the PVT is given in several testing bouts across time. Owing to the time constraints involved in this study, each subject was exposed to the PVT once.
Profile of Mood States (POMS):
The Profile of Mood States (POMS)34 is a self-report measure in which subjects rate themselves on each of 65 adjectives using a 1-5 scale. These 65 responses yield 6 mood state scales which are: Anger-Hostility; Vigor-Activity, Depression-Dejection, Fatigue-Inertia, Tension-Anxiety, and Confusion-Bewilderment. This measure enjoys wide use in sleep research and has been shown to be sensitive to sleep disorder-related mood problems.35,36
Functional Outcome of Sleep Questionnaire (FOSQ):
The Functional Outcome of Sleep Questionnaire (FOSQ) is a self-report measure designed to assess the impact of sleep disorders on daily functioning.37 It has been used in sleep research and appears sensitive to treatment related change.38–40 There are 30 items, which are divided into 5 scales: Activity, Vigilance, General Productivity, Social Outcome, and Intimacy and Sexual Relationships. These scales are summed to make a total score. Higher scores on the FOSQ indicate self-perceived better daily functioning. The total score was used for this analysis.
Patients with diagnosed sleep disorders were offered one of the following treatment plans: (1) OSA patients were treated with nasal continuous positive airway pressure (CPAP) and returned subsequent to diagnosis for repeat NPSG with appropriate titration of CPAP to eliminate apneas, hypopneas, and snoring; (2) Subjects with PLMS were treated with pramipexole 0.375 mg by mouth each night; (3) Those diagnosed with narcolepsy or PTH were treated with modafinil 200 mg by mouth each morning. The patients were not seen in closer follow-up unless there was an adverse event. Thus medications were not titrated up or down.
Three-Month Follow-up
The patients were re-examined after ≥ 3 months of treatment. Those subjects without a sleep disorder were also seen at 3 months. The sleep study, MSLT, and neuropsychologic evaluation were repeated in those patients with a sleep disorder. Those without a sleep disorder participated in the neuropsychologic testing only.
STATISTICAL ANALYSIS
Comparability of demographic and baseline characteristics were summarized by subgroups using means and standard deviations (quantitative data), or frequency of counts (qualitative /categorical data). Parametric t tests for independent samples were used to evaluate group differences when distributions were normal. Categorical data was analyzed using chi square tests. Where small cell sizes precluded the use of chi square, Fisher exact test was employed.
RESULTS
Overview of the Sample
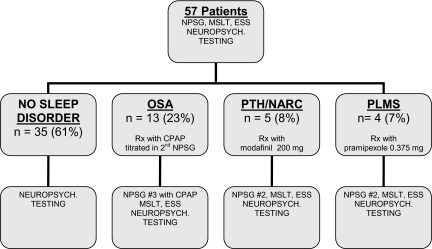
There were 87 subjects initially studied, of whom a total of 57 patients completed the protocol (Figure 1). Of those, 35 subjects (61%) were free of a sleep disorder, 13 (23%) had OSA, 3 (5%) had narcolepsy without cataplexy, 2 (3%) had PTH, and 4 (7%) had PLMS. There were 12 (25%) female and 36 (75%) male participants. Forty-four subjects (77%) were Caucasian, 8 (14%) were African American, and 5 (9%) were Hispanic. Of those who had complete data upon which severity could be determined, 17 subjects (30%) had severe injuries, 3 (5%) had moderate or severe injury, 10 (18%) had moderate injuries, and 5 (9%) had mild injuries. Forty-four (77%) subjects incurred TBI as a result of a motor vehicle accident, 4 (7%) were assaulted, 5 (9%) had a fall, and 4 (7%) were injured by a falling object. The average age, education, and number of months post injury for the entire sample were 38.56 (± 14.75) years, 12.65 (± 1.88) years, and 67.84 (± 126.34) months.
Figure 1.
Flow of study participants. OSA = obstructive sleep apnea; PTH – post-traumatic hypersomnia; NARC = narcolepsy; PLMS = periodic limb movements in sleep; NPSG = nocturnal polysomnographu; MSLT = multiple sleep latency test; CPAP = continuous positive airway pressure.
Did Patients Improve?
We next evaluated the degree to which TBI patients with sleep disorders benefited from treatment. Baseline sleep and demographic data are depicted in Tables 1 and 2. In order to determine if OSA patients were effectively treated, the apnea hypopnea index (AHI), amount of REM sleep, MSLT, and ESS were compared pre and post treatment using a paired samples t test. These comparisons disclosed that the AHI improved dramatically from pretreatment to post treatment (p = 0.001). The amount of REM sleep increased from pre to post treatment (p = 0.04), but there was no change in total sleep time (p = 0.38). There was no significant change in MSLT score (p = 0.66) or ESS (p = 0.43). In order to determine if there had been significant treatment related improvement in those with narcolepsy and PTH, the pre and post treatment MSLT and ESS scores were evaluated using a paired samples t test for the pooled group. These findings disclosed no statistically significant differences from pretreatment to posttreatment on the MSLT or the ESS. However the mean MSLT scores increased from 5.65 ± 1.7 to 9.3 ± 6.9 min in this small group from before treatment to after treatment with modafinil 200 mg daily for narcolepsy or PTH. There were improvements in MSLT scores in one of 3 narcolepsy subjects (from 4.3 to 19 min) and one of 2 PTH subjects (from 6 to 13 min). To determine if there had been a significant treatment-related improvement in those with PLMS, pre and post treatment periodic limb movement index (PLMI) as well as the ESS and MSLT scores were compared using a paired samples t test. These findings revealed a significant decrease in the PLMI from pre (17.7 ± 7.2) to post treatment (1.25 ± 2.5) with 0.375 mg of pramipexole (p = 0.03). There was no significant change in MSLT score from pre (13.1 ± 3.6) to post treatment (13.2 ± 7.7) in this patient group, but these were normal MSLT scores at the outset.
Table 1.
Sleep Study Data for TBI Patients with Sleep Disorders
| OSA |
NAR PTH |
PLM |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aRx |
pRx |
aRx |
pRx |
aRx |
pRx |
|||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| TST | 5.9 | 1.5 | 6.1 | 1.1 | 7.0 | 0.7 | 6.6 | 0.6 | 5.7 | 1.8 | 6.3 | 0.5 |
| ESS | 12.2 | 6.2 | 13.0 | 6.4 | 7.8 | 3.6 | 6.4 | 5.0 | 17.0 | 2.0 | 8.5 | 2.1 |
| %N1 | 15.8 | 12.3 | 12.3 | 8.3 | 11.6 | 3.5 | 11.0 | 6.6 | 8.7 | 2.6 | 8.6 | 4.4 |
| %N2 | 61.4 | 11.7 | 63.7 | 13.6 | 62.2 | 21.6 | 58.4 | 17.7 | 69.8 | 13.0 | 74.8 | 7.6 |
| %N3 | 3.8 | 8.8 | 4.7 | 8.4 | 9.2 | 8.1 | 10.6 | 13.2 | 7.8 | 7.5 | 5.7 | 6.0 |
| %REM | 20.3 | 9.8 | 19.2 | 9.8 | 18.4 | 1.8 | 19.6 | 6.8 | 13.5 | 10.1 | 10.9 | 8.5 |
| SL | 50.9 | 127 | 25.7 | 37.1 | 10.3 | 10.4 | 27.6 | 39.6 | 38.9 | 50.3 | 9.0 | 6.9 |
| SOREM | 1.42 | 3.1 | 0.8 | 2.6 | 2.4 | 3.1 | 0.6 | 0.9 | 3.0 | 6.0 | 2.6 | 5.3 |
| AHI | 31.4* | 21.5 | 3.8* | 3.7 | 0.8 | 1.3 | 1.6 | 1.3 | 1.2 | 1.7 | 2.9 | 3.2 |
| PLMI | 9.9 | 17.1 | 19.8 | 28.8 | 1.8 | 3.5 | 1.8 | 4.0 | 17.7# | 7.2 | 1.3# | 2.5 |
| MSLT | 10.3 | 6.2 | 12.1 | 5.1 | 5.7 | 1.7 | 9.3 | 6.9 | 13.1 | 3.6 | 13.2 | 7.7 |
OSA = obstructive sleep apnea; NAR = narcolepsy; PTH = post-traumatic hypersomnia; PLM = periodic limb movements; aRx = before treatment; pRx = after treatment (with CPAP for OSA, modafinil for NAR/PTH, pramipexole for PLM); M = mean; SD = standard deviation; TST = total sleep time (in hours); ESS = Epworth sleepiness score; %N1 = percent of Stage 1 sleep; %N2 = percent of Stage 2 sleep; %N3 = percent of Stage 3 and 4 (slow-wave or delta) sleep; %REM = percent of stage REM (rapid eye movement) sleep; SE = sleep latency (from time in minutes from lights out to sleep onset) on nocturnal polysomnography; SOREM = number of sleep-onset REM periods on multiple sleep latency test; AHI = apnea-hypopnea index (number of apneas and hypopneas per hour of sleep); PLMI = periodic limb movement index (number of periodic limb movements per hour of sleep); MSLT = MSLT score = mean sleep latency (in minutes) over 5 naps on multiple sleep latency test.
p = 0.001 (before and after treatment with CPAP);
p = 0.03 (before and after treatment with pramipexole)
Table 2.
Demographic Data for the TBI Patients with and without Sleep Disorders
| NO SLEEP DISORDER | SLEEP DISORDER | |
|---|---|---|
| N (%) | N (%) | |
| N | 35 | 22 |
| Sex | ||
| Male | 25 (44) | 16 (11) |
| Female | 10 (17) | 6 (28) |
| Race | ||
| Caucasian | 27 (47) | 17 (30) |
| African American | 5 (9) | 3 (5) |
| Hispanic | 3 (5) | 2 (4) |
| Cause of Injury | ||
| Assault | 2 (4) | 2 (4) |
| Auto/Vehicle | 29 (51) | 15 (26) |
| Fall | 3 (5) | 2 (4) |
| Hit by Falling Object | 1 (6) | 3 (5) |
| CT Scan Findings | ||
| Unknown | 12 (21) | 11 (19) |
| Negative | 6 (11) | 0 (0) |
| Positive | 17 (30) | 11 (19) |
| Brain Injury Severity | ||
| Unknown | 11 (50) | 11 (53) |
| Mild | 5 | |
| Moderate | 2 (12) | 1 (16) |
| Moderate/Severe | 9 (19) | 1 (5) |
| Severe | 8 (19) | 9 (26) |
Outcome Of Sleep Disordered And Non–Sleep Disordered Patients: Neuropsychology And Quality Of Life Measures
In order to examine the relationship between sleepiness, cognitive functioning, mood state, and quality of life, the FOSQ total score, the 6 scales from the POMS, and the average of the fastest 10% of reaction times, the average of the slowest 10% reaction times, and the number of lapses (reaction times > 500 ms) from the PVT were selected for this analysis. The amount of change from pretreatment to post treatment served as the dependent measure in these analyses. The distributions are depicted in Table 3. This analysis was based on 22 sleep disordered and 33 non–sleep disordered patients. The average age for the sleep disorder and non–sleep disorder groups were 42 (± 15.29) years and 36.34 (± 14.18) years respectively. The average years of education was 12.64 (± 2.08) for those with and 12.66 (± 1.76) years without sleep disorders. The individuals with sleep disorders were on average 98.86 (± 165.05) months post injury while those without sleep disorders were 48.32 (± 91.47) months post injury. The distributions of gender, race and cause of injury were all similar between the groups (p > 0.05). There were more patients with severe injuries in the sleep disordered group. There were no differences between the groups in terms of age, education, or number of months post injury (p > 0.05). Glasgow Coma Scale scores (GCS) (p < 0.05) were significantly lower in the sleep disordered group. The results of group comparisons of the outcome measures disclosed: (1) a significant difference in the amount of change in POMS Tension and Anger scales, indicating that the sleep disordered patients had greater reductions in tension and anger than the non sleep disordered patients (p = 0.04); (2) that the sleep disordered patients showed a marginally significant greater reduction in self-reported sleepiness on the ESS (p = 0.05); (3) that the sleep disordered patients did not differ from the non–sleep disordered patients in the amount of change from pre to post treatment on the FOSQ or the PVT measures (all p > 0.05). However, given the number of statistical comparisons performed, these differences are likely the result of chance.
Table 3.
Neuropsychological Test Performance Data for TBI Patients with and without Sleep Disorders
| NON SLEEP DISORDERED |
SLEEP DISORDERED |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| ΔPVT Lapses 2 | −1.90 | 7.87 | −4.00 | 15.67 |
| ΔPVT Fastest 10% RT 3 | 5.45 | 24.97 | 0.73 | 28.86 |
| ΔPVT Slowest 10% RT 4 | −42.34 | 214.48 | −34.27 | 351.77 |
| ΔPOMS Fatigue 2 | −1.24 | 6.46 | 1.50 | 5.07 |
| ΔPOMS Confusion 2 | −1.64 | 4.34 | 0.40 | 7.37 |
| ΔPOMS Tension 2 | −1.68 | 6.37 | 2.05 | 5.43 |
| ΔPOMS Vigor2 | −1.08 | 4.88 | 0.20 | 7.28 |
| ΔPOMS Depression 2 | −2.24 | 10.54 | 2.40 | 11.68 |
| ΔPOMS Anger 2 | −2.48 | 9.64 | 3.70 | 9.98 |
| ΔFOSQ Total Score 3 | 0.36 | 1.99 | −0.75 | 3.12 |
| ΔEpworth | −2.40 | 3.69 | 0.84 | 4.50 |
N for NonOSA group = 16 N for OSA group 19;
N for NonOSA group = 16 N for OSA group 18;
PVT Fastest 10%Reaction Times;
PVT Slowest 10%Reaction Times; PVT = Psychomotor Vigilance Test; POMS = Profile of Mood States; FOSQ = Functional Outscome of Sleep Questionnaire.
Sleepy and Non-Sleepy Patients
There were 12 sleepy and 10 non-sleepy patients who completed the study. There were 16 males and 6 females in the sample. The average age, education and time post injury for the sample was 40.80 (± 14.45), 12.70 (± 2.18), and 75.25 (± 126.18), respectively. The distributions of baseline sleep and demographic data for the 2 groups are depicted in Tables 4 and 5. The average age, education, and time post injury for the sleepy and not sleepy groups were 38 (± 12.86) and 43.20 (± 16.20); 12.30 (± 1.42) and 13.10 (± 2.77); and 66.80 (± 148.61) and 83.70 (± 106.62). The distributions of gender, severity, race, and cause of injury were all similar between the groups (p > 0.05). There were no differences between the groups in terms of age, education, number of months post injury, and GCS. To examine the relationship between sleepiness, cognitive functioning, mood state, and quality of life, the FOSQ total score, the 6 scales from the POMS and the average of the fastest 10% of reaction times, the average of the slowest 10% reaction times, and the number of lapses (reaction times ≥ 500 ms) from the PVT were selected for this analysis. The amount of change from pretreatment to post treatment served as the dependent measure in these analyses. The distributions for demographic and outcome data are depicted in Table 6. Note that there were missing PVT data and thus, this analysis was based on 8 sleepy and 11 non-sleepy patients. The results of these group comparisons disclosed no significant differences between the groups on any of the measures (all p > 0.05).
Table 4.
Sleep Study Data for Sleepy and Non-Sleepy Patients
| NON-SLEEPY | SLEEPY | |||
|---|---|---|---|---|
| N | 10 |
12 |
||
| Total Sleep (h) | 5.81 | 1.52 1 | 6.86 | 0.86 1 |
| Epworth Sleepiness Scale | 12.78 | 11.55 | 11.08 | 4.98 |
| Percent Stage 1 2 | 14.67 | 13.74 | 12.56 | 5.50 |
| Percent Stage 2 3 | 60.11 | 12.55 | 65.63 | 15.50 |
| Percent Stage 3 – 4 4 | 7.90 | 10.02 | 3.96 | 6.46 |
| Percent REM Sleep 5 | 18.92 | 10.52 | 18.36 | 7.46 |
| Apnea Hypopnea Index 6 | 18.85 | 14.58 | 23.16 | 27.05 |
| MSLT Score 7 | 14.77 | 3.95 | 5.53 | 2.01 |
Standard Deviation;
Percentage of total sleep time that is stage 1 sleep;
Percentage of total sleep time that is stage 2 sleep;
Percentage of total sleep time that is stage 3 and 4 sleep;
Percentage of sleep time that is spent in REM sleep;
The number of apnea and hypopnea events per hour of sleep;
The average time to sleep onset across five Multiple Sleep Latency Test naps.
Table 5.
Demographic Data for Sleepy and Non-Sleepy TBI Patients
| SLEEPY |
NOT SLEEPY |
|
|---|---|---|
| N (%) | N (%) | |
| N | 12 | 10 |
| Sex | ||
| Male | 9 (75) | 7 (70) |
| Female | 3 (25) | 3 (30) |
| Race | ||
| Caucasian | 9 (75) | 8 (80) |
| African American | 2 (17) | 1 (10) |
| Hispanic | 1 (8) | 1 (10) |
| Cause of Injury | ||
| Assault | 1 (5) | 1 (5) |
| Auto/Vehicle | 10 (40) | 5 (25) |
| Fall | 0 (0) | 2 (10) |
| Hit by Falling Object | 1 (5) | 2 (10) |
| CT Scan Findings | ||
| Unknown | 6 (50) | 5 (50) |
| Positive | 6 (50) | 5 (50) |
| Negative | 0 | 0 |
| Brain Injury Severity | ||
| Unknown | 6 (50) | 5 (50) |
| Mild | 0 | 0 |
| Moderate | 0 | 1 (10) |
| Moderate/Severe | 0 | 1 (10) |
| Severe | 6 (50) | 3 (30) |
| Sleep Disorder Diagnosis | ||
| Narcolepsy | 3 (25) | 0 |
| OSA 1 | 7 (58) | 6 (60) |
| PLM 2 | 0 | 4 (40) |
| PTH 3 | 2 (17) | 0 |
Obstructive Sleep Apnea;
Periodic Limb Movements in Sleep
Posttraumatic Hypersomnia.
Table 6.
Neuropsychological Test Performance Data for TBI Patients with and without Sleep Disorders
| NOT SLEEPY | SLEEPY | |||
|---|---|---|---|---|
| M |
SD |
M |
SD |
|
| ΔPVT Lapses2 | −0.33 | 2.54 | −8.20 | 21.69 |
| ΔPVT Fastest 10% RT | 8.89 | 33.28 | −5.95 | 24.88 |
| ΔPVT Slowest 10% RT | −120.49 | 202.33 | −39.94 | 470.59 |
| ΔPOMS Fatigue2 | 1.88 | 5.30 | 1.30 | 5.39 |
| ΔPOMS Confusion2 | 2.11 | 6.51 | −1.30 | 5.39 |
| ΔPOMS Tension2 | 2.22 | 4.84 | 2.30 | 6.29 |
| ΔPOMS Vigor2 | 0.80 | 8.24 | −0.40 | 6.68 |
| ΔPOMS Depression2 | 2.66 | 16.44 | 2.90 | 6.41 |
| ΔPOMS Anger2 | 7.44 | 11.46 | 0.80 | 8.24 |
| ΔFOSQ Total Score2 | −1.37 | 3.14 | 0.20 | 3.25 |
| ΔEpworth | 0.80 | 3.91 | 1.62 | 5.19 |
PVT = Psychomotor Vigilance Test; POMS = Profile of Mood States; FOSQ = Functional Outscome of Sleep Questionnaire
DISCUSSION
In this paper, we report the results of a prospective evaluation of unselected traumatic brain injury patients with PSG, MSLT, ESS, and neuropsychological as well as functional outcome measures before and after treatment of OSA, narcolepsy, PTH, and PLMS. An analysis of pre and post treatment results disclosed a polysomnographic reversal of OSA with CPAP and in PLMS with pramipexole. In subjects with OSA there was no demonstrable improvement in excessive daytime sleepiness as defined by the MSLT or ESS after treatment with CPAP, despite polysomnographic resolution of OSA. One of 3 narcolepsy subjects and one of 2 PTH subjects demonstrated resolution of hypersomnia by MSLT with modafinil 200 mg daily. There were no significant changes in measures of mood, quality of life and cognitive performance in TBI patients after treatment of their sleep disorders. There are a number of methodological factors that must be considered in evaluating these results.
Is the MSLT the Right Outcome Measure?
We are not the first investigators to find that the MSLT does not change with treatment. In a trial of modafinil for the treatment of residual excessive daytime sleepiness in obstructive sleep apnea, modafinil had no effect on sleepiness as measured by the multiple sleep latency test; however, significant improvements in alertness were found with the maintenance of wakefulness test.41 In another study,42 142 patients with sleep apnea-hypopnea syndrome (AHI = 10-30) were randomly assigned to receive conservative treatment (sleep hygiene and weight loss) or conservative treatment plus CPAP. There was no significant improvement in MSLT scores in the CPAP group at 3 and 6 months. A trial conducted to assess the efficacy of modafinil (200-400 mg/day) for the treatment of EDS in Parkinson disease failed to show a significant improvement in MSLT scores compared with placebo.43
A review of 75 studies concluded that MSLT and MWT have limited clinical utility for confirming response to treatment in groups of patients with different sleep disorders.44 A meta-analysis45 of randomized controlled trials where CPAP was compared with either a placebo or with conservative management in the treatment of OSA showed that CPAP: (1) significantly reduced subjective daytime sleepiness (ESS), (2) improved objective daytime wakefulness (MWT), but (3) did not affect objective daytime sleepiness (MSLT).
Why is the MSLT Unresponsive to Treatment?
One explanation may be that sleep and wakefulness are 2 separate active processes. The active process of sleep and propensity to fall asleep are measured by MSLT. The active process of wakefulness and the ability to stay awake are measured by MWT. It is possible that CPAP and modafinil treatment did not affect the propensity to fall asleep as measured by MSLT. Both treatments may have enhanced the ability to stay awake, but we did not measure the MWT to obtain this data. In support of this view, the meta-analysis reported above found improvement by MWT in spite of observing no change in MSLT with CPAP treatment.45 On the basis of our study and what has been discussed above, it is possible that the MWT may be a better outcome measure than MSLT in future clinical trials. While there was an improvement of borderline significance (p = 0.05) in ESS, there is good reason to doubt the validity of self-report in this patient population.2
Other Methodological Issues
The sample sizes were small, and, in addition, complete data on severity of injury was not available on all subjects. For this reason we were unable to address the possibility of a relationship between injury severity and outcome of treatment for sleep disorders. We did not have sufficient numbers to evaluate outcomes in narcolepsy and PTH. Significant effects might have been detected with larger sample sizes. In this study we planned to recruit 90 TBI subjects, expecting that approximately 40% would have sleep disorders and excessive daytime sleepiness. In actuality, 47% had sleep disorders, but only 26% had excessive daytime sleepiness as measured by MSLT. Of those who returned for the second study, 39% had a sleep disorder and 21% had excessive daytime sleepiness. There was an attrition rate of 35% between the initial evaluation and the post treatment phase. This is not unusual for research with TBI patients. Future investigations of TBI patients and sleep disorders need to recruit very large numbers of TBI patients in order to adequately sample the heterogeneity of sleep disorders in this population. This would be best and most economically accomplished with large-scale multicenter investigations by collaborative investigators.46
While CPAP treatment of OSA patients was properly titrated in the laboratory, dosages of modafinil and pramipexole were fixed a priori by the study protocol with no accommodation made for individual responses and related dose titration. For this reason, patients with PTH or narcolepsy may have been undertreated and remained sleepy on the 200 mg dose of modafinil, yet might have improved with a higher dose. However, titration of modafinil would have required accurate self-report from patients, which remains problematic in this patient population.2 Perhaps this could have been addressed with periodic MWTs as an aid to modafinil dosage titration. Periodic limb movements improved in all patients with 0.375 mg of pramipexole, but this is a fairly high dose. It is known that some OSA patients have residual hypersomnia despite adequate treatment with CPAP.47,48 Thus, the lack of improvement in MSLT scores in these patients may not be entirely surprising.
We did not find any significant change on the neuropsychological test scores or quality of life measures. It has been reported in some, but not all, studies that some OSA patients show improvement on neuropsychological measures while others do not.47 There may be some permanent deficits in OSA that are not reversed by CPAP.47 It is also possible that neuropsychological and quality of life measures did not improve because of residual sleepiness despite treatment.
This study represents the first attempt at assessing the effectiveness of treatment of sleep disorders in patients with traumatic brain injury. The difficulties encountered in this endeavor illustrate the importance of large multicenter collaborative studies. Alternative methodological tools will be necessary for assessing sleepiness/wakefulness and for capturing the extra cognitive burden produced by sleep disorders in TBI patients as well as assessing any improvement after treatment.
ACKNOWLEDGMENTS
We would like to express our appreciation to Amal Abuelheiga, RPSGT for her assistance with this project.
DISCLOSURE STATEMENT
This study was conceived and initiated by the investigators and fundamentally supported by the Moody Foundation with additional support from Cephalon, Inc. The authors have no other financial conflicts of interest. The study design included the off-label use of modafinil for the treatment of posttraumatic hypersomnia. No other investigational drugs or treatment modalities were used in this study.
REFERENCES
- 1.Zaloshnja E, Miller T, Langlois J, Selassie A. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- 2.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3:349–56. [PMC free article] [PubMed] [Google Scholar]
- 3.Wilde MC, Castriotta RJ, Lai JM, Atanasov S, Masel BE, Kuna ST. Cognitive impairment in patients with traumatic brain injury (TBI) in obstructive sleep apnea. Arch Phys Med Rehabil. 2007;88:1284–8. doi: 10.1016/j.apmr.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med. 2007;3:357–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Watson NF, Dikmen S, Machamer J, Doherty M, Temkin N. Hypersomnia following traumatic brain injury. J Clin Sleep Med. 2007;3:363–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Parcell DL, Ponsford JL, Redman JR, Rajaratnam SM. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch Phys Med Rehabil. 2008;89:843–50. doi: 10.1016/j.apmr.2007.09.057. [DOI] [PubMed] [Google Scholar]
- 7.Rao V, Spiro J, Vaishnavi S, et al. Prevalence and types of sleep disturbances acutely after traumatic brain injury. Brain Injury. 2008;22:381–386. doi: 10.1080/02699050801935260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti C. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–83. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 9.Jha A, Weintraub A, Allshouse A, et al. A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J Head Trauma Rehabil. 2008;23:52–63. doi: 10.1097/01.HTR.0000308721.77911.ea. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall NS, Wong KKH, Liu PY, Cullen SRJ, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 12.Chami HA, Devereux RB, Gottdiener JS, et al. Left ventricular morphology and systolic function in sleep-disordered breathing: the Sleep Heart Health Study. Circulation. 2008;117:2599–607. doi: 10.1161/CIRCULATIONAHA.107.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenfant C. Do sleep disorders have an impact on blood pressure? Am J Ther. 2008;15:345–50. doi: 10.1097/MJT.0b013e31815fd0fe. [DOI] [PubMed] [Google Scholar]
- 14.Hia KM, Young T, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep disordered breathing and non-dipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31:795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macey PM, Kumar R, Woo MA, Valladares EM, YanoGo FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 16.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 17.Ellen RLB, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-King M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 18.Pandi-Perumal SR, Verster JC, Kayumov L, et al. Sleep disorders, sleepiness and traffic safety: a public health menace. Brazilian J Med Biol Res. 2006;39:863–71. doi: 10.1590/s0100-879x2006000700003. [DOI] [PubMed] [Google Scholar]
- 19.Levin HS. Neurobehavioral recovery. J Neurotrauma. 1992;9(Suppl 1):S359–S373. [PubMed] [Google Scholar]
- 20.Levin HS. Head trauma. Curr Opin Neurol. 1993;6:841–6. doi: 10.1097/00019052-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–8. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Dikmen S, Machamer J, Temkin N. Mild head injury: facts and artifacts. J Clin Exp Neuropsychol. 2001;23:729–38. doi: 10.1076/jcen.23.6.729.1019. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 24.American Sleep Disorders Association Report. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 25.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–22. [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: U.S. Government Printing Office; 1968. [Google Scholar]
- 27.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 28.Castriotta RJ, Lai JM. Sleep disorders associated with traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1403–6. doi: 10.1053/apmr.2001.26081. [DOI] [PubMed] [Google Scholar]
- 29.Coleman R. Sleeping and waking disorders: indications and techniques. Menlo Park: Addison Wesley; 1982. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. In: Guilleminault C, ed; pp. 265–95. [Google Scholar]
- 30.American Sleep Disorders Association. International classification of sleep disorders (revised) Rochester, MN: American Sleep Disorders Association; 1997. [Google Scholar]
- 31.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in subjects with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 32.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA subjects with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 33.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267. [PubMed] [Google Scholar]
- 34.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational Testing Service; 1971. [Google Scholar]
- 35.Yu BH, Ancoli-Israel S, Dimsdale JE. Effect of CPAP treatment on mood states in subjects with sleep apnea. J Psychiatr Res. 1999;33:427–32. doi: 10.1016/s0022-3956(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 36.Bardwell WA, Ancoli-Israel S, Dimsdale JE. Types of coping strategies are associated with increased depressive symptoms in subjects with obstructive sleep apnea. Sleep. 2001;24:905–9. doi: 10.1093/sleep/24.8.905. [DOI] [PubMed] [Google Scholar]
- 37.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 38.Gooneratne NS, Weaver TE, Cater JR, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51:642–9. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 39.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 40.Weaver TE, Maislin G, Dinges DF, et al. Self-efficacy in sleep apnea: instrument development and subject perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep. 2003;26:727–32. doi: 10.1093/sleep/26.6.727. [DOI] [PubMed] [Google Scholar]
- 41.Kingshott RN, Vennelle M, Coleman EL, Engleman HM, Mackay TW, Douglas NJ. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of residual excessive daytime sleepiness in the sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:918–23. doi: 10.1164/ajrccm.163.4.2005036. [DOI] [PubMed] [Google Scholar]
- 42.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164:939–43. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- 43.Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson’s disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psych. 2005;76:1636–9. doi: 10.1136/jnnp.2005.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet MH. ACNS clinical controversy: MSLT and MWT have limited clinical utility. J Clin Neurophys. 2006;23:50–8. doi: 10.1097/01.wnp.0000190415.83841.17. [DOI] [PubMed] [Google Scholar]
- 45.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal AM, Cincu R, Joharapurkar SR. Traumatic brain injury and sleep disturbances. J Clin Sleep Med. 2008;4:177. [PMC free article] [PubMed] [Google Scholar]
- 47.Black J. Residual sleepiness in adults with obstructive sleep apnea. Respir Physiol Neurobiol. 2003;136:211–20. doi: 10.1016/s1569-9048(03)00083-1. [DOI] [PubMed] [Google Scholar]
- 48.Hirshkowitz M, Black J. Effect of adjunctive modafinil on wakefulness and quality of life in patients with excessive sleepiness-associated obstructive sleep apnoea/hypopnoea syndrome. CNS Drugs. 2007;21:407–16. doi: 10.2165/00023210-200721050-00004. [DOI] [PubMed] [Google Scholar]