Abstract
Wdr5 is developmentally expressed in osteoblasts and is required for osteoblast differentiation. Mice overexpressing Wdr5 under the control of the mouse α(1)I collagen promoter (Col I-Wdr5) display accelerated osteoblast differentiation as well as accelerated chondrocyte differentiation, suggesting that overexpression of Wdr5 in osteoblasts affects chondrocyte differentiation. To elucidate the molecular mechanism by which overexpression of Wdr5 in the perichondrium regulates chondrocyte differentiation, studies were undertaken using skeletal elements and cultured metatarsals isolated from wild-type and Col I-Wdr5 embryos. FGF18 mRNA levels were decreased in Col I-Wdr5 humeri. Furthermore, local delivery of FGF18 to the bone collar of ex vivo cultures of metatarsals attenuated the chondrocyte phenotype of the Col I-Wdr5 metatarsals. Impairing local FGF action in wild-type metatarsals resulted in a chondrocyte phenotype analogous to that of Col I-Wdr5 metatarsals implicating impaired FGF action as the cause of the phenotype observed. The expression of Twist-1, which regulates chondrocyte differentiation, was increased in Col I-Wdr5 humeri. Chromatin immunoprecipitation analyses demonstrated that Wdr5 is recruited to the Twist-1 promoter. These findings support a model in which overexpression of Wdr5 in the perichondrium promotes chondrocyte differentiation by modulating the expression of Twist-1 and FGF18.
Introduction
Endochondral bone formation is a highly regulated process that is initiated when mesenchymal cells form condensations and ends with the formation of a mineralized skeleton. During endochondral bone formation, mesenchymal cells within the condensations differentiate into proliferating chondrocytes, which in turn differentiate into hypertrophic chondrocytes and form a cartilage template. Cells surrounding this cartilage anlagen differentiate into osteoblasts forming the perichondrium (Karsenty, 1999; Karsenty and Wagner, 2002; Liu et al., 2007; Liu et al., 2002; Marie, 2003; Ohbayashi et al., 2002; Olsen et al., 2000; Wagner and Karsenty, 2001). Studies in genetically modified mice, point to an intricate network of signals that regulate the proliferation and differentiation of growth plate chondrocytes, as well as osteoblast differentiation. Among the local signaling pathways that are known to play a role in these processes are the canonical Wnt signaling (Akiyama et al., 2004; Hartmann, 2006; Hartmann and Tabin, 2000; Hu et al., 2005; Kawakami et al., 2000; Kawakami et al., 1999; Krishnan et al., 2006; Westendorf et al., 2004; Yamaguchi et al., 1999), bone morphogenetic protein (BMP) (Canalis et al., 2003; Ducy and Karsenty, 2000; Hoffmann and Gross, 2001; Kronenberg, 2003), parathyroid hormone related peptide (PTHrP), Indian Hedgehog (Ihh) (Chung et al., 1998; Chung et al., 2001; Karaplis et al., 1994; Karp et al., 2000; Kobayashi et al., 2002; Kronenberg, 2003; Lanske et al., 1999; Schipani et al., 1997; St-Jacques et al., 1999; Vortkamp et al., 1996) and fibroblast growth factor (FGF) (Colvin et al., 1996; Kronenberg, 2003; Marie, 2003; Ornitz, 2005; Wang et al., 1999) pathways. Several studies have demonstrated that the perichondrium regulates cartilage growth, by providing a source of signaling molecules that regulate chondrocyte proliferation and differentiation (Di Nino et al., 2001; Gamer et al., 2008; Hinoi et al., 2006; Long and Linsenmayer, 1998; Marie, 2003; Ornitz, 2005; Vortkamp et al., 1996).
Wdr5, a BMP-2 -induced gene, encodes a protein belonging to the WD repeat family of β-propeller proteins (Gori et al., 2001). These proteins are crucial for numerous cellular functions including signal transduction, mRNA processing, gene regulation and cell cycle regulation (Neer et al., 1994; Smith et al., 1999; Wysocka et al., 2003). Wdr5 is a critical component of the Set/Ash histone methyltransferase complex and is essential for histone H3 lysine 4 (H3K4) trimethylation, a marker associated with transcriptional activation of genes (Wysocka et al., 2005). The finding that silencing of Wdr5 dramatically impairs the expression of several genes including Runx-2, osteocalcin, Wnt1 and Wnt3a in MC3T3-E1 cells (Zhu et al., 2008) points to a critical role for Wdr5 in regulating the expression of genes involved in osteoblast and chondrocyte differentiation.
Wdr5 is developmentally expressed in osteoblasts. Overexpression of Wdr5 in MC3T3-E1 cells dramatically accelerates the program of osteoblastic differentiation, whereas suppression of Wdr5 expression markedly impairs osteoblast differentiation (Gori et al., 2001; Gori et al., 2006; Zhu et al., 2008). Mice overexpressing Wdr5 in osteoblasts, under the control of the mouse α(1) I collagen promoter (Col I-Wdr5), display acceleration of endochondral and intramembranous bone formation (Gori et al., 2006). At embryonic day (E) 14.5, the long bones of Col I-Wdr5 embryos are longer than those isolated from wild-type littermates due to a larger zone of hypertrophic chondrocytes (Gori et al., 2006), implying that Wdr5 overexpression in the perichondrium affects the expression of factors that regulate chondrocyte differentiation in a paracrine manner. Studies were, therefore, undertaken to address the molecular mechanism by which overexpression of Wdr5 in the perichondrium influences chondrocyte differentiation.
Materials and methods
Metatarsal isolation
Hind limbs isolated from E15.5 Col I-Wdr5 embryos and their wild-type littermates were transferred to a 100 μl drop of medium for further dissection. Soft tissues were peeled off the tibia and metatarsals, while holding the femur to stabilize the limb. The phalangeal rudiments of the three middle digits were removed along with the soft tissue, permitting the isolation of the middle three metatarsals. Each metatarsal was then dissected and transferred to a 24 well plate in 500 μl of medium (high glucose DMEM with sodium pyruvate and pyridoxine, supplemented with 1% penicillin/streptomycin, 0.05mg/ml ascorbic acid and 0.25% heat-inactivated FBS). After 24 hours in culture, the rudiments were removed to a 100 μl drop of DMEM in a sterile dish under a dissection microscope. The elements were stabilized with forceps to permit insertion of a Heparin acrylic bead (Sigma Chemical Co, St. Louis, MO) or Affigel blue beads (BioRad, Hercules, CA) below the periosteum of the bone collar (in the middle third of the metatarsal). Heparin acrylic beads (Sigma Chemical Co, St. Louis, MO) were incubated with 1 mg/ml of FGF18 (Preprotech, Rocky Hill, NJ), or 1 mg/ml of BSA for 1 hour at room temperature prior to implantation. To examine the effects of inhibiting the FGF pathway, Affigel blue beads (BioRad, Hercules, CA) were incubated with DMSO (Sigma Chemical Co, St. Louis, MO) in saline with or without 10–100 nM of the FGF inhibitor PD173074 (PD2499 Sigma Chemical Co, St. Louis, MO) (Skaper et al., 2000) for 1 hour at room temperature prior to implantation. The metatarsal explants were then returned to the culture well and incubated for 4 days, at which time the explants were harvested for analyses. Mice were maintained in a virus and parasite-free barrier facility and exposed to a 12 hour light/dark cycle. All studies were approved by the institutional animal care committee.
Histological evaluation
Forelimbs isolated from 14.5 dpc embryos and metatarsals were fixed in 4% paraformaldehyde. After 24 hours the specimens were transferred to a 5% sucrose solution followed by an overnight incubation in 30% sucrose. Specimens were then embedded for frozen sections. Tissue blocks were cut into 5 μm sections for metatarsals and 10 μm for forelimbs and stained with Toluidine Blue or Hematoxylin & Eosin (H&E) to permit phenotypic analyses.
In situ hybridization analyses
Digoxigenin (DIG-) or 35S-UTP labeled probes were used for in situ hybridization as previously described (Gori et al., 2006). Twist-1 and FGF-18 probes used in these studies were kindly provided by Dr. G. Karsenty (Columbia University, New York, NY) and Dr. D.M. Ornitz (Washington University, St Louis. MO), respectively.
BrdU incorporation
Pregnant females were injected intra-peritoneally with 100 mg of BrdU/12 mg of FdU (5-Flouro-2′-deoxyuridine) per g of body weight at E14.5 and sacrificed 1 and 13 hours later. Embryonic forelimbs were then fixed, processed and sectioned using standard procedures. BrdU positive (BrdU+ve) nuclei were detected using a Zymed immunostaining Kit (Zymed, San Francisco, CA) according to the manufacturer’s instructions. BrdU+ve and BrdU-negative (BrdU−ve) nuclei were counted in three similarly sized regions of each the round and flat proliferating areas as well as in the hypertrophic region of forelimbs isolated from Col I-Wdr5 embryos and their wild-type littermates. Data were expressed as percent positive nuclei in the selected areas.
Quantitative Real-Time RT-PCR
Humeri were isolated from E14.5 dpc Col I-Wdr5 embryos and their wild-type littermates and dissected free of adjacent tissue under a dissection microscope. Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative Real-Time RT-PCR was performed using the Opticon DNA Engine System (MJ Research, Waltham, MA). mRNA levels encoding each gene of interest were normalized for actin mRNA in the same sample using the formula of Livak and Schmittgen (Livak and Schmittgen, 2001).
Chromatin immunoprecipitation (ChIP) analyses
MC3T3-E1 cells were plated at a density of 5 × 103 cells/cm2 and cultured for 7 days. Chromatin immunoprecipitation was performed as previously reported (Huebert et al., 2006; Zhu et al., 2008) using 10 μg of α-Wdr5. Real-Time PCR was performed using primers amplifying the Twist-1 promoter (−580/−400). Primers amplifying sequences in the Twist-1 coding region were used to normalize for DNA content and to calculate the enrichment of the promoter sequences relative to control sequences in the ChIP samples using the formula of Livak and Schmittgen as previously reported (Livak and Schmittgen, 2001; Zhu et al., 2008). Twist-1 promoter primers: F 5′-GGTTTCCGACTAGAGGTTT-3′; R 5′-TGTGACAGCAGTAGTGGC-3′; Twist-1 coding region primers: F 5′-TCGGACAAGCTGAGCAA-3′; R 5′-CGGAGAAGGCGTAGCT-3′.
Statistical analysis
Student’s paired t-test was used to evaluate differences between humeri isolated from wild type and Col I-Wdr5 embryos and in MC3T3-E1 cells between ChIP samples and input samples. A p value < 0.05 was considered statistically significant.
Results
Transgenic mice overexpressing Wdr5 in osteoblasts (Col I-Wdr5) display accelerated osteoblast differentiation (Gori et al., 2006). An intriguing observation in these mice is an increase in the length of the long bones, observed as early as E14.5, prior to vascular invasion. Histology of the growth plate of the transgenic mice demonstrated that a significant expansion of the hypertrophic chondrocyte layer accounts for the increased in the length of the long bones (Figure 1A and Gori et al., 2006), pointing to an important role of the Wdr5 transgene in promoting chondrocyte differentiation. Because the size of the hypertrophic chondrocyte layer is regulated by the rate at which proliferative chondrocytes differentiate, BrdU incorporation assay analyses were performed. As shown in Table 1, overexpression of Wdr5 in the perichondrium resulted in a 62% increase in BrdU positive cells in the proliferating chondrocyte layer (round and flat chondrocytes). Pulse-chase experiments demonstrated a 103% increase in BrdU positive cells in the hypertrophic layer 13 hours post-BrdU injection. This suggests that the Wdr5 transgene also accelerates the differentiation of proliferating chondrocytes into hypertrophic chondrocytes. Since the expression of the transgene is specifically restricted to the perichondrium and the expansion of the hypertrophic layer is not observed at E13.5, prior to the appearance of osteoblasts in the bone collar (Gori et al., 2006), these results support the hypothesis that Wdr5 overexpression in the perichondrium causes the chondrocyte phenotype observed in the Col I-Wdr5 mice, by regulating the expression of factors that, in a paracrine fashion, affect chondrocyte proliferation and differentiation.
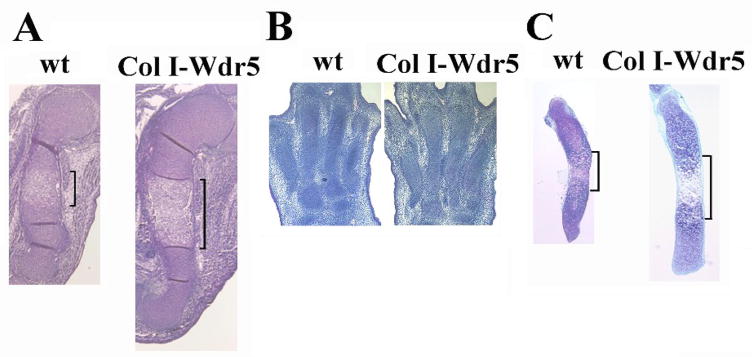
Figure 1. The Col I-Wdr5 transgene accelerates chondrocyte differentiation.
A): Humeri isolated from E14.5 wild-type (wt) and Col I-Wdr5 embryos, B) Paws isolated from E15.5 wild-type (wt) and Col I-Wdr5 embryos, C): metatarsals isolated from E15.5 wild-type (wt) and Col I-Wdr5 embryos and cultured for 4 days. Brackets indicate the expansion of the hypertrophic layer. Representative skeletal elements from at least three wild-type and Col I-Wdr5 embryos are shown.
Table 1.
BrdU incorporation
| % BrdU+ cells | % BrdU+ cells Pulse-chase (13h) |
|||
|---|---|---|---|---|
| wt | Col I-Wdr5 | wt | Col I-Wdr5 | |
| 19.3±1.39 | 31.4±1.79** | Round | 11.4±2.34 | 23.6±4.57* |
| Flat | 26.3±3.07 | 34.54±2.2* | ||
| Hypertrophic | 14.7±2.98 | 29.9±3.94* | ||
BrdU incorporation of chondrocytes in humeri isolated from E14.5 wild-type (wt) and Col I-Wdr5 embryos. n=7 for BrdU incorporation ± S.E.M. and n=3 for pulse-chase experiments ± S.E.M.
=p< 0.05 by Student’T-test.
To determine if an ex vivo culture system would recapitulate the in vivo phenotype, allowing the establishment of a model to permit dissection of the molecular mechanism by which the Wdr5 transgene results in a chondrocyte phenotype, metatarsals were isolated from E15.5 wild-type and Col I-Wdr5 embryos. As shown in Figure 1B, because distal elements mature less rapidly than proximal bones, at E15.5 the metatarsals of the Col I-Wdr5 embryos are undistinguishable from those of their wild-type littermates (Figure 1B). However, after 4 days in culture, metatarsal elements of the Col I-Wdr5 embryos resemble the phenotype seen in the humerus, including increased length and expansion of the hypertrophic chondrocyte layer. To further characterize the chondrocyte phenotype of the humeri isolated from E14.5 embryos and of the metatarsals cultured for 4 days, in situ hybridization analyses were performed for type II collagen (Col II), a specific marker of proliferating chondrocytes, type X collagen (Col X) and osteopontin (OP), markers of hypertrophic chondrocytes. As shown in Figure 2, the cultured metatarsals recapitulated the in vivo chondrocyte phenotype of the Col I-Wdr5 embryos, demonstrating a marked expansion of the hypertrophic chondrocyte layer primarily due to an increase in the domain of the more mature osteopontin expressing hypertrophic chondrocytes. Similar to humeri isolated from Col I-Wdr5 embryos, no differences were observed in the Col II expression domain between metatarsal cultures isolated from wild-type and Col I-Wdr5 embryos. These data demonstrate that, after 4 days in culture, metatarsal rudiments isolated from Col I-Wdr5 and wild type embryos recapitulate the phenotype observed in vivo. Therefore, this culture system provides a powerful tool to dissect the mechanism by which Wdr5 expression in the perichondrium results in the chondrocyte phenotype observed in the Col I-Wdr5 mice.
Figure 2. Molecular analyses of chondrocyte differentiation.
Sections of humeri and metatarsal cultures isolated from wild-type (wt) and Col I-Wdr5 embryos. Type II collagen (Col II), type X collagen (Col X) and osteopontin (OP) antisense riboprobes were digoxigenin (DIG-) labeled or radiolabeled with 35S-UTP to a specific activity of at least 108 cpm/μg of template. Representative skeletal elements from at least three wild-type (wt) and Col I-Wdr5 embryos are shown.
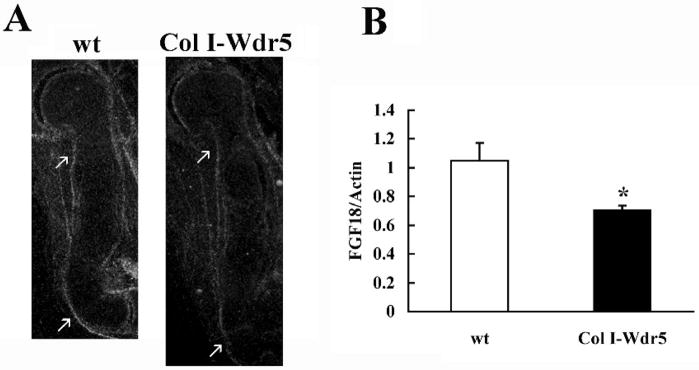
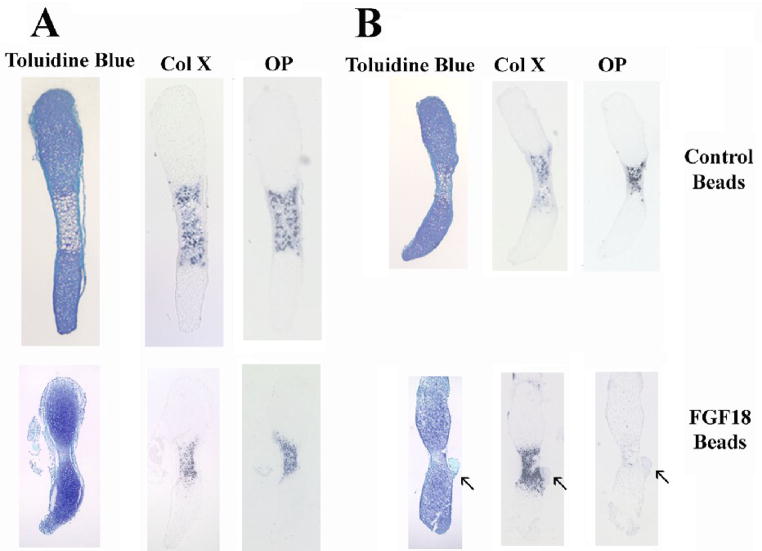
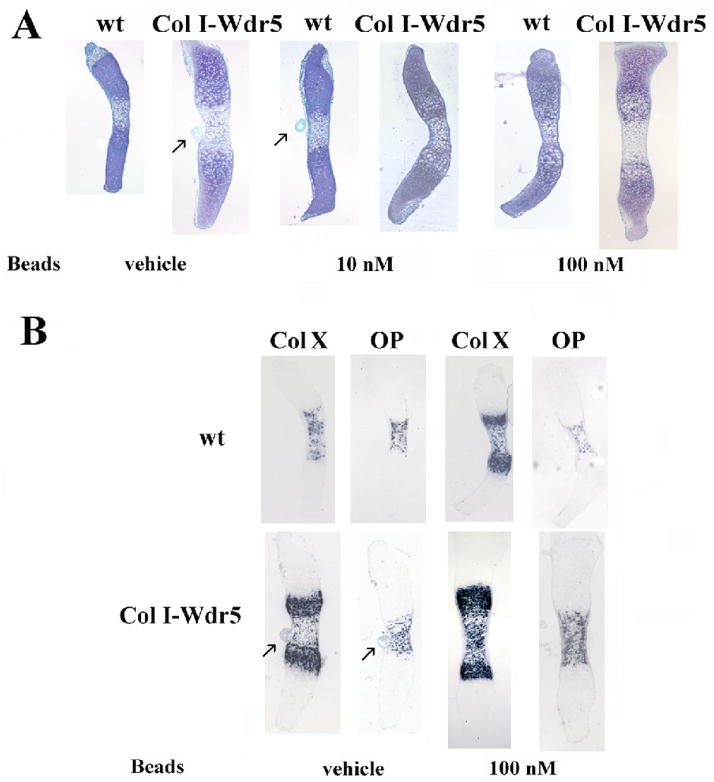
Several signaling pathways regulate chondrocyte proliferation and differentiation. Notably, ablation of FGF18 leads to a phenotype similar to that observed in the Col I-Wdr5 embryos: at E16.5 FGF18 null embryos demonstrate an expansion of the hypertrophic layer, accompanied by an increase in chondrocyte proliferation in the proximal long bones (Liu et al., 2002). Thus, studies were undertaken to determine whether expression of Wdr5 in the perichondrium impairs FGF18 expression. In situ hybridization analyses demonstrate a decrease in FGF18 mRNA expression in perichondrial cells of humeri isolated from Col I-Wdr5 embryos relative to humeri isolated from their wild-type littermates (Figure 3A). Quantitative Real-Time PCR analyses confirmed these data demonstrating a 30% decrease in the level of the mRNA encoding FGF18 in humeri isolated from E14.5 Col I-Wdr5 embryos relative to their wild type littermates (Figure 3B), thereby supporting the hypothesis that overexpression of Wdr5 in the perichondrium promotes chondrocyte differentiation by suppressing FGF18 expression. To explore the hypothesis that the decrease in FGF18 mRNA levels in the perichondrium of Col I-Wdr5 embryos maybe responsible for the chondrocyte phenotype observed in these embryos, experiments were carried out to investigate whether local delivery of FGF18 to the perichondrium of cultured metatarsals isolated from Col I-Wdr5 embryos could prevent expansion of the hypertrophic chondrocyte layer. As shown in Figure 4A, implantation of FGF18 containing beads in the bone collar of Col I-Wdr5 metatarsal cultures resulted in an attenuation of the chondrocyte phenotype, evidenced by a decrease in Col X and OP expression domains in metatarsal cultures implanted with FGF18 coated beads relative to those implanted with BSA coated beads. As expected, wild type metatarsal cultures implanted with FGF18 coated beads displayed a smaller hypertrophic chondrocyte layer evidenced by decreased Col X and OP mRNA expression domains, relative to wild-type metatarsals implanted with BSA coated beads (Figure 4B), demonstrating the effectiveness of the experimental model and the bioactivity of the FGF18. Thus, these experiments indicate that reversing the decreased FGF18 expression in the perichondrium of transgenic mice reverses the chondrocyte phenotype observed in these mice. To further support this hypothesis, beads incubated with or without the FGF inhibitor PD173074 (10 and 100 nM) (Skaper et al., 2000) were implanted into the bone collar of cultured metatarsals isolated from wild-type embryos and their Col I-Wdr5 littermates. As shown in Figure 5A, histological analyses demonstrate that blocking local FGF signaling in the perichondrium of both wild type and Col I-Wdr5 metatarsals results in a dose-related expansion of the hypertrophic chondrocyte layer. To confirm these histological analyses, in situ hybridization analyses were performed using Col X and OP. These studies demonstrate that blocking FGF signaling in the perichondrium of wild-type metatarsal cultures recapitulates the expansion of the hypertrophic chondrocyte layer seen in Col I-Wdr5 metatarsal cultures characterized by an increase in the Col X and OP expression domains (Figure 5B). Inhibition of FGF signaling in Col I-Wdr5 metatarsal cultures further accentuates the phenotype of the expanded hypertrophic chondrocyte layer (Figure 5B) demonstrating that Wdr5 overexpression in the perichondrium impairs, but does not abolish FGF signaling. Similar results were obtained when beads soaked in 10 nM of the inhibitor were used (Figure 5A and data not shown). Thus, these results support the hypothesis that overexpression of Wdr5 in the perichondrium regulates FGF18 expression and are consistent with the hypothesis that impaired FGF18 expression is the cause of the accelerated chondrocyte differentiation seen in Col I-Wdr5 mice.
Figure 3. Wdr5 regulates FGF18 expression.
A) In situ hybridization analysis for FGF18. Sections of humeri isolated from E14.5 wild-type (wt) and Col I-Wdr5 embryos are shown. The FGF18 antisense riboprobe was radiolabeled with 35S-UTP to a specific activity of at least 108 cpm/μg of template. Representative skeletal elements from at least three wild-type and Col I-Wdr5 embryos are shown. Arrows point to FGF18 expression. B) Quantitative Real-Time RT-PCR was performed on mRNA isolated from E14.5 wild-type (wt) (open bars) and Col I-Wdr5 (black bars) humeri. Expression of FGF18 mRNA was normalized to that of actin in the same sample. Values are expressed as the relative expression of the normalized mRNAs levels of the Col I-Wdr5 humeri versus that of wild-type (wt) humeri. Data shown are based on nine independent mRNA isolations ± S.E.M. * = p< 0.05 by Student t-test.
Figure 4. Local delivery of FGF18 to the perichondrium attenuates the chondrocyte phenotype of the Col I-Wdr5 embryos.
Heparin acrylic beads incubated with 1 mg/ml FGF18 or BSA were implanted into the perichondrium of metatarsals isolated from E15.5 Col I-Wdr5 embryos (A) and their wild-type (wt) littermates (B). Metatarsals were then cultured for 4 days. Histological analysis and in situ hybridization analysis for Col X and OP were then performed. Arrows indicate heparin acrylic beads present in some sections. Representative metatarsal cultures from at least six wild-type (wt) and Col I-Wdr5 embryos are shown.
Figure 5. Blocking FGF signaling in the perichondrium recapitulates the Col I-Wdr5 phenotype in metatarsal cultures.
A) Affigel blue beads incubated with10 nM or100 nM of FGF inhibitor or DMSO were implanted into the perichondrium of metatarsals isolated from E15.5 wild-type (wt) and Col I-Wdr5 embryos. Metatarsals were then cultured for 4 days. Histological analyses, using Toluidine Blue staining were then performed. B) In situ hybridization analyses for Col X and OP were performed on metatarsals isolated from E15.5 wild-type (wt) and Col I-Wdr5 embryos implanted with Affigel blue beads incubated with 100 nM of FGF inhibitor or DMSO. Representative metatarsal cultures from at least six wild-type (wt) and Col I-Wdr5 embryos are shown. Arrows point to beads present in some sections.
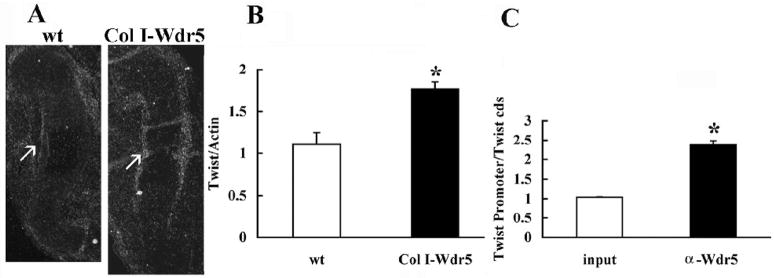
FGF18 is induced by canonical Wnt signaling and Runx-2 (Kapadia et al., 2005; Reinhold and Naski, 2007). Because Wdr5 is required for Runx-2 expression and for canonical Wnt signaling (Zhu et al., 2008), suppression of FGF18 expression by the Wdr5 transgene suggests that Wdr5 overexpression in the perichondrium must regulate the expression of factors that block induction of FGF18 by Runx2 or Wnts. One such candidate, whose expression is restricted to the perichondrium, is Twist-1 (Hinoi et al., 2006; Reinhold et al., 2005). As shown in Figure 6A, in situ hybridization analyses demonstrate that the expression of Twist-1 is increased in the perichondrium and bone collar of humeri isolated from E14.5 Col I-Wdr5 embryos relative to that of their wild type littermates. In addition, quantitative Real-Time PCR showed a significant increase in Twist-1 mRNA in humeri isolated from E14.5 Col I-Wdr5 embryos relative to that of their wild-type littermates (Figure 6B). To determine whether Wdr5 functionally interacts with Twist-1 in vivo, chromatin immunoprecipitation (ChIP) analyses, using the osteoblastic cell line MC3T3-E1, were performed. As shown in Figure 6C, Wdr5 interacts with the Twist-1 promoter region in the context of intact chromatin. Thus, these data demonstrate that overexpression of Wdr5 leads to increased expression of Twist-1 in the perichondrium. Since Wdr5 binds to the Twist-1 promoter in the context of intact chromatin, this suggests that Wdr5 regulates Twist-1 expression. Since Twist-1 suppresses FGF18 expression (Hinoi et al., 2006), our findings support the hypothesis that enhanced Twist-1 expression is responsible for the changes in FGF18 mRNA levels observed in the perichondrium of the Col I-Wdr5 mice. This decrease in FGF18 in turn, promotes chondrocyte proliferation and differentiation during endochondral bone formation.
Figure 6. Wdr5 regulates Twist-1 expression.
A) In situ hybridization analysis for Twist-1. Sections of humeri isolated from E14.5 wild-type (wt) and Col I-Wdr5 embryos are shown. Twist-1 antisense riboprobe was radiolabeled with 35S-UTP to a specific activity of at least 108 cpm/μg of template. Representative humeri from at least three wild-type (wt) and Col I-Wdr5 embryos are shown. Arrows point to the expression of Twist-1. B) Quantitative Real-Time RT-PCR was performed on mRNA isolated from E14.5 wild-type (wt) (open bars) and Col I-Wdr5 (black bars) humeri. Expression of Twist mRNA was normalized to that of actin in the same sample. Values are expressed as the relative expression of the normalized mRNAs levels of the humeri from Col I-Wdr5 embryos versus that of wild-type (wt) humeri. Data shown are based on nine independent mRNA isolations ± S.E.M. * = p< 0.05 by Student t-test. C) Quantitative ChIP analyses demonstrating a specific interaction of Wdr5 with the Twist-1 promoter. Values are shown as enrichment of the promoter sequences normalized for coding region sequences versus that obtained for input samples. Data shown are representative of that obtained with three independent experiments ± S.E.M. *= p<0.05 by Student t-test for ChIP samples versus input samples.
Discussion
The formation of endochondral bones is a complex process that requires intricate regulation of chondrocyte proliferation and differentiation, as well osteoblast differentiation to ensure proper skeletal development (Karsenty and Wagner, 2002; Kronenberg, 2003). Several studies have demonstrated that local signals from the proliferating, prehypertrophic and hypertrophic chondrocytes regulate chondrogenesis and osteogenesis during endochondral bone development. In addition, signaling from the perichondrium has been shown to regulate chondrocyte proliferation and differentiation, implicating coupling between chondrogenesis and osteogenesis during endochondral bone formation (Chung et al., 2001; Di Nino et al., 2001; Hinoi et al., 2006; Karp et al., 2000; Kronenberg, 2003; Long and Linsenmayer, 1998).
We have found that overexpression of Wdr5 in the perichondrium leads to an accelerated rate of chondrocyte maturation. Previous studies have shown that, while endogenous Wdr5 is expressed in proliferating chondrocytes, hypertrophic chondrocytes and osteoblasts, the expression of the Wdr5 transgene is restricted to osteoblasts (Gori et al., 2004; Gori et al., 2006). Furthermore, prior to the differentiation of precursor cells into type I collagen expressing osteoblasts, a time when the Col I promoter driving the transgene is not yet active in the perichondrium (Rossert et al., 1995), Col I-Wdr5 embryos do not display the hypertrophic chondrocyte phenotype (Gori et al., 2006). Thus, these histological and molecular observations point to effects of Wdr5 on chondrocyte proliferation and differentiation, mediated by signaling molecules expressed in the developing bone, which regulate chondrocyte maturation in a paracrine fashion.
Gain-and loss-of-function studies in mice have demonstrated that FGFs and their receptors are crucial regulators of both intramembranous and endochondral bone formation (Kronenberg, 2003; Marie, 2003; Ornitz, 2005). Among the FGFs, FGF18 expression is restricted to the perichondrium and regulates chondrocyte proliferation and differentiation as well as osteogenesis (Liu et al., 2007; Liu et al., 2002). Notably, comparison of the growth plate of Col I-Wdr5 mice with that of FGF18 null mice at E16.5 shows a similar chondrocyte phenotype characterized by increased chondrocyte proliferation and expansion of the hypertrophic layer (Liu et al., 2002), which suggest that Wdr5 might regulate the expression of FGF18. Our results demonstrated decreased FGF18 expression in Col I-Wdr5 mice, suggesting that Wdr5 suppresses FGF18 expression, thereby promoting chondrocyte proliferation and differentiation. Consistent with the hypothesis that impaired FGF18 expression in the perichondrium of the Col I-Wdr5 mice may account for the growth plate phenotype seen in these mice, our studies demonstrated that local delivery of FGF18, via implantation of FGF18 coated beads in the perichondrium of metatarsal elements, attenuate the phenotype of the Col I-Wdr5 metatarsals, whereas wild-type metatarsals cultures implanted with beads soaked with an inhibitor of FGF signaling recapitulate the Col I-Wdr5 chondrocyte phenotype.
The effects of FGF18 on chondrocyte proliferation and differentiation vary depending on the stage of embryonic development examined (Liu et al., 2007; Liu et al., 2002). Notably, studies in the FGF18 null mice demonstrate a smaller hypertrophic chondrocyte layer, accompanied by decreased chondrocyte proliferation and delayed vascular invasion in the paw at E14.5 and E15.5 which is the converse of the phenotype observed in the Col I-Wdr5 mice (Liu et al., 2007). However, at later developmental stages (E16.5) the FGF18 null mice demonstrate an expansion of the hypertrophic chondrocyte layer accompanied by an increase in chondrocyte proliferation in the proximal long bones (Liu et al., 2002). Thus, the growth plate phenotype of the FGF18 null mice at this stage of endochondral bone formation is identical to that of the Col I-Wdr5 mice. The findings that the Col I promoter drives LacZ expression in the humerus between E13.5 and E14.5 (and much later in the distal elements) (Rossert et al., 1995), might explain the difference observed at early stages of endochondral bone formation, between the Col I-Wdr5 mice and the FGF18 null mice. In the latter, FGF signaling is impaired from the time of fertilization, whereas in the former, FGF signaling is unaffected for the first two weeks of embryogenesis.
Since FGF18 is induced by canonical Wnt signaling (Kapadia et al., 2005; Reinhold and Naski, 2007), which is enhanced in the osteoblasts of Col I-Wdr5 mice and by Runx-2 whose expression is regulated by Wdr5 (Gori et al., 2006; Zhu et al., 2008), the observation that FGF18 expression was decreased in Col I-Wdr5 mice seemed somehow paradoxical. This raised the question as to whether overexpression of Wdr5 indirectly suppresses FGF18 expression by enhancing the expression of factors that block the induction of FGF18 by Runx-2 or by the canonical Wnt signaling pathway.
Our studies demonstrate that Wdr5 associates with the Twist-1 promoter and that Twist-1 expression is increased in Col I-Wdr5 mice. Twist-1, a basic helix-loop-helix transcription factor expressed in perichondrial cells but not in chondrocytes, interacts with the Runx-2 DNA binding domain, thereby inhibiting its activity (Bialek et al., 2004). Interestingly, overexpression of Twist-1 in osteoblasts under the control of the mouse α(1) I collagen promoter leads to a hypertrophic chondrocyte phenotype analogous to that seen in the Col I-Wdr5 mice (Hinoi et al., 2006). In these mice, Twist-1 inhibits Runx-2 activity leading to suppression of FGF18 expression, thereby accelerating chondrocyte maturation (Hinoi et al., 2006). Since Wdr5 is essential for histone H3 lysine 4 (H3K4) trimethylation (Dou et al., 2005; Wysocka et al., 2003; Wysocka et al., 2005), one of the histone modifications associated with transcriptional activation of genes, our chromatin immunoprecipitation data suggest that Wdr5 regulates chromatin modifications at the Twist-1 promoter leading to activation of Twist-1 expression. This hypothesis is supported by our findings that Twist-1 expression is significantly increased in Col I-Wdr5 mice. Thus, we propose that by enhancing the expression of Twist-1 in the perichondrium, Wdr5 overexpression results in decreased FGF18 expression thereby promoting chondrocyte differentiation. The increase in Twist-1 expression in Col I-Wdr5 mice may also explain the finding that, despite the decrease in FGF18 expression, Col I-Wdr5 mice display enhanced osteoblast differentiation. In fact, based on the delayed osteoblast differentiation observed in mice with a hypomorphic Twist-1 allele (Hinoi et al., 2006), it is possible that the enhanced expression in Twist-1, observed in the Col I-Wdr5 mice, contributes to enhanced osteoblastogenesis.
Our data do not exclude the possibility that other signaling pathways involved in chondrocyte proliferation and differentiation may contribute to the chondrocyte phenotype of the Col I-Wdr5 transgenic mice. Although further in vivo functional studies, currently underway, are needed to determine at what extent Wdr5 is required to regulate osteoblastogenesis and chondroctye maturation in vivo, the current studies provide evidence for a mechanism by which overexpression of Wdr5 in the perichondrium induces the expression of Twist-1 leading to a decrease in FGF18 expression. This results in accelerated chondrocyte proliferation and differentiation and reinforces the concept that signals from the perichondrium play an important role in the regulation of chondrocyte proliferation and differentiation during endochondral bone development.
Acknowledgments
This work was supported by Grant DK36597 (MD) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–35. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- Chung UI, Lanske B, Lee K, Li E, Kronenberg H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci U S A. 1998;95:13030–5. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107:295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–7. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Di Nino DL, Long F, Linsenmayer TF. Regulation of endochondral cartilage growth in the developing avian limb: cooperative involvement of perichondrium and periosteum. Dev Biol. 2001;240:433–42. doi: 10.1006/dbio.2001.0471. [DOI] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005;121:873–85. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–14. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Ho V, Cox K, Rosen V. Expression and function of BMP3 during chick limb development. Dev Dyn. 2008;237:1691–8. doi: 10.1002/dvdy.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori F, Divieti P, Demay MB. Cloning and characterization of a novel WD-40 repeat protein that dramatically accelerates osteoblastic differentiation. J Biol Chem. 2001;276:46515–22. doi: 10.1074/jbc.M105757200. [DOI] [PubMed] [Google Scholar]
- Gori F, Demay MB. BIG-3, a novel WD-40 repeat protein, is expressed in the developing growth plate and accelerates chondrocyte differentiation in vitro. Endocrinology. 2004;145:1050–1054. doi: 10.1210/en.2003-1314. [DOI] [PubMed] [Google Scholar]
- Gori F, Friedman LG, Demay MB. Wdr5, a WD-40 protein, regulates osteoblast differentiation during embryonic bone development. Dev Biol. 2006;295:498–506. doi: 10.1016/j.ydbio.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–8. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–59. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Bialek P, Chen YT, Rached MT, Groner Y, Behringer RR, Ornitz DM, Karsenty G. Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 2006;20:2937–42. doi: 10.1101/gad.1482906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Gross G. BMP signaling pathways in cartilage and bone formation. Crit Rev Eukaryot Gene Expr. 2001;11:23–45. [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Huebert DJ, Kamal M, O’Donovan A, Bernstein BE. Genome-wide analysis of histone modifications by ChIP-on-chip. Methods. 2006;40:365–9. doi: 10.1016/j.ymeth.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Kapadia RM, Guntur AR, Reinhold MI, Naski MC. Glycogen synthase kinase 3 controls endochondral bone development: contribution of fibroblast growth factor 18. Dev Biol. 2005;285:496–507. doi: 10.1016/j.ydbio.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–89. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–8. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- Karsenty G. The genetic transformation of bone biology. Genes Dev. 1999;13:3037–51. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Wada N, Nishimatsu S, Nohno T. Involvement of frizzled-10 in Wnt-7a signaling during chick limb development. Dev Growth Differ. 2000;42:561–9. doi: 10.1046/j.1440-169x.2000.00545.x. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Wada N, Nishimatsu SI, Ishikawa T, Noji S, Nohno T. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ. 1999;41:29–40. doi: 10.1046/j.1440-169x.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–86. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104:399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lavine KJ, Hung IH, Ornitz DM. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev Biol. 2007;302:80–91. doi: 10.1016/j.ydbio.2006.08.071. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–69. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long F, Linsenmayer TF. Regulation of growth region cartilage proliferation and differentiation by perichondrium. Development. 1998;125:1067–73. doi: 10.1242/dev.125.6.1067. [DOI] [PubMed] [Google Scholar]
- Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene. 2003;316:23–32. doi: 10.1016/s0378-1119(03)00748-0. [DOI] [PubMed] [Google Scholar]
- Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Ohbayashi N, Shibayama M, Kurotaki Y, Imanishi M, Fujimori T, Itoh N, Takada S. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–9. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005;16:205–13. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold MI, Kapadia RM, Liao Z, Naski MC. The Wnt-inducible transcription factor Twist1inhibits chondrogenesis. J Biol Chem. 2005;281:1381–1388. doi: 10.1074/jbc.M504875200. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Naski MC. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. J Biol Chem. 2007;282:3653–63. doi: 10.1074/jbc.M608995200. [DOI] [PubMed] [Google Scholar]
- Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129:1421–32. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs CS, Lee K, Pirro A, Kronenberg HM, Juppner H. Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc Natl Acad Sci U S A. 1997;94:13689–94. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Kee WJ, Facci L, Macdonald G, Doherty P, Walsh FS. The FGFR1 inhibitor PD 173074 selectively and potently antagonizes FGF-2 neurotrophic and neurotropic effects. J Neurochem. 2000;75:1520–27. doi: 10.1046/j.1471-4159.2000.0751520.x. [DOI] [PubMed] [Google Scholar]
- Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–5. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–22. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–32. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Spatz MK, Kannan K, Hayk H, Avivi A, Gorivodsky M, Pines M, Yayon A, Lonai P, Givol D. A mouse model for achondroplasia produced by targeting fibroblast growth factor receptor 3. Proc Natl Acad Sci U S A. 1999;96:4455–60. doi: 10.1073/pnas.96.8.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Burlingame AL, Roeder RG, Brivanlou AH, Allis CD. WDR5 Associates with Histone H3 Methylated at K4 and Is Essential for H3 K4 Methylation and Vertebrate Development. Cell. 2005;121:859–72. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–23. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Zhu ED, Demay MB, Gori F. Wdr5 is essential for osteoblast differentiation. J Biol Chem. 2008;283:7361–7. doi: 10.1074/jbc.M703304200. [DOI] [PubMed] [Google Scholar]