Abstract
Light therapy is increasingly applied in a variety of sleep medicine and psychiatric conditions including circadian rhythm sleep disorders, seasonal affective disorder, and dementia. This article reviews the neural underpinnings of circadian neurobiology crucial for understanding the influence of light therapy on brain function, common mood and sleep disorders in which light therapy may be effectively used, and applications of light therapy in clinical practice.
Citation:
Shirani A; St. Louis EK. Illuminating rationale and uses for light therapy. J Clin Sleep Med 2009;5(2):155-163.
Keywords: Light therapy, circadian disorders, insomnia, mood disorders, physiology
LIGHT AND CIRCADIAN NEUROBIOLOGY
A circadian rhythm is a self-sustaining biological activity oscillating with a periodicity near 24 hours. Circadian rhythms have been detected in living organisms under natural conditions, as well as during isolation in artificial circumstances, including space travel.1 Circadian rhythms tend to move into phase synchrony with environmental rhythms, mainly the day/night cycle. While the persistence of circadian rhythms under experimental decoupling from the environment points to rhythmicity generated by an internal biologic clock, the widespread phase synchronization with the rotation of the planet (circadian from the Latin, meaning about a day) underscores the capability of the biologic clock to reset. We present a summary of the relevant physiologic processes pertinent to understanding the relationship between chronobiology and the light dark (LD) cycle, followed by a discussion of light use as a therapeutic tool in medicine.
The Biologic Clock
Scientific evidence mounting over the last decades has unveiled the location of the mammalian “master biologic clock” in the paired suprachiasmatic nuclei (SCN) of anterior hypothalamus.2 Lesions of the SCN in animal experiments have been linked to disruptions of circadian rhythms of hormones3 and complex behavior such as drinking and locomotion.4 Specific genetic mutations targeting SCN abolish the circadian sleep rhythm.5 Consistent with their clock function, the SCN neurons exhibit an internal rhythmic activity, which is generated by cyclic expression, transcription, and feedback regulation of recently identified genes (CLOCK, PER, and others),6 arrested by the inhibition of protein synthesis,7 and preserved in isolation from the surrounding brain tissue in cell cultures.8 Posttranslational modifications are the proposed mechanism for the periodicity of the biologic clock.9–11 The resulting endogenous circadian period varies in different species and between individual subjects. The average endogenous circadian period of humans is slightly longer than the clock day, measuring approximately 24.2 hours.12
Setting the Biologic Clock
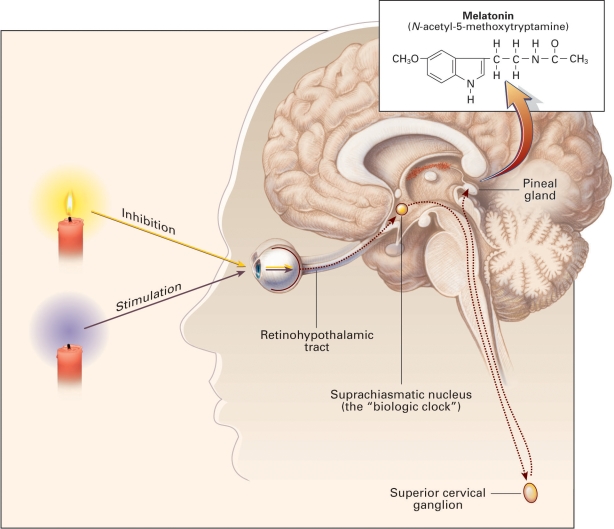
The endogenous circadian period of the biologic clock may lack phase synchrony with the surrounding environment. One would expect that the discrepancy between the 24.2-h intrinsic human circadian period and the 24-h terrestrial day would cause a continuous phase delay relative to the calendar day. However, certain external stimuli, known as zeitgebers (literally, from the German, time-givers), exert a synchronizing effect on the circadian rhythm of biological organisms, producing and sustaining an in-phase temporal progression of the endogenous circadian period and Earth's 24-h rotation. The process of establishing phase synchronization between biological and planetary periodicities, i.e., setting the biologic clock, is referred to as entrainment. Daily entrainment of the biologic clock by environmental zeitgebers prevents inadvertent drifting or divergence from the 24-h day. Although less crucial synchronizing stimuli such as physical exercise have been identified,13,14 the principal entraining zeitgeber of the human endogenous circadian period is the LD cycle.15 The entraining influence of the LD cycle upon the human central nervous system is mediated through the light-sensitive receptors of the retina. Shielding sighted individuals from natural daily light cycles under laboratory conditions decouples the endogenous circadian rhythm from the LD cycle.12 The high prevalence of non-entrained, free-running circadian rhythms in the totally blind population provides further evidence for the crucial role of the LD cycle as a central zeitgeber.16 Although retinal visual photoreceptors (rods and cones) contribute to circadian light entrainment,17 the capacity for entrainment by light remains preserved in absence of functional retinal rods and cones in certain types of blindness.18,19 A distinct, non–image-forming subset of retinal ganglion cells containing the light sensitive pigment, melanopsin, functions in circadian entrainment by transducing light into neural impulses20 that project via the retinohypothalamic tract (RHT) directly to the SCN, the mammalian master biologic clock.20 Retinal afferents to SCN modulate endogenous pacemaker activity and set the biologic clock in phase with the 24-h LD cycle.20 Retinal light exposure tonically activates SCN neurons through release of multiple neurotransmitters, including the excitatory amino acid glutamate from RHT nerve endings,21 resulting in greater SCN neuronal activity during the illuminated portion of the LD cycle. GABAergic efferents from SCN inhibit the activity of the paraventricular hypothalamic nucleus (PVN), ensuring the maintenance of an inverse activation state relative to SCN.22 Diminished nocturnal retinal light exposure lowers tonic stimulation of the SCN and, in turn, GABAergic inhibition of the PVN. The surge in PVN neuronal discharge is transmitted via a circuitous pathway that projects caudally to the upper thoracic intermediolateral cell column, then ascends rostrally as preganglionic sympathetic fibers to the superior cervical ganglion (SCG), and finally continues as postganglionic fibers (nervi conarii) to innervate the pineal gland (Figure 1).23,24 The release of norepinephrine from the postganglionic noradrenergic fibers of SCG promotes the synthesis of melatonin from serotonin in the pineal gland.25 Melatonin is secreted into the blood stream and detected by the melatonin 1 and 2 receptors (MT-1, MT-2) in the SCN, further reducing and modulating its electrical activity in a negative feedback loop.26 In the presence of intact retinal photoreceptors, light inhibits while darkness promotes, melatonin secretion from the pineal gland.25 Under natural conditions, plasma melatonin concentration surges after dusk and returns to low daytime values with the onset of dawn.
Figure 1.
Physiology of Melatonin Secretion. Melatonin (inset) is produced in the pineal gland. The production and secretion of melatonin are mediated largely by postganglionic retinal nerve fibers that pass through the retinohypothalamic tract to the suprachiasmatic nucleus, then to the superior cervical ganglion, and finally to the pineal gland. This neuronal system is activated by darkness and suppressed by light. The activation of α1- and β1-adrenergic receptors in the pineal gland raises cyclic AMP and calcium concentrations and activates arylalkylamine N-acetyltranferanse, initiating the synthesis and release of melatonin. The daily rhythm of melatonin secretion is also controlled by an endogenous, free-running pacemaker located in the suprachiasmatic nucleus. Reproduced from Brzezinski A. Melatonin in humans. N Eng J Med 1997; 336:186-195 (with kind permission from The Publishing Division of the Massachusetts Medical Society, Waltham, Massachusetts).
CLINICAL APPROACH TO THE BIOLOGIC CLOCK
Light therapy targets the biologic (subjective) clock and attempts to reset the phase of the clock's activity relative to the LD cycle and/or influence its amplitude. Since improved quality and quantity of sleep coincides with sleep at subjective night,27 common goals of light therapy include: (1) synchronizing the sleep-wake cycle with the subjective night; (2) shifting the biologic clock phase to facilitate sleep at a desired time of day/night; and (3) advancing the biologic clock phase to attain indirect effects on mood (see discussion below on light therapy of mood disorders). The biologic clock is, however, not equally amenable to phase shifts throughout its circadian period. Successful light therapy, therefore, requires the identification of circadian windows of opportunity for clinical intervention.
Timing of Light Therapy
The responsiveness of the biologic clock (SCN) to an intervention is represented by the magnitude of the ensuing shift in the clock's phase. SCN phase shifts obtained by systematic exposure to a zeitgeber of defined magnitude at various biologic clock times (under exclusion of other zeitgebers) may be measured in minutes and subsequently plotted against the biologic time of exposure as the phase response curve (PRC, Figure 2). The sinusoidal shape of the PRC reveals a remarkable property of the biologic clock: any zeitgeber can yield phase advancement (positive shift), delay (negative shift), or be entirely phase neutral depending on the biologic clock time at which that zeitgeber is administered. Since no direct measures of SCN activity phase exist, the biologic clock time must instead be inferred from indirect markers of SCN activity, such as the plasma melatonin concentration.28 SCN responsiveness to light administration correlates with diurnal changes in the plasma/salivary concentration of melatonin as illustrated by the phase response curve.29,30 Since plasma corticosteroids and core body temperature31 are also under SCN circadian regulation, diurnal fluctuation of these parameters also serve as proxy measures of biologic clock time.32 While the sleep-wake cycle is generally phase synchronized with the biologic clock in healthy subjects who are following a normal circadian rhythm,27 the sleep-wake cycle is not a wholly reliable indicator of biologic clock time, since a reset, phase-shifted biologic clock may not substantially alter the sleep-wake cycle itself.33
Figure 2.
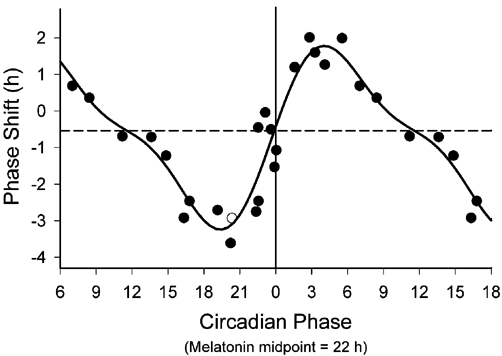
The PRC to the bright light stimulus using melatonin midpoints as the circadian phase marker. Phase advances (positive values) and delays (negative values) are plotted against the timing of the centre of the light exposure relative to the melatonin midpoint on the pre-stimulus constant routine (defined to be 22 h), with the core body temperature minimum assumed to occur 2 h later at 0 h. Data points from circadian phases 6-18 are double plotted. The filled circles represent data from plasma melatonin, and the open circle represents data from salivary melatonin in subject 18K8 from whom blood samples were not acquired. The solid curve is a dual harmonic function fitted through all of the data points. The horizontal dashed line represents the anticipated 0.54 h average delay drift of the pacemaker between the pre- and post-stimulus phase assessments. Reproduced from: Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol 2003; 549: 945–952 (with kind permission of Wiley-Blackwell, Oxford, UK).
Plasma melatonin levels are relatively low throughout the subjective day given tonic suppression of pineal gland activity by the SCN. Dim light melatonin onset (DLMO) refers to a surge in plasma melatonin concentration correlated with diminishing SCN neuronal firing, usually occurring in the first part of the subjective night. Maximal phase delay of the biologic clock can be induced by light administration prior to the core body temperature minimum, or during the melatonin concentration surge (generally in the first half of subjective night). In contrast, maximal phase advancement of the biologic clock is achieved by light administration following the core body temperature minimum or during the plasma melatonin concentration fall (generally in the second half of subjective night).12,32 Due to practical difficulties, measurement of DLMO and core body temperature minimum for optimal timing of therapeutic light administration have not been adopted into routine clinical practice. For instance, core body temperature may be falsely elevated due to physical exercise or ambient temperature, and measuring DLMO would require night time blood/saliva sampling. Simplified algorithms (discussed below) designed to assist clinicians in identifying the subjective day and night (i.e., individual circadian rhythm) and selection of proper timing for light therapy await replacement by more accurate and user-friendly tools.
Types and Dosing of Light Therapy
The biologic valence of light therapy is determined by 2 inherent features—wavelength and intensity. Visible light has an approximate wavelength spectrum of 380 (violet) to 760 (red) nm. Early studies of light generally utilized bright white light (a mixed spectrum of wavelengths similar to day light) to examine light effects on human circadian rhythm. Recent studies, however, have demonstrated that short wavelength blue light (∼ 460 nm) possesses greater phase shifting properties than the rest of the visible light spectrum.34–37 Hence, blue light therapy may obviate the need for high intensities to influence the biologic clock.
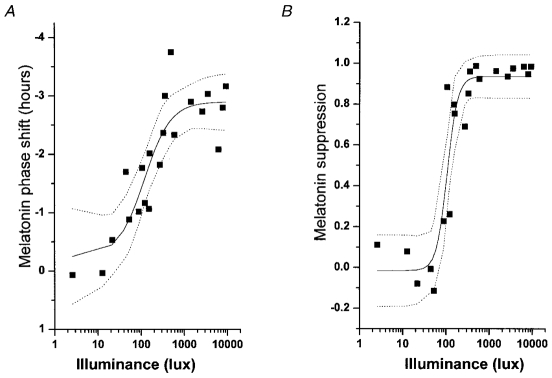
The unit of intensity for visible light is lux. For example, the intensity of sunlight at midday measures over 100,000 lux. Early research in light therapy operated under the assumption that bright white light (7,000-12,000 lux simulating the ambient outdoor light intensity just after dawn) was necessary to produce discernible biologic effects.33,38 Subsequent research, however, proved that much lower intensity sources, such as ordinary room light (≈180 lux) were sufficient to reset the human biologic clock,39 implying that exposure to ordinary room light on the opposite side of the Y-axis of the PRC may interfere with logical design and desired outcome of prescribed light therapy.39–41 One strategy to compensate for the undesired effects of ordinary room light on the SCN is to administer a higher dose (intensity × duration) of therapeutic light. The elongated S-shaped dose-response curve (DRC) correlates the resultant circadian phase shift in minutes with a particular light dose (Figure 3). The DRC is nonlinear, slopes maximally between 50 and 550 lux and plateaus thereafter, indicating that high-intensity stimuli exceeding 550 lux are only slightly more effective than lower intensity stimuli. For instance, the net biologic effect of 9000 lux over 6.5 h may only be twice that of 100 lux over the same period.41 Although possible in principle, it may, therefore, be difficult to overcome the effects of ordinary room light on circadian rhythm by administering a higher intensity light for a longer duration. Moreover, sensitivity of the SCN to various light intensities is dependent on previous light exposure history.42,43 Extended prior exposure to dim light sensitizes the SCN to light, generating greater melatonin suppression following exposure to light of modest intensity, while baseline exposure to high light intensities produces the opposite effect.44,45 A thorough history of the nature, amount, and timing of daily light exposure of each patient may prove essential for the appropriate design and prescription for optimal light therapy.46,47 In some cases, successful therapy may necessitate a combination of light exposure and light restriction by wearing dark goggles (see delayed sleep phase type below) or even employ light restriction solely.48
Figure 3.
Illuminance-response curve of the human circadian pacemaker. The shift in the phase of the melatonin rhythm (A), as assessed on the day following exposure to a 6·5 h experimental light stimulus, has been fitted with a four parameter logistic model using a non-linear least squares analysis. Acute suppression of plasma melatonin (B) during the light exposure also has been fitted with a four parameter logistic model using a non-linear least squares analysis. The logistic models predict an inflection point of the curve (i.e. the sensitivity of the system) at ≈120 lx. Saturation of the phase-shift response is predicted to occur with ≈550 lx and saturation of the melatonin-suppression response is predicted to occur with ≈200 lx. Individual subjects are represented by ▪ the model by the continuous line, and the 95 % confidence intervals by the dotted lines. Reproduced from: Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 2000; 526: 695-702 (with kind permission of Wiley-Blackwell, Oxford, UK).
DISORDERS RESPONSIVE TO LIGHT THERAPY
Light therapy has been used to treat a number of disorders that can be classified in three broad categories: (1) disorders caused by desynchronization between the circadian rhythm, sleep-wake cycle, and the external environment; (2) mood disorders; and (3) disorders that entail elements of both.
Circadian Rhythm Sleep Disorders
Circadian rhythm sleep disorders are misalignments between the timing of an individual's circadian rhythm of sleep propensity and the natural or societal rhythms of the individual's environment. Circadian rhythm sleep disorders may arise when the physical environment is altered relative to internal circadian timing (as in rapid air travel across several time zones) or when intrinsic circadian timing is out of phase with the individual's environment. The hallmarks of all circadian rhythm sleep disorders are tenacious insomnia and/or hypersomnia relative to the environmental clock time.
Circadian rhythm sleep disorder, delayed sleep phase type (DSPT) is common in adolescents and young adults, and is characterized by delayed bedtime and impaired ability to arise early or entrain to a usual daytime work schedule. Attempts to realign the sleep cycle often fail, resulting in sleep-onset insomnia and excessive daytime sleepiness. Advancing the circadian rhythm requires light exposure following the nadir of core body temperature. Exposure to white light of 2500 lux for 2 h in the early morning, combined with light restriction after 16:00 (dark goggles) is an effective treatment for DSPT.49 Alternatively, a light mask offering exposure to gradually escalating light intensities through closed eyelids over the last 4 h of habitual sleep time can be used to advance the circadian rhythm in DSPT patients.50 In a clinical trial, one week of morning blue light therapy succeeded in advancing the dim light melatonin onset by 2.5 h but failed to induce a similar shift in the sleep-wake cycle.51 Despite limited evidence, the American Academy of Sleep Medicine currently considers timed phototherapy as “a rational and effective intervention for DSPT”.52
Circadian rhythm sleep disorder, advanced sleep phase type (ASPT) represents an undesirably early onset of sleep in the evening followed by an excessively early morning awakening. Patients with ASPT, usually older adults, are plagued by inability to stay awake for social events in evening hours. Evening light therapy (prior to core body temperature nadir) can be used to phase delay patients toward a later bedtime. Information on light treatment for this condition is, however, very limited.52–55
Circadian rhythm sleep disorder, nonentrained type is common in blind, but rare in the sighted population. A complete lack of circadian rhythm phase synchronization with the 24-h day enables the sleep-wake cycle to follow an approximate 24.2 h endogenous period length, resulting in a gradual drift of the sleep-wake cycle in successive phase delays over the entire calendar day. Bright light may succeed in entraining a subpopulation of patients who retain functional melanopsin containing retinal ganglion cells. Such patients display intact plasma melatonin suppression in response to bright light exposure, a property that allows the identification of potential therapy candidates.18,56
A mismatch between the work and sleep-wake cycle schedule and the endogenous circadian rhythm gives rise to circadian rhythm sleep disorder, shift work type. Light therapy may assist in realigning the circadian rhythm with the desired work schedule, provided the work shift changes sufficiently infrequently.57 Habitual night shift workers demonstrate improved nocturnal alertness under bright light exposure in the work place, and restriction of daytime light, indicating a sustained biologic clock phase reversal.58,59
Circadian rhythm sleep disorder, jet lag type represents the sudden misalignment of a previously entrained circadian rhythm and a new geographic time zone imposed by rapid transmeridian air travel. Sleep deprivation in the course of travel may further contribute to the sleep-wake disturbance in this condition. Given the endless possibilities in combining the disorder parameters (number of time zones crossed, direction of travel, baseline phase of the circadian rhythm relative to local time, light exposure history, etc.), it is difficult to provide general recommendations for light therapy in jet lag disorder.60 Computer algorithms based on the PRC have been devised to calculate the optimal timing of light therapy.61 A simplified approach to treatment could involve pre-flight phase advancement for eastward travel and phase delay for westward travel. Using this paradigm, a study reported significant benefit in light therapy for jet lag disorder.62 In addition to light exposure, timed light restriction may prove to play an important role in treating this disorder.63
Dementia
Severe disturbances of circadian rhythms due to deteriorating SCN function,64 diminished exposure to zeitgebers (e.g., reduced daily variation in environmental light), and/or impaired visual reception are commonly encountered in elderly institutionalized patients.65 Prolonged wakefulness and wandering at night are compensated by daytime napping. Nocturnal agitation and wandering have been related to the amount and circadian distribution of light exposure.65 In a population with dementia and behavioral problems, daytime exposure to diffuse bright light reduced the variability of rest/activity cycle, as documented by actigraphy.66 Evening bright light administration (19:00-21:00) assisted in consolidating sleep in elderly with Alzheimer disease, sundowning, and nocturnal agitation.67 Morning bright light exposure increased the total nocturnal sleep time in another group of dementia patients without affecting behavior.68 Behavioral parameters improved following morning bright light therapy, yet sleep quality and quantity remained unchanged in another study.69 A rigorous study of demented nursing home residents also failed to improve nocturnal sleep by light therapy, but did not rate the behavioral impact of the treatment.70 In another trial, total agitation ratings in institutionalized patients with severe Alzheimer disease were unchanged following light therapy, although effects on circadian rhythms of these patients were not reported.71 Conflicting results may be attributed to heterogeneity of the studied population with respect to underlying diagnosis, stage of disease, visual impairment and/or methodological questions such as timing of light exposure with respect to core body temperature or baseline light conditions affecting light sensitivity (light history). Additional large controlled, prospective trials are warranted to clarify the value of light therapy in the treatment of behavioral problems accompanying dementia.
Mood Disorders
Mood disorders are highly prevalent and frequently associated with alterations in hypothalamic and pituitary hormones and disturbance of sleep architecture and sleep-wake cycle,72 suggesting a concomitant disruption of biologic clock function. Conversely, the temporal relationship between the sleep-wake cycle and biologic clock phase affects circadian mood variations in healthy individuals in a complex fashion.73
The precise nature of neurobiological actions of phototherapy in mood disorders remains unclear. Paralleling general concepts underlying pharmacotherapy of depressive disorders, one postulated mechanism for the antidepressant effect of light is mediation by biogenic amines, since depletion of tryptophan, the amino acid precursor of the neurotransmitter serotonin, reverses beneficial effects of light therapy on mood.74,75
Reduced retinal light sensitivity secondary to disturbances of the retinal neurotransmitter, dopamine,76 has been proposed as a potential culprit in seasonal affective disorder (SAD).77,78 Exposure to intense light increases retinal dopamine activity and favors day vision over night vision.79,80 Another controversial hypothesis holds that light's antidepressant effects are conferred through phase advancement of the biologic clock.81–84 Phase advancement of the biologic clock may even be implicated in the antidepressant properties of selective serotonin reuptake inhibitors such as fluoxetine, as supported by an in vitro study.85 More complex interactions of antidepressant medications with clock genes in a synergistic or complimentary fashion to light are also possible. For instance, in an animal model, fluoxetine induced the expression of clock genes in brain regions beyond the SCN.86
Phototherapy was introduced in 1984 as a treatment for mood disorders with a seasonal pattern (seasonal affective disorder or SAD).87 Seasonal pattern is a standard DSM-IV-TR specifier for the depressed portion of mood disorders, requiring a temporal relationship between the onset of the depressive episodes and a particular time of year, followed by full remission associated with another time of year, observed over a minimum time span of 2 consecutive years.72 The seasonal pattern specifier may be used to qualify unipolar or bipolar depression. Patients with SAD often display the atypical neurovegetative symptoms of hypersomnia and hyperphagia, 2 positive predictors of response to light therapy.88 The onset of winter depression may also be associated with a tendency to circadian phase delay termed eveningness.89 The classical phototherapeutic approach to SAD uses a morning dose of 5000 lux/h (10,000 lux for 30 min, or 2500 lux for 2 h). As the clinical benefits of light therapy in SAD appear to stem in part from a phase advancement of the circadian rhythm (represented by the earlier DLMO), the proper timing of the administration of the light treatment relative to DLMO is crucial.82 To achieve the best results, light therapy should be administered in the morning hours, approximately 8.5 h after the DLMO.82 In case the measurement of DLMO is not readily available, the following surrogate algorithm may be used. For every half-hour of nocturnal sleep beyond 6 h, light therapy should be scheduled 15 min prior to the habitual awakening time, to a maximum of 1.5 h.90 For instance, a patient who habitually sleeps 8 h (i.e., 4 × 30 min beyond 6 h) has to be awakened 4 × 15 minutes, or 1 h, prior to the usual awakening time to receive light treatment. This is, however, merely a rough estimate based on data obtained from patients with SAD. The same regimen has also been successfully applied to patients with delayed sleep phase disorder and nonseasonal depression.90 Fluoxetine did not offer an advantage over light therapy in SAD, underscoring the value of light therapy as a cost effective first-line treatment in this condition.91
Up to 20% of patients with nonseasonal unipolar depression are treatment resistant to antidepressant medications,92 in part attributable to the inclusion of a subgroup with undiagnosed bipolar disorder in this population.93 Not surprisingly, phototherapy as a sole therapeutic tool, and in combination with other measures, has been studied for treatment of both nonseasonal unipolar and bipolar depressive disorders. In general, the efficacy of light therapy in nonseasonal depression appears to be lower than in SAD.94,95 When used as stand-alone treatment (without concomitant antidepressant medication), the outcome is inconsistent and unpredictable.96–98 Mood improvement following total sleep deprivation may be a positive response predictor to phototherapy in nonseasonal depression.99 Light therapy as an adjuvant measure (combined with pharmacotherapy), however, offers more robust results.98,100–101 Regimens for light administration in non-seasonal depression are similar to those customary for SAD.
Insomnia
Insomnia is the most common sleep-wake related complaint.102 In addition to medical etiologies such as chronic pain or sleep related breathing disorders, the International Classification of Sleep Disorders (ICSD-2) identifies psychological and behavioral factors as the defining features of various insomnia subtypes. Indeed, chronic insomnia has been linked to the future onset of psychiatric disorders, especially depression and anxiety.103–105 On the other hand, certain psychosocial factors that are frequently encountered in the setting of anxiety and depression such as older age, unemployment, lack of habitual exercise, psychological stress, and poor perceived health appear to be associated with insomnia,106–107 justifying a common “symptomatic” treatment approach to insomnia in absence of clear medical or psychiatric etiologies. Moreover, sleep onset insomnia has been correlated with a delayed circadian rhythm, lending further support to the therapeutic use of light in this condition.108
A non-pharmacologic treatment trial of psychophysiological insomnia compared sleep hygiene instructions alone to sleep hygiene instructions coupled with phototherapy, and found that the inclusion of phototherapy was necessary to produce a statistically significant benefit.109 A subsequent trial revealed that the exposure duration of 45 min was superior to 20 min.110 In a population with sleep onset insomnia, exposure to bright morning light reduced sleep latency, insomnia severity, and pre-sleep anxiety, while increasing total sleep time and improving overall daytime functioning.111 These benefits were further associated with a significant phase advancement of DLMO and sleep onset. Melatonin and ramelteon, an anti-insomnia agent, exert their sleep promoting effects presumably through MT-1 and MT-2 agonism. Morning light therapy could similarly improve sleep quality by modulating endogenous melatonin.112–113
Light Application in Clinical Practice
Various light stimuli covering a range of intensities and spectrums have been used for therapeutic intervention, although not all have been adequately tested. The optimal duration of light therapy cycles in various conditions has not yet been established. The majority of published studies of light therapy protocols do not exceed one month duration. Only one trial reviewed for this paper extended over 60 days.110 At the present time, decisions regarding the number of therapeutic cycles and their duration must therefore be deferred to clinical judgment.
Traditionally, investigators have resorted to multiple fluorescent tubes to emit intensities from 2,500 to 12,000 lux for light therapy. The light source is placed 1-3 feet from the patient. A diffusion screen placed over the fluorescent tubes ensures even distribution of light and protects from ultraviolet wavelengths. The patient is instructed to use the light for illumination while performing desk work or reading and to avoid gazing at the light source directly. As the lower portion of the retina appears to have a greater propensity to communicate with the biologic clock,114 positioning the light source above eye level is recommended.
Unsupervised early morning light exposure performed at home raises the question of sleepy patients' compliance. Naturalistic dawn simulation was devised to minimize the need for reliance on the patient's active participation and practically reduce the necessary time investment for the treatment to zero. The patient, while still asleep, is exposed to a light source starting at near complete darkness and growing over approximately 3 h to a maximum intensity of 250 lux, effectively reproducing the natural sleep conditions under a tree cover in the northern hemisphere spring.115 The light source is placed above the head of the bed and faces the pillow. Maximum intensity is programmed to coincide with the habitual waking time and to subsequently fade out rapidly. The benefit is presumably due to the interaction of light with the retinal receptors through the closed eyelids.116 In addition to easing compliance, naturalistic dawn simulation eliminates possible ocular adverse effects due to excessive exposure and/or preexisting retinal disease.
Bright light could pose dangers to patients with known retinal pathology, and in those using photosensitizing medications. Excluding these cases and excessive exposure, however, light therapy overall appears to have a very favorable risk-benefit ratio.117 Although reported in SAD, switching depression to mania and inducing suicidal ideation are rare.118,119 Complaints of decreased sleep, dry mouth, headache, weakness, and fatigue are as commonly reported as in fluoxetine therapy.91 Ocular adverse effects in individuals who were previously free of ocular disease were not detected, even after extended exposure.120 Nevertheless, ophthalmologic evaluation every few years may be a reasonable precaution.
Side effects of light therapy overdose may include agitation, headache, or nausea. Insomnia, particularly initial insomnia, may also be encountered.117 Side effects can be obviated by reducing the light dose (intensity, duration, or both), increasing the distance between the patient and the light source, or by moving morning therapy sessions to a later time or evening treatments to an earlier time to diminish the light efficacy according to the PRC.
Conclusion
Light is the main zeitgeber for the human circadian rhythm. Light exerts its influence on the central nervous system through interaction with retinal ganglion cells. The capacity of light to advance or delay the circadian rhythms of melatonin, core body temperature, and corticosteroids can be utilized for clinical purposes. The main clinical applications of light therapy are in circadian rhythm sleep disorders, dementia, mood disorders, and insomnia. In bright light therapy, 5000 lux/h administered daily over several weeks is usual. Lower light intensities yield similar efficacies, if presented as naturalistic dawn simulation or short wavelength blue light. Given the widespread influences of light upon circadian brain functions, the spectrum of clinical disorders in which light therapy can be considered is likely to expand, with early evidence pointing toward potential efficacy in subsyndromal mood and certain eating disorders.121,122 Large randomized, controlled clinical trials are necessary to identify response predictors and better characterize optimal administration parameters including duration and timing of light therapy. Additionally, clinical trials evaluating the longitudinal efficacy and safety of light therapy for chronic medical conditions would help refine future clinical applications of light therapy. Practical application of light therapy would also be aided by efforts to educate clinicians concerning the scientific basis of light therapy and its clinical applications.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Sulzman FM, Ellman D, Fuller CA, Moore-Ede MC, Wassmer G. Neurospora circadian rhythms in space: a reexamination of the endogenous-exogenous question. Science. 1984;225:232–4. doi: 10.1126/science.11540800. [DOI] [PubMed] [Google Scholar]
- 2.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 3.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 4.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamura H, Miyake S, Sumi Y, et al. Photic induction of mPer1 and mPer2 in Cry-deficient mice lacking a biological clock. Science. 1999;286:2531–4. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 6.Shearman LP, Sriram S, Weaver DR, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–9. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 7.Inouye ST, Takahashi JS, Wollnik F, Turek FW. Inhibitor of protein synthesis phase shifts a circadian pacemaker in mammalian SCN. Am J Physiol. 1988;255:R1055–R1058. doi: 10.1152/ajpregu.1988.255.6.R1055. [DOI] [PubMed] [Google Scholar]
- 8.Bos NP, Mirmiran M. Circadian rhythms in spontaneous neuronal discharges of the cultured suprachiasmatic nucleus. Brain Res. 1990;511:158–62. doi: 10.1016/0006-8993(90)90235-4. [DOI] [PubMed] [Google Scholar]
- 9.Okamura H. Clock genes in cell clocks: roles, actions, and mysteries. J Biol Rhythms. 2004;19:388–99. doi: 10.1177/0748730404269169. [DOI] [PubMed] [Google Scholar]
- 10.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–48. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 11.Busino L, Bassermann F, Maiolica A, et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–4. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 13.Buxton OM, Lee CW, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2004;284:R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- 14.Baehr EK, Eastman CI, Revelle W, Olson SH, Wolfe LF, Zee PC. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1542–R1550. doi: 10.1152/ajpregu.00761.2002. [DOI] [PubMed] [Google Scholar]
- 15.Roenneberg T, Foster RG. Twilight times: light and the circadian system. Photochem Photobiol. 1997;66:549–61. doi: 10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 16.Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127–34. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- 17.Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of suprachiasmatic nucleus neurons to light and dark adaptation. Relative contributions of melanopsin and rod-cone inputs. J Neurosci. 2007;27:9623–31. doi: 10.1523/JNEUROSCI.1391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 19.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–7. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 20.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 21.Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–30. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boudaba C, Szabó K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–60. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparks DL. Anatomy of a new paired tract of the pineal gland in humans. Neuroscience Lett. 1998;248:179–82. doi: 10.1016/s0304-3940(98)00365-6. [DOI] [PubMed] [Google Scholar]
- 24.Teclemariam-Mesbah R, Ter Horst GJ, Postema F, Wortel J, Buijs RM. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J Comp Neurol. 1999;406:171–82. [PubMed] [Google Scholar]
- 25.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–95. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 26.Dubocovich ML. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007;8:34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 28.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 29.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–65. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 31.Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. doi: 10.1016/0006-8993(95)96872-i. [DOI] [PubMed] [Google Scholar]
- 32.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 33.Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–71. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 34.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wave length light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 35.Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wave length light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 36.Wright H, Lack LC, Kennaway DJ. Differential effects of light wavelength in phase advancing melatonin rhythm. J Pineal Res. 2004;36:140–4. doi: 10.1046/j.1600-079x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 37.Cajochen C, Munch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 38.Wever RA. Light effects on human circadian rhythms: a review of recent Andechs experiments. J Biol Rhythms. 1989;4:161–85. [PubMed] [Google Scholar]
- 39.Boivin DB, Czeisler CA. Resetting of circadian melatonin and cortisol rhythms in humans by ordinary room light. Neuroreport. 1998;9:779–82. doi: 10.1097/00001756-199803300-00002. [DOI] [PubMed] [Google Scholar]
- 40.Boivin DB, Duffy JF, Kronauer RE, et al. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–2. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 41.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer WE, Millam JR, Bradley FA. Photostimulation of Japanese quail by dim light depends upon photophase contrast, not light intensity. Biol Reprod. 1988;38:536–43. doi: 10.1095/biolreprod38.3.536. [DOI] [PubMed] [Google Scholar]
- 43.Meyer WE, Millam JR. Plasma melatonin levels in Japanese quail exposed to dim light are determined by subjective interpretation of day and night, not light intensity. Gen Comp Endocrinol. 1991;82:377–85. doi: 10.1016/0016-6480(91)90313-u. [DOI] [PubMed] [Google Scholar]
- 44.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–4. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 45.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 2006;21:394–404. doi: 10.1177/0748730406292391. [DOI] [PubMed] [Google Scholar]
- 47.Rufiange M, Beaulieu C, Lachapelle C, Dumont M. Circadian light sensitivity and rate of retinal dark adaptation in indoor and outdoor workers. J Biol Rhythms. 2007;22:454–7. doi: 10.1177/0748730407305375. [DOI] [PubMed] [Google Scholar]
- 48.Barbini B, Benedetti F, Colombo C, et al. Dark therapy for mania: a pilot study. Bipolar Disord. 2005;7:98–101. doi: 10.1111/j.1399-5618.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase disorder. Sleep. 1990;13:354–61. [PubMed] [Google Scholar]
- 50.Cole RJ, Smith JS, Alcalá YC, et al. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17:89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- 51.Lack L, Bramwell T, Wright H, Kemp K. Morning blue light can advance the melatonin rhythm in mild delayed sleep phase syndrome. Sleep Biol Rhythms. 2007;5:78–80. [Google Scholar]
- 52.Sack R, Auckley D, Auger RR, et al. IV. Circadian rhythm sleep disorders: Part II: advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder and irregular sleep-wake rhythm. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–36. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 54.Lack L, Wright J. The effect of evening bright light in delaying the circadian rhythms and lengthening the sleep of early morning awakening insomniacs. Sleep. 1993;16:436–43. doi: 10.1093/sleep/16.5.436. [DOI] [PubMed] [Google Scholar]
- 55.Singer CM, Lewy AJ. Case report: use of the dim light melatonin onset in the treatment of ASPS with bright light. Sleep Res. 1989;18:445. [Google Scholar]
- 56.Hoban TM, Sack RL, Lewy AJ, et al. Entrainment of a free-running human with bright light? Chronobiol Int. 1989;6:347–53. doi: 10.3109/07420528909056941. [DOI] [PubMed] [Google Scholar]
- 57.Boivin DB, James FO. Light treatment and circadian adaptation to shift work. Ind Health. 2005;43:34–48. doi: 10.2486/indhealth.43.34. [DOI] [PubMed] [Google Scholar]
- 58.Yoon IY, Jeong DU, Kwon KB, Kang SB, Song BG. Bright light exposure at night and light attenuation in the morning improve adaptation of night shift workers. Sleep. 2002;25:351–6. [PubMed] [Google Scholar]
- 59.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptaion to night work. N Engl J Med. 1990;322:1253–9. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 60.Samel A, Wegmann HM. Bright light: A countermeasure for jet lag? Chronobiol Int. 1997;14:173–84. doi: 10.3109/07420529709001154. [DOI] [PubMed] [Google Scholar]
- 61.Houpt TA, Boulos Z, Moore-Ede MC. MidnightSun: Software for determining light exposure phase shifting schedules during global travel. Physiol Behav. 1996;39:561–8. doi: 10.1016/0031-9384(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 62.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel:3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–28. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daan S, Lewy AJ. Scheduled exposure to daylight: a potential strategy to reduce “jet lag” following transmeridian flight. Psychopharmacol Bull. 1984;20:566–8. [PubMed] [Google Scholar]
- 64.Van Someren EJW, Riemersma RF, Swaab DF. Functional plasticity of the circadian timing system in old age: light exposure. Prog Brain Res. 2002;138:205–23. doi: 10.1016/S0079-6123(02)38080-4. [DOI] [PubMed] [Google Scholar]
- 65.Martin J, Marler M, Shochat T, et al. Circadian rhythms of agitation in institutionalized patients with Alzheimer's disease. Chronobiol Int. 2000;17:405–18. doi: 10.1081/cbi-100101054. [DOI] [PubMed] [Google Scholar]
- 66.Van Someren EJW, Kessler A, Mirmiran M, et al. Indirect bright light improves circadian rest-activity disturbances in demented patients. Biol Psychiatry. 1997;41:955–63. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 67.Satlin A, Volicer L, Ross V, et al. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer's dementia. Am J Psychiatry. 1992;149:1028–32. doi: 10.1176/ajp.149.8.1028. [DOI] [PubMed] [Google Scholar]
- 68.Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14:520–5. [PubMed] [Google Scholar]
- 69.Skjerve A, Holsten F, Aarsland D, Bjorvatn B, Nygaard HA, Johansen IM. Improvement in behavioral symptoms and advance of activity acrophase after short-term bright light treatment in severe dementia. Psychiatry Clin Neurosci. 2004;58:343–7. doi: 10.1111/j.1440-1819.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- 70.Ancoli-Israel S, Martin JL, Kripke DF, et al. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50:282–9. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ancoli-Israel S, Martin JL, Gehrman P, et al. Effects of light on agitation in institutionalized patients with severe Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:194–203. [PubMed] [Google Scholar]
- 72.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV TR). 4th ed. Text Revision. Washington DC: American Psychiatric Association; 2000. pp. 345–428. [Google Scholar]
- 73.Boivin DB, Czeisler CA, Dijk DJ, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 74.Neumeister A, Praschak-Rieder N, Besselmann B, Rao ML, Gluck J, Kasper S. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54:133–8. doi: 10.1001/archpsyc.1997.01830140043008. [DOI] [PubMed] [Google Scholar]
- 75.Lam RW, Zis AP, Grewal A, Delgado PL, Charney DS, Krystal JH. Effects of rapid tryptophan depletion in patients with seasonal affective disorder in remission after light therapy. Arch Gen Psychiatry. 1996;53:41–4. doi: 10.1001/archpsyc.1996.01830010043007. [DOI] [PubMed] [Google Scholar]
- 76.Pflug R, Nelson R, Huber S, Reitsamer H. Modulation of horizontal cell function by dopaminergic ligands in mammalian retina. Vision Res. 2008;48:1383–90. doi: 10.1016/j.visres.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lam RW, Levitan RD. Pathophysiology of seasonal affective disorder: a review. J Psychiatry Neurosci. 2000;25:469–80. [PMC free article] [PubMed] [Google Scholar]
- 78.Hébert M, Beattie CW, Tam EM, Yatham LN, Lam RW. Electroretinography in patients with winter seasonal affective disorder. Psychiatry Res. 2004;127:27–34. doi: 10.1016/j.psychres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–9. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gagné AM, Gagné P, Hébert M. Impact of light therapy on rod and cone functions in healthy subjects. Psychiatry Res. 2007;151:259–63. doi: 10.1016/j.psychres.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Lewy AJ, Sack RL. The phase-shift hypothesis of seasonal affective disorder. Am J Psychiatry. 1988;145:1041–2. doi: 10.1176/ajp.145.8.1041c. [DOI] [PubMed] [Google Scholar]
- 82.Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 83.Wirz-Justice A, Graw P, Kräuchi K, et al. Light therapy in seasonal affective disorder is independent of time of the day or circadian phase. Arch Gen Psychiatry. 1993;50:929–37. doi: 10.1001/archpsyc.1993.01820240013001. [DOI] [PubMed] [Google Scholar]
- 84.Meesters Y, Jansen JHC, Beersma DGM, Bouhuys AL, van den Hoofdakker RH. Light therapy for seasonal affective disorder. The effects of timing. Br J Psychiatry. 1995;166:607–12. doi: 10.1192/bjp.166.5.607. [DOI] [PubMed] [Google Scholar]
- 85.Sprouse J, Braselton J, Reynolds L. Fluoxetine modulates the circadian biologic clock via phase advances of suprachiasmatic nucleus neuronal firing. Biol Psychiatry. 2006;60:896–9. doi: 10.1016/j.biopsych.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Uz T, Ahmed R, Akhisaroglu M, et al. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134:1309–16. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 88.Terman M, Terman, JS, Williams JBW. Seasonal affective disorder and its treatments. J Prac Psychiatry Behav Health. 1998;5:287–303. [Google Scholar]
- 89.Murray G, Allen NB, Trinder J. Seasonality and circadian phase delay: prospective evidence that winter lowering of mood is associated with a shift towards eveningness. J Affect Disord. 2003;76:15–22. doi: 10.1016/s0165-0327(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 90.Terman M, Terman JS. Light therapy. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th. Philadelphia: Elsevier/Saunders; 2005. pp. 1424–42. [Google Scholar]
- 91.Lam RW, Levitt AJ, Levitan RD, et al. The Can-SAD Study: A randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry. 2006;163:805–12. doi: 10.1176/ajp.2006.163.5.805. [DOI] [PubMed] [Google Scholar]
- 92.Burrows GD, Norman TR, Judd FK. Definition and differential diagnosis of treatment-resistant depression. Int Clin Psychopharmacol. 1994;9:5–10. doi: 10.1097/00004850-199406002-00002. [DOI] [PubMed] [Google Scholar]
- 93.Inoue T, Nakagawa S, Kitaichi Y, et al. Long-term outcome of antidepressant-refractory depression: the relevance of unrecognized bipolarity. J Affect Disord. 2006;95:61–7. doi: 10.1016/j.jad.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 94.Stewart JW, Quitkin FM, Terman M, Terman JS. Is seasonal affective disorder a variant of atypical depression? Differential response to light therapy. Psychiatry Res. 1990;33:121–8. doi: 10.1016/0165-1781(90)90065-d. [DOI] [PubMed] [Google Scholar]
- 95.Thalén BE, Kjellman BF, Morkrid L, Wisom R, Wetterberg L. Light treatment in seasonal and nonseasonal depression. Acta Psychiatr Scand. 1995;91:352–60. doi: 10.1111/j.1600-0447.1995.tb09794.x. [DOI] [PubMed] [Google Scholar]
- 96.Yamada N, Martin-Iverson MT, Daimon K, Tsujimoto T, Takahashi S. Clinical and chronobiological effects of light therapy on nonseasonal affective disorders. Biol Psychiatry. 1995;37:866–73. doi: 10.1016/0006-3223(94)00221-N. [DOI] [PubMed] [Google Scholar]
- 97.Loving RT, Kripke DF, Elliot JA, Knickerbocker NC, Grandner MA. Bright light treatment of depression for older adults. BMC Psychiatry. 2005;5:41. doi: 10.1186/1471-244X-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Even C, Schröder CM, Friedman S, Rouillon F. Efficacy of light therapy in nonseasonal depression: a systematic review. J Affect Disord. 2008;108:11–23. doi: 10.1016/j.jad.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 99.Fritzsche M, Heller R, Hill H, Kick H. Sleep deprivation as a predictor of response to light therapy in major depression. J Affect Disord. 2001;62:207–15. doi: 10.1016/s0165-0327(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 100.Prasko J, Horacek J, Klaschka J, Kosova J, Ondrackova I, Sipek J. Bright light therapy and/or imipramine for inpatients with recurrent non-seasonal depression. Neuro Endocrinol Lett. 2002;23:109–13. [PubMed] [Google Scholar]
- 101.Martiny K, Lunde M, Unden M, Dam H, Bech P. Adjunctive bright light in non-seasonal major depression: results from clinician-rated depression scales. Acta Psychiatr Scand. 2005;112:117–25. doi: 10.1111/j.1600-0447.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 102.The Gallup Organization. The Gallup study of sleeping habits. Princeton, NJ: Gallup Organization; 1979. [Google Scholar]
- 103.Chang PP, Ford DE, Mead LA, et al. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 104.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 105.Harvey AG. Insomnia: symptom or diagnosis? Clin Psychol Rev. 2001;21:1037–59. doi: 10.1016/s0272-7358(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 106.Kales JD, Kales A, Bixler EO, et al. Biopsychobehavioral correlates of insomnia, V: Clinical characteristics and behavioral correlates. Am J Psychiatry. 1984;141:1371–6. doi: 10.1176/ajp.141.11.1371. [DOI] [PubMed] [Google Scholar]
- 107.Kyuja K, Makoto U, Masako O, Xianchen L, Ryuji O. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41–47. [PubMed] [Google Scholar]
- 108.Morris M, Lack L, Dawson D. Sleep-onset insomniacs have delayed temperature rhythms. Sleep. 1990;10:1–14. doi: 10.1093/sleep/13.1.1. [DOI] [PubMed] [Google Scholar]
- 109.Guilleminault C, Clerk A, Black J, Labanowski M, Pelayo R, Claman D. Nondrug treatment trials in psychophysiologic insomnia. Arch Intern Med. 1995;155:838–44. [PubMed] [Google Scholar]
- 110.Kirisoglu C, Guilleminault C. Twenty minutes versus forty-five minutes morning bright light treatment on sleep onset insomnia in elderly subjects. J Psychosom Res. 2004;56:537–52. doi: 10.1016/j.jpsychores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 111.Lack L, Wright H, Paynter D. The treatment of sleep onset insomnia with bright morning light. Sleep Biol Rhythms. 2007;5:173–9. [Google Scholar]
- 112.Leppamaki S, Meesters Y, Haukka J, Lonnqvist J, Partonen T. Effects of simulated dawn on quality of sleep - a community-based trial. BMC Psychiatry. 2003;3:14. doi: 10.1186/1471-244X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–7. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 114.Glickman G, Hanifin JP, Rollag MD, et al. Inferior retinal light exposure is more effective than superior retinal light exposure in suppressing melatonin in humans. J Biol Rhythms. 2003;18:71–79. doi: 10.1177/0748730402239678. [DOI] [PubMed] [Google Scholar]
- 115.Terman M, Su J. Controlled trial of naturalistic dawn simulation and negative air ionization for seasonal affective disorder. Am J Psychiatry. 2006;163:2126–33. doi: 10.1176/ajp.2006.163.12.2126. [DOI] [PubMed] [Google Scholar]
- 116.Danilenko KV, Wirz-Justice A, Kräuchi K, Weber JM, Terman M. The human circadian pacemaker can see by the dawn's early light. J Biol Rhythms. 2000;15:437–46. doi: 10.1177/074873000129001521. [DOI] [PubMed] [Google Scholar]
- 117.Terman M, Terman JS. Bright light therapy:side effects and benefits across the symptom spectrum. J Clin Psychiatry. 1999;60:799–808. [PubMed] [Google Scholar]
- 118.Schwitzer J, Neudorfer C, Blecha HG, et al. Mania as a side effect of phototherapy. Biol Psychiatry. 1990;28:532–4. doi: 10.1016/0006-3223(90)90489-o. [DOI] [PubMed] [Google Scholar]
- 119.Praschak-Rider N, Neumeister A, Hesselmann B, et al. Suicidal tendencies as a complication of light therapy for seasonal affective disorder. A report of three cases. J Clin Psychiatry. 1997;58:389–92. doi: 10.4088/jcp.v58n0903. [DOI] [PubMed] [Google Scholar]
- 120.Gallin PF, Terman M, Reme CE, Rafferty B, Terman JS, Burde RM. Ophthalmologic examination of patients with seasonal affective disorder, before and after light therapy. Am J Ophthalmol. 1995;119:202–10. doi: 10.1016/s0002-9394(14)73874-7. [DOI] [PubMed] [Google Scholar]
- 121.Rastad C, Ulfberg J, Lindberg P. Light room therapy effective in mild forms of seasonal affective disorder- A randomized controlled study. J Affect Disord. 2008;108:291–6. doi: 10.1016/j.jad.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 122.Braun DL, Sunday SR, Fornari VM, et al. Bright light therapy decreases winter binge frequency in women with bulimia nervosa: a double-blind, placebo-controlled study. Compr Psychiatry. 1999;40:442–8. doi: 10.1016/s0010-440x(99)90088-3. [DOI] [PubMed] [Google Scholar]