Abstract
In rats and primates, the central nucleus of the amygdala (CeN) is most known for its role in responses to fear stimuli. Recent evidence also shows that the CeN is required for directing attention and behaviors when the salience of competing stimuli are in flux. To examine how information flows through this key output region of the primate amygdala, we first placed small injections of retrograde tracers into the subdivisions of the central nucleus and examined inputs from specific amygdaloid nuclei. The amygdalostriatal area and interstitial nucleus of the posterior limb of the anterior commissure (IPAC) were distinguished from the CeN using histochemical markers, and projections to these regions were also described. As expected, the basal nucleus and accessory basal nucleus are the main afferent connections of the central nucleus and transition zones. The medial subdivision of the central nucleus (CeM) receives a significantly stronger input from all regions compared to the lateral core subdivision (CeLcn). The corticoamygdaloid transition zone (a zone of confluence of the medial parvicellular basal nucleus, paralaminar nucleus, and the sulcal periamygdaloid cortex) provides the main input to the CeLcn. The IPAC and amygdalostriatal area can be divided in medial and lateral subregions, and receive input from the basal and accessory basal nucleus, with differential inputs according to subdivision. The pirifom cortex and lateral nucleus, two important sensory interfaces, send projections to the transition zones. In sum, the CeM receives broad inputs from the entire amygdala, whereas the CeLcn receives more restricted inputs from the relatively undifferentiated corticoamygdaloid transition region. Like the CeN, the transition zones receive most of their input from the basal nucleus and accessory basal nucleus, however, inputs from the piriform cortex and lateral nucleus, and a lack of input from the parvicellular accessory basal nucleus, are distinguishing afferent features.
Keywords: extended amygdala, interstitial nucleus of the posterior limb of the anterior commissure, piriform cortex, amygdalostriatal area, fundus striatii
The central nucleus of the amygdala (CeN) is a major output region of the amygdaloid complex. It is best known for its role in mediating autonomic and visceral responses to emotional stimuli through projections to the hypothalamus and brainstem (Hopkins, 1975, Price and Amaral, 1981, Fudge and Haber, 2000). A large literature shows that the CeN mediates the expression of freezing and startle to aversive stimuli (Kapp et al., 1979, Hitchcock and Davis, 1991, Liang et al., 1992, Campeau and Davis, 1995, Salinas et al., 1996, Killcross et al., 1997, Kalin et al., 2001). However, recent studies using reversible inactivation of the CeN also indicate a role in fear learning (Samson et al., 2005, Wilensky et al., 2006). In addition to its role in fear learning and responses, the CeN is required for allocating attention to positive cues when the predictive value changes unexpectedly (when the animal is ‘surprised’) (Holland and Gallagher, 2006, Lee et al., 2006). In support of a role in responses to unpredicted stimuli, the CeN is required for inhibiting approach when a reward is unexpectedly coupled with an aversive stimulus (Kalin et al., 2004, Petrovich et al., 2006, Seamans et al., 2006).
The CeN has two main regions, the lateral core subdivision (CeLcn), and medial subdivision (CeM) (Price et al., 1987, DeOlmos, 1990, Martin et al., 1991), that have specific interconnections. The CeLcn projects in a mainly unidirectional fashion to the CeM, and is an intrinsic modulator of the CeM (Pascoe and Kapp, 1985, Jolkkonen and Pitkanen, 1998, Cassell et al., 1999, Dumont et al., 2002, Huber et al., 2005). Beyond this intrinsic connection, the CeLcn has relatively restricted outputs to the lateral parabrachial nucleus and bed nucleus of the stria terminalis. In contrast, the CeM—which is modulated by the CeLcn--projects broadly outside the amygdala, to targets in midline thalamus, lateral hypothalamus, and brainstem (Hopkins, 1975, Price and Amaral, 1981, Kita and Oomura, 1982, Cassell et al., 1986, Gray and Magnuson, 1987, Gray and Magnuson, 1992, Fudge and Haber, 2000). Transitional regions between the CeN and striatum, known as the amygdalostriatal area and the interstitial nucleus of the posterior limb of the anterior commissure (IPAC), also play a role in emotional responses (deOlmos and Ingram, 1972, LeDoux et al., 1990, Heimer et al., 1997, Shammah-Lagnado et al., 1999, Wang et al., 2002). In rats, these transition zones receive many of the same inputs as the central nucleus, but have some efferent features of the striatopallidal system (Fuller et al., 1987, LeDoux et al., 1990, Zahm, 1998, McDonald et al., 1999, Shammah-Lagnado et al., 1999, Shammah-Lagnado et al., 2001b). Our previous investigation of the amygdalostriatal region in primates indicates that this complex region has medial and lateral sectors that resemble the CeN and striatum, respectively, based on histochemical and cellular features (Fudge and Haber, 2002). However, little is known of the connectivity of this region, or of the zone encompassing the putative IPAC.
Our goal was to determine whether there are differential inputs to the CeM, CeLcn, amygdalostriatal area, and IPAC from specific amygdaloid nuclei in the primate. We first placed small injections of retrograde tracers into the CeN, IPAC and amygdalostriatal area, and analyzed the subdivision-specific distribution of retrogradely labeled cells in discrete amygdala nuclei. Results were then confirmed by anterograde tracing studies.
EXPERIMENTAL PROCEDURES
INJECTION SITES
We placed small injections (40 nL) of Lucifer Yellow conjugated to dextran amine (LY; 10% Molecular Probes, Eugene, OR), fluororuby conjugated to dextran amine (FR; 4%, Molecular Probes); fluorescein conjugated to dextran amine (FS: 10%, Molecular Probes) into the central nucleus, amygdalostriatal area and IPAC as previously described (Fudge et al., 2004). Control injections into the caudal ventral striatum, performed as part of previous studies (Fudge et al., 2004, Fudge et al., 2005b), were used for comparison. Injections of LY, FR, and FS, and tritated amino acids (AA; 1:1 ratio of tritiated proline and leucine: Perkin Elmer, Boston, MA) to be used for anterograde analysis were then placed into various amygdaloid nuclei. Previous studies have indicated that there is no cross reactivity of antibodies to FR, FS, WGA, and LY (Haber et al., 2000).
SURGERIES
Twelve Old world primates (Macaca fascicularis and Macaca nemestrina) weighing between 3.0 and 9.0 kg. were used for these studies (Labs of Virginia, Yemassee, SC; Three Springs Laboratories, Pekaski, PA, and Worldwide Primates, Talahassee, FL). Control data from several injection sites were part of a previous study, as noted above. All experiments were carried out in accordance with National Institutes of Health guidelines. Experimental design and techniques were aimed at minimizing animal use and were reviewed by the University of Rochester Committee on Animal Research. Initial anesthesia was administered by intramuscular injections of ketamine hydrochloride (10 mg/kg). Animals were then intubated, and a deep plane of anesthesia was induced by intravenous infusion of pentobarbital (initial dose 20 mg/kg) and maintained as needed during surgery. A craniotomy was performed and the bone flap removed to visualize cortical surface landmarks. Electrophysiologic mapping was carried out to locate internal structures such as the anterior commissure, boundaries of the striatum and globus pallidus, and the amygdala. After stereotaxic coordinates for the boundaries of these structures were located, the location of the CeM, CeLcn and IPAC was estimated. Small deposits of retrograde tracer were pressure-injected over 10-15 minutes into the CeN using a 0.5 μL Hamilton syringe. Only one injection of each tracer was used per animal. Following each injection, the syringe remained in place for 20 minutes to prevent leakage of tracer up the syringe track. Following analysis of the retrograde tracing studies, injections of FR, LY, FS (40-50 nL) and AA (200 nL) were injected into the amygdala in a separate group of animals to confirm retrograde results. Following injection placement for all animals, the bone flap was replaced, and the overlying musculature and skin sutured. Animals were treated with prophylactic antibiotics and pain medication for 7-10 days post-operatively.
Ten to thirteen days after surgery, animals were deeply anesthetized and killed by perfusion through the heart with 0.9% saline containing 0.5 mL of heparin sulfate (200 mL/minute for 10 minutes), followed by cold 4% paraformaldehyde in a 0.1 M phosphate buffer/30% sucrose solution (100 mL/minute for one hour). The brain was retrieved, placed in fixative overnight, and then sunk in increasing gradients of sucrose (10%, 20%, and 30%). Brains were then cut on a freezing microtome (50 μm sections) and saved in cryoprotectant solution (30% ethylene glycol and 30% sucrose in 0.1 M phosphate buffer) at -20°C (Rosene et al., 1986). Adjacent sections through the striatum, IPAC, and amygdala were selected for labeling for tracer and histochemical markers (see below) to determine subregions of these structures. We used staining with cresyl violet and acetylcholinesterase (AChE), as well as immunocytochemistry for dopamine transporter (DAT), calbindin-D28k (CaBP) and parvalbumin (PV), to determinine boundaries of the various amygdaloid nuclei, and to differentiate CeN subdivisions (Price et al., 1987, Amaral and Bassett, 1989, Jolkkonen et al., 2001b, Fudge and Haber, 2002) (Cote et al., 1991, Heimer et al., 1999, Freedman and Shi, 2001).
IMMUNOCYTOCHEMISTRY
Tracers
Sections were thoroughly rinsed in 0.1 M phosphate buffer (pH 7.2) with 0.3% Triton-X (PB-TX). After treatment with endogenous peroxidase inhibitor for 5 minutes, followed by more rinses, sections were pre-incubated in a blocking solution of 10% normal goat serum in 0.1M PB-TX (NGS-PB-TX) for 30 minutes. Tissue was then placed in primary antisera to LY (1:2000, Molecular Probes, rabbit), FS (1:2000, Molecular Probes, rabbit), and FR (1:1000, Molecular Probes, rabbit) for approximately 96 hours at 4 °C. After thorough rinsing with 0.1 M PB-TX, and pre-incubation with 10% NGS-PB-TX, sections were then incubated in biotinylated secondary anti-rabbit antibody. Tracers were then visualized using the avidin-biotin reaction (Vector ABC Standard kit, Burlingame, CA). Visualization of the tracer wheat-germ agglutinin conjugated to horseradish peroxidase was previously performed for control injection (cases J8WGA, J6WGA, J12WGA, and J4WGA) using similar techniques (Fudge et al., 2004). Additional compartments for each case were also processed for tracer, enhanced with nickel intensification (3, 3’-diaminobenzidine tetrahydrochloride with 1% nickel ammonium sulfate and 1% cobalt chloride, catalyzed by 0.03% hydrogen peroxide for 1-2 minutes) and counterstained with AChE (Geneser-Jensen and Blackstad, 1971) or cresyl violet.
CaBP-immunoreactivity (IR), PV-IR, DAT-IR, and AChE staining
DAT-, CaBP-, and PV-IR, and AchE activity were used to distinguish the CeM and CeLcn, to delineate them from the surrounding transition regions. These markers were chosen based on previous delineations of the CeN subdivisions in rats and primates (Price et al., 1987, Amaral and Bassett, 1989, Cote et al., 1991, Martin et al., 1991, Heimer et al., 1999, Freedman and Shi, 2001, Jolkkonen et al., 2001b, Fudge and Haber, 2002). Sections were thoroughly rinsed, and preincubated in 10% NGS-PB-TX as described above, and then incubated for 96 hours in parvalbumin (Swant, Basel, Switzerland, 1:5000), DAT (1:10,000, Chemicon, Temucula, CA, mouse), or CaBP (Chemicon, 1:10,000, mouse) antisera. Sections were then rinsed, blocked and incubated in secondary anti-mouse biotinylated antibody. Following thorough rinsing DAT, CaBP, and PV proteins were visualized using the avidin-biotin reaction described above. AChE staining was performed using the Geneser technique (Geneser-Jensen and Blackstad, 1971).
AUTORADIOGRAPHY
Sections for autoradiography were mounted on chrome-alum gelatin-coated slides and cleared in xylene 48 hours. Slides were then dipped in Kodak NTB2 photographic emulsion (Kodak, Rochester, NY) and exposed for 4-6 months in a light-tight box at -20°C. Slides were then developed in cold Kodak D19 developer (15 °C) for 2.5 minutes, fixed, washed, and counterstained with cresyl violet.
ANALYSIS
Cases containing retrograde tracer injections that included the optic tract, globus pallidus, or other structures outside the amygdala were excluded from the analysis. Remaining cases were examined for labeling in the striatum and subthalamic nucleus, to further rule out involvement of the striatum and globus pallidus in the injection sites. Placement of the retrograde injection sites within CeN subdivisions was determined by examining 1:8 sections through the amygdala. The distribution of labeled cells in the amygdala was then charted by hand, using camera lucida techniques under brightfield microscopy with a 10x objective. Charts were then transferred into digital form using a drawing tablet interfaced with the program Adobe Illustrator CS2 (Adobe Systems, San Jose, CA). The boundaries of various amygdaloid subregions, along with identifying landmarks such as blood vessels and fiber tracks, was drawn from adjacent sections stained with cresyl violet or AChE. These hand-drawn charts were also entered into the computer and carefully aligned over charts of labeled cells matching landmarks such as blood vessels and fiber tracts for reference. Counterstained sections were used to confirm these data.
The location of anterograde injection sites in specific amygdala nuclei was determined using adjacent sections stained for either AChE or cresyl violet, as well as counterstained sections. The distribution of labeled fibers in the CeN subdivisions and transitional zones was performed using a 20x objective in conjunction with bright-field and dark-field illumination. Hand-drawn charts were created using camera lucida techniques. These were subsequently scanned into the computer using Adobe Photoshop CS2, and imported into an Adobe Illustrator CS2 format. The distribution of labeled fibers in specific CeN subdivisions was evaluated using adjacent sections stained for AChE or cresyl violet, or immunolabeled for DAT and CaBP immunoreactivity. In some cases, additional sections processed for tracer and counterstained with cresyl violet and/or AChE were used to confirm the projection.
RESULTS
Amygdaloid nuclei (Fig. 1)
Fig.1.
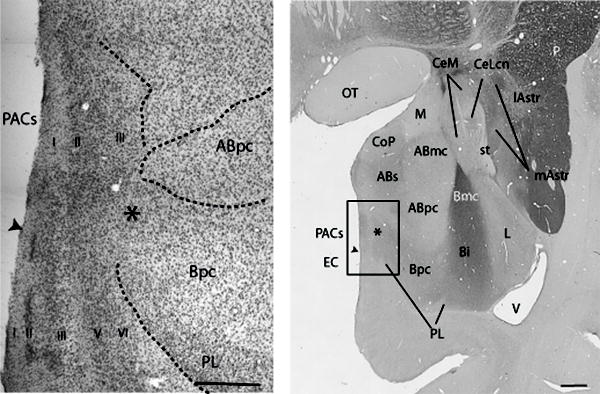
Low power photomicrograph of a coronal section through the amygdala, following AChE staining. Asterisk is located at the corticoamygdaloid transition zone, an undifferentiated region where the PACs, medial Bpc, and PL merge. Bar = 1 mm. Inset: High power photomicrograph of the corticoamygdaloid transition zone, stained with cresyl violet in an adjacent section. Bar = 500 μm.
The nuclear regions of the amygdala outside the CeN are described according to the nomenclature of Price, Amaral, and colleagues (Price et al., 1987, Amaral et al., 1992). The lateral nucleus is composed of dorsal, dorsal intermediate, ventral and ventral intermediate subdivisions which are recognized based on their cellular features and AChE staining (Pitkanen and Amaral, 1998). The basal nucleus is subdivided into magnocellular (Bmc), intermediate (Bi) and parvicellular subdivisions (Bpc), which are discernible based on Nissl and AChE staining differences. AChE activity is highest in the magnocellular subdivision and declines along a gradient in the intermediate and parvicellular subdivisions (Amaral and Bassett, 1989). The paralaminar nucleus (PL), which has been considered part of the parvicellular subdivision of the basal nucleus, is AChE poor, and is best seen with Nissl staining (Fig. 1 inset). This nucleus forms a thin sheet surrounding the basal nucleus rostrally and caudally. The intercalated islands are clusters of small cells interposed between the major nuclei, and are darkly stained in Nissl preparations. The accessory basal nucleus is composed of magnocellular (ABmc), parvicellular (ABpc), and ‘sulcal’ (ABs) subdivisions. The magnocellular and sulcal subdivisions are distinguished by relatively higher AChE staining compared to the parvicellular subdivision. The sulcal subdivision of the accessory basal nucleus is found caudally, and is apposed to the caudomedially adjacent amygdalohippocampal area (also high in AChE). Its inclusion in the amygdalohippocampal area (AHA) has been suggested based on some neurochemical and connectional similarities (Amaral and Bassett, 1989, Pitkanen and Amaral, 1998). The AHA is included in the ‘corticoamygdaloid transition region’ (see below), and is characterized by high AChE staining, similar to the ABs. As it progresses towards the rostral hippocampus, the AHA becomes more laminar and forms a cortical bridge between the amygdala and emerging rostral hippocampus (subiculum and CA1). The more laminar, differentiated character of this ‘bridge’ has lead to a separate designation as the hippocampal-amygdaloid transition area (HATA) (Rosene and Van Hoesen, l987).
The piriform cortex is also found along the medial wall of the temporal lobe, and is anterior to the anterior cortical nucleus (not shown). It is recognized by its thick, tortuous layer II, and moderate AChE activity. While the piriform cortex is not part of the amygdala proper, it is closely associated with the ‘superficial nuclei’ of the amygdala (Carmichael et al., 1994), and is included in the present analysis because of its strong projection to the CeN based on studies in rats (McDonald et al., 1999). The ‘superficial nuclei’ form the medial aspect of the amygdala and include the anterior and posterior cortical nuclei (CoA and CoP, respectively), the medial nucleus, and the periamygdaloid cortex (PAC). Each has distinct cellular features. The periamygdaloid cortex is divided into four zones with varying degrees of differentiation (PAC I-III and PACs). The sulcal subdivision (PACs) is located around the amygdaloid fissure (semiannular sulcus), and is the least differentiated. It merges with the medial parvicellular basal nucleus (including the paralaminar nucleus) and caudoventrally adjacent entorhinal cortex, to form the ‘corticoamygdaloid transition zone’ (asterisk, Fig. 1 and inset). The uniqueness of the ‘corticoamygdaloid transition zone’ in primates has long been recognized (Crosby and Humphrey, 1941, DeOlmos, 1990, Sims and Williams, 1990) (this region has also been referred to as the amygdalopiriform transition region(DeOlmos, 1990)). In contrast to the rostrally adjacent periamygdaloid cortex, which has a clear three-layer organization, the corticoamygdaloid transition zone has a much less organized layering scheme. Along with the loss of clearly defined layers, the area is recognized by clusters of small granule-type cells that occupy its deepest part, apposed to the medial Bpc/paralaminar region. These small cells resemble those in the laterally adjacent paralaminar region (where they are in a more ‘sheet-like’ formation), and in the caudally adjacent entorhinal cortex (olfactory subdivision) where they appear in clusters in layer II of this five-layered cortex. The appearance of a laminar layer II (Amaral et al., 1987, Beall and Lewis, 1992) is used as criteria for the beginning of the entorhinal cortex, olfactory subdivision (Fig. 1 inset, arrowhead).
The CeM, CeLcn, Astr area, and IPAC (Fig. 2, Table 1)
Fig. 2.
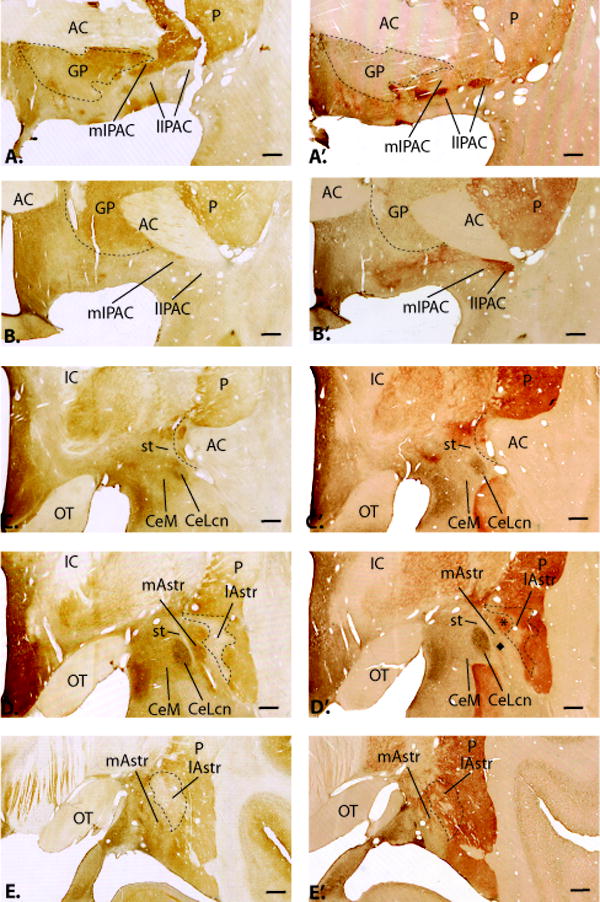
Adjacent coronal sections through the primate transition zones, arranged in rostrocaudal order. A-E. Sections immunoreacted for calbindin-D28k (brown) and bcl-2 (black). A’-E’. Adjacent sections processed for bcl-2-IR (black) and AChE activity (red). The lateral IPAC and lateral amygdalostriatal area have relatively high levels of AChE and very low CaBP-IR compared to the medial IPAC and medial amygdalostriatal area, which have low AChE and moderate to high CaBP-IR. Bars = 1 mm.
Table 1.
| CEM | CELCN | MASTR | MIPAC | LASTR | LIPAC | |
|---|---|---|---|---|---|---|
| AChE | ++ | + | + | + | +++ | +++ |
| DAT-ir | ++ | - | - | - | ++++ | ++++ |
| CABP-ir | ++ | +++ | ++ | ++ | - | - |
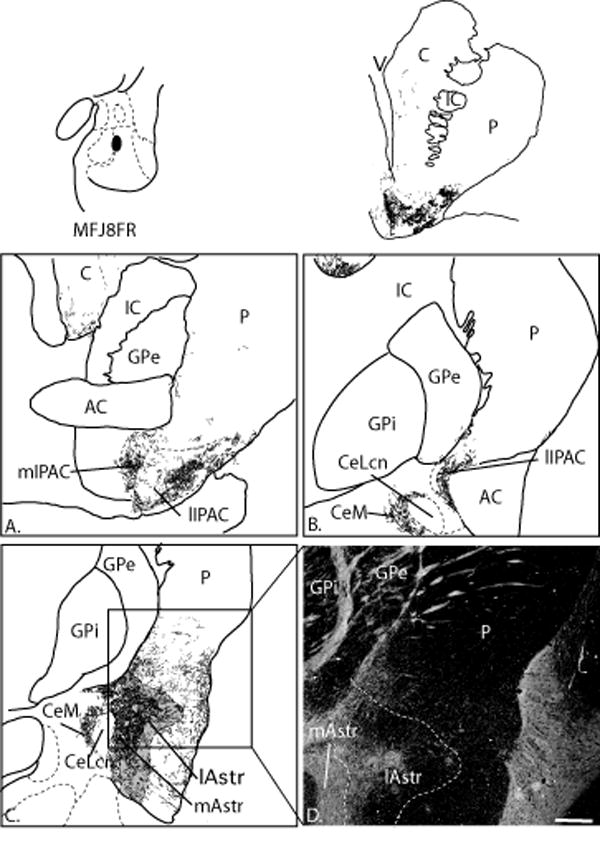
The CeN subdivisions are described using the nomenclature of deOlmos (DeOlmos, 1990) and Martin (Martin et al., 1991) based on work in primates. The amygdalostriatal area is subdivided into medial and lateral areas, based on cellular and histochemical criteria previously described (Fudge and Haber, 2002). The IPAC nomenclature is adapted from Alheid and colleagues, based on histochemical features noted in the rat (Alheid et al., 1995, Shammah-Lagnado et al., 2001a). The anterior amygdaloid area is a diffuse region that lies rostral to the central nucleus.
The CeM is a funnel-shaped, heterogeneous group of cells that sweeps medially around the CeLcn and into the basal forebrain (Fig. 2C, C’-E, E’). It contains moderate AChE activity, with thick AChE-positive passing fibers. The CeLcn, or lateral central core, is an oval structure encapsulated by fibers of the stria terminalis (Fig. 2 C, C’-E, E’). In contrast to the CeM, it contains very low AChE activity and is distinguished by a relative absence of DAT-IR compared to the CeM (DeOlmos, 1990, Sakamoto et al., 1999, Freedman and Shi, 2001, Fudge and Haber, 2002). The CeLcn is also characterized by strong cellular immunoreactivity for bcl-2 (a neuroprotective molecule), a localization which appears unique to higher primate species (seen as dark brown stain in Fig. 2) (Fudge and Haber, 2002, Fudge, 2004). Immediately lateral to the CeLcn is a heterogeneous region we have termed the ‘medial amygdalostriatal area’ (Fig. 2 D, D’-E, E’). This region contains heterogeneous islands of medium-sized neurons that resemble those in the CeLcn and striatum (‘paracapsular islands’) based on histochemical and cellular criteria (DeOlmos, 1990) (Fudge and Haber, 2002). In addition to the medium-sized cells of the paracapsular islands, clusters of intercalated (granular and parvicellular) neurons populate the medial amygdalostriatal area. The heterogeneous cell islands of the medial amygdalostriatal area are embedded in a matrix of low AChE and DAT staining, and moderate CaBP-IR, similar to the CeLcn. The lateral amygdalostriatal area is a broad region marked by low CaBP-IR, and relatively higher AChE and DAT staining IR (Fig 2C, C’-D, D’). Based on this pattern of immunoreactivity, we previously proposed that the lateral amygdalostriatal area is analogous to the shell of the ventral striatum (Fudge and Haber, 2002).
The IPAC is the region surrounding the posterior limb of the anterior commissure based on studies in rodent. A similar region exists in the primate (Fig. 2A, A’-B, B’), where a lateral zone of very high AChE activity and DAT-IR are consistent with descriptions of lateral IPAC in rats (Shammah-Lagnado et al., 2001a). Medial IPAC regions adjacent to the globus pallidus have moderate AChE and DAT levels, also consistent with descriptions in rodents. In addition, we found that the medial and lateral aspect of this IPAC-like region contained relatively high and low levels of CaBP-IR, respectively, paralleling corresponding patterns in the medial and lateral amygdalostriatal area (Table 1). Parvalbumin immunoreactivity, which distinguishes the IPAC from the amygdalostriatal region in rodents (Jolkkonen et al., 2001b), was uniformly low to absent throughout the monkey IPAC, amygdalostriatal area, and CeN subdivisions and was therefore not a useful marker in this species.
RETROGRADE CASES
Injection site placement (Fig. 3A and B)
Fig. 3.
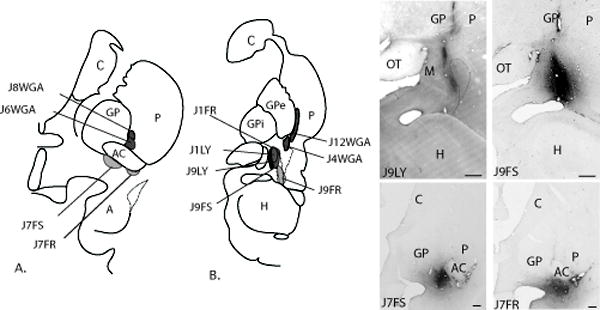
A-B. Schematic of the placement of retrograde injections in the CeN, transition zones, and ventromedial putamen. Photomicrographs show examples of four injection sites. Tract marks reflect nonspecific DAB staining due to gliosis and there is no tracer uptake associated with them. Bars = 1 mm.
There were ten retrograde injection sites. Two retrograde injection sites were confined to the encapsulated AChE-poor lateral core subregion (CeLcn), and did not encroach on adjacent CeM or amygdalostriatal structures (Fig. 3B, cases J1LY and J9FS). One injection site was confined to the CeM (case J9LY), and one injection site included both CeM and CeLcn (case J1FR) (Fig. 3B). Two injection sites were located in the IPAC (cases J7FR and J7FS) (Fig. 3A). The injection site in case J7FS was centered in the medial IPAC, with some involvement of the lateral IPAC. The injection site in case J7FR was rostral and lateral to the injection site in case J7FS. Neither IPAC injection resulted in labeled cells in the subthalamic nucleus, ruling out uptake of tracer in the globus pallidus. One retrograde injection site encompassed the medial and lateral amygdalostriatal area at the level of the caudal amygdala/rostral hippocampus, and has previously been described (case J9FR) (Fudge et al., 2004). Four retrograde tracer injections in the caudal ventromedial putamen were included in the analysis for comparison (J8WGA J6WGA, J4WGA and J12 WGA) (see detailed descriptions (Fudge et al., 2004, Fudge et al., 2005b)).
Distribution of retrogradely labeled cells
CeM: Case J9LY (Fig. 4 A-F) and CeM/CeLcn J1FR (not shown)
Fig. 4.
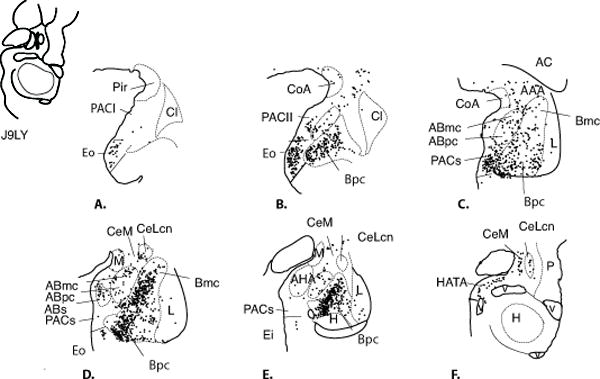
Charts of retrogradely labeled cells in the amygdala following injections in the CeM (Case J9LY, A- F.)
The pattern of labeled cells in the two cases was similar, and case J9LY is described in detail. At rostral levels, the piriform cortex and PAC II contained few labeled cells, however, there were many labeled cells in PAC I and rostral PACs. The Bi and Bpc contained many labeled cells, with a more moderate concentration in the Bmc. Densest concentrations of labeled cells were aggregated in the medial half of the Bi and Bpc, and there was an especially dense concentration of labeled cells in the medial paralaminar region (Fig. 4C and D). The ABmc and Abpc contained moderately dense numbers of labeled cells. Caudally, the ABs and AHA also contained many LY-positive cells (Fig. 4D and F). There were scattered labeled cells in the lateral nucleus (Fig. 4F).
CeLcn: Case J9FS (Fig. 5A-F) and J1LY (not shown)
Fig. 5.
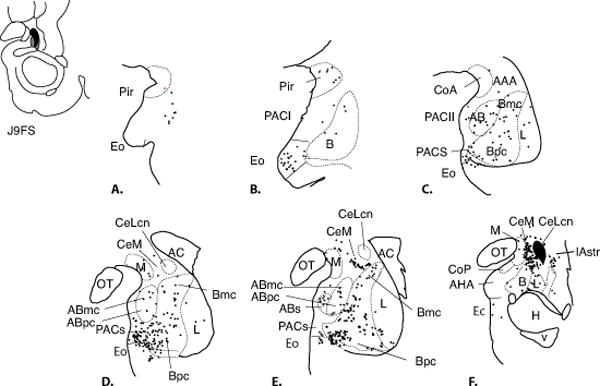
Charts of retrogradely labeled cells in the amygdala following injections in the CeLcn (Case J9FS, A-F.). One dot = one cell.
Overall, injection sites with tracer deposits confined to the CeLcn resulted in fewer labeled cells in the amygdala than those that included the CeM. There were a significant number of labeled cells in PACI and PACs, but few to no labeled cells in the piriform cortex, CoA, or CoP. In the basal nucleus, labeled cells were less heavily concentrated than in cases with injections in the CeM. Within the basal nucleus, the paralaminar nucleus contained the majority of labeled cells (Fig. 5 D, E). The ABmc and ABpc contained a light distribution of labeled cells, however, the rostral ABs had a moderate distribution of FS-positive neurons. There were no labeled cells in the AHA. In the lateral nucleus, there was a light distribution of labeled cells in the ventral subdivision, and scattered labeled cells in the dorsal intermediate subdivision. The CeM and medial amygdalostriatal area contained a number of labeled cells but there were few to no labeled cells in the lateral amygdalostriatal area.
IPAC Medial (Case J7FS, Fig.6A-E)
Fig. 6.
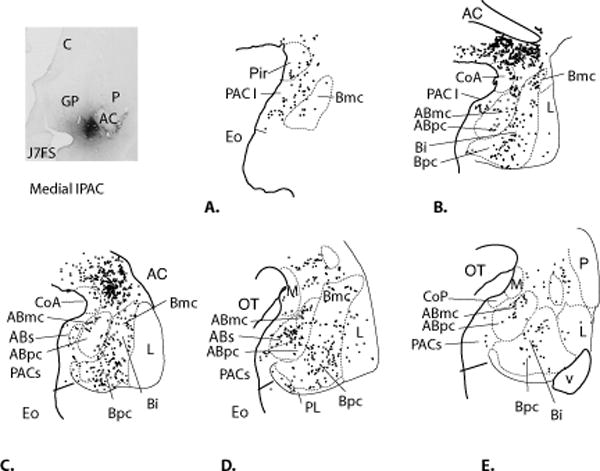
Charts of retrogradely labeled cells in the amygdala following an injection centered in the medial IPAC (Case J7FS, A- E.)
This IPAC injection site was located medial and caudal to the injection in Case J7FR (below), and resulted in a slightly different pattern of labeled cells. The piriform cortex and PAC I contained a light to moderate concentration of labeled cells, with relatively few labeled cells in the rest of the PAC, medial nucleus, CoA or CoP. FS-positive cells were found in all subdivisions of the basal nucleus. The ABmc contained many labeled cells, while the ABpc and ABs had a relatively light distribution of FS-positive cells. Labeled cells were also lightly distributed in the ventrolateral and dorsal intermediate subdivisions of the lateral nucleus (Fig. 6I). The CeM contained a dense concentration of labeled cells, and the CeLcn and medial amygdalostriatal area also contained a moderate number of labeled cells.
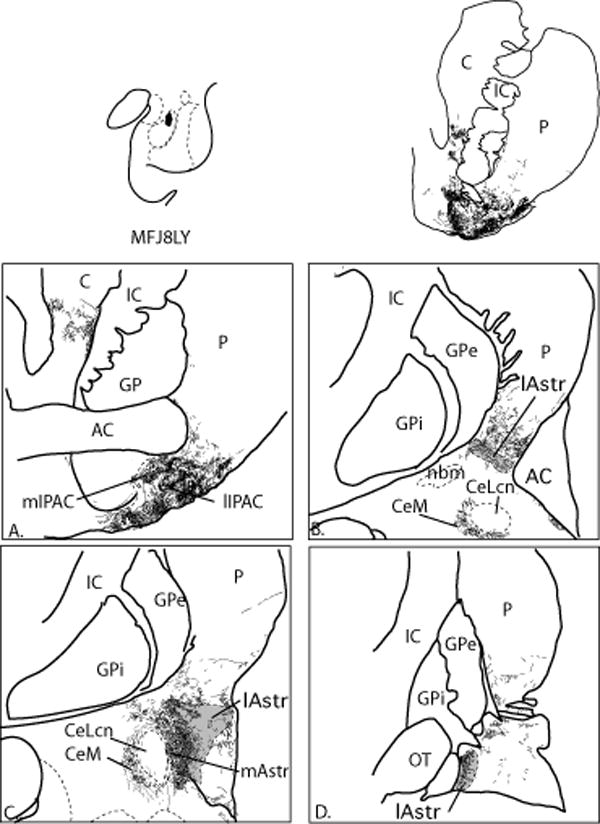
IPAC lateral (Case J7FR, Fig. 7A-E)
Fig. 7.
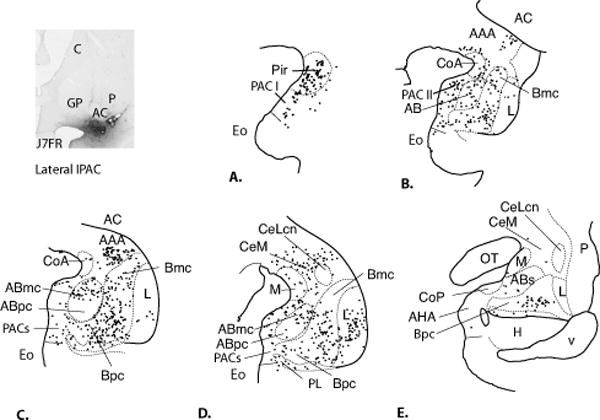
Charts of retrogradely labeled cells in the amygdala following an injection centered in the lateral IPAC (Case J7FR, A-E.). One dot = one cell.
The injection site was centered in the rostral, lateral IPAC, and resulted in a high concentration of labeled cells in the piriform cortex and rostral PAC subdivisions (Fig. 7A, B). The CoA had a moderate number of labeled cells. The medial nucleus contained a light distribution of FR-positive cells, as did the CoP (Fig. 7A, B). The basal nucleus had many labeled cells at all rostrocaudal levels in the Bmc, Bi, and Bpc, similar to case J7FS. The ABmc contained moderate numbers of labeled cells, however, there were few labeled cells in ABpc or ABs. Compared to case J7FS (above, medial IPAC), the lateral nucleus contained a relatively dense distribution of labeled cells localized in the ventral, intermediate subdivision. In contrast, there was a relatively light distribution of labeled cells in the CeM, and few to no labeled cells in the CeLcn, or the amygdalostriatal area, compared to Case J7FS. The AHA was also relatively devoid of labeled cells.
Amygdalostriatal area (Case J9FR, not shown)
This injection site encompassed both the medial (CaBP-positive) and lateral (CaBP-poor) amygdalostriatal area, and the distribution of labeled cells in the amygdala has been reported previously. It is described briefly for comparative purposes. Labeled cells were most densely distributed in the basal nucleus where they were seen in all subdivisions. In the accessory basal nucleus, FR+ cells were confined to the magnocellular subdivision. In contrast to injections into the IPAC, there were relatively few labeled cells in the piriform cortex, CoA, CoP, medial nucleus, or PAC. The CeM had a moderate number of FR positive cells, as did the AAA.
Ventromedial putamen (Cases J8WGA (Fig. 8), J4WGA, J12WGA, and J6WGA (not shown))
Fig. 8.
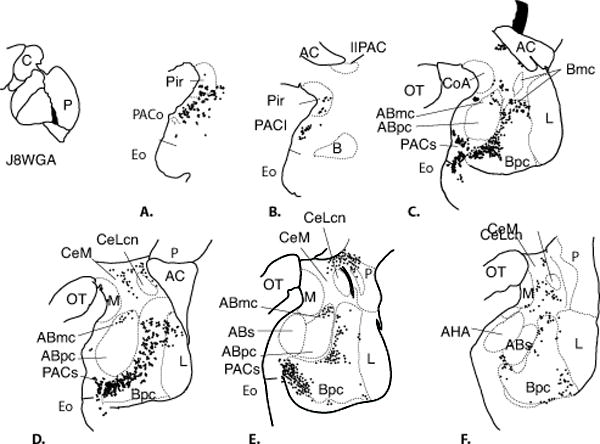
A-F. Distribution of retrogradely labeled cells in the amygdala following an injection of WGA-HRP (Case J8) into the ventromedial putamen at the level of the anterior commissure. One dot = one cell.
The distribution of retrogradely labeled cells in the amygdala was similar in all four cases, therefore the distribution in Case J8WGA is described. Similar to injections in the IPAC, the piriform cortex had a relatively high density of labeled cells (Fig. 8A), as did the rostral PAC (PAC II). However, there were few labeled cells in the CoA, CoP, medial nucleus, or AHA. The basal nucleus contained many WGA-labeled cells, mainly in the medial Bi and Bpc. Labeled cells in the medial Bpc were continuous with labeled cells in the PACs and contiguous entorhinal cortex (olfactory subdivision). The ABmc contained a moderate number labeled cells, but as in the cases with IPAC and amygdalostriatal injections, there were relatively few labeled cells in the ABpc or ABs. The lateral nucleus contained light distributions of labeled cells in the dorsal subdivisions.
ANTEROGRADE CASES
Injection site placement
There were 12 anterograde injection sites in the amygdala, and one in the piriform cortex (Fig. 9A-C). There were seven injection sites in the basal nucleus: three were in the Bmc (cases J12FR, J12LY, and J8FR), and four were in Bpc (cases J15LY J20LY J14FR, and case J15FS). In the Bmc injections, the injection in case J12LY encroached slightly on the CeM, whereas the injection sites in cases J12 FR and J8FR were confined to the Bmc. In the Bpc injections, three injection sites were in the medial half of the Bpc (cases J15LY, J20LY, and J15FS) and one injection site was in the ventrolateral Bpc (case J14FR). In case J15FS, the injection site was situated at the caudal pole of the medial Bpc. Four injection sites were in the AB: two were confined to the ABmc (cases J8LY and J12FS), one injection site included both the ABmc and Abpc (case J21FR), and one injection site was confined to the ABpc (case J18FR). In the caudal amygdala, one large injection site included the AHA and ABs (case J15AA). A tritiated amino acid injection site was centered in the piriform cortex (case J6AA).
Fig. 9.
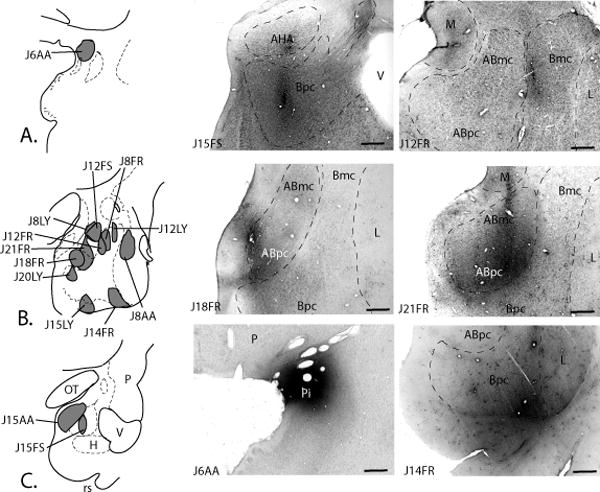
A-C. Schematic of the location of 14 injections of anterograde tracers in the amygdala and piriform cortex, arranged in rostrocaudal order. Photomicrographs show six representative anterograde injection sites. Bars = 1 mm.
Distribution of anterogradely labeled fibers in the CeN and transition zones
Bmc (Case J8FR, (Fig. 10) and J12FR, and J12FS, not shown)
Fig. 10.
A-C. Distribution of anterogradely labeled fibers in the CeN and transition zones following an injection of tetramethylrhodamine (fluororuby) into the basal nucleus, magnocellular subdivision. The distribution of labeled fibers in the rostral striatum is shown for comparison. Gray zones depict the CaBP-poor lateral amygdalostriatal area. D. Darkfield photomicrograph of the boxed area in C., showing labeled fibers in the medial and lateral amygdalostriatal areas and relationship to the putamen. Bar = 500 μm.
The pattern of labeled fibers in all three cases was similar, therefore Case J8FR is illustrated. Fine, labeled fibers containing boutons were densely distributed in the CeM and continued dorsomedially into the basal forebrain (Fig. 10 B-D). Thick, smooth fibers en passant were also seen coursing through the CeM (not charted). The CeLcn contained few labeled fibers of either type. The medial amygdalostriatal area contained a moderate concentration of thin, beaded FR-positive fibers. The lateral amygdalostriatal area also contained many labeled fibers (light gray zone in Fig.10) that extended into CaBP-positive striatal areas of the ventromedial putamen. The lateral IPAC also contained a relatively dense concentration of labeled fibers. The medial IPAC contained a moderate concentration of labeled fibers (Fig 10 B-C). Labeled fibers in the lateral IPAC extended rostrally to overlap the cellular islands in the ventral and lateral shell of the striatum (Fig. 10A).
Medial Bpc (Cases J15LY (Fig. 11A-D), Case J20 (Fig. 11E-F))
Fig. 11.
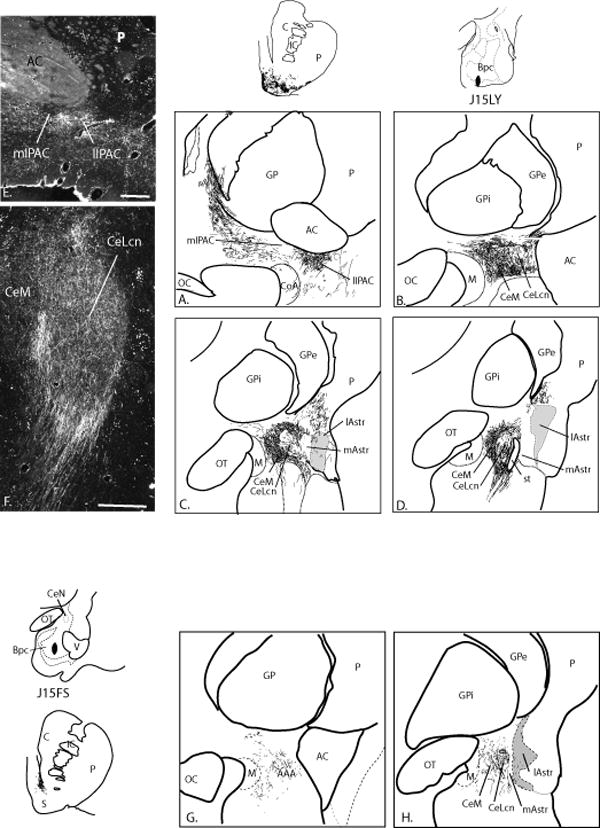
A-D. Distribution of anterogradely labeled fibers in the CeN and transition zones following an LY injection into the medial Bpc and paralaminar nucleus (case J15LY). Gray regions depict the lateral amygdalostriatal area. Labeled fibers occupy the ventral and lateral shell of the nucleus accumbens, which is shown for comparison. E-F. Darkfield photomicrographs of the IPAC (E.) and CeN (F.) in case 20 LY in which the injection site was medial to that in J15LY at the same rostrocaudal level, in the corticoamygdaloid transition region. Bars = 500 μm. G-H. Distribution of anterogradely labeled fibers in the CeN following an FS injection into the caudal Bpc and paralaminar nucleus. There are only scattered labeled fibers in the medial amygdalostriatal area and none in other transition zones. Labeled fibers in the ventral striatum are restricted to the dorsomedial shell.
The injection sites in cases J15LY and J20 LY were at similar rostrocentral levels of the medial Bpc, and resulted in a similar distribution of labeled fibers. Fine caliber, beaded LY-positive fibers following both injections were densely distributed in the CeM at all rostrocaudal levels (Fig. 11 C-E). Labeled fibers also occupied the caudal CeLcn (Fig. 11D, F). The lateral amygdalostriatal area also contained a moderate distribution of labeled fibers, which also extended into the CaBP-positive region of the caudal ventromedial putamen (Fig. 11 C, D). The dorsal paracapsular island of the medial amygdalostriatal area had a dense patch of labeled fibers, however, other medial amygdalostriatal areas were devoid of LY-IR fibers, and there were no labeled fibers in the lateral amygdalostriatal area. The lateral IPAC had a dense distribution of labeled fibers, however, there were few to no labeled fibers in the medial IPAC. Labeled fibers in the lateral IPAC were continuous with LY-positive fibers in the rostral ventromedial putamen and shell of the nucleus accumbens.
Caudal medial Bpc (Case J15FS, Fig 11 G-H)
In contrast to injections in the rostrocentral medial Bpc, case J15FS, with an injection placed in the caudal Bpc resulted in a more restricted pattern of labeled fibers. FS-positive fibers overlapped the CeM and CeLcn as in Cases J15LY and J20LY (Fig 11 G-H). However there were few to no labeled fibers in any of the transitional zones (i.e. IPAC or amygdalostriatal area). Interestingly, the ventral striatum, which has been considered a transition zone between the extended amygdala and striatum by some authors (Heimer et al., 1999, Alheid, 2003), also contained a restricted distribution of labeled fibers compared to cases J15LY and J20LY, which were confined to a small region of the dorsomedial shell.
Lateral Bpc(Case J14FR, not shown)
In contrast to more medially located injections sites, labeled fibers resulting from this injection were mainly distributed in the CeM, with only sparse labeled fibers in the CeLcn. There was a light distribution of FR-positive fibers in the ventral, medial amygdalostriatal area, which avoided the paracapsular islands, and there were no labeled fibers in the lateral amygdalostriatal area. FR-positive fibers in the IPAC were concentrated in a small rostro-lateral patch, and there was a small patch of labeled fibers in the ventral shell region of the ventral striatum.
ABmc (Case J8LY (Fig. 12), and J21FR, J12FS, not shown)
Fig. 12.
A-D. Distribution of anterogradely labeled fibers resulting from an injection confined to the ABmc (case J8LY). Labeled fibers densely innervate the CeM and all four transition zones, but avoid the CeLcn. The distribution of labeled fibers is in the ventral and lateral shell of the ventral striatum is shown for comparison. Labeled fibers also extend into the ventromedial caudate and putamen. Gray zones depict the CaBP-poor lateral amygdalostriatal area.
Following injection sites involving the ABmc, there was a high concentration of labeled fibers in the CeM. Dense concentrations of labeled fibers overlapped both the medial and lateral IPAC (Fig. 12A), continuing rostrally to form a patchy innervation of the shell and core of the ventral striatum. Further caudal, there were many labeled fibers in the medial amygdalostriatal area (Fig. 12C) with a more moderate, patchy distribution of labeled fibers in the lateral amygdalostriatal area and adjacent ventromedial putamen (Fig. 12B-D). The CeM contained a dense distribution of fine, beaded fibers while the CeLcn was relatively devoid of LY-positive fibers.
ABpc (Case J18FR, Fig. 13)
Fig. 13.
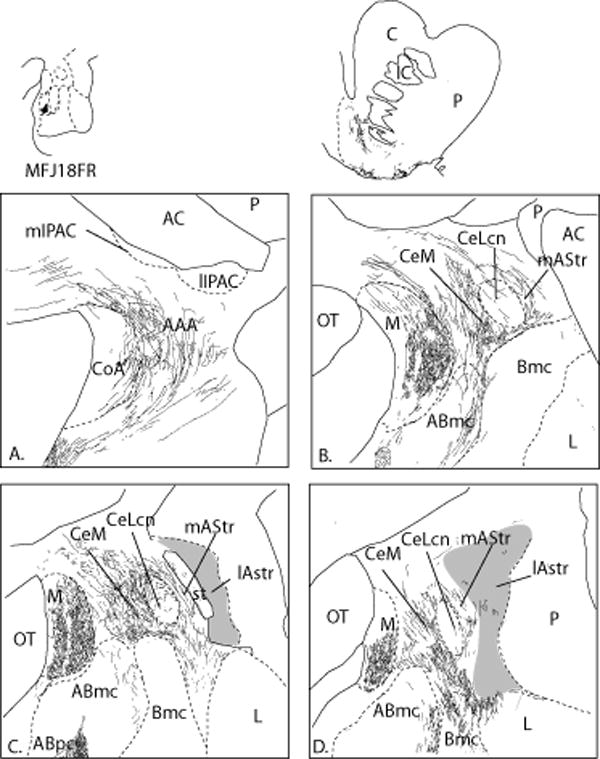
A-D. Distribution of labeled fibers following an FR injection in the parvicellular subdivision of the accessory basal nucleus (case J18FR). Labeled fibers are mainly distributed in the CeM, however, a light to moderate concentration of labeled fibers is seen in the medial amygdalostriatal area. In the ventral striatum, shown for comparison, there is relatively weak labeling in the transition between the core and shell. Gray zones depict the CaBP-poor lateral amygdalostriatal area, which is relatively devoid of labeled fibers.
In general, the concentration of labeled fibers following injections into the Abpc was far less dense than that following injection sites that included the ABmc. The majority of FR-positive fibers were concentrated in the CeM (Fig. 13 B-D), with few to no labeled fibers in the CeLcn. Neither subdivision of the IPAC contained labeled fibers (Fig. 13A). The medial amygdalostriatal area contained a light distribution of FR-positive fibers, which surrounded, but did not overlap, the paracapsular islands. The lateral amygdalostriatal area was relatively devoid of FR-positive fibers.
Lateral nucleus (Case J8AA, not shown)
Silver grains were moderately concentrated over the amygdalostriatal area, with a relatively heavier distribution over the medial than lateral subdivision. The ventral putamen also had a moderate distribution of labeled fibers. The heaviest concentration of labeled fibers, however, was over the nucleus basalis of Meynert, just ventral to the external segment of the globus pallidus. There were no labeled fibers in the CeM, CeLcn, or IPAC.
AHA/ABs (Case J15AA, Fig. 14)
Fig. 14.
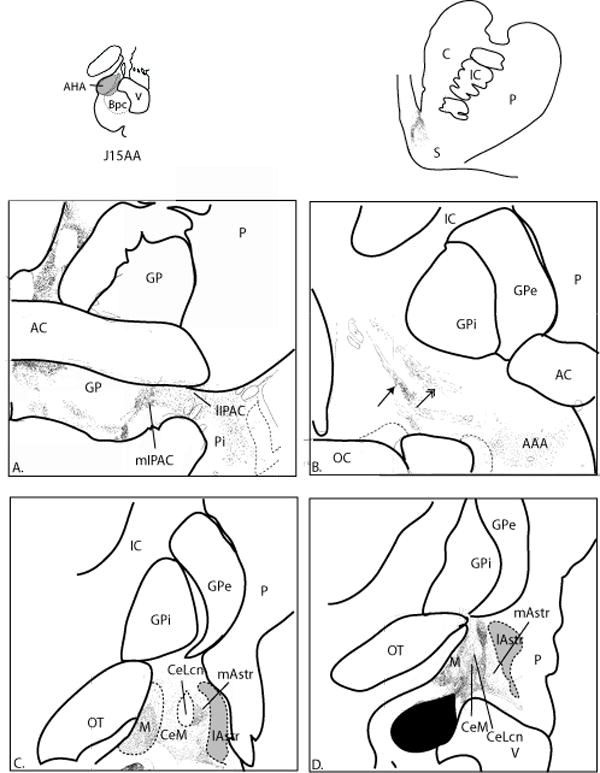
A-D. Distribution of labeled fibers following a large injection into the AHA and ABs. Silver grains are distributed over CeM, medial amygdalostriatal area and medial IPAC. The ventral paracapsular island of the medial amygdalostriatal area has a dense distribution of labeled fibers (C). Gray zones depict the CaBP-poor lateral amygdalostriatal area. Note the dorsal and ventral streams of labeled axons in the sublenticular zone (B) following an injection that labels both the central and medial nucleus of the amygdala. Labeled fibers in the ventral striatum are confined to the dorsomedial shell.
This relatively large injection site was centered in the AHA, with some spread into the ABs. Labeled fibers were moderately concentrated in the CeM, with a sparse distribution in the CeLcn (Fig. 14C, D). There was, however, a dense concentration of labeled fibers over the ventral paracapsular island of the medial amygdalostriatal area. The lateral amygdalostriatal area contained few to no labeled fibers. In the basal forebrain, parallel streams of labeled fibers, one ventral and the other dorsal, could be seen (Fig. 14B, arrows). There was a light to moderate distribution of silver grains in the rostral medial IPAC, and no labeled fibers in the lateral IPAC (Fig. 14A). In the ventral striatum, labeled fibers were restricted to the dorsomedial shell.
Piriform cortex (Case J6AA, not shown)
The majority of labeled fibers were found in the medial and lateral IPAC, and ventromedial putamen at the level of the anterior commissure. The CeM contained a moderate concentration of labeled fibers which diminished significantly at caudal levels, and the CeLcn contained a very light distribution of labeled fibers. There were scant silver grains in medial and lateral amygdalostriatal area.
DISCUSSION
Defining the CeN subdivisions and transition zones in rats and primates
In both rats and primates, the CeM is characterized by heterogeneous neuronal types and moderate AChE staining, while the CeLcn contains medium spiny neurons, and is relatively devoid of AChE activity (present results) (Price et al., 1987, Amaral and Bassett, 1989, DeOlmos, 1990, Sims and Williams, 1990). However, differences exist between species. An important difference is that the ‘intermediate’ subdivision central nucleus does not exist in primates (McDonald, 1982, Price et al., 1987, Sims and Williams, 1990, Martin et al., 1991). Striking neurochemical discontinuities also exist between species, including differing distribution patterns for serotonin transporter-immunoreactivity, 5HT immunoreactivity (Steinbusch, 1981, Freedman and Shi, 2001, Bauman and Amaral, 2005, O’Rourke and Fudge, 2006), norepinephrine transporter (Smith et al., 2006), and neurotensin-immunoreactivity (Cassell et al., 1986, Gray and Magnuson, 1987, Martin et al., 1991).
A key finding of this study is that the CeN transition zones (IPAC and amygdalostriatal areas) found in rats are can also be appreciated in monkey. However, there are also species differences based on some neurochemical and cellular features, as described below. In rats, the entire region flanking the CeLcn (or, “CeL”) laterally has been variously referred to as the ‘amygdalostriatal area’ (LeDoux et al., 1990, Petrovich et al., 1996), ‘area PX’ (Price et al., 1987), area X (Hall and Geneser-Jensen, 1971), and the ‘lateral capsular subdivision’ of the central nucleus (McDonald, 1982, Cassell et al., 1986, Jolkkonen and Pitkanen, 1998). As information on the transition regions has become available, the nomenclature of the region has evolved. More recently, this region in rodent has been parsed into ‘dorsal’ and ‘ventral’ regions based on relatively high AChE and tyrosine hydroxylase (TH) levels in the ‘dorsal’ subdivision, and very low levels of these markers in the ‘ventral’ subdivision (Cassell et al., 1999, Jolkkonen et al., 2001b). The ‘capsular’ designation has been retained for the ventral region, while the term ‘amygdalostriatal region’ is now used for the dorsal region.
Broad similarities between rat and monkey transition zones are found, once the expansion and rotation of the amygdala in monkey is accounted for (Table 1). In primates, the amygdala is rotated medio-ventrally, so that the amygdalostriatal area and ventromedial putamen are located in a medial-to-lateral orientation with respect to each other, rather than the more dorsal-to-ventral orientation seen in the rat. Thus, the medial amygdalostriatal area of human and nonhuman primates is most similar to the ventral ‘capsular’ region in the rat, and like it, contains a matrix of very low AChE and DAT staining. In contrast to the rat, however, the medial amygdalostriatal area contains islands of medium spiny neurons embedded in this matrix (present results) (DeOlmos, 1990, Freedman and Shi, 2001, Fudge and Haber, 2002). These islands, referred to as ‘paracapsular islands’ in the human (DeOlmos, 1990), have differential staining patterns. A relatively large dorsal paracapsular island contains high AChE, DAT- and CaBP-IR, matching that in the ventromedial putamen (Fig. 2D’). In contrast, a similarly large ventral paracapsular island has low levels of AChE and DAT-IR, but moderate CaBP-IR (and bcl-2-IR (Fudge and Haber, 2002)), matching the CeLcn. Based on these neurochemical data, the main dorsal and ventral paracapsular islands may be remnants of the ventromedial putamen and CeLcn, respectively, which become encapsulated and separated from their nearby structures by passing fibers of the stria terminalis. Previous work in primates suggests that these islands are ‘accessory’ to the ‘main’ CeLc, and are continuous with it (DeOlmos, 1990, Brockhaus, l938, Martin et al., 1991). Our data suggest that in monkey the ventral paracapsular island is indeed similar to the CeLcn, while the dorsal island is neurochemically more similar to the ventral putamen. Both paracapsular islands are distinguished from the many irregularly placed clusters of granular and parvicellular neurons (‘intercalated islands’ (Alheid et al., 1995, Heimer et al., 1999)) seen throughout the extended amygdala and amygdala.
The lateral amygdalostriatal area, an irregularly shaped area lateral to the medial amygdalostriatal area, is characterized by intermediate to high AChE activity and DAT-IR, and very low levels of CaBP-IR, which is the pattern typical of the shell of the nucleus accumbens (Fudge and Haber, 2002). In this study we found that the lateral IPAC is indistinguishable from the caudally adjacent lateral amygdalostriatal area (or rostrally adjacent ventrolateral shell of the accumbens) based on the present staining criteria (Table 1). This data suggests that together, the lateral IPAC and the lateral amygdalostriatal area, share neurochemical features of the shell of the nucleus accumbens. Thus, both transition regions may be part of the ‘caudal ventral striatal’ continuum. In contrast, the medial IPAC resembles the medial amygdalostriatal area (albeit lacking the paracapsular islands), with light AChE and DAT-IR and moderate CaBP-IR. In rats a light distribution of PV-IR cells distinguishes the amygdalostriatal area and ventral striatum from the IPAC and CeN (Jolkkonen et al., 2001b, Zahm et al., 2003), however, all of these regions are devoid of PV-IR cell bodies in primates, consistent with previous reports in primates (Waldvogel and Faull, 1993, Holt et al., 1997, Pitkanen and Amaral, 1993).
Afferent inputs to the CeN and transition zones (Table 2)
Table 2.
| CeM | CeLcn | mIPAC | mAstr | lIPAC | lAstr | Caudal Vs | |
|---|---|---|---|---|---|---|---|
| MeN | + | + | + | - | - | - | |
| ABpc | ++ | + | - | + | - | - | - |
| AHA/ABs | ++ | + | ++ | ++ | - | - | - |
| PACI-III | + | + | ++ | - | ++ | ++ | |
| PACs | +++ | +++ | + | - | ++ | - | +++ |
| PL | +++ | +++ | ++ | + | + | - | - |
| medBpc | +++ | +++ | + | + | +++ | ++ | +++ |
| ABmc | +++ | + | +++ | +++ | +++ | +++ | +++ |
| Bmc | +++ | + | ++ | ++ | +++ | ++ | +++ |
| latBpc | +++ | + | + | + | + | + | + |
| Piriform | ++ | + | ++ | + | +++ | + | +++ |
| Lat | - | - | + | +++ | ++ | ++ | + |
Relative density of projections to the CeN subdivisions and transition zones (-, scant, +light, ++moderate, +++dense)
Technical considerations for neuronal tracing studies
The advantage of retrograde studies is that they provide a broad overview of potential afferent structures that can influence specific brain regions. A key concern for retrograde tracing studies, however, is the possibility of tracer uptake and transport by fibers of passage, leading to false positive results (Halperin and LaVail, 1975, Nance and Burns, 1990). This is particularly relevant for studies of extended amygdala components, which lie in or near passing fiber tracts of the ventral amygdalofugal pathway. The ventroamygdalofugal path carries fiber leaving the amygdala which pass through the central nucleus to terminate in the thalamus, hypothalamus, and the brainstem. Injection sites in which there is necrosis or tissue damage at the injection site are most highly prone to fibers of passage problems. We used slow, pressure injections of tracer through small bore pipette tips to reduce this problem (Nance and Burns, 1990, Schmued et al., 1990, Vercelli et al., 2000). Cases subsequently found to have resulting necrosis at site were eliminated from the analysis. We also examined the remaining cases for extraneous cell labeling indicative of uptake by passing fibers. For example, there were no labeled fibers in the mediodorsal thalamus following injections into the any of the extended amygdala structures, indicating that no significant uptake by fibers passing from the amygdala proper--a major afferent to the mediodorsal thalamus via ventral amygdalofugal path (Porrino et al., 1981, Aggleton and Mishkin, 1984, Russchen et al., 1987)--had occurred. Finally, we examined cases where the injection target was ‘missed’ and an injection placed in nearby fiber tracts, such as the anterior commissure or external capsule. These injection sites resulted in very few retrogradely labeled cells in any brain region, suggesting that the fiber of passage problem was negligible. Another problem inherent in retrograde studies of central nucleus and its transition zones is their close proximity to clusters of the cholinergic neurons that populate the basal forebrain. Retrograde tracer injections may spread into cholinergic cell islands that are dispersed throughout the basal forebrain, and result in retrogradely labeled cells. To guide interpretation of the retrograde studies, therefore, complementary anterograde studies are critical.
Anterograde studies are also important to examine the density and scope of terminal fields, since the density of retrogradely labeled cells does not necessarily correspond to the density of the terminal field. Because anterograde tracers are also theoretically subject to the fibers of passage problem as well as false labeling of axon collaterals, careful analysis in conjunction with retrograde studies is also required. Injections within the lateral nucleus, basal nucleus subdivision, and accessory basal nucleus resulted in different patterns of labeling not only in the CeN subdivisions and transition zones, but also with respect to their termination pattern in the ventral striatum (shown for comparison) and other subcortical and brainstem structures. Therefore, false positive labeling by passing fibers or axon collateral does not appear to have played a significant role. Finally, although tritiated amino acids are highly sensitive anterograde tracers, they have the disadvantage that they do not reveal terminal morphology, making interpretation of labeled fibers as passing or terminating inconclusive.
General projections to the CeN
While almost every region of the amygdala sends at least a minor input to the CeN complex, the majority of projections to the CeN originate in the basal nucleus and magnocellular accessory basal nucleus. This finding is consistent with previous studies in rats, cats, and primates (Russchen, 1986, Krettek and Price, 1978, Ottersen, 1982, Aggleton, 1985, McDonald, 1991, McDonald, 1992, Pitkänen et al., 1995, Savander et al., 1995, Savander et al., 1996, Jolkkonen and Pitkanen, 1998, Pitkanen and Amaral, 1998, Shammah-Lagnado et al., 1999, Bonda, 2000). All subdivisions of the basal nucleus and accessory basal nucleus project to the CeN, however, there are differential inputs to the CeN subdivisions (see Differential inputs, below). The lateral nucleus has relatively sparse inputs to either the CeM or CeLcn. Although older studies in rats indicate a lateral nucleus projection to the CeN (Ottersen, 1982), more recent reports indicate that this input is almost exclusively to the ‘capsular’, or ‘amygdalostriatal’ area (Pitkänen et al., 1995, Pitkanen and Amaral, 1998, Jolkkonen et al., 2001b). Several previous reports in primates support the finding that the lateral nucleus projects to the amygdalostriatal area, and not the CeN (Pitkanen and Amaral, 1998, Fudge et al., 2002), consistent with our findings in the present study (see Projections to transition zones, below).
Most ‘olfactory cortical structures’ (namely the piriform cortex, periamygdaloid cortex, and anterior and posterior cortical nuclei) send light to moderate inputs to the CeN. The exception is the PACs, which has a relatively strong input to the CeN based on the retrograde data(cases J9LY, J1FR, J9FS, J1LY). The PACs merges with the medial Bpc in the ‘corticoamygdaloid transition’ zone (Fig. 1), and is the least differentiated subregion of the PAC. Previous anterograde injections encompassing the primate PACs result in strong anterograde labeling in both CeM and CeLcn (VanHoesen, 1981, Aggleton, 1985). While a lack of projections from the PAC to CeN has been reported following anterograde tracer studies in rats (Majak and Pitkanen, 2003), retrograde and anterograde studies indicate a strong input from ‘amygdalopiriform’ cortex in this species (Ottersen, 1982, McDonald et al., 1999, Shammah-Lagnado et al., 1999, Santiago and Shammah-Lagnado, 2005, Jolkkonen et al., 2001a). The rodent amygdalopiriform region is like the primate PACs in that it forms a bridge between the PAC and entorhinal cortex. While it does not merge with the posterior (parvicellular) basal nucleus in rats as in primate, the amygdalopiriform region may have some homologies with the PACs in primates, based on cytoarchitectural characteristics (DeOlmos et al., 1985, DeOlmos, 1990). Indeed, the continuous flow of retrogradely labeled cells from PACs into the entorhinal cortex following CeN injections mirrors findings in the rat (Ottersen, 1982), and supports the idea that the PACs and adjacent entorhinal cortex are closely related (Haug, 1976, Krettek and Price, 1977, Turner et al., 1978, Witter and Groenewegen, 1986, Amaral et al., 1987).
Differential inputs to CeM and CeLcn
Injections that included the CeM resulted in relatively greater densities of labeled cells in the amygdala, compared to injections confined to the CeLcn. This is best illustrated in projections from the basal nucleus, which is a main afferent input to both CeM and CeLcn. While labeled cells were seen in the basal nucleus after all injections into the CeN, there were relatively fewer labeled cells in this nucleus when injections were confined to the CeLcn (cases J1LY and J9FS compared to case J9LY and J1FR). This finding is consistent with previous anterograde studies in rats (Savander et al., 1995) and primates (Bonda, 2000), and with the present anterograde results. Within the basal nucleus, CeLcn inputs mainly originated in the medial Bpc in the ‘corticoamygdaloid transition zone’, based on both retrograde and anterograde data. While anterograde injections in all subdivisions of the basal nucleus resulted in many labeled fibers in the CeM, only injections into the medial Bpc (cases J15LY, J15 FS, J20LY) resulted in a significant density of labeled fibers in the CeLcn as well. This result is consistent with one previous study in the primate describing labeled fibers in both subdivisions of the CeN following injections of tritiated amino acids into the caudal Bpc (termed ‘medial basal nucleus’ in that study) (Aggleton, 1985). In rats, the caudolateral Bpc has strong inputs to the CeN as a whole, and afferents to the lateral subdivision of the central nucleus emanate only from this subregion of the basal nucleus (Savander et al., 1995, Shammah-Lagnado et al., 1999). Conversely, the medial Bpc of rodents has relatively weak inputs to the CeN, similar to the caudolateral Bpc projection in primate. Taken together, these results suggest that there may be some homology between the caudal medial Bpc in primate and caudolateral Bpc in rat.
The unique connectivity of the primate medial Bpc may be related to its unusual cytoarchitectural features. In addition to its indistinct boundary with the PACs, it is also invaded by the paralaminar nucleus. Following retrograde tracer injections into both the CeM and CeLcn, labeled cells were densely concentrated in medial paralaminar nucleus/medial Bpc, and in contiguous deep layer III of the PACs. The existence of the paralaminar nucleus in the Bpc is not seen in rats and cats, where the paralaminar nucleus is mainly associated with the lateral nucleus. In the primate, including humans, the paralaminar nucleus follows the external capsule under the Bpc to merge with the PACs and medial Bpc in the corticoamygdaloid transition zone (Price et al., 1987, DeOlmos, 1990). Whereas the corticoamygdaloid transition zone is among many amygdaloid regions projecting to the CeM, it is one of the few intrinsic inputs to the CeLcn.
The accessory basal nucleus is the other major input to the CeN. However, only the sulcal subdivision of the accessory basal nucleus (ABs) has inputs to both CeN subdivisions based on retrograde studies. Although one anterograde injection that included the ABs resulted in labeled fibers in the CeM and not the CeLcn, this was a relatively caudal injection site that included only a small portion of the ABs (mainly centered in the AHA, Fig. 14). Moreover, labeled fibers were densely concentrated over the ventral paracapsular island, which is histochemically similar to the CeLcn, and may be an ‘accessory’ to it (see above). The magnocellular and parvicellular subdivisions of the accessory basal nucleus mainly direct input to the CeM, and both have relatively sparse projections to the CeLcn. This relationship was confirmed in anterograde studies in which injections into the ABmc and ABpc resulted in labeled fibers that terminate in the CeM, but avoid the CeLcn. The relative absence of ABmc and ABpc projections to the CeLcn has been previously noted in primates (Aggleton, 1985, Bonda, 2000).
Projections to transition zones
Based on retrograde studies, the IPAC and amygdalostriatal area receive their main inputs from the basal and magnocellular accessory basal nuclei, similar to the CeN. Analysis of retrograde injection site data in conjunction with anterograde studies confirmed this, but revealed more precise patterns of innervation. Using histochemical markers for each zone in conjunction with anterograde studies, we found that there is a differential input from specific subdivisions of the basal and accessory basal nuclei.
Injections into the Bmc (cases J8FR, J12FR, and J12LY) resulted in labeled fibers in both medial and lateral IPAC, as well as in both subdivisions of the amygdalostriatal area. Furthermore, labeled fibers from the Bmc extend beyond the transition zones into adjacent ventral striatal regions both rostrally and caudally (see also (Fudge et al., 2004)). In contrast, our injections into the medial and lateral Bpc (cases J15LY, J20LY, and J14FR) –but not the caudal Bpc region captured in Case J15FS--result in relatively dense concentrations of labeled fibers in the lateral IPAC, with relatively fewer inputs to the medial IPAC. The large numbers of labeled cells in the Bpc following the retrograde injection into the medial IPAC (case J7FS) may therefore be due to uptake by cells of the lateral IPAC or cholinergic neurons in the region. On the other hand, our anterograde injections did not encompass the central Bpc where may retrogradely labeled cells were found, making interpretation difficult. Previous studies in primates examining the amygdaloid projection to the ‘substantia innominata’ (which encompasses IPAC, amygdalostriatal area, and magnocellular cholinergic neurons) suggest that the Bpc may project to the region containing the medial IPAC (Irle and Markowitsch, 1986, Russchen et al., 1985a, Russchen et al., 1985b), but further studies examining anterograde labeling in conjunction with histochemical boundaries are necessary. The most caudal injection into the Bpc (Case J15FS, Fig. 9) resulted in labeled fibers in the CeM and CeLcn, with no labeled fibers in the IPAC or amygdalostriatal transition zones. Interestingly though, the dorsomedial shell of the nucleus accumbens contained a very restricted distribution of labeled fibers, raising the question of whether this is a ‘transition zone’ with the extended amygdala, as has been previously suggested (Alheid, 2003),.
With respect to the accessory basal nucleus, the ABmc projects to all four transition regions, while the Abpc sends very light projections to the medial amygdalostriatal area and medial IPAC, and has no input to the lateral IPAC or lateral amygdalostriatal areas. The ABs has relatively greater input to the medial IPAC compared to the lateral IPAC based on our retrograde cases (J7FR and J7FS). An anterograde injection that included the ABs resulted in relatively more labeled fibers in the medial compared to the lateral IPAC, supporting the retrograde results.
In the rat, extensive studies of basal and accessory basal nucleus projections to the IPAC and amygdalostriatal area have produced mixed results (Kelley et al., 1982, Savander et al., 1995, Petrovich et al., 1996, Savander et al., 1996, Shammah-Lagnado et al., 1999, Jolkkonen et al., 2001b), and translating findings to the primate may prove difficult. Pitkanen and colleagues did not demonstrate afferents from the basal nucleus to the amygdalostriatal area, but did find projections to the ‘capsular’ part of the central nucleus (and CeM) (Savander et al., 1995). This latter region appears most similar to the medial amygdalostriatal area based on histochemical staining patterns examined in this study. Using both retrograde and anterograde studies, Shammah-Lagnado et al reported that the posterior basal nucleus projects to the amygdalostriatal region as well as to the IPAC, consistent with the present results (Shammah-Lagnado et al., 1999). There are also mixed findings with respect to accessory basal nucleus projections. In earlier studies, Savander et al. found that the rostral accessory basal nucleus projects to the ‘capsular’ subdivision (and CeM), with few inputs to the lateral core, consistent with the present results (Savander et al., 1996). Petrovich et al. found that while the accessory basal nucleus as a whole projects to the CeM, the anterior accessory basal nucleus (‘BMAa’) has stronger inputs to the ‘fundus striatii’ (IPAC), similar to findings in Shammah-Lagnado’s study of afferents to the IPAC (Shammah-Lagnado et al., 1999). The BMAa also projects to the ‘capsular’ subdivision of the central nucleus whereas the posterior accessory basal nucleus (BMAp) innervates mainly the ‘ventral strip’ of the capsular region, which may be similar to the medial amygdalostriatal area in our study (Petrovich et al., 1996). Thus, it is not clear whether there is homology between subdivisions of the accessory basal nucleus in rats and primates, a point previously noted by Price (Price et al., 1987). Here, we found that the magnocellular accessory basal nucleus (which may correspond to the anterior accessory basal nucleus in rat) has much stronger inputs to the CeM and transition zones relative to the parvicellular subdivision (corresponding to the posterior accessory basal nucleus), in general agreement with rodent studies. On the other hand, while the posterior accessory basal nucleus is reported to project robustly to the shell of the nucleus accumbens in rats (Petrovich et al., 1996), the parvicellular accessory basal nucleus has fairly weak inputs to the nucleus accumbens in the primate (Fudge et al., 2002). Consistent with its relatively weak striatal inputs, the parvicellular accessory basal nucleus also has few inputs to any of the ‘striatal-like’ regions in primates (Russchen et al., 1985a, Fudge et al., 2002, Fudge et al., 2004), including the region encompassing the lateral IPAC and lateral amygdalostriatal area (present results).
The piriform cortex sends a strong input to the IPAC, targeting the lateral subdivisions relatively more than the medial subdivisions. Retrograde injections into the caudoventral putamen at the level of the anterior commissure also resulted in many labeled cells in the piriform cortex, consistent with anterograde results in Case J6AA. This indicates that primary olfactory information is an important sensory input to the CeN’s lateral transition zones with the striatum and to the caudoventral (limbic) striatum near the anterior commissure. These results are generally consistent with rodent studies indicating piriform cortex input to the ventrolateral shell and IPAC region (Fuller et al., 1987, Brog et al., 1993, Shammah-Lagnado et al., 1999).
As mentioned, lateral nucleus input is relatively specific to the transition zones, with few inputs to the CeN. Previous results in the rat (Fuller et al., 1987, Pitkänen et al., 1995, Shammah-Lagnado et al., 1999, Jolkkonen et al., 2001b) and in monkey (Pitkanen and Amaral, 1998, Fudge et al., 2002) support this finding.
Functional considerations
While the central nucleus is an important a relay linking conditioned fear information from the amygdala proper to brainstem centers involved in startle and freezing (Applegate et al., 1983, Hitchcock and Davis, 1991), recent work indicates a much more diverse role in behavior. Indeed, the central nucleus is also involved in forming fear-stimulus associations (Killcross et al., 1997, Wilensky et al., 2006), in suppression of operant behavior linked to aversive outcomes (Killcross et al., 1997, Petrovich et al., 2006, Seamans et al., 2006), in allocating attention to changes in positive predictive stimuli (Gallagher et al., 1990, Holland and Gallagher, 1993), and in surprise-induced enhancements in new learning (Holland and Gallagher, 2006, Lee et al., 2006). Differential inputs to the CeN subterritories suggest some clues as to how the CeN facilitates these flexible responses to appetitive and aversive stimuli.
A basic organizational feature of the CeN is its division into the CeM and CeLcn. The CeLcn projects mainly in a unidirectional fashion to the CeM (Petrovich and Swanson, 1997, Jolkkonen and Pitkanen, 1998), and is proposed to be the inhibitory ‘gatekeeper’ of CeM outputs (Nose et al., 1991, Sun et al., 1994). Stimulation of the CeM, but not the CeLcn, has immediate effects on autonomic and motor functions, via hypothalamic and brainstem projections (Kapp et al., 1982, Applegate et al., 1983, Davis et al., 1994). In the present set of studies we show that the primate amygdala, particularly the basal and accessory basal nuclei, has broad inputs to the CeM, which can rapidly and directly influence behavior through effector sites in the hypothalamus and brainstem. In contrast, the CeLcn is mainly influenced by projections from the ‘corticoamygdaloid transition zone’, namely the medial Bpc, paralaminar nucleus, and PACs.
In monkeys, the corticoamygdaloid transition zone is a specific target for hippocampal inputs, which are thought to mediate emotional context (Rosene and Van Hoesen, 1977, Saunders et al., 1988). Emotional context—i.e. the physical backdrop against which associations between stimuli and their emotional relevance are formed –can be aversive (as in contextual fear conditioning) or positive (as in place preference learning). Evocation of positive or negative contextual memory depends on both hippocampal afferents and their amygdala targets (Phillips and LeDoux, 1992, Fanselow and Kim, 1994). Hippocampal projections to the corticoamygdaloid transition region indicate that memories of the emotional context can have an ongoing modulatory effect on the CeLcn, and its influence as ‘gatekeeper’ of CeM output.
The idea that ‘context’ is a key influence on CeN regulation—particularly via the CeLcn-- is also reflected in the cortico-amygdaloid inputs. Prefrontal cortical inputs to the CeN arise from the agranular and rostral dysgranular insula in human and nonhuman primates (including the agranular insula on the posterior orbital surface (Carmichael and Price, 1994, Ongur et al., 2003)) (Carmichael and Price, 1996, Ghashghaei and Barbas, 2002, Stefanacci and Amaral, 2002, Fudge et al., 2005a). Interestingly, the corticoamygdaloid transition area is reciprocally connected with the anterior insula (Mufson et al., 1981, Amaral and Price, 1984, Carmichael and Price, 1996, Stefanacci and Amaral, 2002). These reciprocally connects regions project to the CeN, particularly the CeLcn (present results) (VanHoesen, 1981, Stefanacci and Amaral, 2002, Mufson et al., 1981, Fudge et al., 2005b). In a complementary manner to the hippocampus, which provides contextual information on the physical environment, the insula provides information on internal context-- namely the composite of bodily sensations previously linked to positive or negative experience (for example, visceral sensations previously associated with sickness or anxiety) (Craig, 2002, Critchley et al., 2004). For example, the anticipation of an emotionally relevant cue—experienced prior to the actual stimulus--elicits insula activation in humans (Chua et al., 1999, Montague and Berns, 2002, O’Doherty et al., 2002, Simmons et al., 2006).
Thus, the CeLcn is influenced by contextual representations of previous experience-- internal via the insula, and external via the hippocampus—through projections from the corticoamygdaloid transition area. The CeLcn’s relatively restricted intrinsic input from the corticoamygdaloid transition region suggests that contextual elements of recent past experience are critical to its ‘gatekeeping’ function on the CeM output. Based on our anatomic findings, the CeLcn’s tonic regulation of the CeM appears to be influenced by afferent systems involved in maintaining representations of internal feeling states and the environmental context in which they were experienced. This organization may help the animal to maintain focused, context-appropriate responses in order to conserve a previously adaptive behavior. In contrast, multiple amygdaloid regions have direct access to the CeM, indicating that tonic control by the CeLcn can be rapidly overridden by novel or immediate changes in the sensory field.
Acknowledgments
This work was supported by MH063291 (J.F.). We gratefully acknowledge the technical assistance of Paul Sonneborn and Kyeesha Becoats in performing histochemical work.
ABBREVIATIONS
- AAA
anterior amygdaloid area
- AB
accessory basal nucleus
- ABmc
accessory basal nucleus, magnocellular subdivision
- ABpc
accessory basal nucleus, parvicellular subdivision
- ABs
accessory basal nucleus, sulcal subdivision
- AC
anterior commissure
- ACA
amygdalo-claustral area
- AHA
amygdalohippocampal area
- Bi
basal nucleus, intermediate subdivision
- Bmc
basal nucleus, magnocellular subdivision
- Bpc
basal nucleus, parvicellular subdivision
- C
caudate nucleus
- CeLcn
central nucleus, lateral core subdivision
- CeM
central nucleus, medial subdivision
- CoA
anterior cortical nucleus
- CoP
posterior cortical nucleus
- EC
entorhinal cortex
- Eo
enthorhinal cortex, olfactory subdivision
- GPe
globus pallidus, external segment
- H
hippocampus
- IC
internal capsule
- IPAC
Interstitial nucleus of the posterior limb of the anterior commissure
- L
lateral nucleus
- lAstr
lateral amygdalostriatal area
- lIPAC
IPAC, lateral subdivision
- M
medial nucleus
- mAstr
medial amygdalostriatal area
- mIPAC
IPAC, medial subdivision
- P
putamen
- PACI-III
periamygdaloid cortex, subdivisions I-III
- PACs
periamygdaloid cortex, sulcal subdivision
- rs
rhinal sulcus
- S
shell
- Str
striatum
- V
ventricle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Aggleton JP. A description of intra-amygdaloid connections in old world monkeys. Experimental Brain Research. 1985;57:390–399. doi: 10.1007/BF00236545. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Mishkin M. Projections of the amygdala to the thalamus in the cynomolgus monkey. J Comp Neurol. 1984;222:56–68. doi: 10.1002/cne.902220106. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Annals of the New York Academy of Sciences. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, DeOlmos CA, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. 2. Academic Press; San Diego: 1995. pp. 495–578. [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: An immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J Comp Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, Inc.; 1992. Anatomical organization of the primate amygdaloid complex; pp. 1–66. [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiology & Behavior. 1983;31:353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Amaral DG. The distribution of serotonergic fibers in the macaque monkey amygdala: an immunohistochemical study using antisera to 5-hydroxytryptamine. Neuroscience. 2005;136:193–203. doi: 10.1016/j.neuroscience.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Beall MJ, Lewis DA. Heterogeneity of layer II neurons in human entorhinal cortex. Journal of Comparative Neurology. 1992;321:241–266. doi: 10.1002/cne.903210206. [DOI] [PubMed] [Google Scholar]
- Bonda E. Organization of connections of the basal and accessory basal nuclei in the monkey amygdala. European Journal of Neuroscience. 2000;12:1971–1992. doi: 10.1046/j.1460-9568.2000.00082.x. see comments. [DOI] [PubMed] [Google Scholar]
- Brockhaus H. Zur normalen aund pahologischen Anatomie des Mandelkerngebietes. J Psychol Neurol. 1938;49:1–136. [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1996;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Annals of the New York Academy of Sciences. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. Journal of Comparative Neurology. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Cote P-Y, Sadikot AF, Parent A. Complementary distribution of calbindin D-28k and parvalbumin in the basal forebrain and midbrain of the squirrel monkey. European Journal of Neuroscience. 1991;3:1316–1329. doi: 10.1111/j.1460-9568.1991.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telencephalon: the nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. Journal of Comparative Neurology. 1941;74:309–347. [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–200. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- DeOlmos JS. Amygdala. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego: 1990. pp. 583–710. [Google Scholar]
- DeOlmos JS, Alheid GF, Betramino CA. Amygdala. In: Paxinos G, editor. The Rat Nervous System. Academic Press; Sydney: 1985. pp. 223–334. [Google Scholar]
- deOlmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. J Comp Neurol. 1972;146:303–333. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Martina M, Samson RD, Drolet G, Pare D. Physiological properties of central amygdala neurons: species differences. Eur J Neurosci. 2002;15:545–552. doi: 10.1046/j.0953-816x.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behavioral Neuroscience. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Shi C. Monoaminergic innervation of the macaque extended amygdala. Neuroscience. 2001;104:1067–1084. doi: 10.1016/s0306-4522(01)00157-9. [DOI] [PubMed] [Google Scholar]
- Fudge J, Haber S. Defining the caudoventral striatum in primates: cytoarchitectural and histochemical features. Journal of Neuroscience. 2002;22:10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005a;490:101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, Danish M, Pannoni VA. Insular and gustatory inputs to the caudal ventral striatum in primates. Journal of Comparative Neurology. 2005b doi: 10.1002/cne.20660. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. Journal of Comparative Neurology. 2004;476:330–347. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamatergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneser-Jensen FA, Blackstad TW. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. Z Zellforsch. 1971;114:460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. Journal of Comparative Neurology. 1987;262:365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E, Geneser-Jensen F. Distribution of acetylcholine esterase and monoamine oxidase in the amygdala of the guinea pig. Zeitschrift fur Zellforschung und Microskopisches Anatomie. 1971;120 doi: 10.1007/BF00335536. [DOI] [PubMed] [Google Scholar]
- Halperin JJ, LaVail JH. A study of the dynamics of retrograde transport and accumulation of horseradish peroxidase in injured neurons. Brain Research. 1975;100:253–269. doi: 10.1016/0006-8993(75)90482-5. [DOI] [PubMed] [Google Scholar]
- Haug FM. Sulphide silver pattern and cytoarchitectonics of parahippocampal areas in the rat. Special reference to the subdivision of area entorhinalis (area 28) and its demarcation from the pyriform cortex. Advances in Anatomy, Embryology & Cell Biology. 1976;52:3–73. [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The Accumbens: Beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9(3):354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Heimer L, De Olmos JS, Alheid GF, Person J, Sakamoto N, Shinoda K, Marksteiner J, Switzer RC. The human basal forebrain. Part II. In: Bloom FE, et al., editors. Handbook of Chemical Neuroanatomy, vol.15: The Primate Nervous System, Part III. Elsevier; Amsterdam: 1999. pp. 57–226. [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behavioral Neuroscience. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Graybiel AM, Saper CB. Neurochemical architecture of the human striatum. J Comp Neurol. 1997;384:1–25. doi: 10.1002/(sici)1096-9861(19970721)384:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Hopkins DA. Amygdalotegmental projections in the rat, cat, and rhesus monkey. Neuroscience Letters. 1975;1:263–270. doi: 10.1016/0304-3940(75)90041-5. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Irle E, Markowitsch HJ. Afferent connections of the substantia innominata/basal nucleus of Meynert in carnivores and primates. Journal fur Hirnforschung. 1986;27:343–367. [PubMed] [Google Scholar]
- Jolkkonen E, Miettinen R, Pitkanen A. Projections from the amygdalo-piriform transition area to the amygdaloid complex: a PHA-l study in rat. Journal of Comparative Neurology. 2001a;432:440–465. doi: 10.1002/cne.1113. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Pikkarainen M, Kemppainen S, Pitkanen A. Interconnectivity between the amygdaloid complex and the amygdalostriatal transition area: a PHA-L study in rat. Journal of Comparative Neurology. 2001b;431:39–58. doi: 10.1002/1096-9861(20010226)431:1<39::aid-cne1054>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. Journal of Comparative Neurology. 1998;395:53–72. doi: 10.1002/(sici)1096-9861(19980525)395:1<53::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiology & Behavior. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D. Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain Research. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJH. The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Kita H, Oomura Y. An HRP study of the afferent connections to rat lateral hypothalamic region. Brain Research Bulletin. 1982;8:63–71. doi: 10.1016/0361-9230(82)90028-4. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. Journal of Comparative Neurology. 1977;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. Journal of Comparative Neurology. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10(4):1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. J Neurosci. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJD, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci. 1992;12(6):2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majak K, Pitkanen A. Projections from the periamygdaloid cortex to the amygdaloid complex, the hippocampal formation, and the parahippocampal region: a PHA-L study in the rat. Hippocampus. 2003;13:922–942. doi: 10.1002/hipo.10134. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol. 1991;309:445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. Journal of Comparative Neurology. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44(1):15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, Inc.; 1992. Cell types and intrinsic connections of the amygdala; pp. 67–96. [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Annals of the New York Academy of Sciences. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Nance DM, Burns J. Fluorescent dextrans as sensitive anterograde neuroanatomical tracers: applications and pitfalls. Brain Research Bulletin. 1990;25:139–145. doi: 10.1016/0361-9230(90)90264-z. [DOI] [PubMed] [Google Scholar]
- Nose I, Higashi H, Inokuchi H, Nishi S. Synaptic responses of guinea pig and rat central amygdala neurons in vitro. Journal of Neurophysiology. 1991;65:1227–1241. doi: 10.1152/jn.1991.65.5.1227. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. see comment. [DOI] [PubMed] [Google Scholar]
- O’Rourke HJ, Fudge JL. Distribution of serotonin-labeled fibers in the primate amygdala: implications for mood disorders. Biological Psychiatry. 2006;60:479–490. doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. Journal of Comparative Neurology. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behavioural Brain Research. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Petrovich G, Ross C, Holland P, Gallagher M. Society for Neuroscience. Society for Neuroscience; Atlanta, GA: 2006. Central but not basolateral amygdala is critical for control of feeding by aversive, conditioned cues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: A PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Research. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Distribution of parvalbumin-immunoreactive cells and fibers in the monkey temporal lobe: The amygdaloid complex. J Comp Neurol. 1993;331:14–36. doi: 10.1002/cne.903310103. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. Journal of Comparative Neurology. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: Projections originating in the lateral nucleus. J Comp Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Hokfelt BT, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 1987. pp. 279–381. [Google Scholar]
- Rosene D, Van Hoesen G. The hippocampal formation of the primate brain. In: Jones E, Peters A, editors. Cerebral Cortex: Further Aspects of Cortical Function, Including HIppocampus. VI. Plenum Press; New York: 1987. pp. 345–456. [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 1986;34(10):1301–1315. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Rourke HO, Fudge JL. Distribution of Serotonin Transporter Labeled Fibers in Amygdaloid Subregions: Implications for Mood Disorders. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT. Cortical and subcortical afferents of the amygdaloid complex. Advances in Experimental Medicine & Biology. 1986;203:35–52. doi: 10.1007/978-1-4684-7971-3_3. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol. 1985a;242:1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Comp Neurol. 1987;256:175–210. doi: 10.1002/cne.902560202. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985b;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Pearson J, Shinoda K, Alheid GF, De Olmos JS. The human basal forebrain. Part I. An Overview. In: Bloom FE, et al., editors. Handbook of Chemical Neuroanatomy, vol.15: The Primate Nervous System, Part III. Elsevier; Amsterdam: 1999. pp. 1–56. [Google Scholar]
- Salinas JA, Parent MB, McGaugh JL. Ibotenic acid lesions of the amygdala basolateral complex or central nucleus differentially effect the response to reductions in reward. Brain Research. 1996;742:283–293. doi: 10.1016/s0006-8993(96)01030-x. [DOI] [PubMed] [Google Scholar]
- Samson RD, Duvarci S, Pare D. Synaptic plasticity in the central nucleus of the amygdala. Rev Neurosci. 2005;16:287–302. doi: 10.1515/revneuro.2005.16.4.287. [DOI] [PubMed] [Google Scholar]
- Santiago AC, Shammah-Lagnado SJ. Afferent connections of the amygdalopiriform transition area in the rat. Journal of Comparative Neurology. 2005;489:349–371. doi: 10.1002/cne.20637. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J Comp Neurol. 1988;271:185–207. doi: 10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- Savander V, Go C-G, LeDoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361:345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- Savander V, Go C-G, LeDoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the accessory basal nucleus. J Comp Neurol. 1996;374:291–313. doi: 10.1002/(SICI)1096-9861(19961014)374:2<291::AID-CNE10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schmued L, Kyriakidis K, Heimer L. In vivo anterograde and retrograde axonal transport of the fluorescent rhodamine-dextran-amine, Fluoro-Ruby, within the CNS. Brain Research. 1990;526:127–134. doi: 10.1016/0006-8993(90)90258-d. [DOI] [PubMed] [Google Scholar]
- Seamans J, Magyar O, Floresco S. Society for Neuroscience Annual Meeting. Society for Neuroscience; Atlanta, GA: 2006. Subcortical regulation of conflict behavior: central nucleus of the amygdala. [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. 1999;94:1097–1123. doi: 10.1016/s0306-4522(99)90280-4. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. Journal of Comparative Neurology. 2001a;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol. 2001b;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36:449–472. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138:703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. Journal of Comparative Neurology. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat--cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Sun N, Yi H, Cassell MD. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol. 1994;340:43–64. doi: 10.1002/cne.903400105. [DOI] [PubMed] [Google Scholar]
- Turner BH, Gupta KC, Mishkin M. The locus and cytoarchitecture of the projection areas of the olfactory bulb in Macaca mulatta. Journal of Comparative Neurology. 1978;177:381–396. doi: 10.1002/cne.901770303. [DOI] [PubMed] [Google Scholar]
- VanHoesen GW. The differential distribution, diversity and sprouting of cortical projection to the amygdala in the rhesus monkey. In: Ben-Ari Y, editor. The Amygdaloid Complex. Elsevier/Norh-Holland Biomedical Press; Amsterdam: 1981. pp. 77–90. [Google Scholar]
- Vercelli A, Repici M, Garbossa D, Grimaldi A. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Research Bulletin. 2000;51:11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Waldvogel HJ, Faull RLM. Compartmentalization of parvalbumin immunoreactivity in the human striatum. Brain Res. 1993;610:311–316. doi: 10.1016/0006-8993(93)91415-o. [DOI] [PubMed] [Google Scholar]
- Wang C, Kang-Park MH, Wilson WA, Moore SD. Properties of the pathways from the lateral amygdal nucleus to basolateral nucleus and amygdalostriatal transition area. Journal of Neurophysiology. 2002;87:2593–2601. doi: 10.1152/jn.2002.87.5.2593. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ. Connections of the parahippocampal cortex in the cat. IV. subcortical efferents. J Comp Neurol. 1986;251:51–77. doi: 10.1002/cne.902520104. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Is the caudomedial shell of the nucleus accumbens part of the extended amygdala? A consideration of connections. Critical Reviews in Neurobiology. 1998;12:245–265. doi: 10.1615/critrevneurobiol.v12.i3.50. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Irving JC, Williams EA. Discrimination of striatopallidum and extended amygdala in the rat: a role for parvalbumin immunoreactive neurons? Brain Research. 2003;978:141–154. doi: 10.1016/s0006-8993(03)02801-4. [DOI] [PubMed] [Google Scholar]


