Summary
Bacterial virus entry and cell wall depolymerization require the breakdown of peptidoglycan (PG), the peptide cross-linked polysaccharide matrix that surrounds bacterial cells. Structural studies of lysostaphin, a PG lytic enzyme (autolysin), have suggested that residues in the active site facilitate hydrolysis, but a clear mechanism for this reaction has remained unsolved. The active site residues and a structural pattern of β-sheets are conserved among lysostaphin homologs (such as LytM of Staphylococcus aureus) and the C-terminal domain of gene product 13 (gp13), a protein at the tail tip of the Bacillus subtilis bacteriophage φ29. gp13 activity on PG and muropeptides was assayed using high performance liquid chromatography, and gp13 was found to be a D,D-endopeptidase that cleaved the peptide cross-link. Computational modeling of the B. subtilis cross-linked peptide into the gp13 active site suggested that Asp195 may facilitate scissile bond activation and His247 is oriented to mediate nucleophile generation. This is the first model of a Zn2+-metallopeptidase and its substrate to our knowledge. Residue Asp195 of gp13 was found to be critical for Zn2+-binding and catalysis by substitution mutagenesis with Ala or Cys. Circular dichroism and particle induced X-ray emission spectroscopy showed that the general protein folding and Zn2+-binding was maintained in the Cys mutant but reduced in the Ala mutant. These findings together support a model where the Asp195 and His247 in gp13 and homologous residues in the LytM and lysostaphin active sites facilitate hydrolysis of the peptide substrate that cross-links PG. Thus, these autolysins and phage entry enzymes have a shared chemical mechanism of action.
Keywords: Bacteriophage, metallopeptidase, autolysin, φ29, gp13
INTRODUCTION
Bacterial cell growth and infection by bacteriophages require remodeling of the peptidoglycan (PG) cell wall. Autolysis is the process of cell wall degradation by self-produced enzymes that normally permit bacterial cell expansion and division. Phages are extremely efficient at crossing the cell wall barrier during infection by both mechanical (phages T4, P22)1–3 and enzymatic (phages GA-1, φ29, K1E, K1–5, P22, PRD1, T4, T5, T7/T3)4–11 means. The latter involves a battery of lysozymes, amidases, transglycosylases and endopeptidases that cleave either the polysaccharide or peptide bonds of the PG, providing access to the lipid bilayer. The PG of Bacillus subtilis, the host of bacteriophage φ29, is depicted in Fig. 1a.
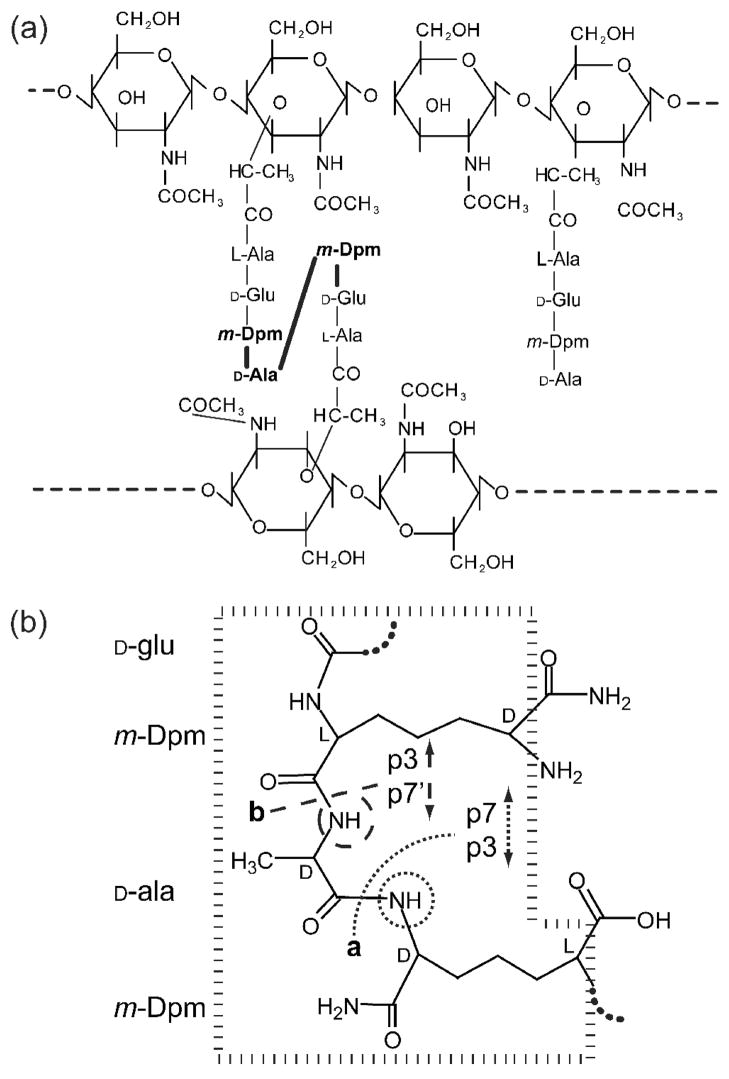
Figure 1. B. subtiliscell wall peptidoglycan structures.
(a) B. subtilis cell wall peptidoglycan is composed of alternating N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) residues with peptide side chains. Peptides can be cross-linked via the D-ala in position 4 and the free ε-amino group of diaminopimelic acid (Dpm). Over 95% of the Dpm free carboxylate groups are amidated31. The predominant cross-linked muropeptide fragment, p21, is two disaccharides cross-linked as shown via their peptide side chains (panel a, left). The activity of gp13 cleaves p21 to produce a disaccharide-tripeptide and a disaccharide-tetrapeptide. This could result from hydrolysis of either of two bonds (panel b, sites a and b) surrounding the cross-link (panel a, bold amino acids and bonds). However, the potential disaccharide-tetrapeptide products differ in the amino acids that have free amino groups (panel b, circles) and thus can be differentiated following modification of these amino groups followed by amino acid analysis (Table 1). The cross-link fragment used for modeling is shown and the chiral centers are indicated as L or D (panel b, dashed box).
Structural and biochemical studies have enlightened autolysin mediated PG remodeling for cell division and lysozyme-mediated phage entry into host bacteria. Atomic structures are available for both the inactive12 and active13 forms of the Zn2+ metallopeptidase LytM of Staphylococcus aureus, a homolog of lysostaphin of Staphylococcus simulans. Lysostaphin is a well-studied metallopeptidase that cleaves the pentaglycine cross-link of the staphylococcal cell wall during autolysis14–19. The atomic structures of the lysostaphin homolog Ale-1 of Staphylococcus capitis20 and LAS protein MepA of Escherichia coli21 have also been determined. However, the precise molecular mechanism for catalysis by the conserved amino acids within LytM, lysostaphin and the lysostaphin-like enzymes remains unknown14, 18. Models and cocrystallized structures of lysostaphin-like peptidases with bound substrates are also not available. Sequence alignments have suggested the presence of lysostaphin-like homologs in phages of both B. subtilis22 and Lactobacillus23, and these are virion components. However, biochemical characterization of these phage proteins has not confirmed the proposed activity.
Bacteriophage φ29 has served as a model for virus assembly mechanisms24, 25. Early studies of the φ29 interaction with the host cell wall revealed that glucosylated teichoic acids rather than a protein serve as the receptor26, 27. Furthermore, the ability of the phage to adsorb to teichoic acids has been shown to reside in the phage tail appendages28. However, adsorption alone is insufficient for infection and progeny production; successful puncture of the cell wall is also required. Here, biochemical study of the cell wall depolymerizing metallopeptidase of bacteriophage φ29 of B. subtilis, gene product 13 (gp13), a homolog of the S. aureus autolysin LytM, is complemented by computational modeling. A possible mechanism of the unified action of phage entry enzymes and autolysins is proposed, based on biochemical and computational characterization of the phage φ29 morphogenetic factor/cell wall depolymerizing enzyme gp1322, 29.
RESULTS
Purified φ29 particles and gp13 cleave the Bacillus subtilis peptidoglycan cross-links
Previous studies localized gp13 to the distal φ29 tail, the site of DNA injection following host cell binding, by immuno-electron microscopy22 and cryo-electron microscopy30. A β-sheet rich C-terminal domain of gp1322, 30 is similar to LytM, the lysostaphin homolog in Staphylococcus aureus, while the N-terminal domain is α-helix rich.
To test the activity of φ29 on the purified peptidoglycan (PG) of B. subtilis (Fig. 1a), purified cell wall sacculi were pseudo-infected with wild-type (wt) φ29(see Methods). It is estimated that there are hundreds of glucosylated teichoic acid φ29 receptors per bacterium26, and each cell has been shown to adsorb ~400 φ2928. Intact PG was largely insoluble and could be pelleted for subsequent muramidase solubilization (see Methods) and high pressure liquid chromatography (HPLC) analysis of muropeptides from the pelleted PG. Following pseudo-infection of sacculi with φ29, there was release of muropeptides into the supernatant that was predominantly p7 (Supplementary Fig. 1d), a disaccharide-tetrapeptide component of the B. subtilis PG (Fig. 1b), while intact PG had little soluble material (Supplementary Fig. 1b). There was also a modest decrease in the cross-linked muropeptide p21 in the φ29 treated insoluble fraction (Supplementary Fig. 1c) compared to the untreated control PG (Supplementary Fig. 1a).
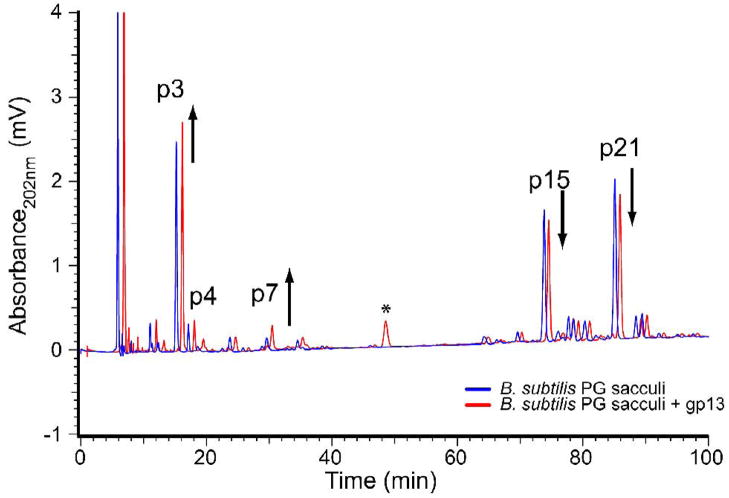
Incubation of sacculi (Fig. 2, blue trace) with purified gp13 (Fig. 2, red trace, offset by 1 min) also revealed a modest decrease in cross-linked species with a corresponding increase in uncross-linked species. gp13 did not cause cleavage of pentaglycine, the lysostaphin substrate in a thin-layer chromatography assay often used for lysostaphin study12 (data not shown).
Figure 2.
gp13 activity on purified B. subtilis peptidoglycan. Mutanolysin digestion of B. subtilis sacculi produces a characteristic profile of peaks (blue line). The untreated sacculi (blue) show the characteristic pattern of cross-linked (>60 min) and uncross-linked (<40 min) muropeptides. Following incubation with purified gp13 protein a modest increase in monomer disaccharide (DS) tripeptide (TriP) and DS tetrapeptide (TP) peaks (under 40 min) is apparent with a slight decrease in cross-linked peaks beyond 60 min (red line). The 43.5 min peak present in the gp13 sample (* red line) is a contaminant from the purified protein solution.
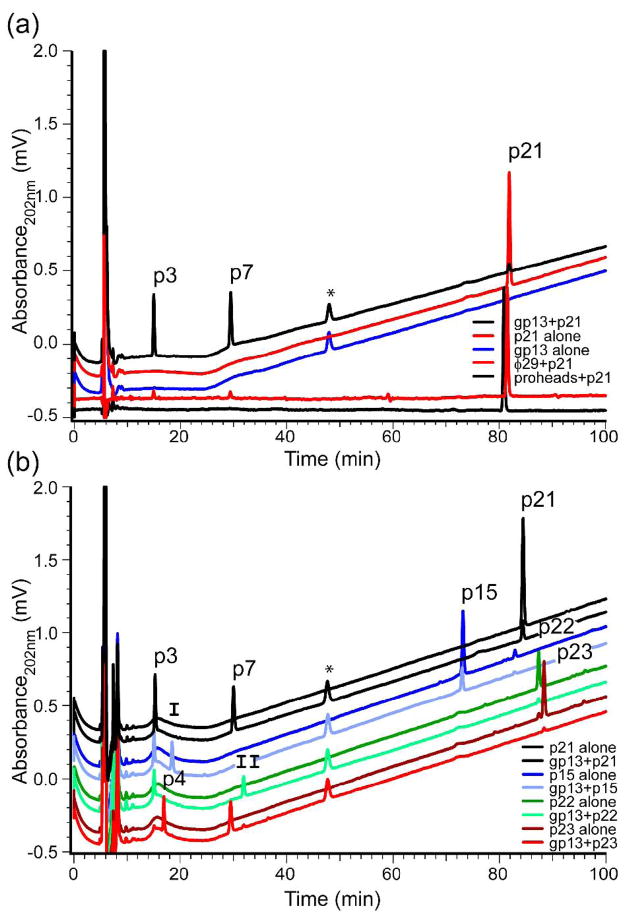
To ensure that gp13 was a tail component with enzymatic activity, HPLC tracking of the dominant cross-linked muropeptide p21 was employed with φ29 virions and gp13 (Fig. 3a). Incubation of φ29 with purified p21 resulted in modest cleavage of p21 to products of smaller molecular mass (Fig. 3a, bottom red line), visualized most clearly on subtraction of the baseline absorption from the raw traces of particles incubated with p21 (Supplementary Fig. 2). Product peaks are at ~15 min and ~30 min (Fig. 3a). The precursor prohead particle, that lacks gp13, does not show the 15 or 30 min peaks (Fig. 3a, bottom black line). This subtle difference suggested that gp13 of the virion tail may have catalytic activity.
Figure 3.
Purified gp13 and φ29 cleaved p21 to the same products, and this activity functions on p15, p22 and p23. Purified p21 alone (a, top red) shows a lone peak at ~81 min, while purified gp13 alone (a, blue) lacks the 81 min peak but contains a 49 min contaminant (*). Incubation of gp13 with p21 (a, top black) shows reduction of p21, with the appearance of two product peaks at 15 min and 30 min. These peaks are present on p21 incubation with φ29 (a, bottom red), but not with gp13-lacking proheads (a, bottom black). These latter two lines are flat because they were produced by subtractions of background runs (Supplementary Fig. 2). (b) Deacetylated (deacetyl) and amidated (amid) forms of the cross-linked muropeptide of B. subtilis are all cleaved to lower molecular weight products. p3: DS-TriPAmid, p4: DSDeacetyl-TriPAmid, p7: DS-TPAmid, p15: DS-TriPAmid-TP-DS, p21: DS-TriPAmid-TPAmid-DS, p22: DS-TriPAmid-TPAmid-DSDeacetyl, p23: DSDeacetyl-TriPAmid-TPAmid-DS, I: DS-TP, II: DSDeacetyl-TPAmid.
Purified cross-linked species were utilized to determine if gp13 contained the enzymatic activity on p21 observed with the virion. gp13 cleaved p21 to products that co-migrated with the φ29 produced products (Fig. 3a, top black line). p15, p21, p22 and p23 (Fig. 3b) are various forms of the cross-linked muropeptide group31. The predominant form, p21, is acetylated on both of the glucosamine residues (Fig. 1a), while p22 and p23 are deacetylated on one of the two glucosamine residues, and p15 is amidated on only one of the Dpm residues. gp13 incubated with each purified cross-linked muropeptide showed substantial product formation (Fig. 3b) to species that migrated at ~15 min and ~30 min. These products had elution times consistent with those of previously identified muropeptides31, 32 (Supplementary Fig. 1a).
φ29 and gp13 cleave the site of the original peptide cross-link formation
Cleavage of the cross-linked peptides may occur at a number of sites: two are illustrated (Fig. 1b). Mass spectrometry analysis of the peak contents that eluted at 15 and 30 min demonstrated masses consistent with p3 and p7 (Fig. 1b, site a) or p3 and p7′ (Fig. 1b, site b) (Table 1). Fluorodinitrobenzene (FDNB) modification of free amino groups in the cleavage products, followed by amino acid analysis, was used to differentiate p7 from p7′ and to determine the specific cleavage site of gp13. p7 and p3 (Fig. 1b, site a) are the products of p21 cleavage by gp13, because only Dpm reacted with FDNB (Table 1). Alanine would have reacted with FDNB if p7′ had been the other product (Fig. 1b, site b).
Table 1.
Muropeptide structure analyses.
| Amino acid analysis (molar ratios) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Muropeptide Name | Muropeptide Structure | m/z (M-H)− |
−FDNB |
+FDNB |
|||||
| Predicted | Observed | Ala | Dpm | Glua | Ala | Dpm | Glua | ||
| p21 | DS-TP-DS-TriP | 1790.8 | 1790.6 | 1.6 | 1.1 | 1 | 1.5 | 0.5 | 1 |
| p3 | DS-TriP | 868.9 | 868.3 | 1.1 | 0.9 | 1 | 1.0 | 0.0 | 1 |
| p7 | DS-TP | 939.4 | 939.3 | 2.0 | 1.1 | 1 | 1.5 | 0.1 | 1 |
Glu was set to 1 for calculation of molar ratios.
Preliminary assessment of product formation kinetics (Supplementary data Fig. 3) suggested that the gp13 solution cleavage rate is slow (~1–2/min), however a similar rate, reported for the phage P22 tail spike endorhamnosidase gp933, is sufficient for infection.
The C-terminal domain of gp13 is active on the peptidoglycan cross-link
Predictions in previous work22, 30 suggested that the gp13 C-terminal domain (CTD) may contain the enzymatic activity on the PG cross-link. To determine if the CTD contains the cross-link-cleaving activity of the protein, the N-terminal domain (NTD) and CTD were expressed separately, purified and tested for their activity in the HPLC assay on the cross-linked muropeptide p21. The NTD is 159 amino acids (aa) and 18.4 kDa, while the CTD is 202 aa and 22.3 kDa (wild-type gp13 is 365 aa and 40.9 kDa) (calculated using ExPASy Protparam34). The mole fraction of the substrate and products are shown for the CTD and NTD alone compared to full-length gp13 (Fig. 4). p3 and p7 were produced by the CTD but not the NTD.
Figure 4. Domain specificity of gp13-mediated cross-link cleavage.
Mass corrected peak area from HPLC analysis of purified N-terminal domain (NTD) and C-terminal domain (CTD) of gp13 incubated with p21. p21 remained uncleaved with the NTD, while product formation was nearly complete with the CTD. p21 (solid bars), p3 (hatched bars), p7 (open bars).
Computational modeling of the gp13-muropeptide complex shows the orientation of the scissile bond
To elucidate the catalytic mechanism underlying the cross-link cleavage mediated by the gp13 CTD and therefore the autolysins such as LytM, the central segment of the p21 muropeptide containing the Dpm-D-ala-Dpm region (Fig. 1b, dashed box) was modeled into the putative active site of gp13. The catalytic binding site of gp13 was identified as the region enclosing the cleft of the gp13 β-sheet domain30 containing a Zn2+cofactor. This region also contains several residues that are highly conserved across LytM and gp13-like proteins of other φ29-related and lactococcal phages (Fig. 5). The original X-ray structure of apo gp13 contains a Zn2+ ion tetracoordinated to D195, H188, H280 and a conserved crystallographic water. The closest oxygen of Aspartate195 (D195) of gp13 is 1.94 Å from Zn2+, and the other oxygen is 2.87 Å from Zn2+. Successful docking of the modeled substrate requires the removal of the zinc bound crystallographic water. Of the 50,000 conformations searched, only seven poses were found, all of which shared a common mode of binding involving the carbonyl oxygen atom of the scissile amide bond positioned within 2.1 to 2.4 Å adjacent to the Zn2+ ion at the original crystallographic water site (Fig. 6a, arrow). The pose with the best docking score is reported here, and the overall model suggests the requirement of water displacement by the potential substrate from the Zn2+ ion as well as chelation of the carbonyl oxygen atom to Zn2+ ion during substrate binding. This mode of binding is supported by a previously solved X-ray structure of latent LytM of Staphylococcus aureus, which showed the Zn2+ ion chelated directly to the carbonyl oxygen atom of the N117 sidechain12. In comparison to the hydrolytic mechanism of another mononuclear zinc metallopeptidase19, 35, the placement of the carbonyl oxygen atom adjacent to the Zn2+ ion is necessary in the activation of the scissile peptide bond of during hydrolysis and the subsequent stabilization of the oxyanion atom of the tetrahedral intermediate. Based on this model, replacement of D195, which is directly chelated to the Zn2+ ion, is expected to alter the overall stabilization effect of the Zn2+ ion and, hence, indirectly affect the overall rate of enzymatic hydrolysis.
Figure 5.
Sequence alignment of gp13 in φ29, B103, GA-1, with LytM and lactococcal phage homologs from bIL285, TP90101, Tuc2009 and ul36. Sequence alignment of φ29 gp13 (top line) compared to other φ29 family members GA-1 and B-103 (lines 2, 3), LytM (line 4) and four lactococcal phages (lines 5–8) over the segment of gp13 containing the Zn2+-binding histidine motif and Asp195 The conserved Zn.2+ chelating residues (φ29 gp13: H188, D195, H280; LytM*: H210, D214, H293) and the two nearby histidines (φ29 gp13: H247, H278; LytM*: H260, H291) have been specifically labeled. The residue number for the first amino acid of each protein is listed in parentheses.
Figure 6.
Modeling of the p21 substrate cleavage target into the putative gp13-Zn2+ containing active site. (a) Modeling of the p21 substrate target (carbon: yellow, oxygen: red, nitrogen: blue, hydrogen: white), containing the peptide bonds of Dpm-Ala-Dpm resulted in energy minimizations that favor orientation of the carbonyl group adjacent to the scissile bond (arrow) as the fourth Zn2+ ligand in gp13. All residues within 5 Å of the model fragment are shown (sticks) with six essential residues as rods. (b) The entire gp13 crystal structure is shown as a ribbon diagram for reference.
The active-site aspartate residue of gp13 binds Zn for polarization
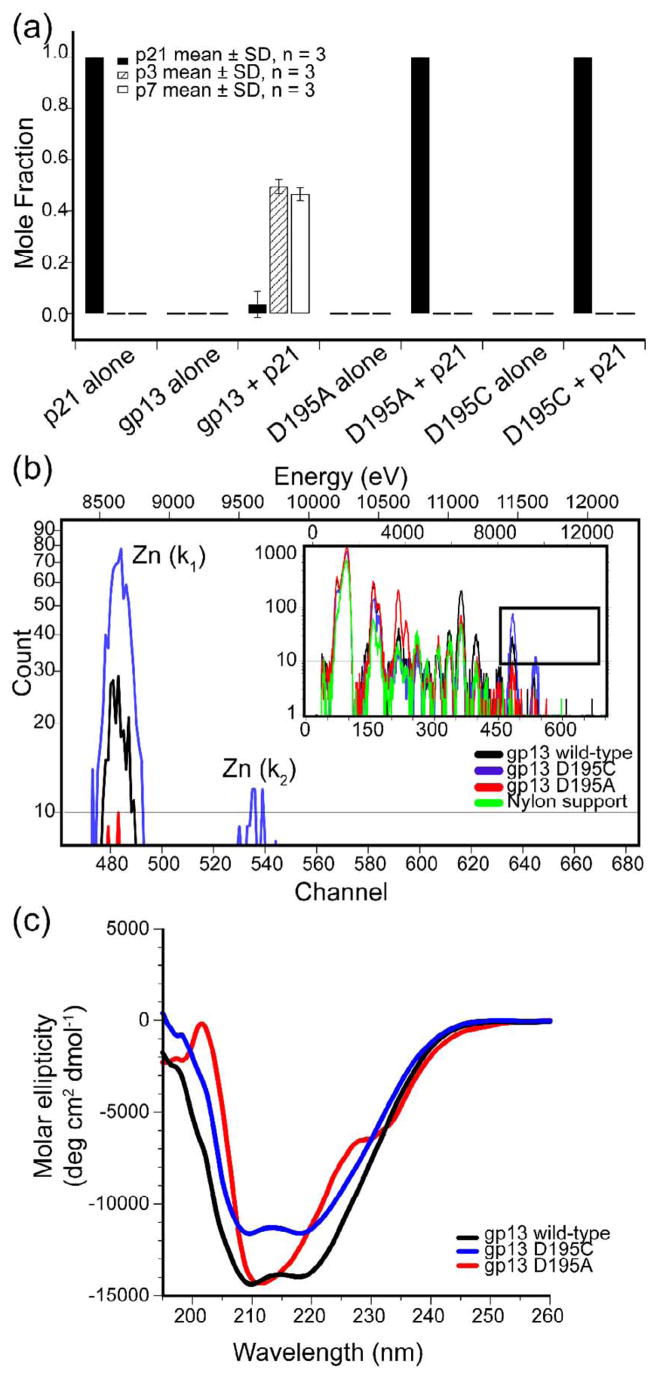
To demonstrate the importance of D195 and its role as part of the catalytic site of gp13, site-directed mutagenesis of the gp13 expression plasmid was performed to generate cysteine and alanine substitutions. The cysteine substitution (D195C) would be expected to maintain Zn2+ binding but may be more electron withdrawing than aspartate36. The alanine substitution (D195A) is expected to block both Zn2+-binding and catalytic activity. Both the D195C and D195A proteins did not show cleavage of p21 to p3 or p7 (Fig. 7a). Thus, D195 was important for enzyme activity.
Figure 7.
gp13 aspartate 195 is involved in Zn2+ binding that is essential for proper protein folding and activity. (a) Mass corrected peak area from HPLC analysis of gp13 point mutants D195C and D195A. p21 (solid bars), p3 (hatched bars), p7 (open bars). (b) Particle Induced X-ray Emission spectra of wild-type gp13 (black) and mutants D195C (blue) and D195A (red). (c) Circular dichroism of wild-type gp13 (black) and mutants D195C (blue) and D195A (red).
To confirm that the D195C change did not prevent Zn2+-binding by gp13, particle induced X-ray emission (PIXE) spectra of wild-type gp13, gp13 (D195C), and gp13 (D195A) were obtained (Fig. 7b). The similarity of the PIXE profiles of the wild type and D195C proteins indicate that the D195C protein bound Zn2+, while the D195A protein did not. Alanine substitution of the homologous aspartate in MepA (D120A) rendered this protein insoluble37. To confirm that gp13 D195A was soluble and to compare the secondary structure folding of wild-type gp13 to both the D195A and D195C proteins, circular dichroism (CD) was employed. CD spectra of gp13 and D195C show the same profile (Fig. 7c) (the vertical shift of D195C may be due to slight differences in protein concentration), while the D195A spectrum was characteristic of substantial disorder of the secondary structure that was absent in wild-type gp13 and gp13 D195C data. These results support the hypothesis that the CTD of gp13 is a PG cross-link-cleaving Zn2+metalloprotease and that D195 is active in binding Zn2+, which contributes to the protein stability, and substrate cleavage.
DISCUSSION
Essential bacterial cell wall PG cleavage that occurs both in growth and cell division and during phage infection is enabled by Zn2+ metallopeptidases. Here, Zn2+ metallopeptidase action of the C-terminal domain (CTD) of the phage φ29 gp13 on B. subtilis PG is documented, biochemical characteristics studied and a computational model of the enzyme-substrate complex presented.
The φ29 virion and gp13 demonstrate cross-link-cleaving capacity
The formation of p3 and p7 cleavage products from the cross-link p21 by gp13 and the gp13-containing virion suggests a mechanism for φ29 entry and confirms the multifunctional roles of gp1322, 29. φ29 gp13-defective mutant particles (sus13(342, 330, 53)) are non-infectious, likely because they rapidly lose the packaged DNA29, 38 even though the gp13 fragments are in the phage22 (sus13(53) contains gp13 by Western blot analysis [Supplementary Fig. 4]). In addition to this DNA retention function, the enzymatic activity of gp13 on PG cross-links (this study) suggest that the few copies of gp13 per virion22, 30 facilitate entry of φ29 through the cross-linked PG layers.
The domain architecture of gp13 is similar to lysostaphin
The PG cross-link-cleaving activity of gp13 resides in the isolated CTD and is independent of the N-terminal domain (NTD) (Fig. 4). In another experiment, particles of the gp13-defective mutant sus13(53), where the CTD of gp13 is truncated and the NTD is intact22, resembled the isolated gp13-NTD; sus13(53) particles were unable to cleave p21 (data not shown). Activity of the CTD is also observed for the PG crosslink-cleaving enzyme LytM of Staphylococcus aureus, where the N-terminus is proposed to be cleaved from the precursor to produce the active enzyme13.
The acetylation of the cross-linked muropeptide does not affect cleavage by gp13 (Fig. 3b). The N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) sugar residues of the PG may be expected to interact with the lysozyme-like NTD of gp1330. A model for enzyme activity of gp13 dimers has been proposed30 where the NTD of one monomer, by binding the NAG-NAM substrate of the PG, aids activity and/or binding of the CTD of the other monomer to a substrate. The absence of dependence on modifications of NAG-NAM for cross-link-cleaving activity of gp13 suggests that either 1) the NTD coordination is independent of acetylation of NAG-NAM, 2) oligomers of NAM-NAG, greater than the two present in the purified muropeptides studied here, are needed for NTD binding or 3) that the NTD does not influence the CTD domain in a coordinated fashion as proposed30. An alternative model could involve the binding of the NTD domain of gp13 that effectively reduces the diffusion coefficient of the gp13 CTD, thereby enhancing the local cleavage of the cross-linked PG in the face of a modest catalysis rate (Supplementary Fig. 3). A similar division of target binding and catalysis between protein domains has been observed for zoocin A, a lysostaphin-like enzyme39.
Dual domain enzymes are known in bacteriophages. As the dual domain endolysins of the phages B3040 and φ1141 enable progeny exit, it is of interest to know if these and other phages also have low copy number virion-associated entry enzymes equivalent in function to gp13 of φ29.
Proposed mechanism of φ29 entry into Bacillus subtilis
Based on current observations and the literature, a model for φ29 entry into B. subtilis is proposed that includes adsorption, PG binding, lysozyme and metallopeptidase mechanisms of PG degradation, membrane puncture and genome injection. Adsorption of the virion to glucosylated teichoic acid surrounding the PG and the likely teichoic acid cleavage by the tail appendages have been discussed previously42. Considering PG cross-link degradation, the CTD of gp13 may be activated much as the CTD of LytM is cleaved away from the N-terminus13, or alternatively, the connected NTD may aid CTD cleavage by limiting diffusion. The in vitro experiments presented here cannot distinguish these in situ events.
After successful localized reduction in PG cross-linking, the φ29 tail knob gp9 may insert into the cell membrane, triggering conformational changes within the tail axis that deliver injection signals to the head-tail connector to initiate genome expulsion. A portion of genome would be propelled by pressure from the packaged DNA43, 44, and subsequent traction of the remaining DNA would continue45.
Zn2+ polarization is essential for catalysis in lysostaphin-like enzymes
D195 of gp13 is essential for catalysis and is superimposed on the LytM aspartate 21422. Support for the essentiality of D195 is found in Ale-1 of S. capitis46 and MepA of E. coli47, where mutation to alanine abrogates function (Ale-1) or renders this expressed protein insoluble and inactive (MepA). However, differentiation between Zn2+-binding for secondary structure determination and Zn2+ polarization for catalysis has not been discussed in other systems. Here, particle induced X-ray emission (PIXE) demonstrated that D195 of gp13 participates as a Zn2+ ligand as suggested by sequence homology22 and the gp13 crystal structure30. Zn2+-binding was demonstrated for the Cys substitution mutant (D195C) by PIXE, but the Ala substitution mutant (D195A) showed appreciably reduced Zn2+signal (Fig. 7b). Although the D195C protein binds zinc, it is inactive for structural and/or electronic reasons. These data suggest that D195 is sufficiently electronegative to facilitate catalysis. Residues in LytM, lysostaphin, Ale-1 and MepA homologous to gp13 D195 may also provide the necessary action for catalysis.
In the current model of hydrolysis by lysostaphin-like enzymes19, gp13-D195 would properly bind Zn2+, polarizing the carbonyl-carbon of the substrate for susceptibility to nucleophilic attack by a hydroxyl group generated from water by a histidine on the opposite site of the active-site (Fig. 8). A hydroxyl nucleophile generated by His247-mediated deprotonation of water most likely facilitates hydrolysis in this geometry because His247 is 3.5 Å from the carbonyl group and is in the same plane while His278 is 0.5 Å further from the carbonyl group and ~45 degrees off axis compared to His247 (Fig. 6). Similar to the previously established model of VanX35, hydrolytic cleavage of the peptide bond occurs after the formation of a tetrahedral intermediate, which forms a bidendate complex with Zn2+ (Fig. 8, step 3). gp13-His247 is homologous to LytM-His260, Ale-1-His200, lysostaphin-His 22522, 48 and MepA-His206 (structural superposition, not shown).
Figure 8.
Proposed hydrolysis reaction of the Bacillus subtilis peptidoglycan cross-link p21 catalyzed by the bacteriophage φ29 gene product 13. Step 1, substrate binding to gp13 active-site, Asp195 mediated polarization of the scissile bond carbonyl carbon and deprotonation of water. Step 2, hydroxyl nucleophile attack of the scissile bond carbon leading to the bidentate complex and tetrahedral intermediate. Step 3, proton removal from His247 by the lone pair on the nitrogen of the scissile bond. Step 4 hydrolysis. Step 5 product release.
gp13-like enzymes in other phages
Other phages encode gp13/lysostaphin-like enzymes that may reflect parallel evolution of PG cross-link cleaving, Zn2+ metallopeptidases. The lactococcal phages and φ29 family members share the His-x(3,6)-Asp and His-x-His motifs (Fig. 5). As infection by the 10^31 phages49 is a frequent genetic transfer event, the likelihood of parallel evolution of entry mechanisms of different phages in conjunction with possible lateral gene transfer from the host warrants further investigation.
METHODS
Phage and protein preparation
Phage φ29 with wild-type (wt) gp13 was prepared using the delayed-lysis fiberless mutant sus8.5(900)14(1241) as described previously22. The gp13 N-terminal domain (NTD), C-terminal domain (CTD), full length and Aspartate 195 mutant gp13 proteins were expressed in either the CodonPlus (Strategene) or the Rosetta (Novagen) strains as previously described30 with modifications. A cocktail of metals (ZnCl2, 1 μM; MnCl2, 0.5 μM; CoCl2, 0.37 μM) was modified from prior work50 and included in the induction medium. EDTA was omitted from all buffers and protease inhibitors. The gp13 C-terminal domain (CTD), and the D195A and D195C mutants were expressed in Rosetta. Cells (OD600nm= 0.5, Klett =44) were grown from overnight cultures with chloramphenicol (34 μg/mL) and ampicillin (100 μg/mL) to select for the Rosetta plasmid and the pTYB1 plasmid, respectively. Cultures were chilled to 0°C in a water/ice bath for 5 minutes prior to IPTG induction at 1 mM and incubated at 20°C overnight in a shaking water bath. Cells harvested by centrifugation were resuspended in 1/20 the original volume of HST (20 mM Hepes, 500 mM NaCl, 0.1% (v/v) Triton-X 100, 4°C) modified from the manufacturer’s guidelines (NEB, Ipswich, MA) to omit the Zn2+chelating agent EDTA, washed of residual media, and resuspended in 30 mL HST with an EDTA-free protease inhibitor mixture (Complete Mini EDTA-free, Roche, Indianapolis, IN) per the manufacturer’s recommendations (1 tablet per 10 mL concentrated cells). The cell concentrate was lysed by two or three passages through a chilled French press and checked for lysis by phase contrast microscopy. The crude lysate was clarified by centrifugation at 4°C and loaded onto a pre-HST buffer-equilibrated 5–8 mL chitin-resin column (NEB) at 4°C and run at a flow rate not exceeding 0.5 mL/min. Self-cleavage of the intein fusion was induced, after a 20 column volume HST wash to remove non-specifically bound material, with HST + 50 mM DTT by room temperature incubation for 36–48 hrs. HST elution with 10 column volumes permitted sufficient protein recovery for subsequent concentration by Centriprep 30 kDa mwco filters (Millipore). Dialysis against TS (20 mM Tris, pH 7.4, 150 mM NaCl) buffer was performed with a 10 kDa mwco Slide-a-Lyzer (Pierce) at 4°C with two 1L exchanges over 4–8 hrs each. Concentrated and buffer exchanged protein was cryo-protected with the addition of sterile glycerol to 10% final and stored at −80°C. The protein was quantified by SDS-PAGE densitometry with BSA standards.
Pfu polymerase (Stratagene) was used in circular PCR to generate the D195A and D195C mutants by modifying the full-length gp13 plasmid pTYB130 using standard conditions and primers as follows:
gp13 D195A forward primer 5′GGAACACTTTGTATAGCCTTTGTGGGCAAAACTGAAAAGTACCC3′ gp13
D195A reverse primer 5′GGGTACTTTTCAGTTTTGCCCACAAAGGCTATACAAAGTGTTCC3′
gp13 D195C forward primer 5′GGAACACTTTGTATATGCTTTGTGGGCAAAACTGAAAAGTACCC3′
gp13 D195C reverse primer 5′GGGTACTTTTCAGTTTTGCCCACAAAGCATATACAAAGTGTTCC3′
Peptidoglycan purification and HPLC analysis
PG from vegetative B. subtilis cells was purified and digested with muramidase (Mutanolysin, Sigma-Aldrich) as previously described31. Muropeptides were reduced with borohydride and separated by HPLC using a NaPO4 buffer and a methanol gradient as described31, 51. Muropeptides in enzyme assay samples were analyzed using the same HPLC system without borohydride reduction. Muropeptides to be purified for use as substrates in enzymatic assays or for structural analyses were further purified using HPLC and a trifluoracetic acid/acetonitrile buffer system32. The structures of purified muropeptides were verified using amino acid analysis52 and mass spectrometry53 as previously described. Muropeptides (250 pMoles) were incubated with gp13 (100 pMoles) in TS (20 mM Tris, pH 7.4, 150 mM NaCl) buffer for 3 hours at 37C and the reaction was halted by acidification with phosphoric acid added to 5% (v/v). Chromatograms were analyzed using PowerChrom (eDaq, Colorado Springs CO). The raw peak areas were converted to molar equivalence to p3 using calculated extinction coefficients54 for muropeptides (p21, p3, p7) and then normalized to mole fraction of total area per run.
Amino acid analysis
Purified putative p7 muropeptide was either modified by fluorodinitrobenzene (FDNB) or left untreated. Samples in 0.1 M NaHCO3 were incubated with 16 mM FDNB for 16 hrs at room temperature in the dark. Both samples were then dried, acid hydrolyzed, and subjected to amino acid analysis as previously described52.
Computational modeling
All modeling and atomic distance measurement was done with the X-ray crystallographic structure of gp1330 using Maestro (Schrödinger, LLC, New York, NY, 2007). The fragment model of the Bacillus subtilis cross-link was built and energy minimized using the OPLS 2005 force field. The docking of the modeled fragment was carried out using the well validated55 and widely used Glide (Schrödinger, LLC, New York, NY, 2007) with default extra precision parameters. The Zn2+ ion, His247 and His278 side chains were defined as potential hydrogen bond donor/acceptor constraints for the docking.
Spectroscopy
Circular dichroism spectra of gp13 and the mutant proteins D195A and D195C (0.1 mg/mL) was conducted from 195 nm to 260 nm at a data pitch of 0.1 nm, acquiring 100 nm/min, with 4 sec response and 1 nm bandwidth using a 0.1 cm cuvette in a Jasco 815. Five measurements were accumulated per sample. Purified proteins were dialyzed against 10 mM potassium phosphate, pH 7.4, buffer using 10 kDa microdialysis cups (Pierce). Protein concentration was assayed by SDS-PAGE densitometry with BSA standards.
Particle induced X-ray emission (PIXE) was conducted with acetone precipitated protein (400 μg) and filter collected on 1 cm2 nylon membranes (Boehringer Mannheim). Briefly, samples were mixed with −20°C acetone to 80% acetone (v/v) and incubated at −20°C for ten min. Visible precipitates were present. Nylon filters with 80% acetone washed samples and dried for at least 10 hrs at 25C were analyzed using a 1.7-MeV Pelletron accelerator (National Electrostatic Corporation) configured to generate a 4.0-MeV He++ ion beam, at a beam current of ~30 nA in a circular beam 1.5–2 mm in diameter. Sample-emitted X-rays were counted and energy analyzed using a Kevex Si(Li) X-ray detector with 5-mm Be window and energy resolution of 145 eV; a capping Al foil also was used to reduce the photon flux to the detector to prevent pile-up. PIXE spectra were acquired using the Hypra program (Charles Evans and Associates) during doses of 40 μC or 20 μC, summed to a total of 80 μC per sample. The Zn Kα1,2 (8.63 keV) line intensity was used to detect and gauge Zn content.
Sequence Alignment
Sequence alignment of LytM [GI:87160448] and gp13 homologs in φ29 family members (φ29 [GI:137932], GA-1 [GI:12248120] and B103[GI:2285516]) was conducted using Clustal W within Align X of the Vector NTI suite (v.10, Invitrogen, Carlsbad, CA). Lactococcal phage related proteins were identified by an ACLAME56, 57 search using gp13. The proteins include bIL285 endopeptidase [GI:13095734], TP901-1 “ORF47” [GI:13786578], Tuc2009 “Minor structural protein 3″ [GI:13487849], and ul36 “structural protein” [GI:21716123]. The alignments to lactococcal phages were added to the gp13-LytM alignment and displayed using AlignX.
Acknowledgments
We thank Dr. John Lipscomb for critical reading of previous versions of the manuscript and Dr. Matthias Bochtler for helpful discussion. We also thank Jessica McElligott for technical assistance and the University of Minnesota Supercomputing Institute for providing the computational resource for the molecular modeling study and the Characterization Facility (which receives partial support from NSF through the NNIN program) for providing the Ion Beam Analysis resources. This work was supported by NIH grant DE003606 to DLA from the NIDCR and GM056695 to DLP from the NIGMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR, NIGMS or the NIH.
Abbreviations
- aa
Amino acid
- NTD
amino-terminal domain
- CTD
carboxy-terminal domain
- Dpm
diaminopimelic acid
- gp
gene product
- HPLC
high performance liquid chromatography
- NAM
N-acetyl muramic acid
- NAG
N-acetyl glucosamine
- PIXE
particle induced X-ray emission
- PG
peptidoglycan
- SDS-PAGE
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- sus
suppressor-sensitive
Footnotes
AUTHOR CONTRIBUTIONS
DNC, YYS, GDH, DLA, DLP designed the experiments, and DNC, YYS, GDH and DLP performed the experiments. DNC, YYS, GDH, DLA and DLP analyzed the data. YX and MGR provided the gp13 plasmid and the purified N-terminal protein used in this study. DNC, YYS, DLA and DLP wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rossmann MG, Mesyanzhinov VV, Arisaka F, Leiman PG. The bacteriophage T4 DNA injection machine. Curr Opin Struct Biol. 2004;14:171–180. doi: 10.1016/j.sbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Kanamaru S, Ishiwata Y, Suzuki T, Rossmann MG, Arisaka F. Control of bacteriophage T4 tail lysozyme activity during the infection process. J Mol Biol. 2005;346:1013–1020. doi: 10.1016/j.jmb.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 3.Andrews D, Butler JS, Al-Bassam J, Joss L, Winn-Stapley DA, Casjens S, Cingolani G. Bacteriophage P22 tail accessory factor gp26 is a long triple-stranded coiled-coil. J Biol Chem. 2005;280:5929–5933. doi: 10.1074/jbc.C400513200. [DOI] [PubMed] [Google Scholar]
- 4.Meijer WJ, Horcajadas JA, Salas M. φ29 family of phages. Microbiol Mol Biol Rev. 2001;65:261–287. doi: 10.1128/MMBR.65.2.261-287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leiman PG, Battisti AJ, Bowman VD, Stummeyer K, Muhlenhoff M, Gerardy-Schahn R, Scholl D, Molineux IJ. The structures of bacteriophages K1E and K1–5 explain processive degradation of polysaccharide capsules and evolution of new host specificities. J Mol Biol. 2007;371:836–849. doi: 10.1016/j.jmb.2007.05.083. [DOI] [PubMed] [Google Scholar]
- 6.Iwashita S, Kanegasaki S. Smooth specific phage adsorption: endorhamnosidase activity of tail parts of P22. Biochem Biophys Res Commun. 1973;55:403–409. doi: 10.1016/0006-291x(73)91101-7. [DOI] [PubMed] [Google Scholar]
- 7.Rydman PS, Bamford DH. Bacteriophage PRD1 DNA entry uses a viral membrane-associated transglycosylase activity. Mol Microbiol. 2000;37:356–363. doi: 10.1046/j.1365-2958.2000.01996.x. [DOI] [PubMed] [Google Scholar]
- 8.Mosig G, Lin GW, Franklin J, Fan WH. Functional relationships and structural determinants of two bacteriophage T4 lysozymes: a soluble (gene e) and a baseplate-associated (gene 5) protein. New Biol. 1989;1:171–179. [PubMed] [Google Scholar]
- 9.Boulanger P, Jacquot P, Plancon L, Chami M, Engel A, Parquet C, Herbeuval C, Letellier L. Phage T5 Straight Tail Fiber Is a Multifunctional Protein Acting as a Tape Measure and Carrying Fusogenic and Muralytic Activities. J Biol Chem. 2008;283:13556–13564. doi: 10.1074/jbc.M800052200. [DOI] [PubMed] [Google Scholar]
- 10.Moak M, Molineux IJ. Role of the gp16 lytic transglycosylase motif in bacteriophage T7 virions at the initiation of infection. Mol Microbiol. 2000;37:345–355. doi: 10.1046/j.1365-2958.2000.01995.x. [DOI] [PubMed] [Google Scholar]
- 11.Molineux IJ. The T7 group. In: Calendar R, editor. The bacteriophages. 2. Oxford University Press; New York, New York: 2006. pp. 277–301. [Google Scholar]
- 12.Odintsov SG, Sabala I, Marcyjaniak M, Bochtler M. Latent LytM at 1.3Å resolution. J Mol Biol. 2004;335:775–785. doi: 10.1016/j.jmb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Firczuk M, Mucha A, Bochtler M. Crystal structures of active LytM. J Mol Biol. 2005;354:578–590. doi: 10.1016/j.jmb.2005.09.082. [DOI] [PubMed] [Google Scholar]
- 14.Surovtsev VI, Fedorov TV, Borozdina MA. Michaelis-menten kinetics for determining enzymatic activity of lysostaphin. Biochemistry (Mosc) 2004;69:754–756. doi: 10.1023/b:biry.0000040199.64244.b4. [DOI] [PubMed] [Google Scholar]
- 15.Browder HP, Zygmunt WA, Young JR, Tavormina PA. Lysostaphin: enzymatic mode of action. Biochem Biophys Res Commun. 1965;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- 16.Schindler CA, Schuhardt VT. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc Natl Acad Sci U S A. 1964;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn EL, Jones DN, Steinhauer BW, Cox F. In vitro activity of lysostaphin. Antimicrobial Agents Chemother (Bethesda) 1966;6:382–388. [PubMed] [Google Scholar]
- 18.Kline SA, de la Harpe J, Blackburn P. A colorimetric microtiter plate assay for lysostaphin using a hexaglycine substrate. Anal Biochem. 1994;217:329–331. doi: 10.1006/abio.1994.1127. [DOI] [PubMed] [Google Scholar]
- 19.Bochtler M, Odintsov SG, Marcyjaniak M, Sabala I. Similar active sites in lysostaphins and D-Ala-D-Ala metallopeptidases. Protein Sci. 2004;13:854–861. doi: 10.1110/ps.03515704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu JZ, Fujiwara T, Komatsuzawa H, Sugai M, Sakon J. Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J Biol Chem. 2006;281:549–558. doi: 10.1074/jbc.M509691200. [DOI] [PubMed] [Google Scholar]
- 21.Marcyjaniak M, Odintsov SG, Sabala I, Bochtler M. Peptidoglycan amidase MepA is a LAS metallopeptidase. J Biol Chem. 2004;279:43982–43989. doi: 10.1074/jbc.M406735200. [DOI] [PubMed] [Google Scholar]
- 22.Cohen DN, Erickson SE, Xiang Y, Rossmann MG, Anderson DL. Multifunctional roles of a bacteriophage φ29 morphogenetic factor in assembly and infection. J Mol Biol. 2008;378:804–817. doi: 10.1016/j.jmb.2008.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenny JG, McGrath S, Fitzgerald GF, van Sinderen D. Bacteriophage Tuc2009 encodes a tail-associated cell wall-degrading activity. J Bacteriol. 2004;186:3480–3491. doi: 10.1128/JB.186.11.3480-3491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson DL, Reilly BE. Morphogenesis of bacteriophage φ29. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: Biochemistry, physiology, and molecular genetics. 2. American Society for Microbiology; Washington, D.C: 1993. pp. 859–867. [Google Scholar]
- 25.Grimes S, Jardine PJ, Anderson D. Bacteriophage φ29 DNA packaging. Adv Virus Res. 2002;58:255–294. doi: 10.1016/s0065-3527(02)58007-6. [DOI] [PubMed] [Google Scholar]
- 26.Young FE. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967;58:2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young FE, Smith C, Reilly BE. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969;98:1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tosi ME, Reilly BE, Anderson DL. Morphogenesis of bacteriophage φ29 of Bacillus subtilis: cleavage and assembly of the neck appendage protein. J Virol. 1975;16:1282–1295. doi: 10.1128/jvi.16.5.1282-1295.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia JA, Carrascosa JL, Salas M. Assembly of the tail protein of the Bacillus subtilis phage φ29. Virology. 1983;125:18–30. doi: 10.1016/0042-6822(83)90060-0. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Y, Morais MC, Cohen DN, Bowman VD, Anderson DL, Rossmann MG. Crystal and cryoEM structural studies of a cell-wall degrading enzyme in the bacteriophage φ29 tail. Proc Natl Acad Sci U S A. 2008;105:9552–9557. doi: 10.1073/pnas.0803787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atrih A, Bacher G, Allmaier G, Williamson MP, Foster SJ. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J Bacteriol. 1999;181:3956–3966. doi: 10.1128/jb.181.13.3956-3966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popham DL, Helin J, Costello CE, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxa U, Steinbacher S, Miller S, Weintraub A, Huber R, Seckler R. Interactions of phage P22 tails with their cellular receptor, Salmonella O-antigen polysaccharide. Biophys J. 1996;71:2040–2048. doi: 10.1016/S0006-3495(96)79402-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The proteomics protocols handbook. Humana Press; Totowa, NJ: 2005. pp. 571–607. [Google Scholar]
- 35.Bussiere DE, Pratt SD, Katz L, Severin JM, Holzman T, Park CH. The structure of VanX reveals a novel amino-dipeptidase involved in mediating transposon-based vancomycin resistance. Mol Cell. 1998;2:75–84. doi: 10.1016/s1097-2765(00)80115-x. [DOI] [PubMed] [Google Scholar]
- 36.Trzaskowski B, Adamowicz L, Deymier P. A theoretical study of zinc(II) interactions with amino acid models and peptide fragments. J of Biol Inorg Chem. 2008;13:133–137. doi: 10.1007/s00775-007-0306-y. [DOI] [PubMed] [Google Scholar]
- 37.Firczuk M, Bochtler M. Mutational analysis of peptidoglycan amidase MepA. Biochemistry (N Y ) 2007;46:120–128. doi: 10.1021/bi0613776. [DOI] [PubMed] [Google Scholar]
- 38.Hagen EW, Reilly BE, Tosi ME, Anderson DL. Analysis of gene function of bacteriophage φ29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J Virol. 1976;19:501–517. doi: 10.1128/jvi.19.2.501-517.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai AC, Tran S, Simmonds RS. Functional characterization of domains found within a lytic enzyme produced by Streptococcus equi subsp zooepidemicus. FEMS Microbiol Lett. 2002;215:133–138. doi: 10.1111/j.1574-6968.2002.tb11382.x. [DOI] [PubMed] [Google Scholar]
- 40.Pritchard DG, Dong S, Baker JR, Engler JA. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology. 2004;150:2079–2087. doi: 10.1099/mic.0.27063-0. [DOI] [PubMed] [Google Scholar]
- 41.Navarre WW, Ton-That H, Faull KF, Schneewind O. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage φ11. Identification of a D-alanyl-glycine endopeptidase activity. J Biol Chem. 1999;274:15847–15856. doi: 10.1074/jbc.274.22.15847. [DOI] [PubMed] [Google Scholar]
- 42.Xiang Y, Morais MC, Battisti AJ, Grimes S, Jardine PJ, Anderson DL, Rossmann MG. Structural changes of bacteriophage φ29 upon DNA packaging and release. EMBO J. 2006;25:5229–5239. doi: 10.1038/sj.emboj.7601386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith DE, Tans SJ, Smith SB, Grimes S, Anderson DL, Bustamante C. The bacteriophage φ29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–752. doi: 10.1038/35099581. [DOI] [PubMed] [Google Scholar]
- 44.Rickgauer JP, Fuller DN, Grimes S, Jardine PJ, Anderson DL, Smith DE. Portal motor velocity and capsid pressure during viral DNA packaging in bacteriophage φ29. Biophys J. 2008;94:159–167. doi: 10.1529/biophysj.107.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Huici V, Salas M, Hermoso JM. The push-pull mechanism of bacteriophage φ29 DNA injection. Mol Microbiol. 2004;52:529–540. doi: 10.1111/j.1365-2958.2004.03993.x. [DOI] [PubMed] [Google Scholar]
- 46.Ramadurai L, Lockwood KJ, Nadakavukaren MJ, Jayaswal RK. Characterization of a chromosomally encoded glycylglycine endopeptidase of Staphylococcus aureus. Microbiology. 1999;145( Pt 4):801–808. doi: 10.1099/13500872-145-4-801. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara T, Aoki S, Komatsuzawa H, Nishida T, Ohara M, Suginaka H, Sugai M. Mutation analysis of the histidine residues in the glycylglycine endopeptidase ALE-1. J Bacteriol. 2005;187:480–487. doi: 10.1128/JB.187.2.480-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramadurai L, Jayaswal R. Molecular cloning, sequencing, and expression of lytM, a unique autolytic gene of Staphylococcus aureus. J Bacteriol. 1997;179:3625–3631. doi: 10.1128/jb.179.11.3625-3631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hendrix RW, Smith MC, Burns RN, Ford ME, Hatfull GF. Evolutionary relationships among diverse bacteriophages and prophages: all the world’s a phage. Proc Natl Acad Sci U S A. 1999;96:2192–2197. doi: 10.1073/pnas.96.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman BJ, Broadwater JA, Johnson P, Harper J, Fox BG, Kenealy WR. Lactose fed-batch overexpression of recombinant metalloproteins in Escherichia coli BL21(DE3): Process control yielding high levels of metal-incorporated, soluble protein. Protein Expression and Purification. 1995;6:646–654. doi: 10.1006/prep.1995.1085. [DOI] [PubMed] [Google Scholar]
- 51.McPherson DC, Popham DL. Peptidoglycan synthesis in the absence of class A penicillin-binding proteins in Bacillus subtilis. J Bacteriol. 2003;185:1423–1431. doi: 10.1128/JB.185.4.1423-1431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.González-Castro MJ, López-Hernández J, Simal-Lozano J, Oruña-Concha MJ. Determination of amino acids in green beans by derivatization with phenylisothiocianate and high-performance liquid chromatography with ultraviolet detection. J Chrom Sci. 1997;35:181–185. [Google Scholar]
- 53.Orsburn B, Melville SB, Popham DL. Factors contributing to heat resistance of Clostridium perfringens endospores. Appl Environ Microbiol. 2008;74:3328–3335. doi: 10.1128/AEM.02629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988;172:451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- 55.Kontoyianni M, McClellan LM, Sokol GS. Evaluation of docking performance: comparative data on docking algorithms. J Med Chem. 2004;47:558–565. doi: 10.1021/jm0302997. [DOI] [PubMed] [Google Scholar]
- 56.Leplae R, Hebrant A, Wodak SJ, Toussaint A. ACLAME: A CLAssification of Mobile genetic Elements. Nucleic Acids Res. 2004;32:D45–49. doi: 10.1093/nar/gkh084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toussaint A, Lima-Mendez G, Leplae R. PhiGO, a phage ontology associated with the ACLAME database. Res Microbiol. 2007;158:567–571. doi: 10.1016/j.resmic.2007.05.002. [DOI] [PubMed] [Google Scholar]