Abstract
Regulation of energy metabolism is controlled by the brain, in which key central neuronal circuits process a variety of information reflecting nutritional state. Special sensory and gastrointestinal afferent neural signals, along with blood-borne metabolic signals, impinge on parallel central autonomic circuits located in the brainstem and hypothalamus to signal changes in metabolic balance. Specifically, neural and humoral signals converge on the brainstem vagal system and similar signals concentrate in the hypothalamus, with significant overlap between both sensory and motor components of each system and extensive cross-talk between the systems. This ultimately results in production of coordinated regulatory autonomic and neuroendocrine cues to maintain energy homeostasis. Therapeutic metabolic adjustments can be accomplished by modulating viscerosensory input or autonomic motor output, including altering parasympathetic circuitry related to GI, pancreas, and liver regulation. These alterations can include pharmacological manipulation, but surgical modification of neural signaling should also be considered. In addition, central control of visceral function is often compromised by diabetes mellitus, indicating that circuit modification should be studied in the context of its effect on neurons in the diabetic state. Diabetes has traditionally been handled as a peripheral metabolic disease, but the central nervous system plays a crucial role in regulating glucose homeostasis. This review focuses on key autonomic brain areas associated with management of energy homeostasis and functional changes in these areas associated with the development of diabetes.
Keywords: brainstem, cannabinoid, glucose, hypothalamus, paraventricular, vagus
Energy homeostasis is largely controlled by the brain, where information reflecting nutritional state is processed, eventually leading to regulatory signals along the autonomic and neuroendocrine axes. Afferent indicators of energy balance include sensory signals (e.g., taste), gastrointestinal (GI) neural signals arising from mechano-and chemoreceptors in the stomach, and metabolic signals detected in blood (e.g., insulin, glucose, leptin). These signals are integrated and processed in central autonomic control centers, located principally in the brainstem and hypothalamus [1]. Treatments proposed for adjusting metabolism are often aimed at modulating neural circuits making up the efferent limb of energy homeostasis, including parasympathetic circuitry related to GI, pancreas, and liver regulation. Likewise, vagal control of the viscera can be compromised by pathology, including diabetes mellitus [2]. Impairment of neuronal signaling can adversely affect autonomic output, resulting in increased feeding, weight gain, and altered hepatic glucose metabolism. Understanding the mechanisms governing parasympathetic output to the viscera may benefit the development of direct therapies for disorders of energy homeostasis. A model of the interactions between hypothalamic and brainstem autonomic nuclei with the periphery is illustrated in figure 1. In order to study these mechanisms, local control of neurons regulating autonomic functions must be understood.
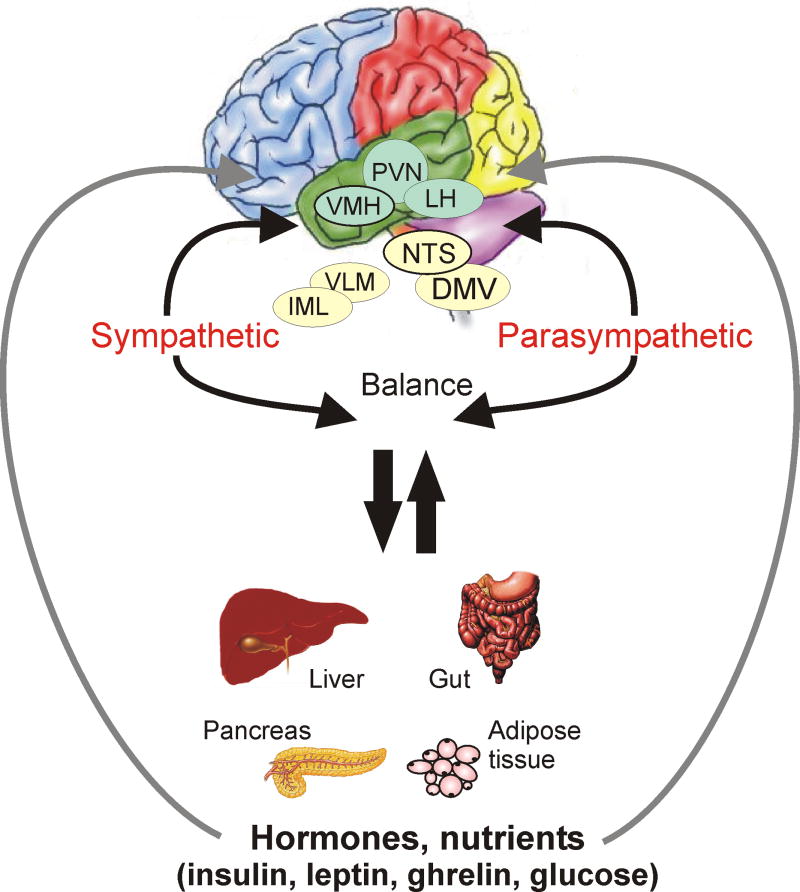
Figure 1.
Schematic of hypothesized parallel brain-digestive system-brain circuits in hypothalamus and brainstem. Hypothalamic nuclei involved in autonomic regulation and feeding make reciprocal connections with neurons in the dorsal vagal complex that regulate visceral function. Within the vagal complex, the NTS receives visceral afferent neural input and projects heavily to vagal motor neurons of the DMV. The NTS is also reciprocally connected to hypothalamic nuclei involved in autonomic regulation, especially the PVN. Both PVN and NTS also project to central sympathetic nervous system regulatory centers. Likewise, both brainstem and hypothalamic areas receive information about peptide and glucose content in the blood. Abbreviations: DMV, dorsal motor nucleus of the vagus; IML, intermediolateral cell column; LH, lateral hypothalamus; NTS, nucleus tractus solitarius; PVN, paraventricular nucleus; VLM, ventrolateral medulla; VMH, ventromedial hypothalamus.
Diabetes mellitus is the most common metabolic disorder in humans and a rather pessimistic prognosis suggests that the disease most likely will be one of the major public health problems in the next decades [3]. Diabetes mellitus is characterized by chronic hyperglycemia due to insulin deficiency in the case of type 1 diabetes or insulin resistance in the case of type 2 diabetes. The loss of pancreatic beta cells in the case of type 1 diabetes can be caused, for example, by viral infection or autoimmune disease. Obesity, often considered as a disorder of energy homeostasis, is strongly associated with risk for many diseases and health conditions such as hypertension, coronary heart disease, stroke, dyslipidemia, and notably, type 2 diabetes.
Diabetes has traditionally been handled as a peripheral metabolic disease. But recently, non-invasive brain imaging techniques providing information on brain anatomy and function have indicated structural and functional abnormalities associated with diabetes. Computed tomography (CT) and magnetic resonance imaging (MRI) studies describe a relationship between diabetes and cerebral atrophy and lacunar infarcts ([4]. Positron emission tomography (PET) and single photon emission CT (SPECT) studies showed regional alterations in cerebral blood flow [4]. Cerebral edema, cerebral hemorrage, or intracranial thrombosis was observed in children with type 1 diabetes and diabetic ketoacidosis [5]. Patients with type 2 diabetes often show white matter hyperintensities which are detectable with brain imaging techniques [6]. All these observations suggest that diabetes has a strong effect on the function of brain, and influencing this system might positively improve the life expectancy and quality of life for diabetic patients.
Areas of the brain that regulate autonomic and neuroendocrine functions have been the subject of increasingly intense analysis in the context of understanding diabetes and other metabolic disorders [7-10]. The central nervous system plays a crucial role in regulating glucose homeostasis, including hepatic gluconeogenesis and glycogenolysis and pancreatic function. These activities are largely mediated by central regulation of the autonomic nervous system, which acts in concert with the hypothalamic-pituitary-adrenal axis to regulate metabolic responses to changes in energy requirements and plasma glucose concentration. In particular, critical autonomic regulatory neurons in the hypothalamus and brainstem are responsible for management of energy homeostasis (Fig. 1), and functional changes in these areas are associated with the development of diabetes [7].
The brainstem dorsal vagal complex (DVC), which includes as primary components the nucleus tractus solitarius (NTS) and dorsal motor nucleus of the vagus nerve (DMV), plays a critical role in the autonomic parasympathetic control of energy homeostasis, through activation of the vagus nerve. Neurons controlling the vagus nerve functionally regulate gastrointestinal motility as well as liver, pancreas, and other organs, and these functions appear to be significantly altered in diabetes (Fig. 2). The NTS is the first site of central synaptic contact for sensory afferent fibers of cranial nerves VII, IX, and X, including viscerosensory vagal afferents. The motor limb of the subdiaphragmatic vagus nerve originates mainly in neurons of the DMV. Neuronal connections within the DVC form the basis for reflex control of visceral parasympathetic function. Thus, for example, activation of mechanoreceptors in the stomach wall by passive distention, active contraction, or chemoactivation of the stomach increases the firing rate in primary vagal afferent fibers, and results in rapid cessation of feeding when the stomach becomes full ([11-16]. Descending input to the DVC, especially from the hypothalamic paraventricular nucleus (PVN), modulates the satiety-producing effects of gastric vagal afferent activation. Activation of the efferent vagus normally acts to upregulate many digestive and other parasympathetic functions, but with appropriate stimulation it can also play a role in negatively modulating metabolic functions, including hepatic gluconeogenesis [17]. Such negative control of vagal output is illustrated in figure 2, where GABA is released onto DMV motor neurons from terminals of preautonomic neurons located mainly in the NTS.
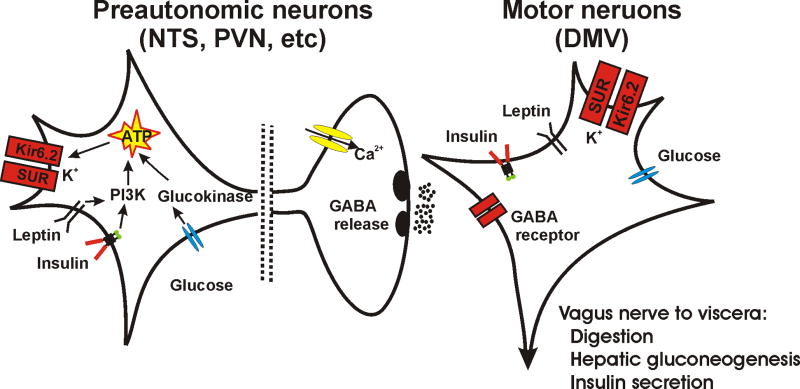
Figure 2.
A simplified diagram illustrating a basic-circuit connection between preautonomic neurons and parasympathetic, preganglionic motor neurons in the context of a model for how energy-regulating factors can influence central autonomic circuits. Somatodendritic KATP channel, with Kir6.2 and SUR subunits are shown in both types of cell. Also shown are leptin and insulin receptors and glucose sensors, all of which can utilize kinases to affect cellular activity. Glucokinase and PI3K are indicated, but AMPK can also mediate some effects. GABA critically regulates motor neuron function and its release is in the DMV is illustrated. General pertinent vagally-mediated visceral effects are listed. Altered glutamate or other neurotransmitter release is omitted for simplicity, but may also occur.
Along with the NTS, the PVN is a key central structure involved in the integrating afferent autonomic information and coordinating neuroendocrine and autonomic motor outputs. The pre-autonomic neurons in PVN receive parasympathetic-related neural information from the NTS, as well as from feeding-related neurons in the ventromedial (VMH) and lateral (LH) hypothalamic nuclei, central hypothalamic areas that can detect circulating indicators of metabolic state such as glucose, leptin, ghrelin, and insulin. Neurons in the vagal complex are also capable of detecting peptide and glucose levels in the blood, so PVN responses to circulating signals can also arise from extrahypothalamic neural sources (Fig. 1). Preautonomic neurons in the PVN are connected to sympathetic and parasympathetic visceral motor circuits through descending projections to the NTS, DMV, ventrolateral medulla, and spinal cord; premotor NTS neurons also participate in autonomic motor regulation via projections to PVN, DMV, and ventrolateral medulla. The PVN and NTS thus receive direct and indirect neural and blood-borne information about metabolic state and digestive system activity, process that information, and directly contribute to coordinated autonomic output to the viscera. These areas thus represent parallel systems for integrating visceral sensory-motor information that is crucial for maintaining metabolic balance.
Brain-responses to metabolic indicators
Although it is well known that select neurons in the CNS respond to changes in plasma glucose concentration - especially hypoglycemia - the role of the brain in the normal regulation of glucose homeostasis is not adequately understood. Neurons in central autonomic areas participate in regulating peripheral glucose metabolism. Chemical or electrical stimulation of LH increases parasympathetic nervous system activation and was shown to reduce blood glucose levels by increasing glycogen synthesis in the liver [18, 19]. To the contrary, stimulation of VMH results in sympathetic nervous system activation and a subsequent rise in blood glucose concentration that was mediated by hepatic glycogenolysis [19]. More selective neurochemical activation of these and other hypothalamic areas often results in less well-segregated responses [20-22], possibly due to the non-selective activation of fiber pathways by electrical stimulation or the more discreet activation of subsets of neurons in these regions by chemical activation. Denervation studies further support the idea that CNS plays a key role in hepatic glucose regulation [17, 23, 24]. Activation of parasympathetic motor output is not normally associated with large changes glucose production by the liver, but systemic insulin-induced suppression of hepatic gluconeogenesis was diminished by approximately half after hepatic branch vagotomy, indicating that the vagus nerve mediates a large portion of the insulin-mediated neural regulation of glucose production [17] (Fig. 2). Effects of metabolic signals on peripheral nervous systems and visceral smooth muscle thus work in concert with effects on central components to modulate visceral functions. In addition to widely-studied peripheral effects on glucose metabolism, insulin can act at receptors in the hypothalamus and possibly also in the DVC to affect parasympathetic motor control.
Leptin and insulin action on KATP channels
In addition to regulation of glucose metabolism, these same brain areas participate in responses to circulating peptides that are critical regulators of energy homeostasis, including insulin and leptin [25]. Leptin receptors have been localized in many hypothalamic and brainstem autonomic nuclei [26]. Application of leptin to neurons of hypothalamic arcuate and ventromedial nuclei decreased the firing rate of action potentials and input resistance [27] in control Sprague-Dawley and lean - but not obese - Zucker rats, which have a mutation of leptin receptor gene, causing hypothalamic insensitivity to leptin. The leptin-sensitive neurons were also sensitive to changes in glucose concentration, and the effects of leptin in these glucose-responsive neurons were prevented with the sulphonylurea, tolbutamide [27]. These observations suggested that the leptin-induced hyperpolarization was due to a current resultant from opening of ATP-sensitive K+ channels (KATP; Fig 2). The lack of leptin response in glucose-responsive neurons from obese Zucker rats indicated that functional leptin receptors are required for hyperpolarizing this brain area and explains the reduced effectiveness of leptin in reducing food intake and body weight gain when injected into the hypothalamus of obese rats [28, 29]. Tolbutamide-sensitive leptin effects were also observed in the dorsal vagal complex. Application of leptin caused a rapid membrane hyperpolarization in half of the NTS neurons sampled and the hyperpolarizing effect of leptin was prevented with tolbutamide or wortmannin, indicating activation of KATP channel via a PI3 kinase-dependent mechanism [30]. The leptin-induced hyperpolarization was also observed in gastric-related DMV neurons, and tolbutamide reversed this effect. Further, leptin reduced the frequency of spontaneous and miniature EPSCs, whereas the IPSCs were unaffected in both NTS and DMV neurons [31]. This suggests that leptin is able to suppress activity of excitatory NTS and DMV neurons that are likely to be involved in regulation of GI tract.
Insulin and leptin can both reduce food intake and body weight. Insulin inhibited hypothalamic glucose-responsive neurons, causing a decreased firing rate, hyperpolarization and increased conductance in control and lean animals, but not in obese rats [32], similar to effects of leptin. Tolbutamide reversed the effect of insulin or glucose on membrane potential, supporting the hypothesis that insulin acted via KATP channels on glucose-responsive neurons of control and lean, but not obese rats. The time course of effects on feeding (hours) and effects on membrane channels (seconds) are dramatically different, implying the link between peptide or glucose infusion and neuronal activity may be far removed or even disconnected from effects on feeding. The effects are nonetheless consistent with previous observations that intracerebroventricular injection of insulin reduces food intake and body weight of lean, but not obese, leptin deficient rats [33]. Notably, effects of leptin on body weight and feeding are also known to occur subsequent to infusion of the peptide into the DVC [34]. These data together with other observations [27, 29] are consistent with the hypothesis that the ability of insulin to activate KATP channels in glucose-responsive neurons and to decrease food intake and body weight in these animals depends on the normal functioning of a brain-leptin pathway. The suggested convergence of leptin and insulin on KATP channels is illustrated in figure 2.
Mice deficient in leptin itself (ob-/ob-) or leptin receptor (db-/db-) become diabetic and obese. Activation of these receptors on neurons, or the subsequent downstream effects of altered neuronal circuitry are apparently involved in regulating metabolic homeostasis. In addition to direct effects of glucose-, insulin-, or leptin-mediated KATP channel modulation on membrane potential and action potential frequency, these factors also influence the release of the classical fast neurotransmitters in the brain, glutamate and GABA.
Glucose-responsive neurons and KATP channels
Glucose-responsive neurons use glucose to regulate their firing rate by altering the activity of the KATP channel [35]. In the last 25 years KATP channels have been found in many different tissues, including pancreatic ß cells and neurons. KATP channels are able to couple cell metabolism to the electrical activity of the neurons and transfer the effects of hormones and neurotransmitters on membrane potential [36]. The KATP channel has two types of subunit, an inwardly rectifying K+-channel subunit (Kir6.2) and a sulphonylurea receptor subunit (SUR) [37]. Both subunits are necessary to form a functional KATP channel (Fig 2).
Uptake and intracellular metabolism of glucose leads to an increase in the ATP/ADP ratio, which promotes ATP binding to the channel complex. This inactivates (closes) the channel, leading to intracellular K+-accumulation followed by membrane depolarization, calcium influx and increased cell firing. When glucose supply is limited, the ATP/ADP ratio falls, the KATP channel opens (activates), and the cell membrane becomes hyperpolarized and neuronal firing decreases [35, 38]. Deletion of Kir6.2 subunits of KATP channel cause failure in glucose mobilization and feeding responses [35], and glucose-responsive neurons in DVC expressed Kir6.2 subunits as well as glucokinase, which was not expressed by glia or in neurons that were not responsive to –glucose [39]. These observations suggest that an intact KATP channel is critical to the functioning of glucose-responsive neurons in the hypothalamus and brainstem.
The KATP channel activator, diazoxide injected into the third cerebral ventricle significantly decreased glucose production of the liver through central stimulation of KATP channels, which lowered blood glucose by inhibiting hepatic glucose production. (Fig.3). The decrease in glucose production was due to inhibition of gluconeogenesis, while the rate of glycogenolysis was not decreased [17]. Application of insulin reproduced the effect of diazoxide. These observations suggested that direct activation of central KATP channels mimic the action of insulin on hepatic gluconeogenesis. Thus, activation of either KATP channels or insulin signaling within the medial hypothalamus is adequate to decrease blood glucose levels through suppression of glucose production by the liver [17]. Blocking of KATP channels with sulphonylureas (e.g., tolbutamide or glibenclamide) prevented the activation of KATP channels by glucose, insulin, and leptin [31, 32, 40]. These experiments suggested that modulation of hypothalamic and/or brainstem KATP channel activity likely plays an important role in regulation of glucose homeostasis. The central autonomic KATP channel may be a therapeutic target for regulating glucose levels in diabetes mellitus.
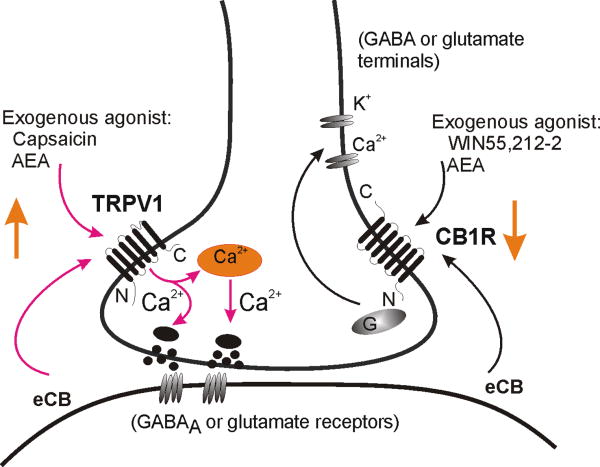
Figure 3.
Schematic model illustrating cannabinoid and vanilloid activity in central autonomic circuits. A synaptic terminal (glutamatergic or GABAergic) is shown, containing TRPV1 and CB1R. Anandamide (AEA) is illustrated as an example of an endogenously synthesized cannabinoid (eCB) agonist that can bind both receptors. Synthesis and retrograde release of eCB from postganglionic neurons is suggested. TRPV1 are non-selective cation channels that are also activated by capsaicin and flux Ca2+, resulting in increased synaptic release. CB1R are G protein-coupled receptors that can also be activated by the synthetic agonist, WIN 55,212-02. Binding CB1R on typically suppresses neurotransmitter release.
In addition to KATP-channels, glucose can alter neuronal function via AMP kinase (AMPK), an enzyme that is regulated by the relative concentrations of AMP and ATP. This is especially true for neurons that are inhibited by glucose, as occurs in the ventromedial hypothalamus [41, 42]. Thus, neurons in the hypothalamus and brainstem can react to changing glucose levels via different mechanisms.
The DVC and glucose sensitivity
In the DVC, activity of neurons could be either increased by glucose or increased by removal of glucose, suggesting responses to both hypo- and hyperglycemia [39, 43]. Most effects were associated with the presence of SUR1, a nearly ubiquitously–expressed KATP channel subunit that also imparts effective KATP channels in hypothalamus, although a hypoglycemia-induced depolarization was attributed to a different mechanism. Effects were associated with expression of Kir6.2 channel protein and more selectively with glucokinase expression [39]. The effects of altered glucose concentration in unidentified vagal complex neurons were thus attributed to effects on KATP-mediated currents in neurons that also expressed glucokinse. Although glucose transporters are expressed in several brain areas, their presence in vascular epithelium may not be necessary in the vagal complex due to the presence of fenestrated capillaries in the NTS, which would allow direct access of the neurons to glucose and other large molecules [44].
The NTS is primarily viscerosensory in nature, receiving direct, obligatory viscerosensory innervation from the organs of the thorax and abdomen. Direct glucose sensing might allow for moment-to-moment monitoring of plasma glucose concentration by the brain. The NTS connects directly with neurons in the hypothalamic PVN that control feeding and digestion as well as with gastrointestinal-, hepatic-, and pancreatic-projecting motor neurons in the DMV and with pre-sympathetic neurons in the ventral brainstem (Fig. 1). Although DMV neurons themselves might sense glucose levels directly, since their dendrites extend well into the NTS [45], elevated glucose in slices resulted a TTX and picrotoxin sensitive outward current in these cells [40] (Fig. 4). Although inconsistent with other studies showing a lack of Cl--dependence to the effect of altered glucose concentration [39], the inhibitory effect of glucose application in DMV motor neurons was prevented by blocking action potentials and was consistent with an increase in Cl- conductance [40]. The effect was therefore hypothesized to be indirect, being due to activation of a local GABAergic circuit. A major inhibitory GABAergic input to gastric-related and other DMV motor neurons arises from the adjacent NTS [46, 47], so these results were consistent with glucose elevation activating GABAergic NTS neurons, which in turn suppressed activity of preganglionic vagal motor neurons (Fig. 2).
This precise arrangement has not been tested directly, but injecting glucose in the DVC also caused a decrease in gastric motility and an increase in intragastric pressure [40]). Consistent with these effects, vagal nerve activity is suppressed in acute hyperglycemia [48], leading to decreased parasympathetic visceral tone. Vagally-mediated gastric regulation is severely compromised in hyperglycemic animals and human patients. Both gastric hyperactivity and gastroparesis are reported, and these are due, at least in part, to vagal dysregulation [49]; gastrointestinal dysfunction in diabetics is often seen in otherwise asymptomatic patients. Vagal involvement in diabetes is also suggested by the finding that hepatic vagal lesions also potently suppress insulin-induced normalization of hepatic gluconeogenesis [17]. Glycemic control of DMV neurons projecting to the pancreas or liver, which are known to regulate insulin and glucose production, respectively, have never been assessed. However, understanding how glucose and GABAergic interact to regulate DMV neurons could aid in normalizing digestive functions and metabolic homeostasis in diabetic patients.
Role of the hypothalamus in diabetes mellitus
The hypothalamus is known as a crucial center for controlling feeding behavior, energy balance and glucose homeostasis. Neurons in the hypothalamus are able to control responses to both hypo-and hyperglycemia via different cellular pathways (i.e,. KATP-mediated excitation and AMPK-mediated inhibition), resulting in glucagon release from the pancreas and secretion of epinephrine [50, 51]. It has long been known that electrical stimulation of the areas of the hypothalamus corresponding to glucose-sensing regions (i.e., VMH, LH) activates glycogen synthase [52], and stimulation of VMH triggers glycogenolysis in the liver [18]. Focal lesions in the area of VMH abolish the hormonal response to systemic hypoglycemia, suggesting the importance and the role of the VMH in the counter-regulatory mechanism [50, 51, 53]. Magnusson and coworkers [54] made the remarkable observation that increased hepatic gluconeogenesis contributes significantly to hyperglycemia in type 2 diabetes. The role of the hypothalamus was further highlighted after Obici and coworkers demonstrated that modulation of lipid metabolism within the hypothalamus causes changes in energy balance and glucose metabolism [55-58].
POMC neurons
Over the last three decades it has become evident that both insulin and leptin activate receptors in several brain areas including the hypothalamus [59-61]. There is increasing evidence suggesting that insulin and leptin penetrate the blood brain barrier (probably indirectly via a transporter) and activate their receptors in several brain areas including the hypothalamus [59-61]. However, just a small portion of peripherally-applied insulin reaches the CNS, and the transport of insulin has a saturable character [50, 62]. Transport is altered during different physiological conditions such as fasting, obesity or diabetes [63]. But the origin of central insulin remains unclear, as other studies assert that insulin is made in the brain [10]. Increased levels of insulin and leptin activate a group of hypothalamic neurons that synthesize the peptide proopiomelanocortin (POMC). Activation of POMC neurons can influence body weight loss, feeding, and energy balance [64, 65]. Consistent with responses to leptin and insulin, POMC neurons also respond to glucose and express KATP channels [66]. In addition to the hypothalamus, a significant number of POMC neurons are located in the NTS, although little is known about their physiology [67]. Parton and coworkers [68] observed that nearly half of the population of hypothalamic POMC neurons was glucose-excited; this may in turn regulate glucose homeostasis via connections with the PVN and/or brainstem. Disruption of glucose sensing in glucose-excited POMC neurons via genetic manipulation of the KATP channels - wherein KATP channels are 250 times less sensitive to closure by ATP – resulted in impaired responses to glucose tolerance tests, [68], which suggests that glucose-sensing by glucose-excited POMC neurons is required for the normal handling of systemic glucose load. The study also provided evidence that the glucose-sensing properties of POMC neurons is diminished with obesity and that the mitochondrial protein uncoupling protein 2 (i.e., UCP2) is involved in this loss of glucose sensitivity. Thus, specific responses in this subset of neurons may represent an important pathogenic component of type 2 diabetes [68]. Responses in other cell groups have yet to be determined.
Other metabolic signals
Roles of histamine, dopamine, and serotonin on hypothalamic control of feeding behavior have been identified in relation to diabetes [53]. In addition, a large number of neuropeptides and circulating hormones have been identified, which can modify ingestion and other homeostatic functions and thus can affect outcomes in diabetes. Over the last 10 years, ghrelin, a 28-amino-acid protein hormone released mainly from the stomach, has garnered a great deal of attention with respect to diabetes because it is thought to stimulate appetite. The hypothalamus and the DVC are the thought to be among the areas affected by ghrelin. Plasma ghrelin levels rise in fasted animals and increased ghrelin tends to increase feeding. These relationships were lost after vagotomy [69], indicating that ghrelin’s effects in the brain are at least partly mediated by vagal connections with the viscera. It is widely appreciated that that ghrelin increases appetite in part by depolarizing hypothalamic orexigenic neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons or by increasing the tonic inhibition exerted by the NPY/AgRP neurons over the POMC neurons [69]. Ghrelin stimulates AMPK in the hypothalamus and increases food intake [25]. Food, glucose deprivation, and administration of an AgRP antagonist can also increase AMPK activity in the hypothalamus. Ghrelin and endogenous cannabinoids increase hypothalamic AMPK activity and leptin or insulin decreases it [25].
Fasting ghrelin concentrations are lower in type 2 diabetic patients compared to non-diabetic controls, and the diminished circulating ghrelin concentration is proportionate to the degree of insulin insensitivity. In type 1 diabetes ghrelin levels are elevated, and the elevation is reduced by insulin treatment. Reduction of ghrelin levels depends on the presence of circulating insulin. In STZ-induced uncontrolled diabetes, hyperphagia is thought to be due to deficient hypothalamic signaling by insulin and leptin, which in turns lead to the activation of NPY/AgRP neurons. Whereas food intake did not increase until three days after STZ administration, plasma ghrelin levels were increased within one day [70]. As hyperphagia developed, plasma ghrelin levels declined. The authors concluded that uncontrolled type 1 diabetes increases both circulating ghrelin levels and sensitivity to ghrelin. These observations support the idea that increased ghrelin signaling contributes to the pathogenesis of diabetic hyperphagia. The exact action of ghrelin to controlling feeding behavior is not clear yet, but evidence suggests that ghrelin has a role in the control of energy balance and body weight in diabetic patients.
Plasticity of neuronal function in diabetes
Emerging evidence suggests that hormonal and nutrient signals acting within the CNS, particularly on nuclei of hypothalamus and brainstem, play a critical role in regulating glucose metabolism. Plasticity in hypothalamic and brainstem circuits regulating metabolism are thus likely to participate in glucose metabolism in diabetes, either by contributing to glucose metabolism dysregulation or by compensating for changes associated with eating and metabolic disorders.
Specific neural pathways involved in autonomic regulation contribute significantly to both normal glucose metabolism and also to altered responses to glucose in models of diabetes. Hyperactivity of dorsal horn neurons in the spinal cord has been associated with diabetic neuropathy in hyperglycemic rats [71], but synaptic changes in these neurons are poorly understood. The frequency of glutamatergic miniature excitatory postsynaptic currents (mEPSCs) was significantly higher in animal models of type 1diabetes than in controls [72]. Preliminary observations likewise indicated an increase in spontaneous EPSC frequency from DMV neurons recorded in acute brainstem slices from diabetic mice [73]. The frequency of GABAergic and glycinergic spontaneous inhibitory postsynaptic currents (sIPSCs) and mIPSCs did not differ in control and diabetic animals in these studies. Application of the GABAB agonist, baclofen produced a greater decrease in frequency of mEPSCs in controls than in diabetic animals [72], but the inhibitory effect on IPSCs was not different. These findings suggest that increased glutamatergic input may contribute to central manifestations of diabetes, including synaptic plasticity and central sensitization in diabetic neuropathic pain.
In addition to indirect effects of glucose on synaptic transmission [40], others have shown that glucose can acutely alter vagal responses and neuronal membrane potential. In addition to acute effects on synaptic transmission observed in the hypothalamus, spinal cord, and brainstem, altered glucose levels lasting for longer periods of time might alter neuronal communication in a manner consistent with altered vagal control of the viscera over time. Whereas acute glucose elevations tend to inhibit vagal output, prolonged hyperglycemia can induce vagally-stimulated insulin release [74] and can increase vagus nerve activity tonically [75]. In patients with type 2 diabetes gastrointestinal problems such as constipation, nausea, and abdominal pain are very common [76]. It is not immediately apparent how this effect over time is consistent with the inhibition of DMV cells produced by acute glucose application [40]. But the putative glucose-induced increase in GABA neuron activity, which in turn enhances GABA release and thus GABAA receptor-mediated Cl--currents in the DMV may, over several days, be associated with long-term, possibly compensatory, plastic changes in synaptic currents over time in the DMV.
The major fast inhibitory neurotransmitter in the brain is GABA. In the NTS and DMV, all fast inhibitory postsynaptic currents (IPSCs), or potentials (IPSPs) are completely blocked by GABAA receptor antagonists, consistent with the prominence of GABA in the regulation of the DVC (Travagli et al., 1991; Smith et al., 1998; Davis et al., 2004). Molecular cloning studies have shown that the GABAA receptor gene family encodes at least 21 different subunits, including α1-6, β1-4, γ1-4, δ, ε, π, θ, and ρ1-3, which are thought to combine as heteropentamers to form pharmacologically distinct receptor isoforms, with α1β2γ2 making up most native synaptic GABAA receptors [77]. In addition to synaptic inhibition, a GABAA receptor-mediated “tonic GABAergic inhibition” (i.e., I-tonic), which is distinct from synaptic inhibition in that I-tonic may result from activation of extrasynaptic GABAA receptors after “spillover” of excess GABA released from terminals [78], has been described in several brain regions. The GABAA receptors mediating I-tonic are high affinity sensors activated by low concentrations of ambient GABA in the extracellular space [79, 80]. Based on their extrasynaptic location and biophysical and pharmacological properties, the subunit composition of the GABAA receptors mediating I-tonic often includes the δ subunit in place of the γ2 subunit, in addition to 2α and 2β subunits [81-83]. In particular, GABAA receptors are highly plastic functionally, being altered by changes in their environment. For example, after seizures in mice, δ subunits are decreased in the dentate gyrus, contributing to increased excitability. However, other subunits (γ2, α4) are increased, and δ subunit expression itself increased in a subset of interneurons [84]. Excessive GABA induced a reduction in α subunit mRNA in cultured embryonic neurons [85] and others have reported region-and subunit-specific changes in GABAA receptors after elevation of brain GABA in vivo [86, 87]. These subunit modifications significantly affect the postsynaptic pharmacology and efficacy of released GABA, particularly at GABAA receptors mediating I-tonic. Thus, chronic glucose-induced enhancement of GABA release in the DMV might similarly alter postsynaptic responses to GABA in a compensatory manner, which could account for some of the vagal hyperresponsiveness seen in diabetic patients. Vagal activity is a crucial component of glucose metabolism and changes in vagal control might participate in long-term dysregulation of glucose metabolism by altering neuronal communication in the brainstem and hypothalamus.
Functional MRI studies have also shown that the hypothalamus is more sensitive to glucose concentration changes in patients with type 1 diabetes than in non-diabetic controls [88]. Ingestion of a glucose solution by type 2 diabetic patients and healthy controls resulted in a prolonged and significant blood oxygen level-dependent decrease in activity in the hypothalamus of healthy subjects, but not in diabetic patients [89]. Thus glucose failed to inhibit hypothalamus neuronal activity in type 2 diabetes. These observations further support the hypothesis that neuronal circuitry is modified in central autonomic regions in patients with type 1or -2 diabetes.
Diabetes and modulated autonomic output
Early studies suggested that i.v. injection of glucose increased efferent activity in the hepatic branch of the vagus nerve in the rat and i.v. administration of insulin suppressed this activity [18]. The effect of central administration of insulin was also tested in rats with hepatic branch vagotomy [17]. Systemic insulin decreased hepatic glucose production in sham-operated animals, but the effect of insulin was significantly diminished by approximately half in animals that underwent hepatic vagotomy. Likewise, the glucose-reducing actions of insulin injected directly into the VMH were eliminated by vagotomy [24]. Efferent vagal control of the liver is therefore required for the inhibition of glucose production following peripheral administration of insulin, although visceral afferent input to the brainstem via the hepatic branch of the vagus nerve may not be necessary. These experiments support the hypothesis that increased circulating insulin levels activate a neuronal hypothalamic-brainstem circuit that is required in order to restrain hepatic gluconeogenesis [17, 23].
Synaptic modulation and diabetes
Vagal afferent activation is a critical satiety signal and initiates the feeling of “fullness” during a meal [90]. Central synaptic circuits controlling the vagus nerve play a role in glucose homeostasis via control of hepatic and pancreatic functions in addition to signaling hypothalamic and other central regulators of metabolic homeostasis. Synaptic balance is altered in the DVC of hyperglycemic/hypoinsulemic mice [73]. In many diseases, altering local synaptic connections chronically, as happens in hyperglycemia, can change responses to future synaptic input. For example, chronic GABA and glutamate imbalance probably contributes to development of some forms of epilepsy [91, 92] and other brain disorders. Thus, altered glucose levels can acutely change GABAergic and glutamatergic synaptic balance in key brain areas. These changes in synaptic balance can, in turn, result in longer-lasting changes in brain function that can outlast the initial stimulus and contribute to alterations in glucose metabolism over a time course that far exceeds the seconds to minutes required to alter neuronal activity acutely.
Analogous to disorders like temporal lobe epilepsy, a “synaptic imbalance” hypothesis proposes that modulation of otherwise normal synaptic control of central autonomic circuits alter parasympathetic function and consequently contributes to diabetes. A recent randomized controlled trial on obese patients showed that participants who underwent adjustable gastric banding surgery were most likely achieve remission from type 2 diabetes and greater weight loss versus control subjects, who participated in a conventional therapy program that included mainly lifestyle modifications [93]. The observation suggests that the degree of weight loss has a critical impact and could be a major driving force of glycemic improvement and diabetes remission in obese patients. It also implies that any contribution to diabetes of altered synaptic regulation in autonomic circuits can be reversed. Thus, controlling feeding and digestive behaviors—functions that are regulated by autonomic circuits in the hypothalamus and brainstem—seem likely to positively contribute to glycemic control. In addition, some diabetic symptoms can begin to remediate prior to significant weight loss, suggesting additional mechanisms beyond weight loss for normalizing glucose. Speculatively, gastric banding and consequently the chronic alteration of gastric mechanoreceptor activity would tend to diminish vagal visceroafferent signaling in the NTS. This might be predicted to stabilize vagal function and act to reset the level of tonic vagal efferent activity. The stabilization of preganglionic vagal motor neuron activity might subsequently affect both hepatic gluconeogenesis and insulin secretion. These effects would be expected to occur rapidly and in addition to any modulation of gastrointestinal function that contributes to altered ingestive behavior. The contribution of central autonomic pathways to maintenance or control of diabetic symptoms is a promising avenue for future research in controlling diabetes. An important requisite of such therapeutic aims is the ability to modulate synaptic inputs pharmacologically.
Involvement of the cannabinoid system in diabetes
Activity of numerous factors acting in brain circuits have been identified as contributors to control of homeostatic functions. Here, we will discuss contribution of one of the most malleable neurochemical systems involved in the brain’s control of metabolism, the cannabinoid (CB) system. A large effort has been extended in the last few years to understand the endocannabinoid system in the context of treating obesity and related metabolic disorders [94], but effects of cannabinoids on neurons controlling energy metabolism are only partially understood.
The behavioral effects on appetite of modulating the CB system are well known [95]. The two best studied endogenously synthesized and released cannabinoid agonists (eCB) are arachidonylethanolamine (anandamide; AEA) and 2-arachidonylglycerol (2-AG). In general terms, type 1CB receptors (CB1R) are activated centrally after brief food deprivation and these tend to upregulate orexigenic and downregulate anorexigenic mediators of food consumption. Several studies have provided evidence that the level of CB1R is high in hypothalamic and brainstem areas involved in regulation of satiety, food intake, and autonomic function [96, 97]. The distribution of these receptors suggests that they could directly regulate the expression of orexigenic and anorexigenic signals and also modulate autonomic circuits [98]. The CB receptors are also present in peripheral cells, including hepatocytes [99], white adipose tissue [100], and pancreatic β-cells [100, 101]. These distributions suggest that peripheral eCB activity is also involved in metabolic control, possibly by regulating insulin levels, glucose uptake, and glucose production [101].
There is growing evidence suggesting that patients with hyperglycemia caused by type 2 diabetes have elevated levels of AEA and 2-AG compared to non-diabetic controls [100, 102]. Elevated plasma eCB levels suggest that the eCBs are overproduced or their metabolism has been decreased. This seems to accompany metabolic and eating disorders and may contribute to the development of abdominal obesity, dyslipidemia and hyperglycemia [95]. In the hypothalamus and brainstem, eCBs have profound effects on synaptic inputs to neurons involved in neuroendocrine and autonomic regulation. Specifically, inhibitory synaptic inputs to both DMV motor neurons [97] and to hypothalamic neuronendocrine neurons [103] are suppressed by CB1R activation, consistent with increased feeding and other known CB effects on energy homeostasis. Figure 3 illustrates a model for actions of cannabinoid activity at the synaptic level in central autonomic circuits.
In addition, eCBs can exert neuro-protective effects in many CNS related disorders, such as cerebral ischemia, stroke, neuronal injury and neurodegenerative diseases, like multiple sclerosis, Huntington`s and Parkinson`s disease [104]. The synthetic cannabinoid HU-210 may have a protective effect in an experimental model of type 1 diabetes [105]. In several brain areas CB1R activation modulates cellular glucose utilization [106]. In animals with streptozotocin-induced type 1 diabetes, the densities of CB1R protein and the specific CB1R binding sites in the hippocampus were increased in nerve terminals and in total membranes, whereas CB1R mRNA expression was decreased [107]. These data suggest that CB1R play a role in diabetic encephalopathy. Another study showed down-regulation of CB1R in PC12 cells treated with high glucose and in sensory dorsal root ganglion neurons from diabetic rats [108]. These data suggested that the elevated plasma glucose that occurs in diabetes mellitus can be associated with decreased expression of CB1R and altered eCB activity.
To complicate the issue, AEA has the ability to activate both CB1R and transient receptor potential vanilloid type 1 and/or 4 (TRPV1, TRPV4) receptors (Fig. 3). Activation of TRPV1 by AEA has an excitatory effect and typically enhances synaptic transmission, but this enhanced release of neurotransmitter can also be inhibited by CB1R activation in some central neurons [109]. CB1R and TRPV1 are co-localized in numerous brain areas, including areas which are responsible for energy and glucose homeostasis [96, 97, 109]. In the dorsal root ganglia of animal models of type 1 diabetes, TRPV1 receptor protein is upregulated [110] and the neurons showed a significant increase in capsaicin-evoked inward currents. This observation coincides with other reported changes in type 1diabetic animals [107, 108], where the increasing glucose levels altered CB1R expression levels. These findings are consistent with the emerging hypothesis that CB1R/TRPV balance in autonomic areas could be crucial regulators of glucose homeostasis and therefore in complicating and/or contributing to effects of diabetes. This altered function can have a strong influence on autonomic output, including hepatic gluconeogenesis but may also provide a possible therapeutic target to better regulate glucose levels in patients with diabetes.
Finally, cannabinoid receptors may act as a substrate for other potent modulators of metabolic function. For example, very recent observations showed that ghrelin and the eCBs stimulate appetite via activation of AMPK. Rimonabant, a CB1R antagonist, can block the effects of ghrelin on feeding behavior, and ghrelin did not have effect on CB1R knockout mice [111]. Interestingly, leptin signalling in the PVN also appears to involve the induction of eCB release and subsequent binding to presynaptic CB1R on glutamate terminals contacting PVN neurons [112]. Ghrelin also increases eCB levels in the hypothalamus and inhibits EPSCs in parvocellular PVN neurons. Based on these observations, an intact eCB signaling pathway appears to be required for the effects of ghrelin on AMPK activity and food intake and for the inhibitory effect of ghrelin and leptin on PVN neurons [111].
Conclusions
Precise regulation of autonomic function and hepatic gluconeogenesis is necessary to maintain appropriate energy sources. Recent observations provide strong evidence in support of an important pathway for brain-liver interactions, which connects lipid and peptide sensing in the hypothalamus and brainstem to hepatic glucose homeostasis [17, 18, 23]. In addition, vagal signaling and direct glucose sensing in the vagal complex could mediate some of the brain’s responses to changes in plasma glucose concentration [40, 43]. Current data support the hypothesis that insulin acts at least partly through central KATP channels to modulate central autonomic centers in the hypothalamus and brainstem to inhibit hepatic glucose production. Many effects of insulin, leptin, and glucose on hypothalamic or other central autonomic circuits require intact vagal outflow through the hepatic branch of the vagus. Neural activity is altered at the level of central synapses in diabetes models, and this is reflected in significantly modified vagal motor function in animal models of diabetes and in patients. Among the numerous modulators that may be targeted to regulate metabolic output, the endogenous cannabinoids represent an emerging system for affecting control over homeostatic functions. To improve health outcomes and quality of life requires early and intensive treatment of diabetes [93]. Modulation of central autonomic circuitry in the hypothalamus and/or brainstem represents a potential therapeutic target for managing glucose metabolism in diabetic patients.
Acknowledgments
Supported by NIH (DK056132) and the University of Kentucky Research Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oomura Y. Input-output organization of the hypothalamus relating to food intake behavior. In: Morgane PJ, Panskepp J, editors. Handbook of the Hypothalamus, Vol 2: Physiology of the Hypothalamus. Vol. 2. Marcel Dekker; New York: 1980. pp. 557–620. [Google Scholar]
- 2.Undeland KA, Hausken T, Gilja OH, Aanderud S, Berstad A. Gastric meal accommodation studied by ultrasound in diabetes. Relation to vagal tone. Scand J Gastroenterol. 1998;33:236–241. doi: 10.1080/00365529850170784. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 4.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006;29:2539–2548. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 5.Wootton-Gorges SL, Glaser NS. Imaging of the brain in children with type I diabetes mellitus. Pediatr Radiol. 2007;37:863–869. doi: 10.1007/s00247-007-0536-8. [DOI] [PubMed] [Google Scholar]
- 6.Jongen C, Biessels GJ. Structural brain imaging in diabetes: a methodological perspective. Eur J Pharmacol. 2008;585:208–218. doi: 10.1016/j.ejphar.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 7.Eikelis N, Esler M. The neurobiology of human obesity. Exp Physiol. 2005;90:673–682. doi: 10.1113/expphysiol.2005.031385. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Tilve D, Stern JE, Tschop M. The brain and the metabolic syndrome: not a wireless connection. Endocrinology. 2006;147:1136–1139. doi: 10.1210/en.2005-1586. [DOI] [PubMed] [Google Scholar]
- 9.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–1231. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 11.Barber WD, Burks TF. Brain stem response to phasic gastric distension. Am J Physiol. 1983;245:G242–248. doi: 10.1152/ajpgi.1983.245.2.G242. [DOI] [PubMed] [Google Scholar]
- 12.Barber WD, Burks TF. Brain-gut interactions: brain stem neuronal response to local gastric effects of substance P. Am J Physiol. 1987;253:G369–377. doi: 10.1152/ajpgi.1987.253.3.G369. [DOI] [PubMed] [Google Scholar]
- 13.Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paintal AS. Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature. 1953;172:1194–1195. doi: 10.1038/1721194a0. [DOI] [PubMed] [Google Scholar]
- 15.Phifer CB, Sikes CR, Hall WG. Control of ingestion in 6-day-old rat pups: termination of intake by gastric fill alone? Am J Physiol. 1986;250:R807–814. doi: 10.1152/ajpregu.1986.250.5.R807. [DOI] [PubMed] [Google Scholar]
- 16.Robinson PH, Moran TH, McHugh PR. Cholecystokinin inhibits independent ingestion in neonatal rats. Am J Physiol. 1988;255:R14–20. doi: 10.1152/ajpregu.1988.255.1.R14. [DOI] [PubMed] [Google Scholar]
- 17.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 18.Niijima A. Blood glucose levels modulate efferent activity in the vagal supply to the rat liver. J Physiol. 1985;364:105–112. doi: 10.1113/jphysiol.1985.sp015733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazu T, Fukuda A, Ban T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature. 1966;210:1178–1179. doi: 10.1038/2101178a0. [DOI] [PubMed] [Google Scholar]
- 20.Honmura A, Yanase M, Saito H, Iguchi A. Effect of intrahypothalamic injection of neostigmine on the secretion of epinephrine and norepinephrine and on plasma glucose level. Endocrinology. 1992;130:2997–3002. doi: 10.1210/endo.130.5.1572308. [DOI] [PubMed] [Google Scholar]
- 21.Iguchi A, Burleson PD, Szabo AJ. Decrease in plasma glucose concentration after microinjection of insulin into VMN. Am J Physiol. 1981;240:E95–100. doi: 10.1152/ajpendo.1981.240.2.E95. [DOI] [PubMed] [Google Scholar]
- 22.Iguchi A, Matsunaga H, Nomura T, Gotoh M, Sakamoto N. Glucoregulatory effects of intrahypothalamic injections of bombesin and other peptides. Endocrinology. 1984;114:2242–2246. doi: 10.1210/endo-114-6-2242. [DOI] [PubMed] [Google Scholar]
- 23.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Szabo AJ, Iguchi A, Burleson PD, Szabo O. Vagotomy or atropine blocks hypoglycemic effect of insulin injected into ventromedial hypothalamic nucleus. Am J Physiol. 1983;244:E467–471. doi: 10.1152/ajpendo.1983.244.5.E467. [DOI] [PubMed] [Google Scholar]
- 25.Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol. 2008;70:513–535. doi: 10.1146/annurev.physiol.70.120806.095256. [DOI] [PubMed] [Google Scholar]
- 26.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 27.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 28.Cusin I, Rohner-Jeanrenaud F, Stricker-Krongrad A, Jeanrenaud B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats. Reduced sensitivity compared with lean animals. Diabetes. 1996;45:1446–1450. doi: 10.2337/diab.45.10.1446. [DOI] [PubMed] [Google Scholar]
- 29.Seeley RJ, van Dijk G, Campfield LA, Smith FJ, Burn P, Nelligan JA, Bell SM, Baskin DG, Woods SC, Schwartz MW. Intraventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Horm Metab Res. 1996;28:664–668. doi: 10.1055/s-2007-979874. [DOI] [PubMed] [Google Scholar]
- 30.Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol. 2006;573:395–412. doi: 10.1113/jphysiol.2006.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology. 2007;148:1868–1881. doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D, Jr, Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–386. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 34.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 35.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 36.Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 38.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucosensing and the K(ATP) channel. Nat Neurosci. 2001;4:459–460. doi: 10.1038/87405. [DOI] [PubMed] [Google Scholar]
- 39.Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira M, Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol. 2001;536:141–152. doi: 10.1111/j.1469-7793.2001.t01-1-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50:168–177. doi: 10.1007/s00125-006-0473-3. [DOI] [PubMed] [Google Scholar]
- 42.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1418–1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 43.Balfour RH, Trapp S. Ionic currents underlying the response of rat dorsal vagal neurones to hypoglycaemia and chemical anoxia. J Physiol. 2007;579:691–702. doi: 10.1113/jphysiol.2006.126094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- 45.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517(Pt 2):521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017:208–217. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi T, Matsuda K, Kono T, Pappas TN. Inhibitory effects of hyperglycemia on neural activity of the vagus in rats. Intensive Care Med. 2003;29:309–311. doi: 10.1007/s00134-002-1580-3. [DOI] [PubMed] [Google Scholar]
- 49.Saltzman MB, McCallum RW. Diabetes and the stomach. Yale J Biol Med. 1983;56:179–187. [PMC free article] [PubMed] [Google Scholar]
- 50.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Sherwin RS. Bringing light to the dark side of insulin: a journey across the blood-brain barrier. Diabetes. 2008;57:2259–2268. doi: 10.2337/db08-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 53.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- 56.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 57.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 58.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 59.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 60.van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature. 1979;282:623–625. doi: 10.1038/282623a0. [DOI] [PubMed] [Google Scholar]
- 61.van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin-binding sites in the rat brain: in vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology. 1979;105:666–673. doi: 10.1210/endo-105-3-666. [DOI] [PubMed] [Google Scholar]
- 62.Margolis RU, Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967;215:1375–1376. doi: 10.1038/2151375a0. [DOI] [PubMed] [Google Scholar]
- 63.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 64.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 65.Tsujii S, Bray GA. Acetylation alters the feeding response to MSH and beta-endorphin. Brain Res Bull. 1989;23:165–169. doi: 10.1016/0361-9230(89)90142-1. [DOI] [PubMed] [Google Scholar]
- 66.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 67.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25:3578–3585. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 69.Higgins SC, Gueorguiev M, Korbonits M. Ghrelin, the peripheral hunger hormone. Ann Med. 2007;39:116–136. doi: 10.1080/07853890601149179. [DOI] [PubMed] [Google Scholar]
- 70.Gelling RW, Overduin J, Morrison CD, Morton GJ, Frayo RS, Cummings DE, Schwartz MW. Effect of uncontrolled diabetes on plasma ghrelin concentrations and ghrelin-induced feeding. Endocrinology. 2004;145:4575–4582. doi: 10.1210/en.2004-0605. [DOI] [PubMed] [Google Scholar]
- 71.Chen SR, Pan HL. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol. 2002;87:2726–2733. doi: 10.1152/jn.2002.87.6.2726. [DOI] [PubMed] [Google Scholar]
- 72.Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol. 2007;579:849–861. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zsombok A, Derbenev AV, Bhaskaran MD, Smith BN. Plasticity of synaptic input to the brainstem in a model of type 1 diabetes. SfN. 2008;481:423. [Google Scholar]
- 74.Laury MC, Takao F, Bailbe D, Penicaud L, Portha B, Picon L, Ktorza A. Differential effects of prolonged hyperglycemia on in vivo and in vitro insulin secretion in rats. Endocrinology. 1991;128:2526–2533. doi: 10.1210/endo-128-5-2526. [DOI] [PubMed] [Google Scholar]
- 75.Ahren B, Sundkvist G, Mulder H, Sundler F. Blockade of muscarinic transmission increases the frequency of diabetes after low-dose alloxan challenge in the mouse. Diabetologia. 1996;39:383–390. doi: 10.1007/BF00400669. [DOI] [PubMed] [Google Scholar]
- 76.Enck P, Rathmann W, Spiekermann M, Czerner D, Tschope D, Ziegler D, Strohmeyer G, Gries FA. Prevalence of gastrointestinal symptoms in diabetic patients and non-diabetic subjects. Z Gastroenterol. 1994;32:637–641. [PubMed] [Google Scholar]
- 77.Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 78.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 80.Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- 81.Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- 84.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montpied P, Ginns EI, Martin BM, Roca D, Farb DH, Paul SM. Gamma-Aminobutyric acid (GABA) induces a receptor-mediated reduction in GABAA receptor alpha subunit messenger RNAs in embryonic chick neurons in culture. J Biol Chem. 1991;266:6011–6014. [PubMed] [Google Scholar]
- 86.Fenelon VS, Herbison AE. In vivo regulation of specific GABAA receptor subunit messenger RNAs by increased GABA concentrations in rat brain. Neuroscience. 1996;71:661–670. doi: 10.1016/0306-4522(95)00492-0. [DOI] [PubMed] [Google Scholar]
- 87.Lindgren S, Simmonds MA. Adaptation of the GABAA-receptor complex in rat brain during chronic elevation of GABA by ethanolamine O-sulphate. Br J Pharmacol. 1987;91:617–625. doi: 10.1111/j.1476-5381.1987.tb11255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musen G, Simonson DC, Bolo NR, Driscoll A, Weinger K, Raji A, Theberge J, Renshaw PF, Jacobson AM. Regional brain activation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab. 2008;93:1450–1457. doi: 10.1210/jc.2007-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vidarsdottir S, Smeets PA, Eichelsheim DL, van Osch MJ, Viergever MA, Romijn JA, van der Grond J, Pijl H. Glucose ingestion fails to inhibit hypothalamic neuronal activity in patients with type 2 diabetes. Diabetes. 2007;56:2547–2550. doi: 10.2337/db07-0193. [DOI] [PubMed] [Google Scholar]
- 90.Burks TF, V HV. Gastrointestinal Motility. Raven Press; New York: 1980. [Google Scholar]
- 91.Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- 92.Winokur RS, Kubal T, Liu D, Davis SF, Smith BN. Recurrent excitation in the dentate gyrus of a murine model of temporal lobe epilepsy. Epilepsy Res. 2004;58:93–105. doi: 10.1016/j.eplepsyres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 94.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 95.Matias I, Di Marzo V. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J Endocrinol Invest. 2006;29:15–26. [PubMed] [Google Scholar]
- 96.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 97.Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 101.Juan-Pico P, Fuentes E, Bermudez-Silva FJ, Javier Diaz-Molina F, Ripoll C, Rodriguez de Fonseca F, Nadal A. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium. 2006;39:155–162. doi: 10.1016/j.ceca.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 102.Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baker D, Pryce G, Giovannoni G, Thompson AJ. The therapeutic potential of cannabis. Lancet Neurol. 2003;2:291–298. doi: 10.1016/s1474-4422(03)00381-8. [DOI] [PubMed] [Google Scholar]
- 105.Dagon Y, Avraham Y, Link G, Zolotarev O, Mechoulam R, Berry EM. The synthetic cannabinoid HU-210 attenuates neural damage in diabetic mice and hyperglycemic pheochromocytoma PC12 cells. Neurobiol Dis. 2007;27:174–181. doi: 10.1016/j.nbd.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 106.Freedland CS, Whitlow CT, Smith HR, Porrino LJ. Functional consequences of the acute administration of the cannabinoid receptor antagonist, SR141716A, in cannabinoid-naive and - tolerant animals: a quantitative 2-[14C]deoxyglucose study. Brain Res. 2003;962:169–179. doi: 10.1016/s0006-8993(02)03999-9. [DOI] [PubMed] [Google Scholar]
- 107.Duarte JM, Nogueira C, Mackie K, Oliveira CR, Cunha RA, Kofalvi A. Increase of cannabinoid CB1 receptor density in the hippocampus of streptozotocin-induced diabetic rats. Exp Neurol. 2007;204:479–484. doi: 10.1016/j.expneurol.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 108.Zhang F, Hong S, Stone V, Smith PJ. Expression of cannabinoid CB1 receptors in models of diabetic neuropathy. J Pharmacol Exp Ther. 2007;323:508–515. doi: 10.1124/jpet.107.128272. [DOI] [PubMed] [Google Scholar]
- 109.Derbenev AV, Monroe MJ, Glatzer NR, Smith BN. Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci. 2006;26:9666–9672. doi: 10.1523/JNEUROSCI.1591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–627. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- 111.Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]