Abstract
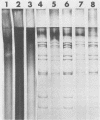
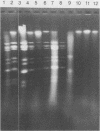
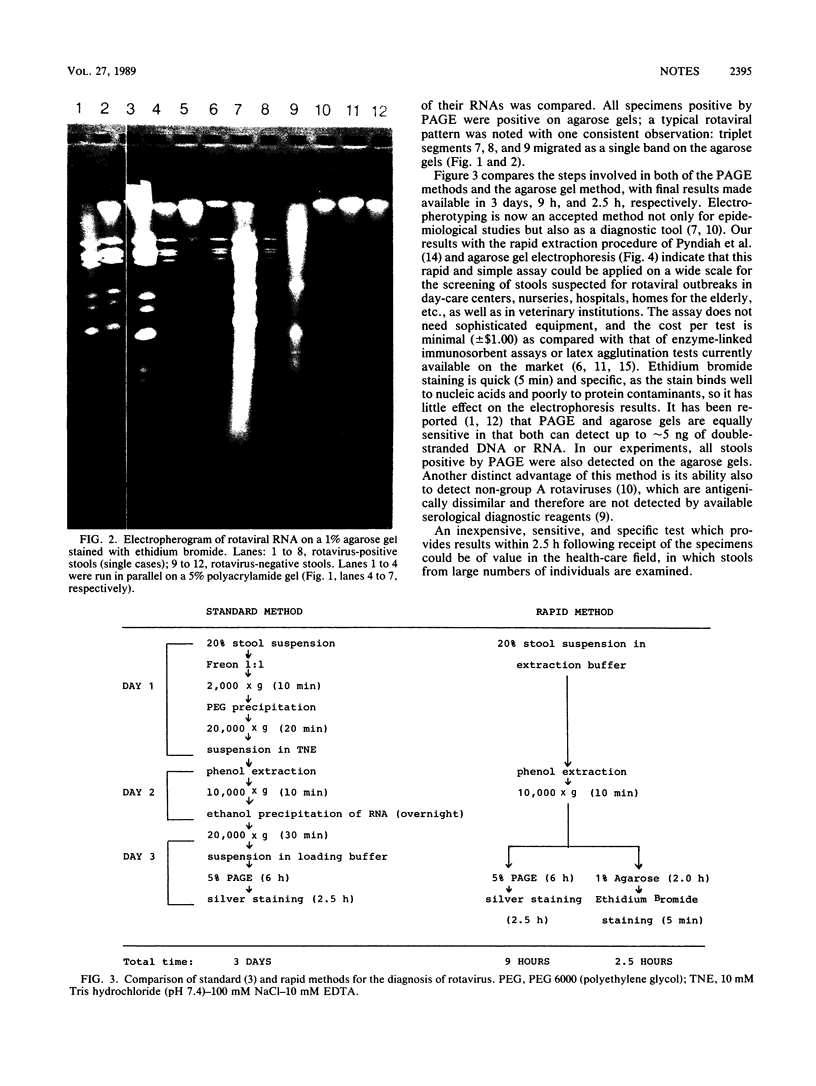
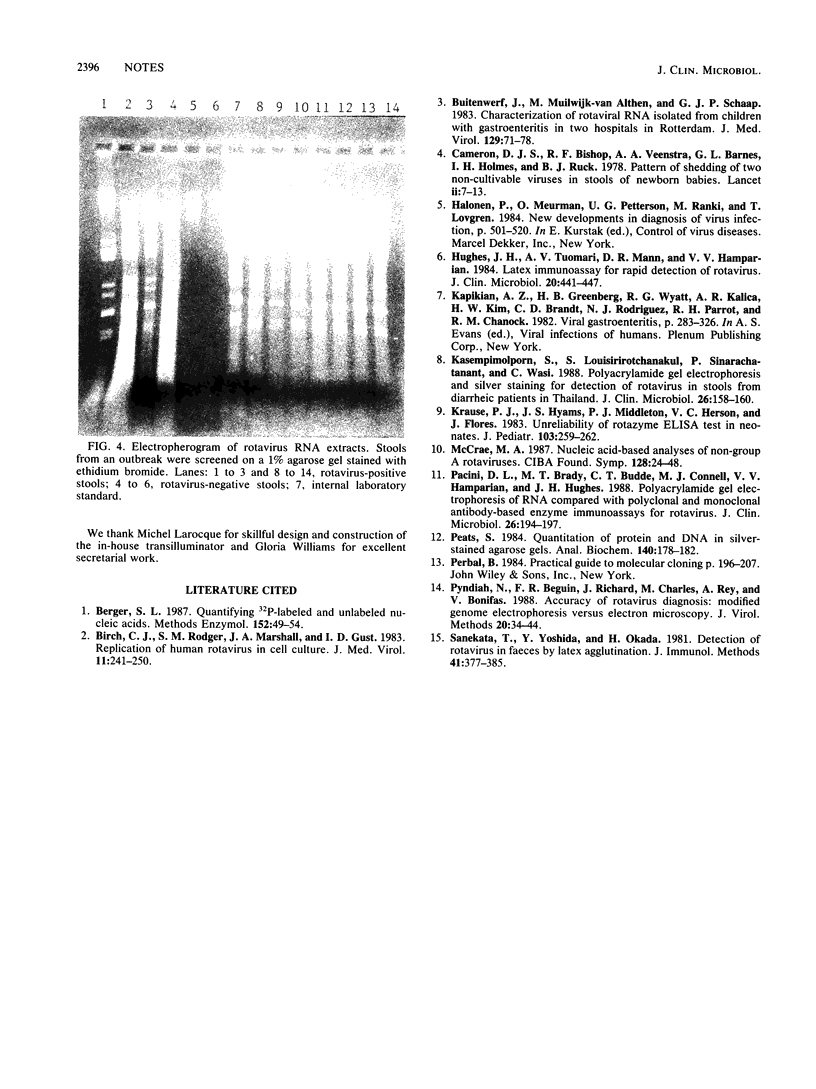
The early diagnosis of human rotavirus infection is essential for effective patient management and infection control. We report here a rapid, easy-to-perform, and inexpensive test for rotavirus detection. The viral RNA is extracted directly from the stools and electrophoresed on 1% agarose gels. Currently available immunoassays for routine diagnostic purposes are directed at the common group A-specific antigen. As reports become available on human gastroenteritis caused by the atypical or novel rotaviruses, this technique presents an added advantage in that it can detect both group A and non-group A rotaviruses.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L. Quantifying 32P-labeled and unlabeled nucleic acids. Methods Enzymol. 1987;152:49–54. doi: 10.1016/0076-6879(87)52009-2. [DOI] [PubMed] [Google Scholar]
- Birch C. J., Rodger S. M., Marshall J. A., Gust I. D. Replication of human rotavirus in cell culture. J Med Virol. 1983;11(3):241–250. doi: 10.1002/jmv.1890110307. [DOI] [PubMed] [Google Scholar]
- Buitenwerf J., Nuilwijk-van Alphen M., Schaap G. J. Characterization of rotaviral RNA isolated from children with gastroenteritis in two hospitals in Rotterdam. J Med Virol. 1983;12(1):71–78. doi: 10.1002/jmv.1890120108. [DOI] [PubMed] [Google Scholar]
- Cameron D. J., Bishop R. F., Veenstra A. A., Barnes G. L., Holmes I. H., Ruck B. J. Pattern of shedding of two noncultivable viruses in stools of newborn babies. J Med Virol. 1978;2(1):7–13. doi: 10.1002/jmv.1890020103. [DOI] [PubMed] [Google Scholar]
- Hughes J. H., Tuomari A. V., Mann D. R., Hamparian V. V. Latex immunoassay for rapid detection of rotavirus. J Clin Microbiol. 1984 Sep;20(3):441–447. doi: 10.1128/jcm.20.3.441-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasempimolporn S., Louisirirotchanakul S., Sinarachatanant P., Wasi C. Polyacrylamide gel electrophoresis and silver staining for detection of rotavirus in stools from diarrheic patients in Thailand. J Clin Microbiol. 1988 Jan;26(1):158–160. doi: 10.1128/jcm.26.1.158-160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. J., Hyams J. S., Middleton P. J., Herson V. C., Flores J. Unreliability of Rotazyme ELISA test in neonates. J Pediatr. 1983 Aug;103(2):259–262. doi: 10.1016/s0022-3476(83)80361-8. [DOI] [PubMed] [Google Scholar]
- McCrae M. A. Nucleic acid-based analyses of non-group A rotaviruses. Ciba Found Symp. 1987;128:24–48. doi: 10.1002/9780470513460.ch3. [DOI] [PubMed] [Google Scholar]
- Pacini D. L., Brady M. T., Budde C. T., Connell M. J., Hamparian V. V., Hughes J. H. Polyacrylamide gel electrophoresis of RNA compared with polyclonal- and monoclonal-antibody-based enzyme immunoassays for rotavirus. J Clin Microbiol. 1988 Feb;26(2):194–197. doi: 10.1128/jcm.26.2.194-197.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peats S. Quantitation of protein and DNA in silver-stained agarose gels. Anal Biochem. 1984 Jul;140(1):178–182. doi: 10.1016/0003-2697(84)90150-7. [DOI] [PubMed] [Google Scholar]
- Pyndiah N., Béguin R., Richard J., Charles M., Rey A., Bonifas V. Accuracy of rotavirus diagnosis: modified genome electrophoresis versus electron microscopy. J Virol Methods. 1988 May;20(1):39–44. doi: 10.1016/0166-0934(88)90038-9. [DOI] [PubMed] [Google Scholar]
- Sanekata T., Yoshida Y., Okada H. Detection of rotavirus in faeces by latex agglutination. J Immunol Methods. 1981;41(3):377–385. doi: 10.1016/0022-1759(81)90199-x. [DOI] [PubMed] [Google Scholar]