Abstract
The current study was designed to explore the use of behavioral (i.e., accuracy and reaction times) and electrophysiological measures (i.e., event-related potentials) to assess the impact of a family-based preventive intervention for preschool-aged, maltreated children in foster care. These measures were recorded during a computerized flanker task designed to assess cognitive control and response monitoring. The sample was recruited from a larger randomized efficacy trial of Multidimensional Treatment Foster Care for Preschoolers (MTFC-P) and included foster children assigned to the intervention condition (n = 10), foster children assigned to a services-as-usual comparison condition (n = 13), and low-income, nonmaltreated community children (n = 11). The children’s behavioral and electrophysiological performance on the task was generally consistent with previous research with adults and older children. There were no group differences on the behavioral measures of cognitive control or response monitoring. Notably, however, group differences were observed on the electrophysiological measures of response monitoring. Specifically, the foster children who received services as usual were significantly less responsive to performance feedback about errors than the foster children who received the intervention and the nonmaltreated children. Applications of this methodology and implications of the results for future prevention research are discussed.
Keywords: foster care, intervention, event-related potentials, cognitive control, response monitoring
Young maltreated children in foster care display numerous disparities in cognitive, academic, and psychosocial functioning compared to their nonmaltreated peers (Clausen, Landsverk, Ganger, Chadwick, & Litrownik, 1998; Pears & Fisher, 2005a; Zima et al., 2000). Rates of disruptive behavior and attentional problems are particularly high (Clausen et al., 1998; Pilowsky, 1995). For many foster children, these difficulties appear to stem from early adverse experiences, including neglectful and/or abusive care and repeated caregiver disruptions. There is increasing acknowledgement that the prevention of negative outcomes among foster children and other at-risk populations might be facilitated by understanding the impact of early adverse experiences on the development of important neurobiological systems (Fishbein, 2000; Gunnar, Fisher, & the Early Experience, Stress, and Prevention Network, 2006). Characterizing foster children’s difficulties in terms of alterations in these neurobiological systems might allow for more precise explanatory models and might identify targets for interventions. Moreover, neurobiological measures collected within the context of randomized efficacy trials can serve as indicators of intervention effectiveness (e.g., Brotman et al., 2007; Fisher, Stoolmiller, Gunnar, & Burraston, 2007).
Cognitive control, the regulation of information processing to satisfy task demands, and response monitoring, the evaluation of information processing in satisfying task demands, have been viewed as complementary cognitive processes that are essential to adaptive functioning (Cohen, Aston-Jones, & Gilzenrat, 2004; van Meel, Oosterlaan, Heslenfeld, & Sergeant, 2005; van Veen & Carter, 2002). These cognitive processes are involved in aligning cognitive strategies and behaviors with desired outcomes. It is believed that the prefrontal cortex (PFC) is responsible for the allocation of cognitive control and that the anterior cingulate cortex (ACC) serves a critical role in response monitoring and the recruitment of additional control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Cohen et al., 2004; van Veen & Carter, 2002). Anatomical, electrophysiological, and behavioral evidence suggests that the PFC and ACC continue to mature into adulthood (Davies, Segalowitz, & Gavin, 2004; Greenough, Black, & Wallace, 1987; Welsh, Pennington, & Groisser, 1991). Additionally, the functional connectivity (or coherence) of these brain regions demonstrates maturational changes across childhood (Barry et al., 2004; Thatcher, Walker, & Giudice, 1987). Because of the protracted developmental course of these brain regions and their connections, it has been speculated that these regions might be influenced by early adverse experiences (Black, 1998; De Bellis, 2001).
Although there has been limited research examining the neurobiological bases of foster children’s difficulties, there is indirect evidence to suggest that deficits in cognitive control and response monitoring play a role. First, specific aspects of cognitive control, such as selective attention and inhibitory control, seem to be negatively affected by early adverse experiences, including maltreatment and repeated caregiver disruptions (Beers & De Bellis, 2002; Lewis, Dozier, Ackerman, & Sepulveda, 2007). Similarly, research with children and adolescents has suggested that the PFC and ACC are impacted by early adverse experiences (Carrion et al., 2001; De Bellis, Keshavan, Spencer, & Hall, 2000). Second, impairments in cognitive control and response monitoring have been implicated in conduct disorder and attention deficit/hyperactivity disorder (ADHD; Casey et al., 1997; Toupin, Déry, Pauzé, Mercier, & Fortin, 2000; van Meel et al., 2005). Impaired functional connectivity among frontal regions of the brain has also been observed among children with ADHD (Murias, Swanson, & Srinivasan, 2007).
In the current study, a computer-administered flanker task (Eriksen & Eriksen, 1974) was employed to assess cognitive control and response monitoring. This task, which requires participants to selectively attend and respond to target stimuli in the presence of interfering stimuli, has a number of strengths. First, it is suited for young children because it requires only minimal language abilities and allows for trial-by-trial performance feedback (McDermott, Perez-Edgar, & Fox, 2007). Second, behavioral measures and electrophysiological measures of brain activity can be examined. Prior research has shown that these measures can provide divergent information about group differences (Harter, Anllo-Vento, Wood, & Schroeder, 1988; Karayanidis et al., 2000; Moser, Hajcak, & Simons, 2005). For example, Karayanidis and colleagues found that, although behavioral measures did not distinguish the children with ADHD from the comparison children, electrophysiological measures differentiated the two groups. Third, the neural substrates of this task are well understood; specifically, in neuroimaging studies with adults, the ventrolateral PFC and ACC have been shown to be activated during this task (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Hazeltine, Poldrack, & Gabrieli, 2000).
Similar patterns of performance on behavioral measures of cognitive control and response monitoring have been observed in adults and older children on the flanker task. Although adults are more accurate and faster than children, both groups demonstrate greater difficulty on trials with highly interfering stimuli (i.e., incongruent trials) than on trials with minimally interfering stimuli (i.e., congruent trials). This index of cognitive control, referred to as the “flanker interference effect,” is characterized by a higher error rate and slower reaction time on incongruent trials than on congruent trials (Enns & Akhtar, 1989; Ridderinkhof, van der Molen, Band, & Bashore, 1997). Adults and children also tend to respond more slowly to trials after incorrect responses than to trials after correct responses (Davies et al., 2004; Luu, Collins, & Tucker, 2000). This pattern of behavior, known as the “response monitoring effect,” has been viewed as a compensatory strategy for improving performance on upcoming trials.
In the current study, electrophysiological measures of response monitoring were evaluated using event-related potentials (ERPs). An ERP is a transient voltage fluctuation that is generated by large populations of localized neurons and that is time-locked to a discrete event such as the participant’s response. In contrast to neuroimaging data, ERP data have excellent temporal resolution (in milliseconds) and provide information about the temporal sequence of cognitive processes. Studies using the flanker task with adults and older children have identified two response-locked ERP components: the error-related negativity (ERN) and the error-positivity (Pe; Davies et al., 2004; van Veen & Carter, 2002; Yeung, Botvinick, & Cohen, 2004). The ERN is characterized as a frontocentral negative deflection occurring approximately 50–150 ms after an incorrect response and is believed to reflect initial response monitoring (Davies et al., 2004; van Veen & Carter, 2002; Yeung et al., 2004). Source localization has indicated that the ERN is most likely generated in the caudal ACC (Dehaene, Posner, & Tucker, 1994; van Veen & Carter, 2002). The Pe, a positive deflection that is maximal at parietal sites, follows the ERN temporally (van Veen & Carter, 2002). It appears to reflect additional response monitoring, specifically subjective error evaluation, and to be generated in the rostral ACC and superior parietal cortex (Herrmann, Rommler, Ehlis, Heidrich, & Fallgatter, 2004; van Veen & Carter, 2002).
An additional aspect of response monitoring can be examined during the flanker task through the inclusion of trial-by-trial performance feedback. Feedback of this nature provides an opportunity to explore participants’ responses to external information about their performance and to examine a feedback-locked ERP component called the feedback-related negativity (FRN). The FRN, which is believed to be generated in the ACC, is a frontocentral negative deflection peaking approximately 300 ms after negative feedback (Luu, Tucker, Derryberry, Reed, & Poulsen, 2003; van Meel et al., 2005). The FRN is hypothesized to reflect the activity of a large neural system of error detection that encompasses response monitoring and motivation.
In the current study, behavioral and electrophysiological performance on the flanker task was examined for three groups: foster children assigned to an intervention condition, foster children assigned to a services-as-usual comparison condition, and low-income, nonmaltreated children. In conducting this study, we had two objectives. The first objective was to examine patterns of behavioral and electrophysiological performance in young children. Consistent with prior research with adults and older children, the children were expected to display the flanker interference effect and response monitoring effect on behavioral measures and to demonstrate defined ERP components, including the ERN, the Pe, and the FRN. The second objective was to conduct a preliminary investigation of the effects of early adverse experiences and a subsequent intervention on the children’s behavioral and electrophysiological performance. As is noted above, cognitive control and response monitoring appear to be adversely affected by early adverse experiences. However, although the plasticity of these cognitive processes following exposure to early adverse experiences has not been investigated, we hypothesized that the intervention, which was designed to increase foster children’s regulatory abilities by training foster parents to provide contingent feedback, would positively impact the children’s cognitive control and response monitoring as reflected by their behavioral and electrophysiological performance. Thus, although both groups of foster children were expected to perform more poorly than the nonmaltreated children, the foster children who received the intervention were expected to perform more similarly to the nonmaltreated children than the foster children who received services as usual on the behavioral and electrophysiological measures.
Method
Participants
The sample included 46 children (age range = 4.87–6.99 years, M = 5.94 years, SD = 0.68 years; 25 males) drawn from a larger randomized efficacy trial of a preventive intervention for foster children. (In contrast to efficacy trials, studies involving electrophysiological measures tend to have smaller sample sizes [e.g., Karayanidis et al., 2000; Moser et al., 2005].) The efficacy trial included three groups of children: foster children who received the intervention (Multidimensional Treatment Foster Care for Preschoolers [MTFC-P] group), foster children who received services as usual (regular foster care [RFC] group), and low-income, nonmaltreated children who lived with their biological parents (community comparison [CC] group). The children were selected from the efficacy trial because they were within the targeted age range. From the sample of 46 children, 5 children (2 MTFC-P, 2 RFC, and 1 CC) were excluded because of poor behavioral performance, 3 children (2 MTFC-P and 1 CC) were excluded because of technical issues during collection of the electroencephalogram (EEG) data, and 4 children (2 MTFC-P, 1 RFC, and 1 CC) were excluded because of excessive artifact in the EEG data or an inadequate number of ERP trials for certain trial types. The resulting analytical sample was 34 children (10 MTFC-P, 13 RFC, and 11 CC). This sample did not significantly differ from the efficacy trial sample on sex or age, Pearson χ2(1, N = 177) = 0.23, ns, Cramér’s V = 0.04, and F(1, 175) = 0.14, ns, η2 = 0.00, respectively. Additionally, these two samples did not differ in terms of foster care placement history, including age at first entry into foster care, length of time in foster care, and number of transitions in care, F(1, 115) = 0.01, ns, η2 = 0.00, F(1, 115) = 0.15, ns, η2 = 0.00, and F(1, 115) = 0.01, ns, η2 = 0.00, respectively. Thus, the analytic sample appeared to be representative of the efficacy trial sample.
Demographic and foster care placement information for the analytic sample is presented in Table 1. The three groups did not differ in terms of sex or age, Pearson χ2(2, N = 34) = 0.57, ns, Cramér’s V = 0.13, and F(2, 31) = 0.15, ns, η2 = 0.01, respectively. Also, there were no significant differences between the MTFC-P and RFC groups on age at first entry into foster care, length of time in foster care, or number of transitions in care, F(1, 21) = 0.06, ns, η2 = 0.00, F(1, 21) = 1.28, ns, η2 = 0.06, and F(1, 21) = 0.22, ns, η2 = 0.01, respectively. Although the children in the current study had been in the efficacy trial for various lengths of time, this length of time did not differ across the three groups, F(2, 31) = 0.17, ns, η2 = 0.01. At the time of the current study, 4 children (1 MTFC-P and 3 RFC) had been reunified with their biological parents and 2 children (1 MTFC-P and 1 RFC) had been adopted.
Table 1.
Basic Demographic and Foster Care Placement Information
| MTFC-P (n= 10) | RFC (n= 13) | CC (n= 11) | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age (years) | 6.08 | 0.57 | 5.92 | 0.68 | 5.99 | 0.76 |
| Age at first entry into foster care (years) | 3.48 | 1.69 | 3.33 | 1.26 | ||
| Length of time in foster care (months) | 17.73 | 7.72 | 23.41 | 14.30 | ||
| Number of transitions in care | 4.70 | 3.20 | 5.31 | 3.04 | ||
| Length of time since entry into efficacy trial (months) | 16.78 | 7.19 | 18.42 | 12.78 | 19.50 | 10.51 |
Note. Number of males per group: MTFC-P = 6, RFC = 6, and CC = 5.
Intervention
The MTFC-P intervention is designed to reduce behavioral difficulties and increase regulatory abilities through the provision of a consistent, contingent environment (Fisher, Ellis, & Chamberlain, 1999). MTFC-P is delivered via a multidisciplinary team (i.e., foster parents, foster parent consultants, behavioral specialists, and family therapists). Prior to placement, the foster parents are trained to provide high rates of reinforcement for positive behaviors and effective consequences for negative behaviors. After placement, the foster parents are given extensive support through 24-hr crisis intervention as needed, daily telephone contact, and weekly support groups. The children receive services from behavioral specialists in their homes and preschools and attend weekly therapeutic playgroup sessions that address developmental, behavioral, and social issues. The family therapists provide the services necessary for successful reunifications with biological parents or successful transitions to adoptive families or other long-term placements. MTFC-P has been shown to reduce the risk of disruption in subsequent permanent placements and increase secure attachment-related behaviors (Fisher, Burraston, & Pears, 2005; Fisher & Kim, 2007).
Flanker Task
The flanker task employed in the current study includes red and green circles as stimuli and trial-by-trial performance feedback. It has been used previously with young children (McDermott et al., 2007). Throughout the task, a small fixation point is displayed in the center of the computer screen. For each trial, a warning cue is presented for 200 ms before a horizontal row of five 1-in. circles, with the central circle directly above the fixation point, is shown for 700 ms. The task comprises congruent trials, which consist of five red circles or five green circles, and incongruent trials, which consist of a central red circle flanked by green circles or a central green circle flanked by red circles. A 30:70 ratio (congruent trials:incongruent trials) is used. Participants are required to respond within 1100 ms. Performance feedback, which consists of a 1-in. face, is then presented for 1050 ms; a smiling face indicates a correct response and a frowning face indicates an incorrect response. The intertrial interval varies in length from 0 to 500 ms. The 20-min task consists of three blocks of 60 trials each.
In the current study, the STIM stimulus presentation system (James Long Company, Caroga Lake, NY) was used to control the task presentation and to record the behavioral and electrophysiological measures for each trial. The children sat approximately 24 in. from a 14-in. computer monitor and held a button box with a red pushbutton and a green pushbutton. Prior to beginning the task, color vision, color familiarity, and comprehension of task terminology were assessed. The children were instructed to press the button that corresponded with the color of the central circle regardless of the color of the flanking circles. They were told to respond quickly and correctly. The children completed eight practice trials to ensure task comprehension.
Electrophysiological Recording and Analysis
Prior to collecting EEG data, a calibration file was collected by running a 50-μV, 10-Hz calibration signal through all channels. The EEG signals were recorded using a Lycra cap fitted with tin electrodes in accordance with the International 10–20 System (Jasper, 1958). The recordings were taken from the mastoids (M1 and M2), three midline sites (Fz, Cz, and Pz), two frontal sites (F3 and F4), and one anterior frontal site (Afz), with C4 serving as the ground. All EEG recordings were referenced to Cz. One channel of electrooculogram was recorded with two Beckman mini-electrodes placed vertically above and below the left eye. The electrode impedances were tested to ensure that each site had an impedance reading of 10 KΩ or less.
The EEG signals were amplified through SA Instrumentation Bioamps (bandpass = 0.1–100 Hz) digitized at 512 Hz with an IOtech Daqbook A/D converter and were re-referenced using average mastoid configuration. The signals were artifact scored using the ERP Analysis System (James Long Company, Caroga Lake, NY). Epochs containing signals +/− 200 μV were excluded from analyses, and artifact due to eye movement was regressed using the ERP Analysis System. Trials with reaction times of less than 200 ms or with errors of omission were excluded from analyses. The EEG data were then time-locked to the appropriate event and were quantified separately for correct and incorrect trials. The children were required to have at least 10 artifact-free trials for each trial type. All data channels were baseline corrected using the appropriate time window. The EEG data were then digitally refiltered with a 15-Hz low-pass filter.
The ERN and the Pe were time-locked to the response and were corrected using a baseline window of −600 to −400 ms relative to the response. The ERN was identified as the maximum negative peak at −20 to 120 ms, and the Pe was identified as the maximum positive peak at 50 to 175 ms relative to the response. In addition to the FRN, visual inspection of the grand average ERP waveforms revealed two early feedback-locked components: the N1 and the P2. The N1, the P2, and the FRN were corrected using a baseline window of −1850 to −1650 ms relative to the feedback. The N1 was identified as the maximum negative peak at 50 to 150 ms, the P2 was identified as the maximum positive peak at 160 to 260 ms, and the FRN was identified as the maximum negative peak at 280 to 480 ms relative to the feedback.
Statistical Analyses
Because of the sample size, it was important to examine the behavioral and electrophysiological measures for extreme values (i.e., values more than 3 standard deviations above or below the mean) and to ensure that these extreme values did not have undue influence on the results. There were no extreme values observed for the behavioral or response-locked ERP measures. However, one child in the MTFC-P group demonstrated extreme values for a number of the feedback-locked ERP measures. When this child was excluded from the analyses examining the feedback-locked ERP components, the pattern of results was the same as the pattern of the results obtained from the analyses that included all of the children. Thus, to preserve power, the data from this child were retained for subsequent analyses.
Repeated measures analyses of variance (ANOVAs) were conducted to examine the behavioral and electrophysiological measures. The Greenhouse-Geiser correction for sphericity was applied as was recommended by Vasey and Thayer (1987). The reported degrees of freedom were not corrected; however, epsilons (ε) are reported. Post hoc paired comparisons using Fisher’s least significant difference were conducted for significant main effects and interactions.
Results
Behavioral Measures
Flanker interference effect
To assess the flanker interference effect on accuracy, percentage of errors of commission was compared using a repeated measures ANOVA with trial type (congruent and incongruent trials) as the within-subjects factor and group (MTFC-P, RFC, and CC) as the between-subjects factor. As is seen in Table 2, the main effect of trial type was significant, with more errors committed on incongruent trials (M = 26%) than on congruent trials (M = 22%). The main effect of group and the interaction between trial type and group were not significant. (Descriptive data for the behavioral measures across trial types are presented by group in Table 3.)
Table 2.
Repeated Measures Analyses of Variance for the Behavioral Measures
| Source | F | Partial η2 |
|---|---|---|
| Flanker interference effect | ||
| Errors of commission | ||
| Trial typea | 11.48*** | 0.27 |
| Groupb | 0.47 | 0.03 |
| Trial Type × Groupb | 2.37 | 0.13 |
| Reaction times | ||
| Trial typea | 4.53* | 0.13 |
| Groupb | 0.68 | 0.04 |
| Trial Type × Groupb | 0.15 | 0.01 |
|
| ||
| Response monitoring effect | ||
| Reaction times | ||
| Preceding trial accuracya | 15.15**** | 0.33 |
| Groupb | 0.75 | 0.05 |
| Preceding Trial Accuracy × Groupb | 0.24 | 0.02 |
Note. Epsilons (ε) are not presented in this table because they were not below 1.0.
df = 1, 31.
df = 2, 31.
p < .05.
p < .01.
p < .005.
p < .001.
Table 3.
Errors of Commission (%) and Average Reaction Times (ms) for Each Group Across Trial Types
| MTFC-P (n = 10) | RFC (n = 13) | CC (n = 11) | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Flanker interference effect | ||||||
| Errors of commission | ||||||
| Congruent trials | 22.00 | 11.16 | 25.46 | 10.72 | 18.69 | 10.12 |
| Incongruent trials | 24.69 | 11.23 | 27.28 | 10.77 | 26.41 | 10.84 |
| Reaction times | ||||||
| Congruent trials | 674.62 | 104.75 | 647.84 | 95.08 | 627.91 | 123.12 |
| Incongruent trials | 695.22 | 118.87 | 666.30 | 74.46 | 638.34 | 108.84 |
|
| ||||||
| Response monitoring effect | ||||||
| Reaction times | ||||||
| After correct trials | 676.17 | 111.18 | 647.52 | 81.94 | 618.78 | 105.95 |
| After incorrect trials | 712.19 | 131.17 | 673.70 | 70.40 | 658.71 | 140.20 |
The flanker interference effect on average reaction time was assessed using a repeated measures ANOVA with trial type as the within-subjects factor and group as the between-subjects factor. The main effect of trial type was significant, with slower reaction times for incongruent trials (M = 667.16 ms) than congruent trials (M = 650.78 ms). Neither the main effect of group nor the interaction between trial type and group was significant.
Response monitoring effect
To examine the response monitoring effect, average reaction time was compared using a repeated measures ANOVA with preceding trial accuracy (after correct and after incorrect trials) as the within-subjects factor and group as the between-subjects factor. The main effect of preceding trial accuracy was significant, with slower average reaction times following incorrect responses (M = 678.76 ms) than following correct responses (M = 648.67 ms). The main effect of group and the interaction between preceding trial accuracy and group were not significant.
Electrophysiological Measures
Response-locked ERP components
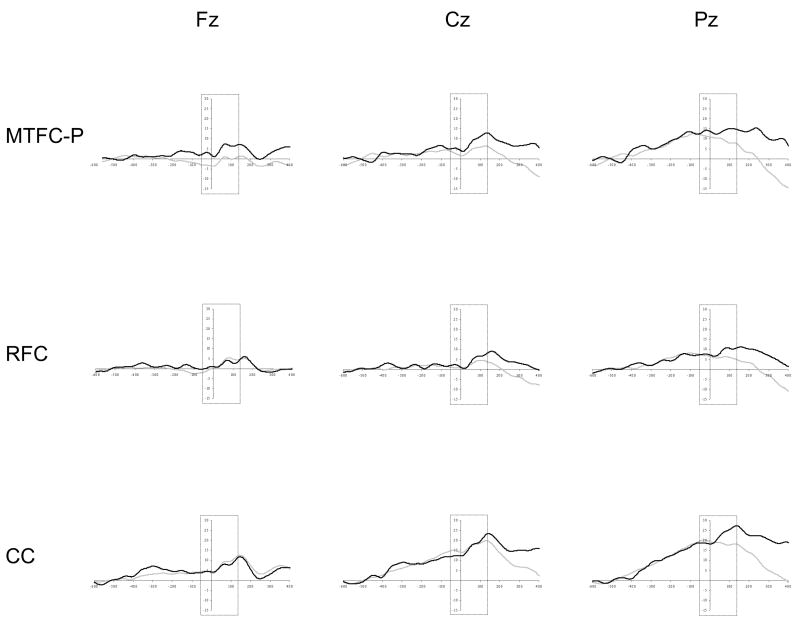
The amplitude of the ERN was assessed using a repeated measure ANOVA with trial accuracy (correct and incorrect trials) and electrode site (Fz, Cz, and Pz) as the within-subjects factors and group as the between-subjects factor. As is displayed in Table 4, the main effect of trial accuracy and the interaction between trial accuracy and electrode site were not significant. There was, however, a main effect of electrode site. The ERN was more negative at Fz (M = −0.34 μV) than at Cz (M = 4.13 μV) and at Pz (M = 9.60 μV). The ERN was also more pronounced at Cz than at Pz. The main effect of group was significant, with the CC group (M = 10.20 μV) differing from the MTFC-P group (M = 2.41 μV) and the RFC group (M = 0.79 μV). There was a significant interaction between electrode site and group. The simple main effect of electrode site was significant for the MTFC-P, RFC, and CC groups. However, the ERN was more pronounced at Fz than at Cz and at Pz and was more pronounced at Cz than at Pz for the MTFC-P group (Fz M = −2.30 μV; Cz M = 0.99 μV; Pz M = 8.53 μV) and the CC group (Fz M = 3.13 μV; Cz M = 11.80 μV; Pz M = 15.67 μV). The amplitude of the ERN at Fz and at Cz, which both differed from the amplitude at Pz, did not differ from each other for the RFC group (Fz M = −1.84 μV; Cz M = −0.40 μV; Pz M = 4.62 μV). The interaction between trial accuracy and group was not significant. (Descriptive data and waveforms for the response-locked ERP components across trial types are presented by group in Table 5 and Figure 1.)
Table 4.
Repeated Measures Analyses of Variance for the Electrophysiological Measures
| Source | F | ∈ | Partial η2 |
|---|---|---|---|
| Response-locked ERP components | |||
| ERN | |||
| Trial accuracya | 0.02 | 1.00 | 0.00 |
| Electrode sitec | 50.36**** | 0.87 | 0.62 |
| Trial accuracy × Electrode sitec | 1.68 | 0.84 | 0.05 |
| Groupb | 5.66** | 0.27 | |
| Trial Accuracy × Groupb | 0.79 | 1.00 | 0.05 |
| Electrode Site × Groupd | 3.22* | 0.87 | 0.17 |
| Pe | |||
| Trial accuracya | 7.68** | 1.00 | 0.20 |
| Electrode sitec | 15.62**** | 0.84 | 0.34 |
| Trial Accuracy × Electrode Sitec | 4.84* | 0.82 | 0.14 |
| Groupb | 4.74* | 0.23 | |
| Trial Accuracy × Groupb | 0.24 | 1.00 | 0.02 |
| Electrode Site × Groupd | 2.16 | 0.84 | 0.12 |
|
| |||
| Feedback-locked ERP components | |||
| N1 | |||
| Trial accuracya | 29.64**** | 1.00 | .49 |
| Electrode sitec | 6.27*** | 0.69 | 0.17 |
| Trial Accuracy × Electrode Sitec | 4.37* | 0.68 | 0.12 |
| Groupb | 5.82** | 0.27 | |
| Trial Accuracy × Groupb | 7.06*** | 1.00 | 0.31 |
| Electrode Site × Groupd | 2.90* | 0.69 | 0.16 |
| P2 | |||
| Trial accuracya | 32.44**** | 1.00 | 0.51 |
| Electrode sitec | 11.64**** | 0.84 | 0.27 |
| Trial Accuracy × Electrode Sitec | 3.15 | 0.58 | 0.09 |
| Groupb | 0.61 | 0.04 | |
| Trial Accuracy × Groupb | 7.74*** | 1.00 | 0.33 |
| Electrode Site × Groupd | 2.49 | 0.84 | 0.14 |
| FRN | |||
| Trial accuracya | 41.35**** | 1.00 | 0.57 |
| Electrode sitec | 1.78 | 0.84 | 0.05 |
| Trial Accuracy × Electrode Sitec | 5.51* | 0.73 | 0.15 |
| Groupb | 1.80 | 0.10 | |
| Trial Accuracy × Groupb | 5.11* | 1.00 | 0.25 |
| Electrode Site × Groupd | 1.94 | 0.84 | 0.11 |
df = 1, 31.
df = 2, 31.
df = 2, 62.
df = 4, 62.
p < .05.
p < .01.
p < .005.
p < .001.
Table 5.
Average Amplitude (μV) of ERP Components for Each Group Across Trial Types
| MTFC-P (n = 10) | RFC (n = 13) | CC (n = 11) | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Response-locked ERP components | ||||||
| ERN | ||||||
| Correct trials | 2.06 | 9.50 | 0.52 | 7.31 | 10.97 | 6.32 |
| Incorrect trials | 2.75 | 7.35 | 1.07 | 7.07 | 9.44 | 7.54 |
| Pe | ||||||
| Correct trials | 9.87 | 12.87 | 10.06 | 8.10 | 19.66 | 7.51 |
| Incorrect trials | 13.80 | 9.81 | 12.16 | 8.06 | 22.76 | 9.06 |
|
| ||||||
| Feedback-locked ERP components | ||||||
| N1 | ||||||
| Correct trials | −6.94 | 4.22 | −5.32 | 3.55 | −6.65 | 3.16 |
| Incorrect trials | −15.14 | 7.98 | −5.67 | 3.61 | −13.30 | 6.37 |
| P2 | ||||||
| Correct trials | 12.22 | 9.22 | 8.16 | 5.83 | 8.08 | 4.58 |
| Incorrect trials | 2.61 | 11.85 | 7.60 | 5.46 | 1.64 | 7.27 |
| FRN | ||||||
| Correct trials | −5.42 | 10.18 | −5.13 | 6.44 | −7.08 | 4.76 |
| Incorrect trials | −18.50 | 15.01 | −8.21 | 6.24 | −16.09 | 5.70 |
Figure 1.
Grand average waveforms for the response-locked ERP components (region within the dashed lines) for each group at Fz, at Cz, and at Pz for correct (gray line) and incorrect trials (black line). Note. Responses were made at 0 ms, and the baseline window was at −600 to −400 ms relative to the response.
The amplitude of the Pe was assessed using a repeated measures ANOVA with trial accuracy and electrode site as the within-subjects factors and group as the between-subjects factor. The main effect of trial accuracy was significant, with greater amplitude in response to incorrect trials (M = 16.24 μV) as compared to correct trials (M = 13.20 μV). In addition, there was a significant main effect of electrode site. The amplitude of the Pe was more positive at Cz (M = 15.97 μV) and at Pz (M = 17.46 μV) than at Fz (M = 10.74 μV). Furthermore, the interaction between trial accuracy and electrode site was significant. The Pe was more positive for incorrect trials than for correct trials at Cz (correct M = 14.04 μV; incorrect M = 17.51 μV) and at Pz (correct M = 14.73 μV; incorrect M = 19.76 μV). In contrast, the Pe for correct and incorrect trials did not differ significantly at Fz (correct M = 10.56 μV; incorrect M = 10.94 μV). There was also a significant main effect of group. The amplitude of the Pe was greater for the CC group (M = 21.21 μV) than the MTFC-P group (M = 11.83 μV) and the RFC group (M = 11.11 μV). The interaction between trial accuracy and group and the interaction between electrode site and group were not significant.
Feedback-locked ERP components
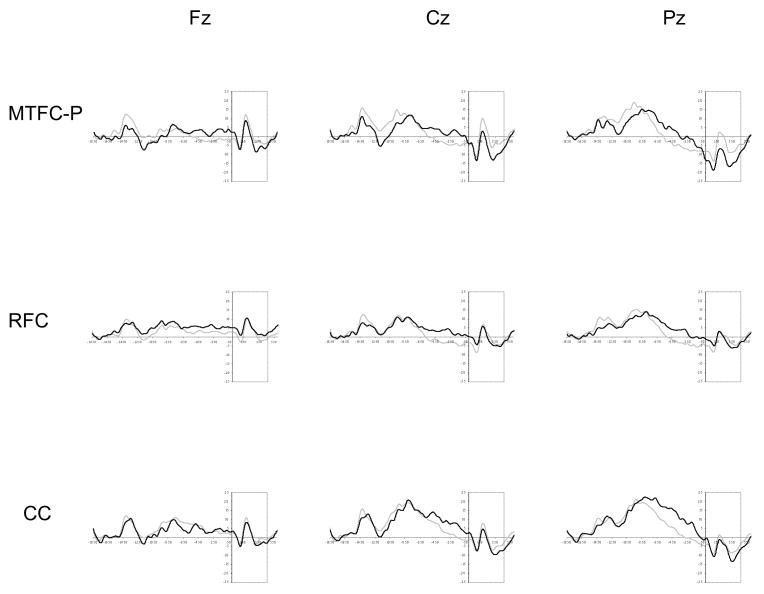
The amplitude of the N1 was analyzed using a repeated measures ANOVA with trial accuracy and electrode site as the within-subjects factors and group as the between-subjects factor. As is seen in Table 4, the main effect of trial accuracy was significant, with a more negative amplitude for incorrect trials (M = −11.37 μV) than for correct trials (M = −6.03 μV). There was also a significant main effect of electrode site. The amplitude of the N1 was more negative at Cz (M = −9.58 μV) and at Pz (M = −9.18 μV) than at Fz (M = −7.75 μV). The interaction between trial accuracy and electrode site was significant. The amplitude of the N1 was more negative for incorrect trials than for correct trials at Fz (correct M = −5.93 μV; incorrect M = −9.24 μV), at Cz (correct M = −6.92 μV; incorrect M = −11.68 μV), and at Pz (correct M = −5.83 μV; incorrect M = −11.85 μV). Although the N1 was more pronounced for incorrect trials at all sites, this difference was more defined at Cz and at Pz. In addition, there was a significant main effect of group. The MTFC-P group (M = −11.04 μV) and the CC group (M = −9.97 μV) displayed a more pronounced N1 than the RFC group (M = − 5.50 μV). The interaction between trial accuracy and group was significant. The amplitude of the N1 differed for correct and incorrect trials for the MTFC-P and CC groups but did not significantly differ for correct and incorrect trials for the RFC group. There was also a significant interaction between electrode site and group. The simple main effect of electrode site was significant for the MTFC-P group (Fz M = −9.08 μV; Cz M = −12.30 μV; Pz M = −11.74 μV) and the CC group (Fz M = −8.49 μV; Cz M = −10.36 μV; Pz M = −11.07 μV) but was not significant for the RFC group (Fz M = −5.67 μV; Cz M = −6.09 μV; Pz M = −4.72 μV). (Descriptive data and waveforms for the feedback-locked ERP components across trial types are presented by group in Table 5 and Figure 2.)
Figure 2.
Grand average waveforms for the feedback-locked ERP components (region within the dashed lines) for each group at Fz, at Cz, and at Pz for correct (gray line) and incorrect trials (black line). Note. The feedback stimuli were presented at 0 ms, and the baseline window was ay −1850 to −1650 ms relative to the feedback stimuli.
The amplitude of the P2 was assessed using a repeated measures ANOVA with trial accuracy and electrode site as the within-subjects factors and group as the between-subjects factor. The main effect of trial accuracy was significant, with a more positive amplitude for correct trials (M = 9.49 μV) than for incorrect trials (M = 3.95 μV). There was also a significant main effect of electrode site. The amplitude of the P2 was greater at Fz (M = 8.01 μV) and at Cz (M = 7.19 μV) than at Pz (M = 4.96 μV). The interaction between trial accuracy and electrode site, the main effect of group, and the interaction between electrode site and group were not significant. There was a significant interaction between trial accuracy and group. The amplitude of the P2 significantly differed for correct and incorrect trials for the MTFC-P and CC groups.
The amplitude of the FRN was analyzed using a repeated measures ANOVA with trial accuracy and electrode site as the within-subjects factors and group as the between-subjects factor. There was a significant main effect of trial accuracy, with a more negative amplitude for incorrect trials (M = −14.27 μV) than for correct trials (M = −5.88 μV). The main effect of electrode site was not significant. However, there was a significant interaction between trial accuracy and electrode site. The amplitude of the FRN was more negative for incorrect trials than for correct trials at Fz (correct M = −6.57 μV; incorrect M = −11.84 μV), at Cz (correct M = −6.15 μV; incorrect M = −15.05 μV), and at Pz (correct M = −4.82 μV; incorrect M = −14.46 μV). Although the FRN was more pronounced for incorrect trials at all electrode sites, this difference was more defined at Cz and at Pz. Neither the main effect of group nor the interaction between electrode site and group was significant. There was a significant interaction between trial accuracy and group. The amplitude of the FRN differed for correct and incorrect trials for all three groups. However, this difference was more defined for the MTFC-P and CC groups.
Discussion
In the current study, behavioral and electrophysiological performance on the flanker task, a measure of cognitive control and response monitoring, was compared for foster children assigned to an intervention condition, foster children assigned to a services-as-usual comparison condition, and low-income, nonmaltreated children living with their biological parents. In conducting this study, we had had two objectives: to determine whether this sample of young children demonstrated similar patterns of performance on the task as adults and older children and to examine the impact of early adverse experiences and a preventive intervention on the children’s performance.
Consistent with prior research (Enns & Akhtar, 1989; Ridderinkhof et al., 1997), the children in all three groups demonstrated the flanker interference effect, an index of cognitive control, by committing more errors and responding slower on incongruent trials than on congruent trials. The children also displayed the response monitoring effect, which reflects a compensatory strategy for improving performance, by responding slower after committing errors. Overall, these results indicate that the flanker task was effective in eliciting the expected pattern of behavioral performance for all three groups of children. It is noteworthy that the foster children exhibited comparable patterns of behavioral performance to the nonmaltreated children, in light of the cognitive, academic, and psychosocial disparities that have been observed among foster children (Clausen et al., 1998; Pears & Fisher, 2005a, Zima et al., 2000).
The results for the ERN and the Pe, which reflect response monitoring, also paralleled the findings of previous studies (Davies et al., 2004; van Veen & Carter, 2002). The Pe for all three groups of children was maximal at the central and parietal sites and was more defined for incorrect trials than correct trials. In addition, the ERN was most pronounced at the frontal site. However, the negative deflection of the ERN was very small, and the ERN was not more negative in response to incorrect trials compared to correct trials. Although inconsistent with prior studies with adults (van Veen & Carter, 2002; Yeung et al., 2004), the current results resembled previous findings with young children who displayed the response monitoring effect and a robust Pe but not a pronounced ERN (Davies et al., 2004). To explain these seemingly contradictory results, Davies and colleagues noted that the ERN is generated by a late maturing region of the ACC and that the Pe is at least partially generated by an earlier maturing posterior region. The result of the current study and the Davies et al. study suggest that different aspects of response monitoring and the underlying brain regions have distinct developmental courses.
The inclusion of trial-by-trial performance feedback also permitted the examination of the children’s responsivity to external feedback. The FRN, which was diffusely distributed across the electrode sites, was more pronounced for incorrect trials than correct trials. Additionally, two early feedback-locked components were identified. The amplitude of the N1, which was maximal at the central and parietal sites, and the amplitude of the P2, which was maximal at the frontal and central sites, both varied according to feedback type. Interestingly, these early feedback-locked components were observed in a study with older children using a guessing paradigm (van Meel et al., 2005). The authors noted that these components, which likely reflect attentional and categorization processes, had not been described in the adult literature. The results of the current study and the van Meel et al. study further highlight the need for research delineating the developmental course of response monitoring.
Interestingly, there were significant group differences on all of the feedback-locked ERP components. For the foster children who received the intervention and nonmaltreated children, the amplitudes of the N1, the P2, and the FRN were significantly different in response to negative feedback compared to positive feedback. In contrast, for the foster children who received services as usual, the amplitude of these components did not vary according to the type of feedback. Overall, these results indicate that the foster children who received services as usual were not as responsive to external feedback as the other children. Furthermore, the similarity between the foster children who received the intervention and the nonmaltreated children also suggests a potential intervention effect on children’s responsivity to external feedback. These results suggest that foster children may be less sensitive and have difficulty responding to environmental input, particularly external feedback. However, the results of the current study suggest that targeted interventions have the potential to ameliorate these difficulties.
The current results also indicate that behavioral and electrophysiological measures collected during the same task might not provide convergent information. Consistent with prior research (Harter et al., 1988; Karayanidis et al., 2000), group differences on the electrophysiological measures were observed in the absence of group differences on the behavioral measures. It has been argued that this pattern of results represents a subtle deficit in cognitive processing that behavioral measures are not sensitive enough to detect (Harter et al., 1988; Karayanidis et al., 2000). Consistent with this speculation, the electrophysiological measures in the current study suggest that the foster children who received services as usual have a subtle deficit in response monitoring. Although this subtle deficit in response monitoring was not apparent in the foster children’s behavioral performance on a focused task with explicit feedback in a laboratory setting, this deficit might have a profound impact on the children’s daily functioning. Indeed, this subtle deficit in response monitoring might be particularly pronounced when foster children perform tasks involving the integration of multiple cognitive processes in complex social and academic settings where they receive less explicit feedback about their performance from their parents, teacher, and peers. Overall, this pattern of results underscores the importance of conducting multilevel assessments when examining important cognitive processes. Additionally, these results suggest that behavioral measures might not be adequately sensitive to group differences in some cases. Because most screening instruments for at-risk children are behavioral in nature, subtle deficits in cognitive processing might be overlooked. Thus, these results could have important implications for prevention research and social policy.
There were several limitations of the current study. First, the sample size was somewhat small. However, studies involving electrophysiological measures tend to have smaller sample sizes (e.g., Karayanidis et al., 2000; Moser et al., 2005), and it is challenging to obtain electrophysiological measures with young at-risk populations. This limitation was mitigated by the replication of prior research findings and the presence of significant group differences on the electrophysiological measures. Second, performance was not assessed prior to the intervention. It is possible, therefore, that the two groups of foster children differed in terms of their electrophysiological performance prior to the intervention. However, the children were randomly assigned to their condition, and the two groups of foster children did not differ on other cognitive or psychosocial measures prior to the intervention (Pears & Fisher, 2005a, 2005b). Moreover, the intervention was specifically designed to increase the consistency and contingency of the foster parents’ feedback to their children, and the intervention effect on children’s responsivity to external feedback is consistent with this focus. Third, concurrent measures of the children’s cognitive, academic, and psychosocial functioning were not assessed. These measures might have provided insight into the impact of individual differences in cognitive control and response monitoring on children’s daily functioning.
Several directions for future research are highlighted by the results of this study. First, it will be important to replicate the current results, particularly the results suggesting an intervention effect on response monitoring. Currently, behavioral and electrophysiological performance on the flanker task is being assessed in a randomized efficacy trial of a preventive intervention designed to promote school readiness in kindergarten-aged foster children (Pears, Fisher, Heywood, & Bronz, 2007). This efficacy trial addresses the limitations of the current study by including a larger sample size, an assessment before and after the intervention, and measures of the children’s daily functioning. Second, researchers have become increasingly interested in the functional connectivity among frontal regions of the brain as altered coherence has been observed among children with ADHD (Murias et al., 2007). This line of research might also elucidate some of the difficulties of foster children.
In conclusion, the results of the current study provide evidence that the flanker task can be successfully employed with young children to assess cognitive control and response monitoring. To date, there have been few, if any, ERP studies with young foster children. This is understandable given the sensitivity required to assess this population. The results of this study provide an optimistic, albeit preliminary, note. They suggest that targeted preventive interventions might impact the neurobiological functioning of at-risk children. Additionally, the results emphasize the importance of assessing behavioral and electrophysiological performance together to reveal subtle differences in cognitive processing. Lastly, the current study highlights the need for further research with at-risk populations.
Acknowledgments
Support for this research was provided by the following grants: MH059780 and MH065046, NIMH, U.S. PHS; MH046690, NIMH and ORMH, U.S. PHS; and DA017592, NIDA, NIH, U.S. PHS. The authors express thanks to the staff and families of the Multidimensional Treatment Foster Care for Preschoolers program, to Kristen Greenley for project management, to Peter Marshall and James Long for technical assistance, and to Matthew Rabel for editorial assistance.
Contributor Information
Jacqueline Bruce, Oregon Social Learning Center and Center for Research to Practice.
Jennifer Martin McDermott, University of Wisconsin.
Philip A. Fisher, Oregon Social Learning Center and Center for Research to Practice.
Nathan A. Fox, University of Maryland.
References
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Rushby JA. Age and gender effects in EEG coherence: I. Developmental trends in normal children. Clinical Neurophysiology. 2004;115:2252–2258. doi: 10.1016/j.clinph.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- Black JE. How a child builds its brain: Some lessons from animal studies of neural plasticity. Preventive Medicine. 1998;27:168–171. doi: 10.1006/pmed.1998.0271. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Archives of General Psychiatry. 2007;64:1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Clausen JM, Landsverk J, Ganger W, Chadwick D, Litrownik A. Mental health problems of children in foster care. Journal of Child and Family Studies. 1998;7:283–296. [Google Scholar]
- Cohen JD, Aston-Jones G, Gilzenrat MS. A systems-level perspective on attention and cognitive control: Guided activation, adaptive gating, conflict monitoring, and exploitation versus exploration. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford Press; 2004. pp. 71–90. [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Spencer S, Hall J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. American Journal of Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Enns JT, Akhtar N. A developmental study of filtering in visual attention. Child Development. 1989;60:1188–1199. [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of target letters in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Fishbein D. The importance of neurobiological research to the prevention of psychopathology. Prevention Science. 2000;1:89–106. doi: 10.1023/a:1010090114858. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Burraston B, Pears KC. The Early Intervention Foster Care Program: Permanent placement outcomes from a randomized trial. Child Maltreatment. 2005;10:61–71. doi: 10.1177/1077559504271561. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Ellis BH, Chamberlain P. Early Intervention Foster Care: A model for preventing risk in young children who have been maltreated. Children’s Services: Social Policy, Research, and Practice. 1999;2:159–182. [Google Scholar]
- Fisher PA, Kim HK. Intervention effects on foster preschoolers’ attachment-related behaviors from a randomized trial. Prevention Science. 2007;8:161–170. doi: 10.1007/s11121-007-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Development. 1987;58:539–559. [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA the Early Experience Stress and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Harter MR, Anllo-Vento L, Wood FB, Schroeder MM. Separate brain potential characteristics in children with reading disability and attention deficit disorder: Color and letter relevance effects. Brain and Cognition. 1988;7:115–140. doi: 10.1016/0278-2626(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JDE. Neural activation during response competition. Journal of Cognitive Neuroscience. 2000;12:118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Rommler J, Ehlis A, Heidrich A, Fallgatter AJ. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Cognitive Brain Research. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Karayanidis F, Robaey P, Bourassa M, De Koning D, Geoffroy G, Pelletier G. ERP differences in visual attention processing between attention-deficit hyperactivity disorder and control boys in the absence of performance differences. Psychophysiology. 2000;37:319–333. [PubMed] [Google Scholar]
- Lewis E, Dozier M, Ackerman J, Sepulveda S. The effect of placement instability on adopted children’s inhibitory control abilities and oppositional behavior. Developmental Psychology. 2007;43:1415–1427. doi: 10.1037/0012-1649.43.6.1415. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Fox NA. Variations of the flanker paradigm: Assessing cognitive skills in young children. Behavior Research Methods. 2007;39:62–70. doi: 10.3758/bf03192844. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42:261–268. doi: 10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Murias M, Swanson JM, Srinivasan R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cerebral Cortex. 2007;17:1788–1799. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears KC, Fisher PA. Developmental, cognitive, and neuropsychological functioning in preschool-aged foster children: Associations with prior maltreatment and placement history. Journal of Developmental and Behavioral Pediatrics. 2005a;26:112–122. doi: 10.1097/00004703-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Pears KC, Fisher PA. Emotion understanding and theory of mind among maltreated children in foster care: Evidence of deficits. Development and Psychopathology. 2005b;17:47–65. doi: 10.1017/s0954579405050030. [DOI] [PubMed] [Google Scholar]
- Pears KC, Fisher PA, Heywood CV, Bronz KD. Promoting school readiness in foster children. In: Saracho ON, Spodek B, editors. Contemporary perspectives on social learning in early childhood education. Charlotte, NC: Information Age; 2007. pp. 173–198. [Google Scholar]
- Pilowsky D. Psychopathology among children placed in family foster care. Psychiatric Services. 1995;46:906–910. doi: 10.1176/ps.46.9.906. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molan MW, Band GPH, Bashore TR. Sources of interference from irrelevant information: A developmental study. Journal of Experimental Child Psychology. 1997;65:315–341. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Toupin J, Déry M, Pauzé R, Mercier H, Fortin L. Cognitive and familial contributions to conduct disorder in children. Journal of Child Psychology and Psychiatry. 2000;41:333–344. [PubMed] [Google Scholar]
- van Meel CS, Oosterlaan J, Heslenfeld DJ, Sergeant JA. Telling good from bad news: ADHD differentially affects processing of positive and negative feedback during guessing. Neuropsychologia. 2005;43:1946–1954. doi: 10.1016/j.neuropsychologia.2005.03.018. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative-developmental study of executive function: A window on prefrontal function in children. Developmental Neuropsychology. 1991;7:131–149. [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zima BT, Bussing R, Freeman S, Yang X, Belin TR, Forness SR. Behavior problems, academic skill delays and school failure among school-aged children in foster care: Their relationship to placement characteristics. Journal of Child and Family Studies. 2000;9:87–103. [Google Scholar]