Summary
Leptin is a protein hormone that acts within the hypothalamus to suppress food intake and decrease body adiposity, but it is increasingly clear that the hypothalamus is not the only site of leptin action, nor food intake the only biological effect of leptin. Instead, leptin is a pleiotropic hormone that impinges on many brain areas, and in doing so alters food intake, motivation, learning, memory, cognitive function, neuroprotection, reproduction, growth, metabolism, energy expenditure, and more. This diversity of function also means that a dysregulation of leptin secretion and signaling can have far reaching effects. To date research on leptin signaling has focused primarily on the hypothalamus, and the result is a relative lack of information regarding the impact of leptin signaling and leptin resistance in non-hypothalamic areas, despite a growing literature implicating leptin in the regulation of neuronal structure and function in the hippocampus, cortex and other brain areas associated with cognition.
Keywords: hypothalamus, food intake, neuroprotection, hippocampus, cognitive function
Introduction
The discovery of the protein hormone leptin ushered in an era in which a variety of neural circuits, both within and outside the hypothalamus, have been implicated in the regulation of food intake. Like many hormones, the initial description of leptin as a regulator of food intake and body adiposity focused the study of leptin biology, but it is now clear that leptin does not produce a singular effect (i.e, a reduction in food intake). This review will highlight the pleiotropic effects of leptin on the brain, first reviewing the role of hypothalamic leptin signaling in the regulation of energy homeostasis, and then toward more recent data implicating leptin signaling in reward, motivation, learning and memory and neuroprotection. These latter effects appear to reflect leptin action in areas outside the hypothalamus, and indicate that leptin may have effects unrelated to its role as a signal of body adiposity.
Leptin as an adiposity signal that acts in the hypothalamus
In both humans and animals, physiological mechanisms monitor body adipose mass and react to changes in energy balance by altering ingestive behavior and energy expenditure to buffer against drastic changes in body adiposity and restore body weight and adiposity once the nutritional challenge dissipates [1–3]. The protein hormone leptin represents an afferent signal within this homeostatic mechanism, with leptin being produced and secreted by adipocytes via mechanisms that are sensitive to both the chronic level of body adipose mass as well as current metabolic status [4–7]. Of the brain areas mediating leptin action, the hypothalamic arcuate nucleus (ARC) is most closely associated with leptin’s effects on energy homeostasis. The ARC contains at least two populations of leptin-sensitive neurons. The first population contains the potent orexigenic peptides Neuropeptide Y (NPY) and Agouti-Related Protein (AgRP). Central injection of either NPY or AgRP leads to a profound increase in food intake, and acute ablation of the NPY/AgRP neurons in adult animals leads to significant hypophagia and weight loss [8, 9]. Thus NPY/AgRP neurons represent important drivers of food intake, and leptin’s action to suppress food intake is mediated, at least in part, by its ability to inhibit these neurons. Adjacent to NPY/AgRP neurons are a population of neurons containing proopiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART). POMC is a large precursor protein which is processed into a variety of smaller products, notably alpha-melanocyte stimulating hormone (α-MSH). Central injection of α-MSH or its stable analog Melanotan II (MTII) produces a marked suppression of food intake, while genetic deletion of POMC results in an obese phenotype [10, 11]. Selective deletion of leptin receptors within POMC neurons also results in an obese phenotype [12], although the effect is much less robust than loss of POMC or deletion of leptin receptors in all neurons. Thus POMC neurons exert a tonic inhibitory influence on food intake, and leptin’s action to suppress food intake is at least partly mediated by an inhibition of POMC neurons. A model has therefore emerged in which these two populations of neurons exert opposing effects on feeding behavior and energy metabolism, and simultaneously respond in an opposing fashion to leptin.
The mechanism by which leptin regulates these neurons involves changes in both gene expression and acute membrane activity [13–15]. These observations suggest that leptin engages multiple signaling pathways to impact neuronal function, and recent data clearly supports this observation [16]. The leptin receptor is a type 1 cytokine receptor traditionally proposed to signal through the Janus Kinase – Signal Transducer and Activator of transcription (Jak-Stat) pathway. Jak-Stat signaling is critical for leptin action on ARC neurons; leptin induces Stat3 activation in both POMC and NPY neurons [17–19] and loss of leptin’s ability to activate Stat3 leads to obesity [20, 21]. However, leptin also engages other intracellular pathways, including ERK, PI3K and cAMP/PDE3B [22, 23]. Of these, PI3K signaling has received the most attention, as loss of PI3K signaling attenuates leptin-induced inhibition of food intake, regulation of neuropeptide expression, and stimulation of sympathetic nervous system activity [22, 24, 25].
It should also be noted that these arcuate neurons respond to a variety of signals in addition to leptin. Insulin also acts in the brain to suppress NPY neurons and stimulate POMC neurons [26, 27]. Direct brain insulin injections suppress food intake, while selective deletion of brain insulin receptor leads to obesity [28, 29]. Thus insulin and leptin have overlapping physiological effects, and this overlap is mimicked by a degree of overlap in the intracellular signaling pathways they regulate, although this regulation and interaction appears to be highly complex. In addition to leptin and insulin these neuronal populations also detect and respond to a variety of additional nutrient signals, including hormonal signals such at ghrelin and PYY [30, 31], as well as fuels such as glucose, fatty acids and amino acids [32–36].
While the arcuate nucleus has received the most attention as a site mediating leptin’s regulation of food intake, leptin appears to also act in additional brain areas both within and outside the hypothalamus. Recent work indicates that leptin activates at least one population of neurons (SF-1 containing neurons) within the ventromedial nucleus (VMN), and that specific deletion of leptin receptor in SF-1 neurons increases food intake and body adiposity [37, 38]. These data are consistent with previous work demonstrating effects of exogenous leptin on VMN neurons and reductions in food intake in response to local VMH leptin injection [39, 40], although the latter studies are complicated by the close anatomical proximity of the VMN to the ARC. There is also strong evidence that leptin suppresses food intake by acting directly in areas of the caudal brainstem, in particular a population of neurons within the nucleus of the solitary tract (NTS). The NTS is classically associated with satiety and meal termination, and local injections of leptin into the brainstem produce reductions in food intake that are similar in magnitude to injections into the forebrain [41, 42].
Lastly, it should it should also be recognized that the suppression of food intake is only one component of leptin’s overall effect on energy homeostasis. In essence, the primary effect of brain leptin signaling could be viewed as a reduction in body adiposity, with the suppression of food intake (energy intake) being one component in an array of physiological changes that collectively lead to reduced body adiposity [43, 44]. One example is the acute effect of leptin on sympathetic outflow and energy expenditure. Relatively early on in the study of leptin it was recognized that the reduction in body weight induced by leptin involved more than changes in food intake [45], with leptin-deficient animals exhibit increased energy efficiency such that excess adiposity occurs even when hyperphagia is prevented [46]. These observations were extended when it was clearly described that leptin increases energy expenditure and fat oxidation [47–49], at least in part via an activation of the sympathetic nervous system [50, 51], and a resultant activation of brown adipose tissue (BAT) [52–54], although leptin decreases body adiposity and increases energy expenditure even in the absence of BAT-derived UCP1 [55]. These observations are consistent with leptin regulating sympathetic outflow and metabolism in other tissues, particularly skeletal muscle and adipose tissue [49, 51, 54, 56, 57].
In addition to an activation of the sympathetic nervous system, leptin also regulates energy homeostasis is via effects on neuroendocrine hormone secretion [58], particularly the secretion of thyroid hormone, gonadotropins, growth hormone, and ACTH. Leptin acts locally in the hypothalamus to stimulate TRH neurons and TRH expression [59] [60, 61], indicating that leptin regulation metabolic rate includes a stimulatory effect on the thyroid axis [62]. Although somewhat less direct, leptin also alters energy balance by regulating reproduction. Decreases in body adiposity and periods of negative energy balance are associated with impaired reproductive function in a variety of species [63], and this effect is principally mediated by an inhibition of hypothalamic gonadotropin releasing hormone (GnRH) secretion [64–66]. By acting in the hypothalamus, leptin hastens the onset of puberty in mice [67–69] and improves reproductive function in settings of negative energy balance or leptin deficiency [70–72]. Likewise, leptin acts directly within the brain to stimulate gonadotropin secretion [73–75], possibly via the inhibition of NPY neurons [76–78]. Leptin deficient humans exhibit impaired reproduction and a failure to attain puberty, and this deficit is fully reversed by leptin replacement [79].
When taken together, these data provide a model for the neural basis of leptin-dependent regulation of energy homeostasis. The traditional view is thus one in which leptin, acting as an adiposity signal, regulates a defined populations of neurons in the hypothalamus to alter food intake and other components of energy homeostasis. While an abundant literature supports the core of this model, it is also apparent that leptin has a more diverse effect on the brain, with implications not only on the regulation of food intake, but on brain areas and neural systems that might be independent of the regulation of energy homeostasis.
Leptin and food intake: Reward and motivation
It is well recognized that nutritional status influences motivated behavior. For instance, food deprivation increases motivation to attain rewarding stimuli, including food, drugs of abuse, and electrical stimulation of specific brain areas [80–82]. These observations collectively demonstrate that negative energy balance enhances the desire for rewarding stimuli. Interestingly, leptin attenuates the effects of negative energy balance in these models [83–85], indicating that leptin does not simply act in the brain to reduce the consumption of food, but that it also acts more generally to reduce the motivation to acquire rewards. Although areas of the hypothalamus (i.e. lateral hypothalamus) are associated with these responses, areas outside the hypothalamus are also involved. The mesolimbic dopamine system is critical for the motivation to obtain pleasurable stimuli [86, 87]. Dopaminergic projections from the ventral tegmental area to the nucleus accumbens are particularly important, as manipulation of this dopamine system powerfully influences instrumental performance for and consumption of drugs or food [88–90]. Leptin directly impinges on this system, acting in the VTA to suppress food intake and modulate dopamine neurons [91–93], and also may indirectly influence this system via effects in the hypothalamus. Thus leptin-dependent suppression of food intake involves brain areas associated with the anticipation, memory or motivation, as supported by available data indicating that leptin alters behaviors related to food intake but independent of the actual consumption of food [83, 94]. Lastly, brain imaging studies in leptin deficient humans have highlighted the profound effect that the absence of leptin has on the brain, with leptin deficient humans responding more strongly to food cues (i.e., images of foods) than normal individuals [95], and leptin treatment altering the neuronal response to food cues in a variety of brain areas [96, 97]. Collectively, these data provide a unique view of the expanding role for leptin, and demonstrate that leptin exerts significant effects on food intake via actions via mechanisms that occur prior to and separate from the actual consumption of food, and that these effects are mediated by brain areas that are distinct from the hypothalamus.
Leptin as a signal promoting neuronal survival and cognition
Upon its discovery, leptin was heralded as a hormone acting in the brain as a signal of body adipose mass. Yet in addition to regulating food intake and body weight homeostasis, leptin also acts within brain areas that are unrelated (at least directly) to an effect on energy homeostasis. Within these brain areas leptin alters neuronal/synaptic function and structure, and influences neuronal survival and proliferation. The result of these effects appears to be an improvement in learning, memory and other forms of cognition and a resistance to insults which impair cognitive performance.
Perhaps the first evidence for a role for leptin in cognition is the recognition that leptin receptors are expressed widely throughout the brain. Although leptin receptors, particularly the signaling form of the receptor (Long form – LepRb) are densely expressed within medial areas of the hypothalamus, leptin receptors are also expressed in many brain areas, including regions associated with learning and memory, such as various cortical regions and the hippocampus [98–102]. Another clue to the role of leptin in the regulation of these brains areas stems from the clear effects that both diet and obesity have on cognitive function. Variations in caloric intake or diet composition can produce changes in gene expression within areas of the hippocampus and cortex [103], indicating that these brain areas are not insulated from variations in nutritional or metabolic status. Similarly, it is increasingly apparent that alterations in nutritional status can alter cognitive function, and in particular that settings of obesity are associated with decreases in cognitive function [104]. Based on these and other observations, the key question therefore is whether variations in leptin signaling contribute to changes to metabolic or nutritionally induced alterations in neuronal or cognitive function within areas outside the hypothalamus.
Effects on neuronal/synaptic function
Within these non-hypothalamic areas, leptin acts in part by directly regulating neural function, including local effects at the synapse [105]. In rodent models of genetic leptin or leptin receptor deficiency, hippocampal neurons (CA1) exhibit impaired development of long term potentiation (LTP) and depression (LTD), and an associated reduction in CaMK II activity [106]. Consistent with this observation, leptin treatment of hippocampal neurons stimulates CaMK II phosphorylation and facilitates the development of LTP [107]. These changes in LTP and LTD are influenced by leptin dependent regulation of NMDA receptor function [108, 109], as leptin enhances NMDA-induced increases in intracellular calcium levels and facilitates NMDA receptor-mediated synaptic transmission [108]. Leptin treatment in neonatal animals altered the expression of NR1, NR2B, synapsin 2A and synaptophysin in the hippocampus [110]. In addition to altering NMDA receptors, there is also evidence that leptin regulates the function of large-conductance, calcium activated K+ channels (BK channels) [111, 112]. Lastly, these effects of leptin are not limited to hippocampal neurons, as leptin receptors are expressed in cerebellar neurons at the both the somatic plasma membrane and the synapse, and treatment of these neurons with leptin facilitated NR2B NMDA receptor mediated calcium influx [113].
Just as leptin regulates hypothalamic neurons via a variety of signaling pathways, evidence suggests that leptin uses multiple signaling pathways to regulate non-hypothalamic neurons. For leptin dependent effects on synaptic function and activity, a large abundance of data implicates leptin-dependent activation of phosphotidylinositol 3-kinase (PI3K) signaling. PI3K is implicated in leptin-dependent regulation of LTP and LTD, NMDA receptors, and actin cytoskeleton dependent clustering of BK channels [108, 109, 111, 112]. Yet other signaling systems are also implicated, including leptin activation of MAPK and Src signaling [108, 113]. Taken together, the above data indicates that leptin directly regulates the signaling and synaptic activity of neurons within the hippocampus, cortex and cerebellum. It is thus highly likely that these signaling effects contribute to the cognitive and behavioral effects of leptin that will be discussed below.
Effects on neuronal structure and plasticity
In addition to direct effects on neuronal/synaptic function, leptin also appears to influence neurons by altering their structure and plasticity. Initial evidence for this effect stemmed from the observation that rodent models of leptin deficiency exhibit smaller brains, that these alterations in brain development are evident in utero, and that leptin replacement in these models serves to increase brain size, protein and DNA [114–117]. More recent studies provide evidence for neuroproliferative effects within the fetal cortex, and indicate that leptin is associated with a maintenance and differentiation of neural stem cells, glial-restricted progenitor cells and/or neuronal lineage cells [118]. Interestingly, similar observations have been made in humans, where fasting plasma leptin levels in normal humans are correlated with grey matter volume in several brain areas [119], and leptin replacement in leptin deficient humans leads to gross changes in brain structure, particularly an increase in grey matter volume in discrete areas [120]. These very provocative observations thus provide strong evidence that leptin, and particularly the absence of leptin, has fundamental effects on brain structure.
In addition to these general effects of leptin, there have been more specific observations of leptin dependent changes in neuronal structure in a variety of brain areas, including the hypothalamus. Leptin deficiency appears to reduce the density of axonal projections from leptin sensitive neurons, and this structural defect can be restored by leptin treatment during a critical period of neonatal life [121]. Consistent with this observation, leptin acts on arcuate nucleus explants to promote neurite outgrowth [121]. Similar observations have also been made within areas of the hippocampus, cortex and cerebellum. Leptin was shown to act in cerebellar neurons to promote neurite outgrowth and increase the complexity of the neurite arbor [122], in hippocampal cells to enhance the motility and density of dendritic filopodia [123], and in cortical neurons to stimulate growth cone morphogenesis [124]. Lastly, there is also evidence in the hypothalamus that leptin influences the number of both inhibitory and stimulatory synaptic inputs onto neurons within the arcuate nucleus. Mice that are deficient for leptin exhibit a skewed distribution of synaptic input onto feeding related neurons, and leptin treatment induces a relatively rapid reorganization of the synaptic input to these ARC neurons [125]. In summary, these data collectively support a model in which leptin acts to influence neuronal structure and plasticity. As such, these changes provide an additional means by which leptin might regulate neuronal function.
Effects on neuronal survival and proliferation
It has long been known that various growth factors act on neurons to protect against neurodegeneration and cell death [126], and a rather large literature has accumulated specifically implicating leptin as a neuroprotective signal [127]. Within a variety of non-neural cells, including cancer cells, leptin has been show to inhibit apoptotic cell death [128–132]. In neurons in vitro, leptin appears to attenuate cell death induced by the removal of serum or neurotrophines [133, 134], to improve cell survival in models of ischemic stroke [135, 136], to protect against glutamatergic excitotoxicity [133, 137], to protect against oxidative stress [133], and to promote the proliferation of hippocampal progenitor cells [138]. These in vitro effects of leptin are replicated by in vivo experiments demonstrating that leptin attenuates the loss of dopamine neurons in a chemically induced model of Parkinson’s disease [139], that leptin deficient mice are more sensitive to middle cerebral artery occlusion (MCAO) but that leptin treatment decreases infarct volume and animal recovery following MCAO [135, 140], and that leptin reduces symptoms of chemically induced epileptic seizures [141]. These effects of leptin appear to stem from leptin’s activation of intracellular signaling pathways associated with growth factor signaling, including the activation of Stat3, PI3K/Akt and ERK/MAPK [133–136, 138, 139, 141]. Interestingly some evidence also implicates an effect of leptin on the NF-KappaB/c-Rel signaling pathway, as the ability of leptin to improve function following MCAO was attenuated in c-Rel deficient mice, and leptin stimulated the antiapoptotic Bcl-xl in cortical neurons via a c-Rel dependent mechanism [140]. Taken together, these data provide compelling support for leptin as a neuroprotective signal, although the physiological implications of this relationship are not fully clear. Are physiological increases (or decreases) in leptin relevant to neuronal health? Are these effects in any way related to leptin’s role as a nutritional signal? Additional work is clearly required to test whether leptin’s neuroprotective actions have any role in neurodegenerative processes, particularly those related to nutrition and metabolism.
Effects on cognition and behavior
The above observations provide strong support for a role for leptin in a variety of brain areas and signaling systems that appear, at least on the surface, to be unrelated to its role in energy homeostasis. In addition, these molecular and cellular examples for leptin-dependent effects on synaptic function, neuronal structure, and neuroprotection also provide a mechanistic basis for the remarkable observation that leptin acts within the brain to support and promote cognitive function. The relationship between leptin and cognitive function exists on multiple levels. First, correlational evidence provides a link between altered, particularly reduced, circulating leptin levels and impaired cognition in humans [142–144], with examples including a correlation between high leptin and improved cognition in the elderly and an association between low leptin levels and impaired learning and memory in HIV infected men. Secondly, genetic models of leptin suggest that leptin deficiency impairs cognitive performance [106, 145–147]. The most provocative of these is the human example, where a leptin deficient individual with reduced cognitive performance at baseline exhibited a marked improvement in neurocognitive tests following leptin treatment [145]. Lastly, there is also direct evidence for an effect of leptin treatment to improve cognitive performance in rodents [107]. These experiments particularly implicate the hippocampus, as local injections of leptin into the hippocampus improved memory retention [148], although at least one experiment has failed to detect an effect of hippocampal leptin on spatial memory [149].
While these observations provide a direct link between leptin and cognition, it is currently unclear whether these behavioral effects of leptin are mediated by discrete brain regions (hippocampus vs cortex), or whether these cognitive changes are mediated by any or all of the diverse effects of leptin on synaptic function, neuronal structure and plasticity, and neuroprotection. Nevertheless, these observations provide an exciting new direction for leptin research, and clearly indicate that leptin is much more than an adiposity signal that acts within the hypothalamus to regulate food intake.
Implications for leptin resistance
Shortly after the discovery of leptin, it was recognized that leptin deficiency was not a common cause of obesity. Instead, most obese individuals exhibit high circulating leptin levels coincident with ongoing hyperphagia [5]. Obese humans and animals are also relatively unresponsive to exogenously administered leptin, and this syndrome of reduced sensitivity to endogenous and exogenous leptin has been termed leptin resistance [150]. Leptin resistance appears to occur on at least two fronts, with the first being a reduced relative transport of circulating leptin across the blood brain barrier [151], and the second a reduction in intracellular signaling downstream of the leptin receptor. The existence of leptin resistance, at least within the hypothalamus, is thus well accepted, and considerable research effort continues to focus on hypothalamic resistance, both to define the cellular mechanisms underlying leptin resistance and to determine the role of hypothalamic leptin resistance in the development of obesity [152–155]. Yet absent within this discussion is the consideration that leptin resistance within non-hypothalamic areas might also occur, and that this resistance, by altering neuronal function within brain areas such as the cortex and hippocampus, might also alter behavior and cognition.
The study of leptin resistance has focused primarily on the failure of exogenous leptin reduce food intake and activate key signaling molecules (Stat3) within neurons of the hypothalamic arcuate nucleus. This ARC-specific leptin resistance is well described, and occurs in response to high fat diets, age, pregnancy and lactation, seasonal variations, and even chronic leptin exposure [153, 156–161]. But does leptin resistance occur in brain areas besides the arcuate nucleus? In addressing the question of region-specific leptin resistance, Munzberg et al., [162] compared leptin-induced phosphorylation of Stat3 in a variety of brain areas in mice fed a high diet, while Ladyman et al., [163] conducted a similar study in pregnant, leptin resistant rats. These experiments detected a marked reduction of leptin-dependent Stat3 phosphorylation in the ARC in leptin resistant animals, but persistent leptin signaling within other brain areas both within and outside the hypothalamus. The conclusion was therefore that leptin resistance occurs specifically in the hypothalamic arcuate nucleus, and to date few if any studies have followed up on these observations by focusing on leptin resistance in non-hypothalamic areas. However, it should be noted that these anatomical studies were based solely on leptin activation of Stat3, and did not account for changes in other signaling pathways. Yet the majority of studies indicate that leptin-dependent changes in neuroprotection, proliferation, synaptic function and structural plasticity are mediated by pathways such as the PI3K/Akt pathway and ERK/MAPK signaling [108, 109, 111–113, 123, 133–136, 138, 139, 141]. Thus, it remains possible that leptin signaling is altered within these brain areas, despite a normal or persistent regulation of Stat3. Available evidence already exists to suggest that leptin resistance may vary based on biological endpoint. For instance, there is evidence that leptin-dependent effects on sympathetic outflow may persist despite an attenuation of leptin’s suppression of food intake [164, 165]. These data therefore provide support for the hypothesis that leptin signaling and leptin resistance vary across brain areas.
Although virtually no work has specifically tested whether leptin resistance develops within brain areas associated with learning, memory or other aspects of cognitive function, a collective consideration of the work reviewed above provides a foundation for this hypothesis. First, increasing age, obesity and metabolic dysfunction each negatively impact these brain areas and lead to impaired cognitive function. Second, leptin signaling, at least within the hypothalamus, is impaired coincident with age, obesity and metabolic dysfunction. Third, animals with genetically impaired leptin signaling show defects in cognitive function. Fourth, exogenous leptin administration improves cognitive function and protects neurons against damage and degeneration, including an effect of leptin when directly administered into the hippocampus. Taken together, these observations indicate that leptin signaling (or the loss thereof) in the cortex or hippocampus is well positioned to contribute to age and/or diet-associated declines in cognitive function. However, future studies must be designed to clearly test whether and when leptin resistance develops in these non-hypothalamic areas, and then to determine if maintenance of leptin signaling ameliorates the effects of diet or age.
Conclusions
Leptin has received considerable attention as a nutritional signal acting in the hypothalamus to suppress food intake. Yet leptin is a pleiotropic hormone, impinging on a variety of brain areas to influence satiety, motivation, learning, memory, cognitive function, reproduction, growth, metabolism, and additional effects not discussed here [166–168]. This diversity of function indicates that the dysregulation of leptin secretion and signaling that occurs in settings of both undernutrition and obesity can have far reaching effects. While to date research on leptin resistance has focused primarily on the hypothalamus and emphasized obesity and diabetes, it is increasingly apparent that leptin also acts within areas such as the cortex and hippocampus to influence neuronal function and promote cognition. As such, alterations in leptin signaling within these non-hypothalamic brain areas may provide a unique mechanism linking obesity and diabetes to impaired cognitive function.
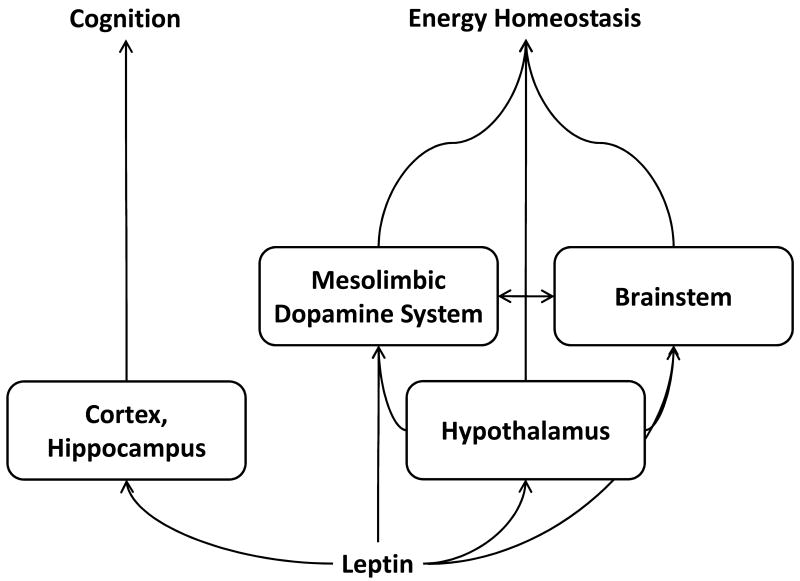
Figure 1. Leptin sensitive brain areas in the regulation of energy homeostasis and cognition.
Leptin-dependent regulation of energy homeostasis is mediated by a complex interaction of brain areas which contribute to leptin’s suppression of feeding behavior and stimulation of energy expenditure. Contrastingly, relatively little is known about leptin’s potential role in the regulation of cognition, but there is clear evidence for a direct effect of leptin within areas of the cortex and hippocampus. It remains unclear whether an interaction exists between those areas influencing energy homeostasis and those influencing cognition, but the existence of well established neuroanatomical connections indicate that such interactions are likely.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris RB, Kasser TR, Martin RJ. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. J Nutr. 1986;116:2536–2546. doi: 10.1093/jn/116.12.2536. [DOI] [PubMed] [Google Scholar]
- 2.Sims EA, Danforth E, Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- 3.Sims EA, Goldman RF, Gluck CM, Horton ES, Kelleher PC, Rowe DW. Experimental obesity in man. Trans Assoc Am Physicians. 1968;81:153–170. [PubMed] [Google Scholar]
- 4.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 5.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 6.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 7.Ostlund RE, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- 8.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 9.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 10.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 11.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 12.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46 :2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 15.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 16.Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 17.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 18.Hakansson ML, Meister B. Transcription factor STAT3 in leptin target neurons of the rat hypothalamus. Neuroendocrinology. 1998;68:420–427. doi: 10.1159/000054392. [DOI] [PubMed] [Google Scholar]
- 19.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 20.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 21.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 22.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 23.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 24.Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab. 2005;289:E1051–1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- 25.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 26.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woods SC, Seeley RJ. Insulin as an adiposity signal. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S35–38. doi: 10.1038/sj.ijo.0801909. [DOI] [PubMed] [Google Scholar]
- 28.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 29.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 30.Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 31.Riediger T, Bothe C, Becskei C, Lutz TA. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 2004;79:317–326. doi: 10.1159/000079842. [DOI] [PubMed] [Google Scholar]
- 32.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 33.Levin BE. Glucosensing neurons do more than just sense glucose. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S68–72. doi: 10.1038/sj.ijo.0801916. [DOI] [PubMed] [Google Scholar]
- 34.Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–171. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 36.van den Top M, Spanswick D. Integration of metabolic stimuli in the hypothalamic arcuate nucleus. Prog Brain Res. 2006;153:141–154. doi: 10.1016/S0079-6123(06)53008-0. [DOI] [PubMed] [Google Scholar]
- 37.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 38.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Sherwin RS. The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes. 1997;46:150–152. doi: 10.2337/diab.46.1.150. [DOI] [PubMed] [Google Scholar]
- 40.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 41.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 42.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 43.Harris RB. Leptin--much more than a satiety signal. Annu Rev Nutr. 2000;20:45–75. doi: 10.1146/annurev.nutr.20.1.45. [DOI] [PubMed] [Google Scholar]
- 44.Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–347. doi: 10.1146/annurev.physiol.65.092101.142622. [DOI] [PubMed] [Google Scholar]
- 45.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 46.Coleman DL. Increased metabolic efficiency in obese mutant mice. Int J Obes. 1985;9(Suppl 2):69–73. [PubMed] [Google Scholar]
- 47.Hwa JJ, Fawzi AB, Graziano MP, Ghibaudi L, Williams P, Van Heek M, Davis H, Rudinski M, Sybertz E, Strader CD. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol. 1997;272:R1204–1209. doi: 10.1152/ajpregu.1997.272.4.R1204. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Hartzell DL, Rose BS, Flatt WP, Hulsey MG, Menon NK, Makula RA, Baile CA. Metabolic responses to intracerebroventricular leptin and restricted feeding. Physiol Behav. 1999;65:839–848. doi: 10.1016/s0031-9384(98)00243-1. [DOI] [PubMed] [Google Scholar]
- 49.Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, Unger RH. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 51.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cusin I, Zakrzewska KE, Boss O, Muzzin P, Giacobino JP, Ricquier D, Jeanrenaud B, Rohner-Jeanrenaud F. Chronic central leptin infusion enhances insulin-stimulated glucose metabolism and favors the expression of uncoupling proteins. Diabetes. 1998;47:1014–1019. doi: 10.2337/diabetes.47.7.1014. [DOI] [PubMed] [Google Scholar]
- 53.Scarpace PJ, Matheny M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am J Physiol. 1998;275:E259–264. doi: 10.1152/ajpendo.1998.275.2.E259. [DOI] [PubMed] [Google Scholar]
- 54.Commins SP, Marsh DJ, Thomas SA, Watson PM, Padgett MA, Palmiter R, Gettys TW. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology. 1999;140:4772–4778. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- 55.Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology. 2006;147:2468–2480. doi: 10.1210/en.2005-1216. [DOI] [PubMed] [Google Scholar]
- 56.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 57.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahima RS. Leptin and the neuroendocrinology of fasting. Front Horm Res. 2000;26:42–56. doi: 10.1159/000061014. [DOI] [PubMed] [Google Scholar]
- 59.Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 60.Fekete C, Kelly J, Mihaly E, Sarkar S, Rand WM, Legradi G, Emerson CH, Lechan RM. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology. 2001;142:2606–2613. doi: 10.1210/endo.142.6.8207. [DOI] [PubMed] [Google Scholar]
- 61.Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 63.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Bronson FH. Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology. 1986;118:2483–2487. doi: 10.1210/endo-118-6-2483. [DOI] [PubMed] [Google Scholar]
- 65.Foster DL, Olster DH. Effect of restricted nutrition on puberty in the lamb: patterns of tonic luteinizing hormone (LH) secretion and competency of the LH surge system. Endocrinology. 1985;116:375–381. doi: 10.1210/endo-116-1-375. [DOI] [PubMed] [Google Scholar]
- 66.McShane TM, Keisler DH. Effects of dietary energy on ovarian function, estrogen suppression of luteinizing hormone and follicle-stimulating hormone, and competency of the gonadotropin surge. Biol Reprod. 1991;45:486–492. doi: 10.1095/biolreprod45.3.486. [DOI] [PubMed] [Google Scholar]
- 67.Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- 68.Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest. 2000;105:749–755. doi: 10.1172/JCI8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 71.Gruaz NM, Lalaoui M, Pierroz DD, Englaro P, Sizonenko PC, Blum WF, Aubert ML. Chronic administration of leptin into the lateral ventricle induces sexual maturation in severely food-restricted female rats. J Neuroendocrinol. 1998;10:627–633. doi: 10.1046/j.1365-2826.1998.00247.x. [DOI] [PubMed] [Google Scholar]
- 72.Wade GN, Lempicki RL, Panicker AK, Frisbee RM, Blaustein JD. Leptin facilitates and inhibits sexual behavior in female hamsters. Am J Physiol. 1997;272:R1354–1358. doi: 10.1152/ajpregu.1997.272.4.R1354. [DOI] [PubMed] [Google Scholar]
- 73.Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- 74.Henry BA, Goding JW, Tilbrook AJ, Dunshea FR, Clarke IJ. Intracerebroventricular infusion of leptin elevates the secretion of luteinising hormone without affecting food intake in long-term food-restricted sheep, but increases growth hormone irrespective of bodyweight. J Endocrinol. 2001;168:67–77. doi: 10.1677/joe.0.1680067. [DOI] [PubMed] [Google Scholar]
- 75.Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- 76.Barker-Gibb ML, Scott CJ, Boublik JH, Clarke IJ. The role of neuropeptide Y (NPY) in the control of LH secretion in the ewe with respect to season, NPY receptor subtype and the site of action in the hypothalamus. J Endocrinol. 1995;147:565–579. doi: 10.1677/joe.0.1470565. [DOI] [PubMed] [Google Scholar]
- 77.McDonald JK, Lumpkin MD, DePaolo LV. Neuropeptide-Y suppresses pulsatile secretion of luteinizing hormone in ovariectomized rats: possible site of action. Endocrinology. 1989;125:186–191. doi: 10.1210/endo-125-1-186. [DOI] [PubMed] [Google Scholar]
- 78.Morrison CD, Daniel JA, Hampton JH, Buff PR, McShane TM, Thomas MG, Keisler DH. Luteinizing hormone and growth hormone secretion in ewes infused intracerebroventricularly with neuropeptide Y. Domest Anim Endocrinol. 2003;24:69–80. doi: 10.1016/s0739-7240(02)00206-0. [DOI] [PubMed] [Google Scholar]
- 79.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- 81.Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- 82.Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav. 2001;73:229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- 84.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 85.Shalev U, Yap J, Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 87.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 88.Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 92.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 93.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 94.Getchell TV, Kwong K, Saunders CP, Stromberg AJ, Getchell ML. Leptin regulates olfactory-mediated behavior in ob/ob mice. Physiol Behav. 2006;87:848–856. doi: 10.1016/j.physbeh.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 95.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104:18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Couce ME, Burguera B, Parisi JE, Jensen MD, Lloyd RV. Localization of leptin receptor in the human brain. Neuroendocrinology. 1997;66:145–150. doi: 10.1159/000127232. [DOI] [PubMed] [Google Scholar]
- 99.Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71:187–195. doi: 10.1159/000054536. [DOI] [PubMed] [Google Scholar]
- 100.Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7:2635–2638. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- 101.Shioda S, Funahashi H, Nakajo S, Yada T, Maruta O, Nakai Y. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci Lett. 1998;243:41–44. doi: 10.1016/s0304-3940(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 102.Ur E, Wilkinson DA, Morash BA, Wilkinson M. Leptin immunoreactivity is localized to neurons in rat brain. Neuroendocrinology. 2002;75:264–272. doi: 10.1159/000054718. [DOI] [PubMed] [Google Scholar]
- 103.Martin B, Pearson M, Brenneman R, Golden E, Keselman A, Iyun T, Carlson OD, Egan JM, Becker KG, Wood W, 3rd, Prabhu V, de Cabo R, Maudsley S, Mattson MP. Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males. PLoS ONE. 2008;3:e2398. doi: 10.1371/journal.pone.0002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 107.Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 108.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. J Neurochem. 2005;95:396–405. doi: 10.1111/j.1471-4159.2005.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walker CD, Long H, Williams S, Richard D. Long-lasting effects of elevated neonatal leptin on rat hippocampal function, synaptic proteins and NMDA receptor subunits. J Neurosci Res. 2007;85:816–828. doi: 10.1002/jnr.21173. [DOI] [PubMed] [Google Scholar]
- 111.Shanley LJ, Irving AJ, Rae MG, Ashford ML, Harvey J. Leptin inhibits rat hippocampal neurons via activation of large conductance calcium-activated K+ channels. Nat Neurosci. 2002;5:299–300. doi: 10.1038/nn824. [DOI] [PubMed] [Google Scholar]
- 112.O'Malley D, Irving AJ, Harvey J. Leptin-induced dynamic alterations in the actin cytoskeleton mediate the activation and synaptic clustering of BK channels. FASEB J. 2005;19:1917–1919. doi: 10.1096/fj.05-4166fje. [DOI] [PubMed] [Google Scholar]
- 113.Irving AJ, Wallace L, Durakoglugil D, Harvey J. Leptin enhances NR2B-mediated N-methyl-D-aspartate responses via a mitogen-activated protein kinase-dependent process in cerebellar granule cells. Neuroscience. 2006;138:1137–1148. doi: 10.1016/j.neuroscience.2005.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 115.Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256:600–602. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- 116.Udagawa J, Hashimoto R, Suzuki H, Hatta T, Sotomaru Y, Hioki K, Kagohashi Y, Nomura T, Minami Y, Otani H. The role of leptin in the development of the cerebral cortex in mouse embryos. Endocrinology. 2006;147:647–658. doi: 10.1210/en.2005-0791. [DOI] [PubMed] [Google Scholar]
- 117.Udagawa J, Nimura M, Kagohashi Y, Otani H. Leptin deficiency causes pycnotic change in fetal cingulate cortical cells. Congenit Anom (Kyoto) 2006;46:16–20. doi: 10.1111/j.1741-4520.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 118.Udagawa J, Hatta T, Hashimoto R, Otani H. Roles of leptin in prenatal and perinatal brain development. Congenit Anom (Kyoto) 2007;47:77–83. doi: 10.1111/j.1741-4520.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- 119.Pannacciulli N, Le DS, Chen K, Reiman EM, Krakoff J. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci Lett. 2007;412:248–253. doi: 10.1016/j.neulet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 121.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 122.Oldreive CE, Harvey J, Doherty GH. Neurotrophic effects of leptin on cerebellar Purkinje but not granule neurons in vitro. Neurosci Lett. 2008;438:17–21. doi: 10.1016/j.neulet.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 123.O'Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35:559–572. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, Nisoli E. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem. 2006;281:12950–12958. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]
- 125.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 126.Signore AP, Zhang F, Weng Z, Gao Y, Chen J. Leptin neuroprotection in the CNS: mechanisms and therapeutic potentials. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang BL. Leptin as a neuroprotective agent. Biochem Biophys Res Commun. 2008;368:181–185. doi: 10.1016/j.bbrc.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 128.Fujita Y, Murakami M, Ogawa Y, Masuzaki H, Tanaka M, Ozaki S, Nakao K, Mimori T. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128:21–26. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoda MR, Keely SJ, Bertelsen LS, Junger WG, Dharmasena D, Barrett KE. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br J Surg. 2007;94:346–354. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- 130.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimabukuro M, Wang MY, Zhou YT, Newgard CB, Unger RH. Protection against lipoapoptosis of beta cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci U S A. 1998;95:9558–9561. doi: 10.1073/pnas.95.16.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 133.Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283:1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- 134.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- 135.Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- 136.Zhang F, Chen J. Leptin protects hippocampal CA1 neurons against ischemic injury. J Neurochem. 2008;107:578–587. doi: 10.1111/j.1471-4159.2008.05645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dicou E, Attoub S, Gressens P. Neuroprotective effects of leptin in vivo and in vitro. Neuroreport. 2001;12:3947–3951. doi: 10.1097/00001756-200112210-00019. [DOI] [PubMed] [Google Scholar]
- 138.Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:34479–34491. doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- 140.Valerio A, Dossena M, Bertolotti P, Boroni F, Sarnico I, Faraco G, Chiarugi A, Frontini A, Giordano A, Liou HC, De Simoni MG, Spano P, Carruba MO, Pizzi M, Nisoli E. Leptin Is Induced in the Ischemic Cerebral Cortex and Exerts Neuroprotection Through NF-{kappa}B/c-Rel-Dependent Transcription. Stroke. 2008 doi: 10.1161/STROKEAHA.108.528588. [DOI] [PubMed] [Google Scholar]
- 141.Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, Weisenfeld AE, Wong M, Yamada KA. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest. 2008;118:272–280. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gunstad J, Spitznagel MB, Keary TA, Glickman E, Alexander T, Karrer J, Stanek K, Reese L, Juvancic-Heltzel J. Serum leptin levels are associated with cognitive function in older adults. Brain Res. 2008;1230:233–236. doi: 10.1016/j.brainres.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 143.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang JS, Letendre S, Marquie-Beck J, Cherner M, McCutchan JA, Grant I, Ellis R. Low CSF leptin levels are associated with worse learning and memory performance in HIV-infected men. J Neuroimmune Pharmacol. 2007;2:352–358. doi: 10.1007/s11481-007-9093-z. [DOI] [PubMed] [Google Scholar]
- 145.Paz-Filho GJ, Babikian T, Asarnow R, Esposito K, Erol HK, Wong ML, Licinio J. Leptin replacement improves cognitive development. PLoS ONE. 2008;3:e3098. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ohta R, Shigemura N, Sasamoto K, Koyano K, Ninomiya Y. Conditioned taste aversion learning in leptin-receptor-deficient db/db mice. Neurobiol Learn Mem. 2003;80:105–112. doi: 10.1016/s1074-7427(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 147.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 148.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 149.Paulus K, Schulz C, Lehnert H. Central nervous effects of leptin and insulin on hippocampal leptin and insulin receptor expression following a learning task in Wistar rats. Neuropsychobiology. 2005;51:100–106. doi: 10.1159/000084167. [DOI] [PubMed] [Google Scholar]
- 150.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab. 2003;285:E10–15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- 152.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 153.Ladyman SR. Leptin resistance during pregnancy in the rat. J Neuroendocrinol. 2008;20:269–277. doi: 10.1111/j.1365-2826.2007.01628.x. [DOI] [PubMed] [Google Scholar]
- 154.Morrison CD. Leptin resistance and the response to positive energy balance. Physiol Behav. 2008;94:660–663. doi: 10.1016/j.physbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scarpace PJ, Zhang Y. Elevated leptin: consequence or cause of obesity? Front Biosci. 2007;12:3531–3544. doi: 10.2741/2332. [DOI] [PubMed] [Google Scholar]
- 156.Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–1117. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 157.Pal R, Sahu A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology. 2003;144:3789–3798. doi: 10.1210/en.2002-0148. [DOI] [PubMed] [Google Scholar]
- 158.Krol E, Duncan JS, Redman P, Morgan PJ, Mercer JG, Speakman JR. Photoperiod regulates leptin sensitivity in field voles, Microtus agrestis. J Comp Physiol [B] 2006;176:153–163. doi: 10.1007/s00360-005-0037-8. [DOI] [PubMed] [Google Scholar]
- 159.Krol E, Tups A, Archer ZA, Ross AW, Moar KM, Bell LM, Duncan JS, Mayer C, Morgan PJ, Mercer JG, Speakman JR. Altered expression of SOCS3 in the hypothalamic arcuate nucleus during seasonal body mass changes in the field vole, Microtus agrestis. J Neuroendocrinol. 2007;19:83–94. doi: 10.1111/j.1365-2826.2006.01507.x. [DOI] [PubMed] [Google Scholar]
- 160.White CL, Whittington A, Barnes MJ, Wang ZQ, Bray G, Morrison CD. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and independent mechanisms. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 163.Ladyman SR, Grattan DR. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology. 2004;145:3704–3711. doi: 10.1210/en.2004-0338. [DOI] [PubMed] [Google Scholar]
- 164.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 165.Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. Differential mechanisms and development of leptin resistance in A/J versus C57BL/6J mice during diet-induced obesity. Endocrinology. 2003;144:1155–1163. doi: 10.1210/en.2002-220835. [DOI] [PubMed] [Google Scholar]
- 166.Harle P, Straub RH. Leptin is a link between adipose tissue and inflammation. Ann N Y Acad Sci. 2006;1069:454–462. doi: 10.1196/annals.1351.044. [DOI] [PubMed] [Google Scholar]
- 167.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 168.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]