Introduction
It is common to see poor drug responses or resistance after repeated treatments of non-targeted chemotherapy in patients with advanced malignancies, but the underlying causes are still not well understood. One rationale for studying cancer-initiating or cancer stem cells is to potentially determine if there is a rare subset of cells responsible for this drug resistance problem and to unravel the molecular mechanisms that govern their existence[1, 2]. This review will provide a critical analysis of current research in pancreatic cancer related to cancer stem cells with a model that may provide a clearer understanding of the process of tumorigenesis and progression using pancreatic ductal adenocarcinoma (PDAC) as an example. A historical background will set the stage for comparing and contrasting recent reports and hopefully offer a better perspective of the cancer stem cell field in the context of PDAC.
Historical Background
Origin of Cancer Stem Cells
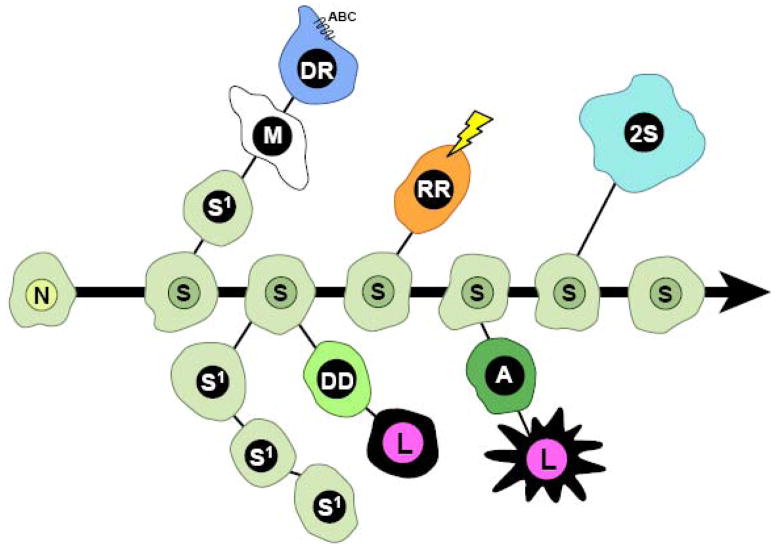
The theory of neoplastic disease, originating from developmentally undifferentiated stem cells, was first proposed by the cytological work of pathologist Julius Cohnheim in 1867[3]. His interpretation of karyotypic chromosomal differences between epithelial and mesenchymal tumors contributed to the characterization and understanding of tumor metastasis, which led to the belief that neoplasia, was a ‘stem cell disorder’. During this era, one of the more prominent proposals came from Boveri’s hypothesis: ‘oncogeny by chromosomal mutation’[4]. The idea favored chromosomal number normalization and tumor evolution through accumulation of precise mutations and selective growth. Boveri’s hypothesis led to the reemergence of the ‘stem-line concept’ as proposed by O. Winge in 1930[5]. Koch’s[6] postulate states that primary tumors are heterogeneous and can be enriched for highly metastatic sublines through in vivo selection, as demonstrated using peritoneal injections of Flexner Joblin rat carcinoma cells that led to highly metastatic ascites tumors. This metastasis model did not reflect the entire process from initiation of tumorigenesis to malignancy, and hence was not widely accepted upon its publication [7]. In the early 1950s Klein and coworkers developed a new model based on serial transplantation in vivo that could give rise to primary tumors and metastasis [8–12]. These studies were the first observations of the heterogeneous nature of tumor cell populations, which suggested stem-like plasticity and multipotency in relation to neoplasia. The establishment of several ‘tumor stem-lines’ allowed researchers to address Boveri’s hypothesis and Winge’s ‘stem-line concept’. The ‘stem-line’ concept postulated that tumors originate from a normal stem cell where karyotypic changes determined the stages of malignancy. This concept was revisited through experimentation by Makino, Levan, and Hauschka [reviewed by Hauschka[13] and Foulds[14]], who used ascites tumors (developed by Klein and Klein) to show that nonmalignant stem cells (NSC) initially contained karyotypic constancy and normality, but subsequently underwent chromosomal alterations caused by environmental factors (i.e., carcinogens, radiation, and toxic compounds)[15] to produce a main stem line (termed S) which was driven by ‘endomitotic reduplication’ of chromosomes[16–19] (Figure 1). This stage was then followed by progression into a secondary stem line S1, which was deemed competent for transition to several cell fates synonymous to stem cell differentiation[20, 21]. Further environmental selective pressures then cause chromosomal re-selection and progression through ‘ascendant’ mechanisms to an invasive state (termed M) resulting in malignancy[22]. The M state maintains plasticity for metastasis or transition to a drug resistant line (DR) after accumulation of specific mutations. The S stem line could also produce a radiation resistant line (RR) by acquiring chromosomal changes caused by severe DNA damage and exhibiting selective growth advantage. Finally, a variant of the S stem line, a 2S polyploid line, retained plasticity for alternative tumor progression that allowed for further evolution of tumors[23](Figure 1).
Figure 1. The Hauschka Model of Tumor Progression through Stem Lines.
Adapted from Hauschka in 1961. [13] N = normal cell, S = main stem-line, S1 =secondary stem line, M = malignant stem cell, DR = multidrug resistant stem cell with upregulated ABC transporter pumps, RR = radiation resistant stem cell, 2S = polyploid stem cell variant, DD = drug- dependent mutant, A = antigenic mutant; and L = mutations lethal for individual tumor cells. A detailed discussion of this model is described in the original publication [13].
‘Minimal Deviation’
Karyotypic characterization of the tumor stem-line concept, using transplantable tumor lines in metastatic ascites tumors, allowed phenotypic identification of changes in the early stages of malignancy. Dunning and Novikoff developed a series of hepatoma cell lines, by providing carcinogens in the food to rats[24–26]. These cells displayed a less transformed phenotype based on karyotypic characterizations. This led to the development of a rat hepatoma line[27], termed ‘Morris hepatoma No. 5123’[28], that was similar to normal regenerative liver cells[29–35]. The No. 5123 transplantable hepatoma cell line had a slow growth rate and low metabolic activity nearly identical to normal liver cells. The heterogeneity of this population indicated both self-renewing (undifferentiated) and differentiated cells similar to normal stem cell phenotypes which led to the term ‘minimum deviation’ to signify an early stage of tumorigenesis and malignancy[36] as stated by the Boveri’s hypothesis: oncogeny by chromosomal mutation. These findings suggested that tumors arising from a common progenitor can give rise to primary tumors that can further progress to malignancy with ‘minimum deviation’ from the parental stem cell of origin, creating a model to study early stage malignancy and cancer stem cell differentiation.
Clonal Evolution of a Tumor
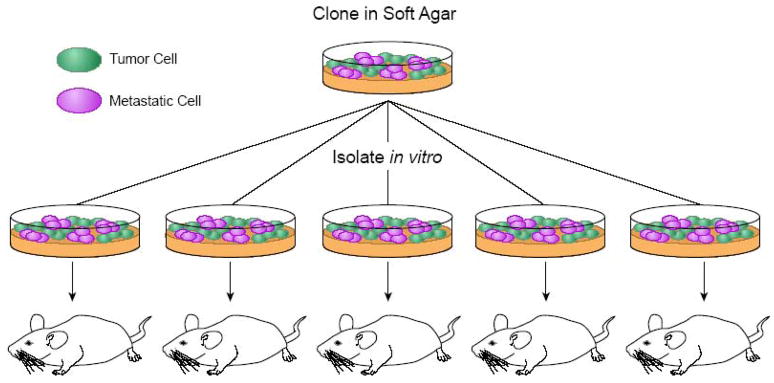
Although prior studies demonstrated tumor heterogeneity, it did not address a ‘rare’ but highly malignant cell within a tumor or whether the cell evolved by in vivo selection caused by microenvironmental differences. In 1967, Nowell’s hypothesis, suggested clonality and stem-line variation within a tumor, either by mitotic errors or environmental selective pressure during malignancy, alluding to a common ‘unicellular origin’[37]. Fidler employed a model of lung tumorigenesis and metastasis using a B16 melanoma cell line to address Nowell’s hypothesis and demonstrated that engraftment was a rare event occurring in about one in a thousand events[38]. This result suggested that tumor formation and metastasis was not only a rare but perhaps, also a random (chance) event. Further experiments, demonstrated that primary cells injected into mice maintained a constant increase of nodules in the lungs that progressively became more malignant compared to the original parental cell line after successful rounds of in vivo selection. From this it was determined that highly metastatic cells did not occur by random stochastic events rather; they reflected a coordinated selective process for invasiveness, lymphocytic recruitment, rapid growth advantage, and increased angiogenic mediators[39–41]. Fidler also set up a series of experiments designed to determine whether the heterogeneity of the primary tumor cell line was due to the preexisting nature of tumorigenesis or whether the cells re-established themselves according to the tumor microenvironment to confer metastatic potential. Fidler postulated that heterogeneity due to the origin of the tumor should give varied tumor incidence, however if heterogeneity occurs due to exogenous influences then the tumor incidence should be equal to that of the line of origin. He selected cells based on colony formation in soft agar and then injected them into mice to compare to the parental cell line after several cycles (Figure 2). These experiments showed that a non-isogenic cell line gave rise to clones with varying degrees of tumorigenicity and metastasis. This validated the idea of metastasis being a coordinated and selective event rather than a random stochastic event[42, 43]. These findings were consistent with Nowell’s hypothesis of heterogeneous tumor stem-lines giving rise to a varied population of sublines by preexisting mechanisms possibly due to mitotic errors or accumulation of precise mutations to the stem cell of origin.
Figure 2. The Fidler Method of Metastatic Clone Isolation.
Adapted from Fidler [42,43]. Tumor cells from a non-isogenic B16 melanoma line are grown in soft agar and clones are picked for mouse injections. After 18 days, lung metastases are isolated and the procedure is repeated to enrich for a highly metastatic cell population. This method was used to determine whether the heterogeneity of the primary tumor cell line was due to the preexisting metastatic cells or whether the cells re-established metastatic cells based on the tumor microenvironment. These studies showed that metastasis was a coordinated and selective event rather than a random stochastic event.
Label Retaining Cells
Stem cell stability, balance of stem cell pools and protection from somatic mutations are some of the reasons that cells may evolve mechanisms for protection from standard chemotherapeutic agents. As such, at least two plausible hypotheses have been proposed; stem cells maintain pools within tissues as required during turnover or tissue damage, and stem cells retain their parental DNA strand during asymmetric division (differentiation) to increase lifespan and protect against DNA replication error prone mutations, historically referred to as the Cairns’ hypothesis[44].
Almost universally, non-targeted chemotherapeutic drugs affect cancer cells based on proliferation compared to normal generally slowly or non-dividing cells. However, if a subset of cancer cells are held in a state of ‘quiescence’ (G0) either by stimulus deprivation or inhibition of cell cycle entry it could potentially diminish drug responses and may eventually permit tumor recurrence after the therapy regimen ends. This does not include resistance, as ‘resistance’ is defined as the genetic tolerance of chemotherapeutics in proliferating cells. A ‘rare’ slow cycling stem cell that stalls in G0 or rarely enters the cell cycle was traditionally identified by pulse-chase experiments with tritiated thymidine (H3-thymidine or 3HTdR)[45]. This is referred to as the ‘label-retaining cell’ (LRC) as has been reported in one case of cancer stem cells[46]. It has been shown that cellular dormant cancer cells are G0-G1 arrested and therefore may explain the occurrence of label retention at the earliest point of tumorigenesis. Tumor dormancy occurs when a primary tumor or disseminated tumor cell has entered G0 and has delayed its ability to exit or reenter the cell cycle. After drug treatment the bulk tumor is often reduced, but a dormant population may survive and exist as minimal residual disease that can reemerge years later by mechanisms that are not well understood. Metastatic events may occur due to activation of dormant disseminated tumors cells at distal organs, and it is this event that may increase the morbidity and mortality associated with advanced malignancies. There are three cellular mechanisms of tumor dormancy: immunosurveillance, angiogenic switching, and cellular dormancy [reviewed by Aguirre-Ghiso[47]]. Cellular dormancy may be the most relevant as this is where primary or disseminated tumor cells are dependent on their microenvironment and held at G0-G1 arrest. An unknown biochemical change in the local environment releases these populations from arrest. The observation of label-retaining cells within a tumor may be explained by cellular dormancy, and its asymmetric transition to transit amplification may depend on the biochemical change in the tumor stem cell niche, yet to be defined, similar to normal stem cells[48, 49]. An understanding of the G0 dormant state of label-retaining cells may allow characterization of why tumor cells evade therapy and potentially provide insights on how to induce dormant cancer cells out of the G0 state and make them vulnerable to conventional anti-proliferative therapeutic agents.
The ‘Side Population’
The demand for therapeutic regenerative medicine fueled the discovery of the ‘side population’ (SP), which was initially used to identify the pluripotent hematopoietic stem cell compartment within the bone marrow[50]. These cells have the potential to repopulate their own ‘self-renewing’ pool and manage hematopoietic stores, or differentiate into lineage specific lymphoid and myeloid cells to replenish the turnover of cells within the immune system. One idea was that these isolated ‘side population’ stem-like cells evolved mechanisms to avoid natural xenobiotic toxicity by expressing ATP-dependent cell surface pumps referred to as ABC (ATP binding cassette) transporters[51, 52]. Since the expression and activity of ABC transporters are elevated in normal stem cells this led to testing if these pumps were able to efflux lipophilic fluorescent DNA intercalating dyes. Thus cells that were able to efflux dyes have been termed the ‘side population’ cells, since they are identified in the fluorescence activated cell sorter as cells that do not retain dye (Figures 3 and 5). Similarly to normal cells, in almost all primary human cancers there are a subset of cells that have either upregulated their transporters or retained the expression of the transporters from the cell of origin based on their ability to efflux dyes. This provided additional evidence that a subset of cancer cells may be similar to side population normal stem cells and these rare cancer cells may be important in the sustained growth of cancer as well as evasion from chemotherapeutic agents. Analysis of SP cells from acute myelogenous leukemia cells (AML) co-labeled with cells carrying normal HSC markers was reported to correlate with poor patient outcome[53, 54]. In the case of chemoresistance, it was shown that several cancers accumulate mutations or genetic changes to increase pump activity, predominantly the MDR-1 transporter and to a lesser degree the MDR-3 pump[52]. Chemotherapeutic drugs such as paclitaxel and vincristine are substrates for these transporters, shedding light on at least one of the underlying mechanisms that may be important in relapse or poor initial drug responses. ABC transporters have become a target for therapeutic development in cancer. In one case, verapamil a calcium channel blocker normally used for hypertension was used to inhibit ABC pumps active on cancer cells, rendering them vulnerable to mitoxantrone (a toposiomerase II inhibitor) toxicity[1]. These findings support the concept that a subset of a heterogeneous population of cancer cells initially contain or have the capacity to acquire a conserved molecular mechanism of survival against xenobiotics or chemotherapeutics agents by expressing dye effluxing ABC transporters. What is much less clear is if the presence of this one marker “SP” is sufficient to be classified as a cancer stem cell.
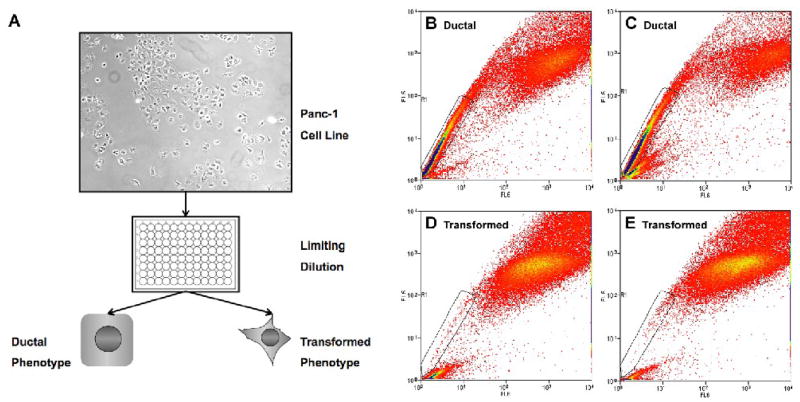
Figure 3. Selection Process to Establish Panc-1 Subclones.
(A) Panc-1 cells are plated by limiting dilution into a 96-well plate and cultured for two weeks at which time clones were selected based on a ductal or transformed phenotype. Characterization of Panc-1 Subclones. Panc-1 clones (B) B10 tumorigenic (ductal), (C) C4 tumorigenic/mildly metastatic (ductal), (D) C5 tumorigenic/highly metastatic (transformed), (E) F9 tumorigenic/highly metastatic (transformed) were analyzed by FACS for their SP content.
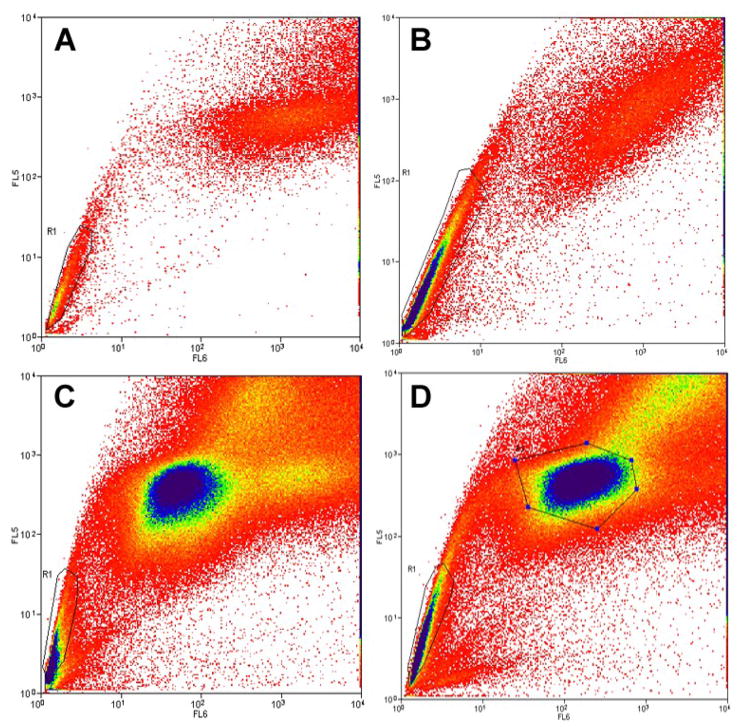
Figure 5. Enrichment of SP by serial sub-culture in vitro.
The ‘side population’ assay. Live cells are stained with Hoechst 33342 dye, gated for live cells, and dual emission wavelengths from the dye are plotted on a log scale. The ‘R1’ gate designates the ‘side population’ (SP). (A) SP fraction from Panc-1 cells make up 10% of the total population, were sorted onto dishes, (B) cultured for one week and reanalyzed, resulting in an increase of the SP fraction to 50%. Identification of SP within isolated MP. Panc-1 cells were sorted for SP and MP fractions, (C) resulting in 10% SP. MP cells from this sort were plated onto dishes. (D) SP analysis of MP the plated MP fraction after sub-culture for four weeks resulted in identification of 5% SP either by residual or by conversion.
Cancer Stem Cells and Tumor Initiators
The observation that a rare cell within tumors can initiate a malignant phenotype is due to a conserved primordial cellular mechanism equivalent to its normal stem cell counterpart is a rediscovered paradigm shift in the cancer field. The concept is that self-renewal is the maintenance of a rare cancer stem cell and the more committed or differentiated cells make up the bulk of a tumor. It is becoming widely believed that the molecular and cellular characterization of these cells may be critical in understanding tumorigenicity and metastatic disease and the elusive nature of cancer stem cells.
The first identification of CSCs was demonstrated by reconstitution of AML in immunodeficient mice using cells isolated based on CD34+/CD38− markers from acute myeloid leukemia (AML) patients. The engraftment demonstrated self-renewal by expansion of CD34+/CD38− and differentiation by asymmetric division to blast cells, eosinophils, and basophils and was referred to as the ‘tumor-initiating cell’. Ensuing reports showed that these purified populations of tumor-initiating cells were pluripotent and could establish a heterogenic hierarchy after engraftment, implying that transformation to leukemia occurs in a more primordial hematopoietic stem cell rather than a more committed or differentiated cell[55]. The next successful demonstration of cancer stem cells was in brain tumors where CD133+ (Prominin 1) expressing cells, referred to as brain tumor stem cells (BTSC), were isolated from patients and assayed in vitro for self-renewal and differentiation. The established method of culture and spheroid formation allowed the identification of self-renewal by expansion of the CD133+ cells and differentiation by asymmetric division to astrocytes, neurons, and oligodendrocytes[56]. Both of these results determined that cells isolated based on specific cell surface markers were able to retain their plasticity from the stem cell of origin. In addition, the rare cells could initiate xenograft tumors with considerably fewer cells compared to cells without the cancer stem cell “markers”. These findings appear to validate ‘Boveri’s hypothesis: ‘oncogeny by chromosomal mutation’ and the concept of ‘minimal deviation’ where genetically altering mutations in brain tumors or genetic fusions (Bcr-Abl) in leukemic cells occur earlier in the stem cells lineage thereby retaining their plasticity, as proposed by Morris and Potter in the 1960’s. A connection between dye effluxing ‘SP’ cells and these cell surface markers have not been clarified.
Pancreatic Stem Cells
There are three major mediators of pancreatic development that determine cell fate of the progenitors from the foregut endoderm. The initial commitment of the endoderm to the premature pancreatic bud involves the loss of sonic hedgehog pathway activation and an increase expression of the homeobox gene PDX-1[57]. Final establishment of a complete organ involves stem cell fates to four major cell types by loss of notch signaling. Each unique cell type increases expression of a protein, which correlates with its differentiation from the stem cell of origin or lineage specificity. Each cell type carries a different marker, for example, the islet cells produce insulin, acinar cells express amylase, ductal cells express DBA (Dolichos Biflorus Agglutinin), and centroacinar cells express HES1 (Hairy and Enhancer of Split I) an indication of stem cell phenotype [58–67]. Although the pancreas has limited regenerative capacity, and there has been no report of primordial cells present, it has been suggested (Stanger et al. 2005) that centroacinar cells are the precursors to acinar and ductal cells [57], and may act as a transition cell prior to complete differentiation. Due to their self-renewal and differentiation capacity, they may be the target cells of origin for PDAC.
Pancreatic Cancer Stem Cells
The idea that a stem-like cancer cell may be the cause of chemoresistance and relapse in patients with pancreatic cancer carries considerable impact in the development of specific and more effective therapeutics. Several reports have made considerable contributions to the understanding of pancreatic cancer and most have alluded to stem-like characteristics either in vitro or in vivo. Cancer stem cells have taken front stage in attempting to understand the mechanism behind the elusive character of a cancer cell and significant efforts have been made to identify these ‘rare’ cells, from primary solid tumor tissues of patients with pancreatic cancer as well as from pancreatic tumor cell lines.
Patient Samples
Isolation of a putative pancreatic cancer stem cell was first reported utilizing a series of cell surface markers from human PDAC tissues samples that had tumor-initiating properties in immunodeficient mice[68]. The guidelines used were previously established by identifying CD44+/CD24low/ESA+ breast cancer cells as having tumor-initiating characteristics in mice. However, pancreatic tumor initiators were shown to have a slightly different marker profile. Subcutaneous xenograft injections in the flank produced tumors at a density of 100 CD44+/CD24+/ESA+ cells per injection that phenotypically resembled the human tumor of origin. Several combinations of these markers were tested to verify the maximum tumor-initiating capacity amongst these populations. The researchers also utilized two other models, a peritoneal injection model where cells were injected into the abdomen of the animal, and an orthotopic model where cells were directly injected into the tail portion of the pancreas. The peritoneal model resulted in tumor initiation of CD44+/CD24+/ESA+ cells at a frequency of 500 cells injected, and the orthotopic model initiated tumors at a frequency of 5000 cells injected as compared to CD44−/CD24−/ESA− cells (which did not produce tumors when 5000 cells were introduced). The central question is if this is sufficient evidence for the pancreatic stem cell hypothesis of cancer. Based on Nowell’s hypothesis and Fidler’s work, these variations are justified by clonal selection and not necessarily by their rarity or stemness. In normal stem cells, there may be differences in the rate of cellular division between parental cells and their progeny. However, cell cycle analysis of these distinct populations did not differ in rates of proliferation (e.g. not quiescent) indicating that the so-called ‘progeny’ have not truly differentiated from the tumor-initiating parental cell, but may have altered their cell surface marker profile based on extracellular cues. What remains a mystery is if the cell cycle has not changed between each population, then how can they differ in tumor initiation?
In an attempt to demonstrate that these isolated populations retained their plasticity, peritoneal tumor tissue was stained with differentiation markers such as S100P and stratifin. If the CSCs had retained their ability to differentiate, then the stained peritoneal tumors should have indicated a hierarchy as seen in previous CSC reports. This was not robustly supported in the data presented. The staining appeared to be a preexisting expression of differentiation markers, as compared to the tissue of origin, and may not have been mechanistically related to the loss of CD44/CD24/ESA. Therefore the heterogeneity may be due to the clonal evolution of the tumor as suggested by Fidler and Nowell and not necessarily due to the stem cell of origin. The purpose of these experiments, in general, is to justify the ability of single cells to have the plasticity to initiate a tumor and differentiate into distinct progeny contributing to an observable tumor hierarchy. Based on the data reported, there seems to be no hierarchy, just a correlation of reconstitution.
Finally, quantitative PCR of the developmental signaling molecule, sonic hedgehog (SHH) was shown to be amplified nearly 50-fold in the highly tumorigenic population. What was not shown is whether the data reflected activation or enrichment from sorting. Sonic hedgehog is a soluble extracellular ligand of PTC and therefore dependent on the cells of the tumor microenvironment for secretion. An ideal demonstration of stem cell pathway regulation would have been to quantify PTC/Smo target gene Gli to show bona fide activation. The increase in SHH message can be interpreted as an indication of an autocrine loop, however without Gli expression data one cannot determine whether the pathway is active. While the authors of this article[68] made an attempt to identify a putative pancreatic cancer stem cell, the data presented when examined carefully can have multiple interpretations and not all are consistent with the current views of a cancer stem cell.
Cell Lines
There are three reports of stem-like characteristics within established pancreatic cancer cell lines. The first report uses historical markers, ABCG2+ from leukemia and CD133+ from glioblastoma, to screen a select panel of cells lines for stem-like characteristics[69]. In particular, ABCG2 correlates with the ‘side population’ phenotype in cells isolated from the bone marrow (reviewed in introduction). In this report, it was shown by relative RNA expression that all five pancreatic cancer cell lines contained ABCG2 mRNA. Surface immunostaining of ABCG2 and analysis by FACS resulted in 2–7% positive staining of the total population from each cell line. Actually, detailed analysis of the secondary ‘only’ IgG2b, as well as the negative control cell line SaOS2 FACS profiles indicated a significant background that may have contributed to the positive ABCG2 staining. Thus the 2–7% positive staining result is only slightly above background and possibly insignificant. The authors of this work concluded that none of the pancreatic cancer cell lines contained an SP phenotype by FACS analysis. However this is contrary to our own observations (Figure 5a) as well as those reported by Zhou et al. [70]. Despite failing to detect SP, the report makes the correlation that if a control cell line such as MCF-7 cells contains an SP phenotype with detectable ABCG2 expression then the detection of ABCG2 in pancreatic cancer cells implies an SP phenotype. It is possible that not all fluorescence activated cell sorters can detect the SP phenotype, therefore these findings would have to be confirmed by using a more sensitive instrument before drawing a negative conclusion of these cell lines. Following this, expression of CD133+ mRNA was shown to be moderate in two pancreatic cell lines, PancTu1 and A818-6, of which 2% of the total population stained CD133+ positive by FACS analysis as compared to the positive control cell line SaOS2. This may be significant, as CD133+ has been used for the isolation of brain and colon cancer stem cells. However, further in vivo experiments would have to be conducted to determine any biological significance.
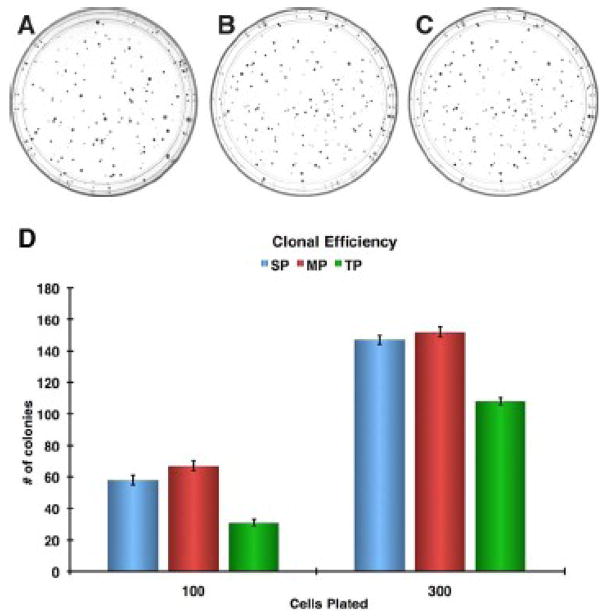
The second report uses spheroid formation to demonstrate stem-like characteristics in vitro[71]. The Panc-1 cell line was used to establish spheroids with serum (SCM) and serum-free medium with supplements commonly used to culture stem cells (SFM). The data indicated that a single cell could generate an adherent spherical cluster that was capable of being continuously propagated in SFM. Although common practice is to show spheroid formation of a three dimensional (3D) non-adherent free floating body, synonymous to embryoid bodies from ES cells, it may be that these two dimensional (2D) structures are representative of clusters rather than spheroids. Since the SP phenotype correlates with stem cells, the clusters were treated with Hoechst 33342 dye to test their dye efflux capacity by ABC transporters. Panc-1 cells grown in SCM contained clusters that were heterogeneous for dye efflux while cells grown in SFM gave rise to colonies that varied in their ability to efflux dye and responses to verapamil. The variation in dye efflux between clusters from the clonal data is consistent with our own findings where cells were plated at clonal density (Figure 3A) and clones were isolated for SP analysis (Figure 3 B–E). Indeed the Panc-1 cell line is heterogeneous for the SP phenotype. In support of this, a reverse cloning experiment, where isolated SP (side population), MP (main population), TP (total population) cells were plated at clonal density showed no clear difference in clonal efficiency (Figure 4). This indicates that each population has similar cloning efficiencies but lacks phenotypic clonal heterogeneity within each purified population (SP, MP, TP). The dye efflux activity in these clusters indicates a conserved trait from the stem cell of origin and can be referred to as ‘minimally deviated’ as suggested by Nowell and Potter 1961[28, 36, 72]. Since there are no phenotypically distinct progeny observed in these clusters, variation in degrees of dye efflux between colonies may be due to the clonal evolution of the tumor similar to the findings of Nowell and Fidler[37, 42] and not necessarily stem-like mechanisms. Additionally, the difference in tumorigenicity was tested between cells grown in serum free and serum containing medium by intraperitoneal injections into nude mice. The serum free grown cells were more tumorigenic, however, this may not be due to the stemness of these cells, but caused by the mitogenic culture conditions containing bFGF and EGF.
Figure 4. Colony Formation Assay of Stem-like Fractions in Panc-1 cells.
The independent growth potential of a single cell from the SP, MP, and TP fractions were tested (A–C), by plating 100 and 300 in triplicate directly from an SP FACS sort and cultured for 14 days, colony counts of SP, MP, and TP fraction at 100 and 300 cells plated per plate (D).
The third report uses appropriate instrumentation to identify a bona fide SP phenotype in the Panc-1 cell line[70]. The population was shown to have ~10% SP that was completely abrogated by verapamil inhibition as well as an observable dye efflux capacity in 2D culture by microscopy. However the authors did not report any biological function (e.g. increased tumorigenicity) as shown in other cell types such as enrichment of the SP phenotype by serial passage nor did they attempt to identify whether the SP and MP were exclusively pure of each other as shown in our own observations (Figure 5). Real-time gene expression analysis of ABCB1 and ABCG2 mRNA demonstrated a possible 4-fold difference in pump activity between SP and main population (MP) or non-SP cells. Historically, ABC transporters are thought to be evolutionarily conserved pumps for the protection of stem cells against xenobiotics[51]. In most cases they are upregulated in cancers to efflux vinca alkaloid and taxane drugs as a form of resistance. In this article [70] the authors report that the presence of SP confers resistance to gemcitabine, a commonly used chemotherapeutic drug for treatment of PDAC. Treatment of cells with gemcitabine increased the SP fraction 3-fold indicating that the non-SP fraction was being targeted by gemcitabine. This is consistent with previous reports indicating that a slower cycling SP cell would be more resistant to a nucleoside analog[73]. If gemcitabine were acting as a substrate then this would be the first report of a nucleoside analog being pumped by an ABC transporter so further investigation of these findings are needed.
These three reports have considerable descriptive evidence of stem-like behaviors in the Panc-1 pancreatic cancer cell line. The most common feature is the ability to efflux Hoechst 33342 dye by ABC transporters, which implicates ABCG2, reported in hematopoietic stem cells, and ABCB1, upregulated in several cancers, as the exact contributors of the SP phenotype. However, these studies did not report previous observations of an ABC transporter that is highly expressed in the Panc-1 cell line, ABCC1 or MRP-1, which may be the pump that drives the SP phenotype. The reports demonstrate that a verapamil sensitive pump confers resistance to vinca alkaloids and in rhodamine 123 accumulation assays[74–76]. Western blot analysis showed that ABCB1/P-gp is not present at all, but MRP-1 is present. It is for this reason that qPCR analysis of ABC transporters may require copy number quantification rather than relative expression. Further experiments by knockdown of these transporters are required to definitively implicate their activity as related to the SP phenotype.
Metastasis
It is important to not only identify markers of cancer stem cells but also if or how cancer stem cells become metastatic. In an attempt to isolate pancreatic CSCs, Hermann et al. [77], discovered a rare subset of highly tumorigenic and metastatic cells located in the invasive border zone of pancreatic cancer tissues. Using a CSC marker identified in glioblastoma CSCs, CD133+, and CXCR4, a marker of cell migration, a panel of human PDAC tissue samples was stained for co-localization. A rare subset of double positive cells was localized to the invasive front of the tumor. Tumor initiation and serial transplantation experiments using CD133+ and CD133− cells isolated from patient samples indicated that CD133+ cells initiated tumors using an inoculation of 500 cells while CD133− did not give rise to tumors. Unlike brain CSCs, where CD133+ spheroids can be differentiated into multi-lineage progeny, CD133+ pancreatic cancer cells grown in spheroids become enlarged upon exposure to differentiation conditions, but they show no clear morphological change to a different cell type. Further experiments using two cells lines that were selected for high and low degrees of metastasis were sorted based on CD133 and CXCR4. The results indicated that both cell lines contained CD133+ cells and could initiate tumors, but only CD133+/CXCR4+ double positive cells within the metastatic cell line could give rise to tumors that disseminated in the blood stream. This correlated well with metastasis found in patients. Interestingly, when these tumors were stained for the epithelial cell marker, cytokeratin, the CD133+ cells were negative but the CD133− cells stained positive. Coincidentally the loss of cytokeratin may be an indication of epithelial to mesenchymal transition (EMT), which is a hallmark of metastasis. Recently a report demonstrated that when highly tumorigenic cancer cells undergo EMT they begin to express tumor-initiator markers[78]. It is possible that these CD133+ cells have undergone EMT and may explain their tumor initiating and highly metastatic phenotype. Finally, one of the purposes of studying CSCs is their resistance to standard chemotherapy. Cell cycle analysis revealed CD133+ cells were slower dividing than CD133− cells, indicating an effect that confers resistance against a nucleoside analog drug such as gemcitabine.
Finally, a PTEN knockout in the mouse pancreas served as a model of pancreatic ductal adenocarcinoma (PDAC). The investigators observed an expansion of centroacinar cells, increased expression of HES1, and decreased expression of amylase from the surrounding tissue. The expansion of centroacinar cells eventually progressed to PDAC[57]. These findings support the idea that centroacinar cells are the source of stem cells of the acinar and ductal cells and perhaps the precursor cells that are transformed prior to PDAC. However, other cell surface markers or dye effluxing capability was not reported.
Telomerase Therapy in Cancer Stem Cells
Pancreatic adenocarcinoma often presents in its late stages and is almost universally unresponsive to many conventional therapies [79]. In many cases, after attempts to treat the disease, the tumor becomes chemoresistant resulting in the poor prognosis of most pancreatic cancer patients. There have been many studies characterizing the cause of this resistance. One common reason is the upregulation of ABC transporter pumps that rapidly eject vinca alkaloids or taxanes from the cell. The findings that pancreatic cancers becomes resistant to gemcitabine is quite rare, but in the case of nucleoside analogs, which are not substrates for ABC transporters, the cause of resistance may be an increase in Bcl-XL expression that can allow incorporation of normal nucleosides but not toxic analogs[80]. The central question is if a small ‘rare’ subset of stem-like cancer cells retains their transporter activity or anti-apoptotic genes that will eventually dominate the content of the tumor.
There are several reasons why tumors recur, such as poor delivery of chemotherapeutics or rapid efflux of vinca alkaloids and taxanes via ABC transporters. The use of gemcitabine has become the primary treatment for pancreatic cancer when surgical resection is not indicated, however the response has been at best modest[81]. Gemcitabine is a nucleoside analog that is incorporated into DNA in substitution of cytidine, therefore, inducing apoptosis by genomic instability [82]. This drug is more effective than classical drugs because it is not a substrate for ABC transporters, its resistance is rare, which would imply that the cause of recurrence is either due to poor drug delivery or that a quiescent stem-like cell is able to evade treatment.
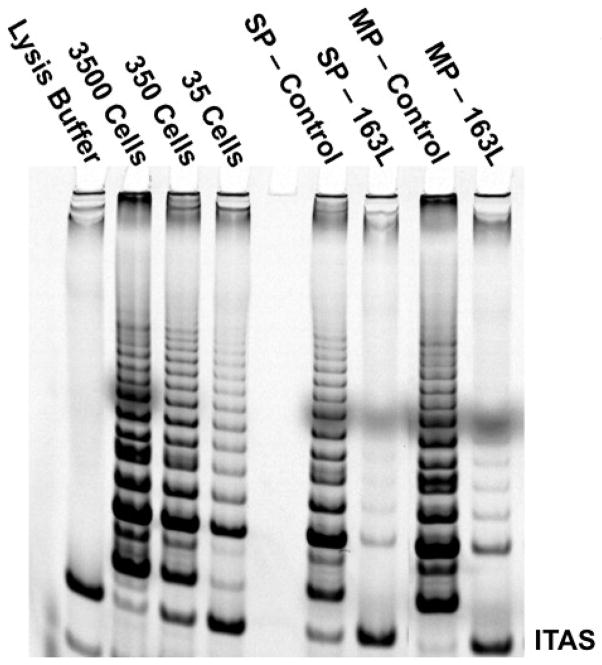
Telomerase (hTERT), a cellular reverse transcriptase, has been shown to be active in greater than 90% of all tumors [83] and has a cell cycle component to its expression [84]. The telomerase holoenzyme is made up of two core components necessary to synthesize telomeric DNA (TTAGGG) to the end of chromosomes, the catalytic protein subunit (hTERT) and the functional or template RNA (hTR or hTERC). Most stem cells have moderate to low telomerase activity and are generally quiescent [85]. It has been shown that telomeres become progressively shorter over time in normal cells that divide (these cells generally are telomerase silent or low expressing). When telomerase is repressed in proliferating cancer cells the cells die and in vivo the tumors regress [86]. The idea that telomerase contributes to the immortality of cancer cells is well established, but it is not as well known if the CSCs also express telomerase activity or if cancer stem cells become quiescent similarly to normal stem cells. If cancer stem cells are quiescent, then telomerase should not be highly expressed. Telomerase has been implicated in most solid tumors of epithelial origin. Several attempts to use standard reverse transcriptase inhibitors have shown they are too toxic to the host using doses required for telomerase inhibition for clinical applications [87–89]. Recent advancements in oligonucleotide chemistry have allowed the design of a competitive telomerase template antagonist (13-mer thio-phosphoamidate backbone conjugated to a palmitoyl (C16)). This compound, GRN163L, has a longer cellular half-life at 37°C and increased potency against their targets over other oligonucleotide backbones and has minimal toxicity to the host [86]. The use of thio-phosphoamidate backbones in oligonucleotides as well as lipidation has proven to be well tolerated in pre-clinical applications [90–93]. The recent development of a telomerase inhibitor using this technology has prompted its application to many cancers in early stage clinical trials [94]. In this case the telomerase oligonucleotide (GRN163L) was not used as an antisense inhibitor to hTERT messenger RNA, but targeted to bind the template region of the functional RNA (hTERC). Thus the GRN163L telomerase inhibitor acts as a competitive telomerase template inhibitor. Panc-1 cells sorted by the SP assay demonstrate robust telomerase activity that is inhibited when treated with GRN163L (Figure 6). The SP fraction had slightly less telomerase activity as shown by the complete retention of internal telomerase activity standard (ITAS) compared to the MP (main population). The reduced activity in the SP population could be explained if the SP are initially cycling slower than the MP compartment. Irrespective, both fractions (SP and MP) expressed robust telomerase activity and were sensitive to the GRN163L treatment as shown by almost complete inhibition of the TRAP ladder. This demonstrates that pancreatic cancer SP cells are not completely quiescent. Thus, a major difference between normal and cancer stem cells may be that cancer stem cells cannot become completely quiescent.
Figure 6. Telomerase Activity in Cancer Stem Cells and Inhibition using GRN163L.
Panc-1 cells were sorted by SP-FACS onto a 6-well culture dish and cultured for three days in the presence or absence of GRN163L drug and assayed for telomerase activity.
Telomerase inhibition is a novel and almost universal tumor target and has been the subject of numerous preclinical studies and recently clinical studies. Since SP cells may not be quiescent but cycle slower than MP cells, this may help explain why SP cells tolerate gemcitabine treatment. The response to GRN163L shows that the mode of action is independent of the rate of cell cycle and validates the use of competitive inhibitors to telomerase for targeting pancreatic putative cancer stem cells. The anti-proliferative effects of gemcitabine have been well characterized and approved for use against pancreatic cancer, but due to enhancement of anti-apoptotic genes the tumor response is very low. The development of a competitive template inhibitor of telomerase provides new approaches to anti-cancer therapy. It may be that the increase of anti-apoptotic genes during gemcitabine treatment are resolved by telomerase inhibition by reduction of the expression of these genes due to ‘uncapping’ of the telomeres (uncapped telomeres create a DNA damage signal), and therefore suggests telomerase as a ‘hinge-pin’ for targeting bulk tumor cells and perhaps cancer stem cells. In a practical sense, reducing the bulk tumor mass with gemcitabine in combination with GRN163L may provide for more durable responses.
Finally, the characterization of the development of the pancreas has revealed two additional targetable stem cell regulatory pathways for differentiation. The first was the hedgehog pathway where loss of hedgehog signaling led to pancreatic bud development [95–97]. Several reports show a reactivation or retention of hedgehog signaling after transformation giving rise to tumors ‘trapped’ in the process of self-renewal [98–100]. Second was the notch pathway where loss of notch signaling gave rise to endocrine and exocrine cells [59–67]. Several reports show a reactivation or retention of notch signaling after transformation to PDAC [101, 102]. If these pathways are intact and responsive, then targeting the mediators within these pathways may be beneficial and in combination with telomerase inhibitors may provide new therapeutic approaches. One approach may be a depletion model where self-renewal driven by an active notch pathway is diminished using a small molecule inhibitor against γ-secretase, the enzyme that generates the notch intracellular domain (NICD) activator [103–105], or by using cyclopamine, a competitive inhibitor of SHH binding to the PTC receptor in the hedgehog pathway [106]. The combination of these inhibitors along with the telomerase inhibitor, GRN163L may prove to be beneficial.
Conclusion
This review attempted to draw parallels with historical findings as compared to recent reports of stem-like characteristics in the pancreatic cancer field. The descriptions of chromosomal aberrations implicating neoplasia as a ‘stem cell disorder’ by Julius Conheim[3] marked the earliest known characterization of the origin of cancer stem cells. However, these observations laid dormant until Boveri’s hypothesis of oncogeny by chromosomal mutations[4] that was further described by O. Winge’s stem line concept[5]. This led to the development of new animal models by Koch[6], Klein and Klein[8–12] that allowed the discoveries of tumor heterogeneity and detailed elucidation of the stem line concept by Makino, Levan and Hauschka[13, 16–21, 23]. Further studies by Dunning and Novikoff led to the understanding of the earliest neoplasia events termed ‘minimal deviation’ to describe the earliest state of the transformed stem cell[24, 25, 72]. Finally, the observations by Fidler and Nowell[37–43, 107] led to the understanding of the clonal evolution of a tumor from a transformed stem cell. These historic findings implicate a primordial adult stem cell as the origin of cancer and provide a solid rationale for more contemporary investigations into the biological mechanisms involved in chemoresistance and tumor relapse.
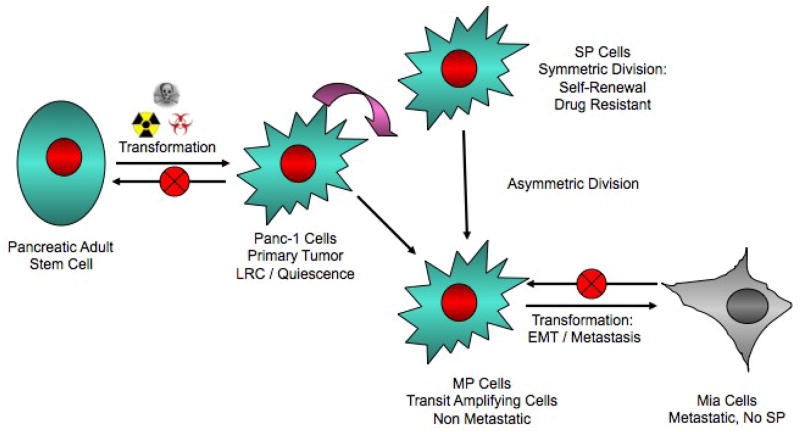
In cells obtained from pancreatic cancer patients, a defined set of markers have been reported by Li and coworkers[68] that can identify a population of cells that are highly tumorigenic, can self-renew, and differentiate synonymously to normal stem cells. However, these studies did not take into account that what is being called ‘self-renewal’ can also be interpreted as an expansion due to overexpression of cyclin D1 and differentiation can be interpreted as an oncogenic-induced expression of differentiation markers. Therefore, these recent experiments mechanistically recapitulate work already reported by Fidler and Nowell but with more detailed markers in identifying tumor-initiators and not necessarily cancer stem cells. Additionally, others have reported that pancreatic cancer cell lines exhibit dye efflux capacity in vitro[69–71]. This could be a marker reflecting the ‘minimal deviation’ retained from the stem cell of origin as similarly shown by Morris[32–34], but these studies did not demonstrate any significant in vivo biological function and therefore the reports remain anecdotal. Another group reported[77] that a highly metastatic cell population expresses cancer stem cell/tumor initiator markers that have lost their epithelial cell marker, cytokeratin, but retain some plasticity similar to stem cells. This report is apparently based on recent reports that cells undergo EMT express cancer stem cell/tumor initiator markers, are highly metastatic, and lose their cytokeratin expression. Finally, a report demonstrated that loss of PTEN specifically in the pancreas induced expansion of the centroacinar cells that eventually progressed to malignancy and was the first evidence that a primordial adult stem cell may be the origin of PDAC [57]. Taking these results along with some of the data presented in this review, we have devised a model that may explain the cell of origin and stem-like plasticity of these cells that gives rise to PDAC (Figure 7).
Figure 7. Pancreatic Cancer Stem Model.
A pancreatic adult stem cell, possibly centroacinar cells, undergoes mutagenic transformation and takes on a state of ‘minimal deviation’, which may have label retaining cell (LRC) properties. This cell can upon appropriate stimulation can either self-renew/symmetrically divide similar to SP drug resistant cells or divide asymmetrically divide giving rise to MP cells. The MP cells makeup the bulk of the tumor and are non-metastatic, but with further transformation/secondary mutations can induce EMT and may clonally evolve the tumor to malignancy.
Pancreatic cancer has proven to be elusive to most chemotherapeutic drugs. In the case of vinca alkaloids and taxanes the ABC transporters are most likely responsible. In the case of nucleoside analogs it may be the inherent slow dividing nature of subset of cancer cells that make them resistant to these drugs. What is not discussed in any of the reports above is the label retaining (LRC) properties of putative cancer stem cells. LRC’s are thought to be slow growing perhaps quiescent cells and thus may be prime candidates for targeting, as they are elusive to most antiproliferative drugs[46]. However, our studies show that telomerase activity (hTERT) is the universal gatekeeper of immortality, and both drug resistant/slow-dividing cells and nonresistant/rapid dividing cells express hTERT and therefore validates telomerase activity inhibition as a universal target for bulk tumor cells as well as putative cancer stem cells. Being that telomerase inhibitors may be implicated in the uncapping of telomeres and inducing a chronic DNA damage response, it is plausible to assume that this targeting strategy may also evade Bcl-XL anti-apoptotic events[80] and therefore have multiple levels of effects.
Based on historic findings and current reports one can evolve a series of guidelines that may be robust for the isolation and study of cancer stem cells as follows. First, markers should be identified to isolate transformed cells that initiate a tumor and retain plasticity synonymous to the primordial adult stem cell of origin (e.g. self-renewal and differentiation) similar to reports of Dick and Dirks[55, 56]. Second, the isolated cells should have a ‘minimally deviated’ phenotype, in terms of karyotype and enzyme function as suggested by Morris and originally proposed by Boveri and Winge. Third, the purified population of cells should have expression of evolutionarily conserved stem cell genes such as ABC transporters and telomerase. Fourth, since tumors cells are not normal stem cells it is not clear if a quiescent population as shown by label retention will be important but it should be assessed. Fifth, these putative cancer stem cells should have intact and responsive developmentally regulated signaling pathways (e.g. sonic hedgehog, Wnt, Notch, TGF) that are potentially targetable. Finally, this population should retain the ability to undergo clonal evolution as suggested by Fidler and Nowell, and further progress through EMT to malignancy. The compliance with of these criteria will lead to an extensive knowledge of putative cancer stem cells that will reveal significant exploitable therapeutic targets.
Acknowledgments
We thank Geron Corporation (Menlo Park, CA) for providing the GRN163L reagent and the NCI SPORE in Gastrointestinal Cancer Grant P50CA12729701 for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 2.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 3.J. Cohnheim, (1867).
- 4.Boveri TH. Frage der Entstehung maligner Tumoren. Jena: Gustav Fischer. 1914 [Google Scholar]
- 5.Winge O. Zytologische Untersuchungen uber die Natur maligner Tumoren II. Teerkarzinome bei Mausen Ztschr Zellforsch u mikroskop Anat. 1930;10:683–735. [Google Scholar]
- 6.Koch FE. Zur der Metastazenbildung bei Impftumoren. Z Krebsforsch. 1939;48:495. [Google Scholar]
- 7.Klein G. Use of the Ehrlich ascites tumor of mice for quantitative studies on the growth and biochemistry of neoplastic cells. Cancer. 1950;3:1052–1061. doi: 10.1002/1097-0142(1950)3:6<1052::aid-cncr2820030616>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Klein G. Conversion of solid into ascites tumours. Nature. 1953;171:398–399. doi: 10.1038/171398a0. [DOI] [PubMed] [Google Scholar]
- 9.Klein G, Klein E. The transformation of a solid transplantable mouse carcinoma into an “ascites tumor”. Cancer Res. 1951;11:466–469. [PubMed] [Google Scholar]
- 10.Klein E, Klein G. Differential survival of solid tumor cells after inoculation into established ascites tumors. Cancer Res. 1954;14:139–144. [PubMed] [Google Scholar]
- 11.Klein E. Gradual transformation of solid into ascites tumors permanent difference between the original and the transformed sublines. Cancer Res. 1954;14:482–485. [PubMed] [Google Scholar]
- 12.Klein G, Revesz L. Quantitative studies on the multiplication of neoplastic cells in vivo. I. Growth curves of the Ehrlich and MC1M ascites tumors. J Natl Cancer Inst. 1953;14:229–277. doi: 10.1093/jnci/14.2.229. [DOI] [PubMed] [Google Scholar]
- 13.Hauschka TS. The chromosomes in ontogeny and oncogeny. Cancer Res. 1961;21:957–974. [PubMed] [Google Scholar]
- 14.Foulds L. The natural history of cancer. Journal of chronic diseases. 1958;8:2–37. doi: 10.1016/0021-9681(58)90039-0. [DOI] [PubMed] [Google Scholar]
- 15.Walpole AL. Initiation and Promotion in Carcinogenesis. Ciba Found Symp on Carcinogenesis. 1959:41–50. [Google Scholar]
- 16.Makino S. Further evidence favoring the concept of the stem cell in ascites tumors of rats. Ann N Y Acad Sci. 1956;63:818–830. doi: 10.1111/j.1749-6632.1956.tb50894.x. [DOI] [PubMed] [Google Scholar]
- 17.Levan A. Chromosome studies on some human tumors and tissues of normal origin, grown in vivo and in vitro at the Sloan-Kettering Institute. Cancer. 1956;9:648–663. doi: 10.1002/1097-0142(195607/08)9:4<648::aid-cncr2820090404>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Levan A. Chromosomes in cancer tissue. Ann N Y Acad Sci. 1956;63:774–792. doi: 10.1111/j.1749-6632.1956.tb50892.x. [DOI] [PubMed] [Google Scholar]
- 19.Levan A, Hauschka TS. Endomitotic reduplication mechanisms in ascites tumors of the mouse. J Natl Cancer Inst. 1953;14:1–43. [PubMed] [Google Scholar]
- 20.Hauschka TS, Levan A. Cytologic and functional characterization of single cell clones isolated from the Krebs-2 and Ehrlich ascites tumors. J Natl Cancer Inst. 1958;21:77–135. [PubMed] [Google Scholar]
- 21.Hauschka TS. Correlation of chromosomal and physiologic changes in tumors. Journal of cellular physiology Supplement. 1958;52:197–233. doi: 10.1002/jcp.1030520411. [DOI] [PubMed] [Google Scholar]
- 22.Schultz J. Malignancy and the genetics of the somatic cell. Ann N Y Acad Sci. 1958;71:994–1008. doi: 10.1111/j.1749-6632.1958.tb46818.x. [DOI] [PubMed] [Google Scholar]
- 23.Makino S. The role of tumor stem-cells in regrowth of the tumor following drastic applications. Acta - Unio Internationalis Contra Cancrum. 1959;15(Suppl 1):196–198. [PubMed] [Google Scholar]
- 24.Dunning WF, Curtis MR, Maun ME. The effect of added dietary tryptophane on the occurrence of 2-acetylaminofluorene-induced liver and bladder cancer in rats. Cancer Res. 1950;10:454–459. [PubMed] [Google Scholar]
- 25.Novikoff AB. A transplantable rat liver tumor induced by 4-dimethylaminoazobenzene. Cancer Res. 1957;17:1010–1027. [PubMed] [Google Scholar]
- 26.Armstrong MI, Gray AE, Ham AW. Cultivation of 4-dimethylaminoazobenzene induced rat liver tumors in yolk sacs of chick embryos. Cancer Res. 1952;12:698–701. [PubMed] [Google Scholar]
- 27.Morris HP, Sidransky H, Wagner BP, Dyer HM. Some characteristics of transplantable rat hepatoma No. 5123 induced by ingestion of N-(2-fluorenyl) phthalamic acid. Cancer Res. 1960;20:1252–1254. [PubMed] [Google Scholar]
- 28.Potter VR, Pitot HC, Ono T, Morris HP. The comparative enzymology and cell origin of rat hepatomas. I. Deoxycytidylate deaminase and thymine degradation. Cancer Res. 1960;20:1255–1261. [PubMed] [Google Scholar]
- 29.Wheeler GP, Alexander JA, Hill DD, Morris HP. Activities of DNA nucleotidyltransferases and other enzymes in cell-free preparations from hepatomas of different growth rates. Cancer Res. 1966;26:2470–2480. [PubMed] [Google Scholar]
- 30.Sugimura T, Ikeda K, Hirota K, Hozumi M, Morris HP. Chemical, enzymatic, and cytochrome assays of microsomal fraction of hepatomas with different growth rates. Cancer Res. 1966;26:1711–1716. [PubMed] [Google Scholar]
- 31.Lea MA, Morris HP, Weber G. Comparative biochemistry of hepatomas. VI. Thymidine incorporation into DNA as a measure of hepatoma growth rate. Cancer Res. 1966;26:465–469. [PubMed] [Google Scholar]
- 32.Morris HP. Studies on the development, biochemistry, and biology of experimental hepatomas. Adv Cancer Res. 1965;9:227–302. doi: 10.1016/s0065-230x(08)60448-0. [DOI] [PubMed] [Google Scholar]
- 33.Morris HP, Wagner BP. The development of “MINIMAL DEVIATION” hepatomas. Acta - Unio Internationalis Contra Cancrum. 1964;20:1364–1366. [PubMed] [Google Scholar]
- 34.Morris HP, Dyer HM, Wagner BP, Miyaji H, Rechcigl M. Some aspects of the development, biology and biochemistry of rat hepatomas of different growth rate. Adv Enzyme Regul. 1964;2:321–333. doi: 10.1016/s0065-2571(64)80023-6. [DOI] [PubMed] [Google Scholar]
- 35.Dyer HM, Gullino PM, Morris HP. Tryptophan pyrrolase activity in transplanted “MINIMAL-DEVIATION” hepatomas. Cancer Res. 1964;24:97–104. [PubMed] [Google Scholar]
- 36.Potter VR. Transplantable animal cancer, the primary standard. Cancer Res. 1961;21:1331–1333. [PubMed] [Google Scholar]
- 37.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 38.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- 39.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–224. [PubMed] [Google Scholar]
- 40.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. European journal of cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- 41.Fidler IJ. Selection of successive tumour lines for metastasis. Nature New Biol. 1973;242:148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- 42.Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38:2651–2660. [PubMed] [Google Scholar]
- 43.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 44.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 45.Lark KG, Consigli RA, Minocha HC. Segregation of sister chromatids in mammalian cells. Science. 1966;154:1202–1205. doi: 10.1126/science.154.3753.1202. [DOI] [PubMed] [Google Scholar]
- 46.Zhang HB, Ren CP, Yang XY, Wang L, Li H, Zhao M, Yang H, Yao KT. Identification of label-retaining cells in nasopharyngeal epithelia and nasopharyngeal carcinoma tissues. Histochem Cell Biol. 2007;127:347–354. doi: 10.1007/s00418-006-0251-9. [DOI] [PubMed] [Google Scholar]
- 47.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talpaz M, Estrov Z, Kantarjian H, Ku S, Foteh A, Kurzrock R. Persistence of dormant leukemic progenitors during interferon-induced remission in chronic myelogenous leukemia. Analysis by polymerase chain reaction of individual colonies. J Clin Invest. 1994;94:1383–1389. doi: 10.1172/JCI117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 52.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 53.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, Andreeff M, Goodell MA. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 54.Feuring-Buske M, Hogge DE. Hoechst 33342 efflux identifies a subpopulation of cytogenetically normal CD34(+)CD38(−) progenitor cells from patients with acute myeloid leukemia. Blood. 2001;97:3882–3889. doi: 10.1182/blood.v97.12.3882. [DOI] [PubMed] [Google Scholar]
- 55.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 56.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 57.Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, Depinho RA, Wu H, Melton DA, Dor Y. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 59.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 60.Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 61.Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15–25. doi: 10.1016/j.mod.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 63.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 64.Kadesch T. Notch signaling: the demise of elegant simplicity. Curr Opin Genet Dev. 2004;14:506–512. doi: 10.1016/j.gde.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 66.Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- 67.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 69.Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. HBPD INT. 2007;6:92–97. [PubMed] [Google Scholar]
- 70.Zhou J, Wang CY, Liu T, Wu B, Zhou F, Xiong JX, Wu HS, Tao J, Zhao G, Yang M, Gou SM. Persistence of side population cells with high drug efflux capacity in pancreatic cancer. World J Gastroenterol. 2008;14:925–930. doi: 10.3748/wjg.14.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gou S, Liu T, Wang C, Yin T, Li K, Yang M, Zhou J. Establishment of clonal colony-forming assay for propagation of pancreatic cancer cells with stem cell properties. Pancreas. 2007;34:429–435. doi: 10.1097/MPA.0b013e318033f9f4. [DOI] [PubMed] [Google Scholar]
- 72.Nowell PC, Morris HP, Potter VR. Chromosomes of “minimal deviation” hepatomas and some other transplantable rat tumors. Cancer Res. 1967;27:1565–1579. [PubMed] [Google Scholar]
- 73.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen H, Zhang S, Morris ME. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. Journal of pharmaceutical sciences. 2003;92:250–257. doi: 10.1002/jps.10283. [DOI] [PubMed] [Google Scholar]
- 75.Tseng E, Kamath A, Morris ME. Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pharm Res. 2002;19:1509–1515. doi: 10.1023/a:1020460700877. [DOI] [PubMed] [Google Scholar]
- 76.Miller DW, Fontain M, Kolar C, Lawson T. The expression of multidrug resistance-associated protein (MRP) in pancreatic adenocarcinoma cell lines. Cancer Lett. 1996;107:301–306. doi: 10.1016/0304-3835(96)04384-4. [DOI] [PubMed] [Google Scholar]
- 77.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maitra A, Hruban RH. Pancreatic Cancer. Annu Rev Pathol. 2007 doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354–362. doi: 10.1159/000065068. [DOI] [PubMed] [Google Scholar]
- 81.Mercalli A, Sordi V, Formicola R, Dandrea M, Beghelli S, Scarpa A, Di Carlo V, Reni M, Piemonti L. A preclinical evaluation of pemetrexed and irinotecan combination as second-line chemotherapy in pancreatic cancer. Br J Cancer. 2007;96:1358–1367. doi: 10.1038/sj.bjc.6603726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kornmann M, Butzer U, Blatter J, Beger HG, Link KH. Pre-clinical evaluation of the activity of gemcitabine as a basis for regional chemotherapy of pancreatic and colorectal cancer. Eur J Surg Oncol. 2000;26:583–587. doi: 10.1053/ejso.2000.0951. [DOI] [PubMed] [Google Scholar]
- 83.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 84.Zhu X, Kumar R, Mandal M, Sharma N, Sharma HW, Dhingra U, Sokoloski JA, Hsiao R, Narayanan R. Cell cycle-dependent modulation of telomerase activity in tumor cells. Proc Natl Acad Sci USA. 1996;93:6091–6095. doi: 10.1073/pnas.93.12.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol. 2006;18:254–260. doi: 10.1016/j.ceb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Gellert GC, Dikmen ZG, Wright WE, Gryaznov S, Shay JW. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast Cancer Res Treat. 2006;96:73–81. doi: 10.1007/s10549-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 87.Hayakawa N, Nozawa K, Ogawa A, Kato N, Yoshida K, Akamatsu K, Tsuchiya M, Nagasaka A, Yoshida S. Isothiazolone derivatives selectively inhibit telomerase from human and rat cancer cells in vitro. Biochemistry. 1999;38:11501–11507. doi: 10.1021/bi982829k. [DOI] [PubMed] [Google Scholar]
- 88.Strahl C, Blackburn EH. The effects of nucleoside analogs on telomerase and telomeres in Tetrahymena. Nucleic Acids Res. 1994;22:893–900. doi: 10.1093/nar/22.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strahl C, Blackburn EH. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol Cell Biol. 1996;16:53–65. doi: 10.1128/mcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akiyama M, Hideshima T, Shammas MA, Hayashi T, Hamasaki M, Tai YT, Richardson P, Gryaznov S, Munshi NC, Anderson KC. Effects of oligonucleotide N3′-->P5′ thio-phosphoramidate (GRN163) targeting telomerase RNA in human multiple myeloma cells. Cancer Res. 2003;63:6187–6194. [PubMed] [Google Scholar]
- 91.Herbert BS, Gellert GC, Hochreiter A, Pongracz K, Wright WE, Zielinska D, Chin AC, Harley CB, Shay JW, Gryaznov SM. Lipid modification of GRN163, an N3′-->P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005;24:5262–5268. doi: 10.1038/sj.onc.1208760. [DOI] [PubMed] [Google Scholar]
- 92.Herbert BS, Pongracz K, Shay JW, Gryaznov SM, Shea-Herbert B. Oligonucleotide N3′-->P5′ phosphoramidates as efficient telomerase inhibitors. Oncogene. 2002;21:638–642. doi: 10.1038/sj.onc.1205064. [DOI] [PubMed] [Google Scholar]
- 93.Pruzan R, Pongracz K, Gietzen K, Wallweber G, Gryaznov S. Allosteric inhibitors of telomerase: oligonucleotide N3′-->P5′ phosphoramidates. Nucleic Acids Res. 2002;30:559–568. doi: 10.1093/nar/30.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, Wright WE, Shay JW. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65:7866–7873. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- 95.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 97.Hebrok M. Hedgehog signaling in pancreas development. Mech Dev. 2003;120:45–57. doi: 10.1016/s0925-4773(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 98.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 99.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 100.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 102.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 103.Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, Foreman KE. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi’s sarcoma tumor cells. Oncogene. 2005;24:6333–6344. doi: 10.1038/sj.onc.1208783. [DOI] [PubMed] [Google Scholar]
- 104.van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends in molecular medicine. 2005;11:496–502. doi: 10.1016/j.molmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 105.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 106.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 107.Nicolson GL, Brunson KW, Fidler IJ. Specificity of arrest, survival, and growth of selected metastatic variant cell lines. Cancer Res. 1978;38:4105–4111. [PubMed] [Google Scholar]