Abstract
Introduction:
Providing smokers with biologically based evidence of smoking-related disease risk or physical impairment may be an effective way to motivate cessation.
Methods:
Smokers were recruited for a free health risk assessment and randomized to receive personally tailored feedback based on their lung functioning, carbon monoxide (CO) exposure, and smoking-related health conditions or generic information about the risks of smoking and personalized counseling based on their diet, body mass index, and physical activity. All (n = 536) were advised to quit smoking and offered access to a free telephone cessation program. Participants were surveyed immediately after intervention and 1 month later to assess the impact on various indices of motivation to quit.
Results:
Immediately posttreatment, experimental participants rated themselves as more likely to try to quit (p = .02) and reported a greater mean increase in their motivation to quit than controls (p = .04). At 1-month follow-up, however, we found no significant group differences on any motivational indices. In post-hoc analyses comparing smokers in the experimental group with and without lung impairment, persons with impaired lung functioning had a greater change from baseline in posttreatment motivation to quit (adjusted p = .05) and perceived risk of developing a smoking-related disease (p = .03) compared with persons with no lung impairment, but we found no significant treatment effect on any motivational indices at 1 month.
Discussion:
The results suggest that the intervention had a small, temporary effect, but we found no clear evidence that the intervention increased motivation to quit smoking during the first month postintervention.
Introduction
To reduce smoking prevalence, we not only must provide treatment for smokers who are ready to quit but also must develop interventions that can enhance motivation for quitting and promote the use of effective cessation treatment programs. There is no single best strategy for motivating behavior change, but many leading health behavior change theories (e.g., the health belief model, health decision model, and protection motivation theory; Eraker, Kirscht, & Becker, 1984; Janz & Becker, 1984; Rogers, 1983) suggest that behavior change is induced in part by one's perceived disease susceptibility and a desire to avoid disease (Weinstein, 1993). Thus, increasing one's awareness of personal risk or harm caused by unhealthy habits could, theoretically, increase motivation for behavior change.
Following this reasoning, many researchers have suggested that providing smokers with biologically based evidence (i.e., biomedical evidence) of smoking-related disease risk or physical impairment may be an effective way to motivate cessation (Lerman, Orleans, & Engstrom, 1993; Lerman et al., 1997; Marteau & Lerman, 2001; McClure, 2001). Whether this strategy works, however, is not clear. Several literature reviews concluded that too few studies of acceptable methodological quality have been conducted to draw any firm conclusions (Bize, Burnand, Mueller, & Cornuz, 2007; McClure, 2001; Wilt, Niewoehner, Kane, MacDonald, & Joseph, 2007). Much of the literature reflects observational studies or randomized experiments in which the intervention design confounded the risk assessment and provision or intensity of cessation counseling, thereby preventing examination of the independent effect of the biomedical risk assessment (Wilt et al., 2007).
In addition, many studies have focused on smoking abstinence as their main outcome. Although abstinence is the ultimate intervention goal, it may be an unreasonable indicator of treatment impact when little or no action-oriented cessation treatment is provided, as has often been the case. A more reasonable indicator of motivational intervention impact is change in one's motivation to quit smoking. This can be manifest in a number of ways, including stated intent to quit, use of treatment services, or actual quit attempts. Finally, the published results have been mixed and do not clearly support the use of personalized risk assessments for motivating smoking cessation (Bize et al., 2007). The present study was designed to overcome some of the key limitations found in previous work and to add to the existing literature base.
Get PHIT! (a Proactive Health Intervention for Tobacco-Users) compared the effects of a personally tailored, biologically based motivational intervention to those of a generic motivational intervention for smoking cessation. The biologically based motivational treatment included feedback on participants’ carbon monoxide (CO) exposure (expired CO and estimated carboxyhemoglobin [COHb] levels), pulmonary functioning assessed via portable office-based spirometry, and self-reported smoking-related symptoms.
CO level and lung functioning were chosen for the biomedical risk assessment for several reasons. First, the assessment of each is fairly straightforward and inexpensive, particularly when portable spirometry is used. Abnormal results on both tests can be clearly linked with smoking, creating a “teachable moment” for intervention; however, even in the absence of impaired lung functioning, there is a teachable opportunity to advise smokers about the potential risks of continued smoking and benefits of quitting. Also, the question remains whether CO assessment and spirometry screening are effective cessation aids. The National Lung Health Education Program concluded that “spirometry testing probably enhances smoking cessation rates” (Ferguson, Enright, Buist, & Higgins, 2000) and routine use of office-based spirometry has been called for with smokers (Bohadana, Nilsson, & Martinet, 2005), but a recent empirical review concluded that “available evidence is insufficient to determine whether obtaining spirometric values and providing that information to patients improves smoking cessation” (Wilt et al., 2007). Finally, we were interested in creating an intervention that could be standardized and delivered across a variety of community settings. The portability, ease of administration, and ability to link standardized health messages to cutoff values on these measures allowed us to achieve these goals.
We hypothesized that providing smokers with personalized, biomedical risk information would increase their motivation to quit compared with smokers advised to quit without this information and that the effect on motivation would be mediated in part by participants’ perceived smoking-related disease susceptibility. Because positive effects of the intervention could be undermined by high levels of emotional distress resulting from the biomedical risk assessment (Lerman et al., 1997; McClure, 2001), we also monitored the emotional impact of the intervention as a secondary outcome. All outcomes were assessed immediately following the counseling and at 1-month postintervention.
Methods
Setting and participants
All activities were reviewed and approved by the Group Health Institutional Review Board. Study enrollment began in March 2005 and ended September 2007.
Smokers were recruited throughout western Washington State using a variety of outreach strategies including health plan records, data from the Washington State Quitline, and a purchased mailing list of smokers (McClure, Richards, Westbrook, Pabiniak, & Ludman, 2007). Likely smokers were mailed a study invitation letter and then called to be screened for interest and eligibility. Ads also were placed in local media, public clinics, and other local venues. Interested smokers were invited to call to learn more and be screened for eligibility. Individuals eligible based on the phone screening were scheduled for an in-person appointment.
Smokers were eligible if at the baseline appointment they were 18 years or older, could read and write in English, were not currently receiving cessation treatment, had no significant physical or mental impairments that prevented use of a computer or phone or impaired their ability to comprehend the counseling, and reported no medical contraindications for spirometry assessment (e.g., recent heart attack or chest surgery). Participation also was limited to smokers with elevated expired CO levels consistent with current smoking (≥10 p.p.m.; SRNT Subcommittee on Biochemical Verification, 2002) and who either smoked an average of 15 cigarettes/day for the past year or smoked at least 10 cigarettes/day but had smoked for 10 years or more. The latter criterion was added to accommodate lighter smokers who had a significant history of smoking. Eligible smokers completed a computer-based survey and were automatically randomized to treatment. All participants were offered a free personalized health risk assessment and advice on changes they could make to improve their health. The intervention was presented as a free health screening, not as a smoking cessation program, and recruitment materials emphasized that people did not have to be interested in quitting smoking to participate.
Intervention
Participants in both groups completed a health risk screening; received a written, personalized health risk report and brief (∼20 min) counseling based on the assessment results; were advised to quit smoking; were given self-help smoking cessation materials (Clearing the Air; National Cancer Institute, 2003); and were given access to a free telephone smoking cessation counseling program that they could enroll in anytime in the next 12 months free of charge if they decided to quit smoking. The telephone cessation program was provided by Free & Clear, Inc., Seattle, WA.
The content of the written health risk report and focus of the brief counseling varied by treatment group. Control reports summarized participants’ intake of whole grain foods, physical activity, body mass index, years smoked, and average number of cigarettes per day. Controls were counseled to make relevant behavior changes, including quitting smoking, but this recommendation was based on generic information about the risks of smoking (e.g., smoking increases one's risk for heart disease). It was not linked with any personal assessment data. Participants also were instructed how to access the free phone counseling program when they were ready to quit smoking.
Experimental reports addressed the association between smoking and various smoking-related conditions (e.g., heart disease, cancer, cataracts, and gum disease), the impact of smoking on lung functioning, the association between smoking and CO exposure, and the health effects of chronic and acute CO exposure. Participants’ self-reported smoking-related symptoms (e.g., persistent cough) and diagnosed medical conditions, CO level, and spirometry results were summarized in the report and linked with clear advice to quit smoking and instructions on how to access cessation treatment, when ready to quit. CO level was assessed using a Bedfont MicroIII monitor, which provided expired CO level and an estimated COHb level based on expired CO. Participants were presented their CO levels and normative levels for nonsmokers.
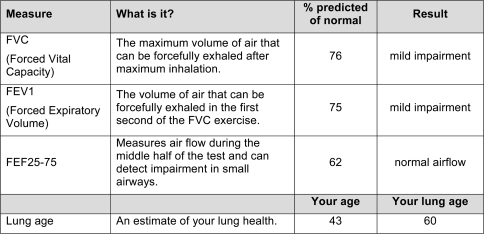
Lung functioning was assessed with a Jaeger SpiroPro portable spirometer. Three measures were assessed: forced vital capacity (FVC), or the maximum volume of air that can be forcefully exhaled after maximal inhalation; forced expiratory volume (FEV1), or the volume of air that can be forcefully exhaled in the first second of the FVC exercise; and FEF25–75, a measure of airflow during the middle portion of the test and indicator of small airway obstruction. Performance was measured in terms of the percent predicted of normal using standardized adjusted algorithms.
Each experimental report explained the relation between smoking and asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD), and other lung disease and the purpose of spirometric testing. Participants completed at least two tests and the best values from each were presented in tabular form along with a brief description of each outcome measure (FEV1, FVC, and FEF 25–75) and a qualitative interpretation of the test results based on standard cutoff values reflecting normal functioning, mild impairment, moderate impairment, or severe impairment for FVC and FEV1, and normal versus reduced airflow for FEF 25–75 (see Figure 1). The goal was not to make a clinical diagnosis but to provide standardized feedback based on objective test measures. Cutoff values were established by a consulting pulmonologist. If FEV1 percent predicted was less than 80%, suggesting possible impairment, lung age also was calculated (Morris & Temple, 1985) and presented.
Figure 1.
Example of spirometry results table from experimental group report.
This feedback was designed to create a teachable moment, regardless of one's lung functioning. Each report stated either that the spirometric test results were currently normal and now would be a good time to consider quitting because continued smoking is linked with lung damage in the future or that the results were suggestive of impaired lung functioning. Either way, participants were informed that now was a good time to consider quitting smoking. This message was reinforced by the counselor.
Finally, each experimental report included a graph depicting average decline in FEV1 over time for a never-smoker, a smoker who quits at age 45, one who quits at age 65, and one who never quits, based on epidemiological data. The graph, adapted from Fletcher and Peto (1977), was used to reinforce that it is never too late to quit smoking. If one has reduced lung functioning, quitting can help preserve functioning and prevent further decline; and if one does not have impaired functioning, the graph illustrates the projected decline in functioning over time with continued smoking. The graph was presented to all experimental participants to reinforce discussions about the importance of quitting smoking. Protocol adherence and treatment fidelity were monitored over the course of the study via direct observation or tape recording of approximately 10% of the counseling sessions. All monitoring was performed by a doctorate-level psychologist (EL). Additional training was provided, as necessary, throughout the study to protect against intervention drift.
Assessment
Participants were surveyed at baseline (pretreatment), immediately posttreatment, and again at 1-month follow-up. Our primary outcome of interest was motivation to quit at 1 month. To characterize the behavioral and intentional aspects of this construct, multiple indices of motivation for quitting smoking were assessed: enrollment in the provided phone counseling program within 1 month of treatment, as determined by objective treatment records; a self-report of a 24-hr quit attempt since the intervention contact; a self-report of seriously considering quitting smoking in the next 30 days; and self-reported motivation for quitting. Motivation for quitting was assessed using a 5-point Likert scale ranging from “not at all” to “extremely.” Additionally, a composite index of motivation was calculated at 1-month postintervention by assigning all abstainers a score of 6 on the Likert motivation scale. Thus, the new index reflected motivation to quit among both smokers and nonsmokers at 1-month follow-up. Abstinence was defined as a self-report of no smoking, even a puff, in the past 7 days.
Our secondary outcomes included motivation to quit assessed immediately following the intervention, as well as self-reported intent to use the provided treatment materials, the impact on participants’ perceived disease susceptibility postintervention, and the emotional impact of the intervention. These variables were chosen based on their hypothesized roles in mediating treatment outcome or their importance as secondary outcomes. For example, perception of disease risk, or disease susceptibility, has been shown to influence the adoption of protective health behaviors (Brewer et al., 2007) and is an important potential treatment mediator. Emotional distress is a potential adverse effect of the intervention (McClure, 2001) and important secondary outcome.
To assess perceived disease susceptibility, participants were asked how likely it was that they would be diagnosed with a smoking-related disease in their lifetime and how likely it was that they would develop lung disease such as COPD, emphysema, or cancer. To assess the emotional impact of the counseling, participants were asked posttreatment how upset they were by the information they just received. Response options for each of these items ranged from “not at all” to “extremely” on a 5-point scale. Mood also was assessed pre- and postintervention using the Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988).
Additional assessment measures included participant demographics, smoking history, the Fagerström Test for Nicotine Dependence (Fagerström, Heatherton, & Kozlowski, 1990), stage of change for smoking cessation (Prochaska & DiClemente, 1983; Prochaska et al., 1994), perceived severity of lung disease, likelihood of using the provided treatment resources, likelihood of trying to quit as a result of the intervention, likelihood of quitting smoking in the next 30 days, and self-efficacy for quitting smoking. To assess self-efficacy, participants were asked how confident they were that they could quit smoking for good, ranging from “not at all” to “extremely” confident on a 5-point Likert scale (Audrain et al., 1997; Orleans et al., 1991; Rimer & Orleans, 1994). Perceived severity and perceived likelihood for each outcome described above also were rated on a 5-point Likert scale ranging from “not at all” to “extremely.”
Sample flow
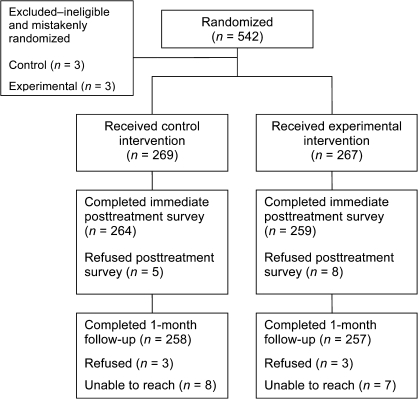
The sample flow is depicted in Figure 2. A total of 542 participants were randomized to treatment, although six participants’ CO levels did not meet the eligibility cutoff, and they were removed from the sample postrandomization and preanalysis, leaving 536 participants. Completion rates for the posttreatment and 1-month surveys were 98% and 96%, respectively.
Figure 2.
Overview of screening, enrollment, randomization, and follow-up data collection.
Data analyses
Descriptive statistics were used to characterize participants by treatment group. Groups were compared using t tests for means and chi-square tests for percentages. To examine the impact of the intervention on each treatment group's motivation to quit smoking, we computed change from baseline to follow-up. Ordinal and continuous outcomes were compared using mean change and t tests used to get unadjusted p values. Adjusted p values were obtained for the motivational outcomes using regression analyses, controlling for the baseline value of each outcome and relevant baseline covariates selected a priori based on their theoretical relationship with motivation to quit (footnote for list of covariates, see Table 2). Binary outcomes were compared using a McNemar's chi-square test to compare the change in the percent “yes” from baseline to follow-up, and adjusted p values were obtained using logistic regression controlling for the baseline value of each outcome and relevant covariates. Consistent with a McNemar's chi-square analysis, this analysis was limited to those whose status on the outcome changed between baseline and follow-up. For a few outcomes, no baseline measure was available (e.g., if enrolled in phone counseling). For these outcomes, groups were compared using means or percentages rather than change from baseline. Similar unadjusted analyses were used to examine change in secondary outcomes of interest. A similar analytic plan was used for post-hoc comparison of persons with and without lung impairment in the experimental group.
Table 2.
Change in motivational indices from baseline to follow-up
| Motivational index | Control group | Experimental group | p value | Adjusted p valuea |
| Motivation to quitb | ||||
| Baseline: mean | 3.50 | 3.48 | .80 | |
| Postintervention: change from baseline | 0.11 | 0.25 | .04 | .09 |
| One month: change from baselinec | 0.03 | −0.05 | .35 | .20 |
| Considering quitting in 30 days | ||||
| Baseline: % | 52.6 | 53.8 | .79 | |
| Postintervention: change from baseline | −2.00 | 1.70 | .21 | .15 |
| One month: change from baselinec | −4.50 | −5.80 | .99 | .82 |
| Mean motivation to quit indexd,e | 3.63 | 3.53 | .30 | .06 |
| Percent made a quit attempte | 19.0 | 22.5 | .31 | .72 |
| Percent enrolled in phone-based programe | 15.2 | 11.6 | .22 | .24 |
Note. All measures were assessed among smoking and nonsmoking participants at each time point, unless otherwise noted.
Adjusted for baseline value of each variable plus baseline measures of self-efficacy for quitting, perceived likelihood of quitting in next 30 days, number of times ever quit smoking, Fagerström Test for Nicotine Dependence score, perceived smoking-related disease susceptibility, and perceived smoking-related disease severity.
Likert scale ranging from 1 = “not at all” to 5 = “extremely.”
Analyses limited to continuing smokers only (n = 485) at the 1-month follow-up.
Likert scale ranging from 1 to 6, where nonsmokers at 1 month have been assigned a score of 6.
Assessed at 1-month follow-up only.
Results
Participants
Baseline characteristics are presented in Table 1. As was our objective, smokers were recruited across the continuum of readiness to quit. Mean expired CO level was 26.5 p.p.m., and 37% of the experimental group had lung functioning indicative of at least mild impairment based on their FEV1, FVC, or FEF 25–75 scores (26.6% had impaired FVC performance, 33.3% had impaired FEV1 scores, and 13.1% had impaired FEF 25–75 performance). No significant intervention group differences were observed on baseline measures. Among experimental participants with impaired spirometric performance, the average age was 50.9 years and the average estimated lung age was 79.8 years.
Table 1.
Demographic characteristics of study sample at baseline
| Characteristics | Overall (%) | Control (%) | Experimental (%) | p value |
| Female | 53.2 | 52.8 | 53.6 | .86 |
| White | 82.4 | 80.9 | 83.8 | .37 |
| Education | ||||
| Some college or greater | 75.5 | 78.1 | 72.9 | .17 |
| Medical insurance | 92.9 | 92.9 | 92.9 | .98 |
| Insurance coverage for tobacco treatment | 42.0 | 42.4 | 41.5 | .70 |
| Stage of change | .99 | |||
| Precontemplation | 24.8 | 25.1 | 24.5 | |
| Contemplation | 50.8 | 50.6 | 50.9 | |
| Preparation | 24.4 | 24.3 | 24.5 | |
| Considering quitting in 30 days | 53.2 | 52.6 | 53.8 | .79 |
| Lung impairmenta | ||||
| Impaired FVC | ||||
| Impaired FEV1 | ||||
| Impaired FEF25–75 | ||||
| M | M | M | p value | |
| Age (years) | 50.8 | 50.6 | 50.9 | .74 |
| Cigarettes per day | 20.7 | 20.6 | 20.8 | .79 |
| FTND | 5.12 | 5.22 | 5.03 | .27 |
| Prior quit attempts | 10.1 | 9.7 | 10.5 | .58 |
| Expired carbon monoxide | 26.5 | 26.7 | 26.3 | .63 |
| Self-efficacy for quittingb | 2.96 | 2.89 | 3.04 | .08 |
| Perceived severity of lung diseaseb | 4.40 | 4.40 | 4.40 | .95 |
Note. FTND, Fagerström Test for Nicotine Dependence. Baseline values of primary and secondary outcomes are presented in Tables 2 and 3.
Defined as spirometry performance indicative of impairment on FEV1, FVC, or FEF25–75. Experimental participants only.
Likert scale ranging from 1 = “not at all” to 5 = “extremely.”
Because smokers were recruited through both proactive and reactive means, we compared baseline motivation to quit by each recruitment source to see if interest in quitting smoking varied across these groups. We found no significant differences.
Treatment impact by intervention group
Motivation for quitting and behavior change.
Self-reported motivation to quit increased significantly immediately following intervention in both the experimental and control groups (p < .001 and p = .02, respectively), but the increase was greater in the experimental group than among controls (+0.25 vs. +0.11; p = .04 and adjusted p = .09; see Table 2). Among continuing smokers at 1 month, motivation to quit was similar to baseline levels in both intervention groups.
The percentage of people considering quitting in the next 30 days increased relative to baseline at immediate posttreatment follow-up in the experimental group (from 53.8% to 55.5%) and decreased in the control group (from 52.6% to 50.6%), but these differences were not statistically significant (see Table 2). At 1 month, the percentage of continuing smokers considering quitting in the next 30 days decreased in both groups to below baseline levels, but the change did not vary by treatment group.
No significant group differences were observed at 1 month on the calculated motivation index, which included continuing smokers and quitters, the percentage who enrolled in the provided phone treatment program, or the percentage who made a quit attempt (see Table 2).
Emotional impact of counseling.
Compared with controls, experimental participants rated the information they received as more upsetting (p = .0001; Table 3). Despite this difference, mean scores for both groups were low and most participants rated their feedback as either “not at all” or only “a little” upsetting (60.5% experimental vs. 88.2% control). Experimental participants displayed greater, but not statistically significant, improvement in their positive affect immediately following intervention (+2.8 experimental vs. +2.0 control, p = .08). Control subjects exhibited a greater change in negative affect (+0.2 experimental vs. −0.7 control, p = .04).
Table 3.
Intervention group differences on secondary outcomes
| Control group | Experimental group | p value | |
| How upsetting was information: meana | 1.40 | 2.22 | .0001 |
| PANAS positive affectb | |||
| Baseline: mean | 29.0 | 30.3 | .053 |
| Postintervention: change from baseline | 2.00 | 2.80 | .08 |
| PANAS negative affectb | |||
| Baseline: mean | 16.2 | 15.4 | .18 |
| Postintervention: change from baseline | −0.70 | 0.20 | .04 |
| Likelihood will try to quit smoking as a result of intervention | |||
| Postintervention: meana | 3.59 | 3.81 | .02 |
| Likelihood will use provided materials to try to quit | |||
| Postintervention: meana | 3.85 | 3.95 | .23 |
| Perceived risk of lung diseasea | |||
| Baseline: mean | 3.48 | 3.49 | .89 |
| Postintervention: change from baseline | 0.07 | −0.04 | .10 |
| One month: change from baseline | |||
| Perceived risk of smoking-related diseasea | |||
| Baseline: mean | 3.64 | 3.64 | .97 |
| Postintervention: change from baseline | 0.04 | −0.05 | .19 |
| One month: change from baseline | −0.17 | −0.20 | .70 |
Note. All measures were assessed among smoking and nonsmoking participants at each time point.
Likert scale ranging from 1 = “not at all” to 5 = “extremely.”
Scale range = 10–50, where lower scores represent less affect.
Because overall scale scores can obscure subtle emotional distress caused by the intervention, we also examined the percentage of participants in each group who rated themselves as “quite a bit” or “extremely” distressed, upset, or scared on individual PANAS negative affect scale items. Groups did not differ in the proportion who described themselves as distressed (5.6% experimental vs. 8.2% control, p = .24), upset (3.4% experimental vs. 4.8% control, p = .39), or scared (8.2% experimental vs. 10.8% control, p = .32). The combined scale scores and individual item analyses suggest that the experimental intervention was not emotionally distressing.
Intent to use provided services.
Most participants (70.2%) said posttreatment that they were “very” or “extremely” likely to use the resources provided to try to quit smoking. Group differences were not significant (see Table 3).
Likelihood will try to quit as a result of intervention.
Most participants (62.3%) thought that they were “very” or “extremely” likely to try to quit smoking as a result of the intervention. Likelihood was rated significantly higher among the experimental group (p = .02).
Impact on perceived disease susceptibility.
Groups' perceived risk of developing a lung disease, such as COPD or lung cancer, increased from baseline to posttreatment among control subjects and decreased slightly among experimental participants, but the difference in the change between groups was not statistically significant (see Table 3). Similar results were observed for perceived risk of developing a smoking-related disease. We did not conduct additional analyses to test for the hypothesized effect of disease risk as a mediator of treatment outcome, since the intervention did not have a significant impact on motivation to quit smoking and was not associated with perceived disease risk at 1 month, two conditions necessary for mediation (Holmbeck, 1997).
Post-hoc analyses
Bednarek et al. (2006) found that smokers informed that they had evidence of airway obstruction on spirometric screening were more likely to quit smoking by 1-year follow-up, suggesting that this information increased smokers’ motivation or ability to quit. To test for a similar effect on our motivation outcomes, we compared persons in the experimental condition with evidence of lung impairment to those with normal lung functioning. Relevant baseline and follow-up comparisons are presented in Table 4. Notably, perceived risk of developing a lung disease and perceived risk of developing a smoking-related disease increased immediately posttreatment among persons with impaired lung functioning and decreased among those with normal functioning, but this change was only significant for perceived risk of developing a smoking-related disease (p = .03). Perceived disease risk declined for both groups by 1 month, and the change was not significant. Motivation to quit increased more posttreatment among impaired smokers than unimpaired smokers (+0.37 vs. +0.19, adjusted p = .05) but returned to near baseline levels in both groups by 1 month. With the exception of the percentage that made a quit attempt by 1-month follow-up, the other motivational indices trended toward more positive change in the impaired group, but all differences were small and none were statistically significant.
Table 4.
Comparison of experimental participants with and without impaired lung functioning on primary and secondary outcomes
| Outcome | Not impaired (n = 168) | Impaired (n = 99) | p value | Adjusted p valuea |
| Motivation to quitb | ||||
| Baseline: mean | 3.45 | 3.52 | .60 | |
| Postintervention: change from baseline | 0.19 | 0.37 | .07 | .05 |
| One month: change from baselinec | −0.09 | 0.01 | .40 | .47 |
| Considering quitting in 30 days | ||||
| Baseline: % | 52.1 | 56.6 | .48 | |
| Postintervention: change from baseline | −0.70 | 5.80 | .18 | .59 |
| One month: change from baselinec | −6.50 | −4.60 | .59 | .61 |
| Mean motivation to quit indexd | 3.49 | 3.63 | .29 | .71 |
| Percent made a quit attempt | 23.1 | 21.9 | .82 | .92 |
| Percent enrolled in phone-based program | 8.9 | 16.2 | .08 | .46 |
| Likelihood will try to quit smoking as result of intervention | ||||
| Postintervention: mean | 3.74 | 3.92 | .18 | |
| Likelihood will use provided materials to try to quit | ||||
| Postintervention: mean | 3.96 | 3.95 | .94 | |
| Perceived risk of lung diseaseb | ||||
| Baseline: mean | 3.41 | 3.63 | .06 | |
| Postintervention: change from baseline | −0.10 | 0.06 | .12 | |
| One month: Change from baseline | 0.08 | −0.03 | .68 | |
| Perceived risk of smoking diseaseb | ||||
| Baseline: mean | 3.58 | 3.74 | .17 | |
| Postintervention: change from baseline | −0.13 | 0.10 | .03 | |
| One month: change from baseline | −0.29 | −0.07 | 0.51 | |
| PANAS positive affecte | ||||
| Baseline: mean | 29.63 | 31.37 | .06 | |
| Postintervention: change from baseline | 2.73 | 2.98 | .70 | |
| PANAS negative affecte | ||||
| Baseline: mean | 15.37 | 15.43 | .94 | |
| Postintervention: change from baseline | −0.25 | 0.97 | .06 | |
| How upsetting was informationb | ||||
| Postintervention: mean | 1.99 | 2.60 | .0001 | |
Note. PANAS, Positive and Negative Affect Scale; All measures were assessed among smoking and nonsmoking participants at each time point, unless otherwise noted.
Primary outcome model adjusted for baseline value of each variable plus baseline measures of self-efficacy for quitting, perceived likelihood of quitting in next 30 days, number of times ever quit smoking, Fagerström Test for Nicotine Dependence score, perceived smoking-related disease susceptibility, and perceived smoking-related disease severity.
Likert scale ranging from 1 = “not at all” to 5 = “extremely.”
Analyses limited to continuing smokers only (n = 485) at the 1-month follow-up.
Likert scale ranging from 1 to 6, where nonsmokers have been assigned a score of 6 assessed at 1 month.
Scale range = 10–50, where lower scores represent less affect.
Discussion
Researchers have suggested that providing smokers with personalized feedback about the health risks of their smoking may help promote cessation (Lerman et al., 1993, 1997; Marteau & Lerman, 2001; McClure, 2001), but the research to date does not allow for definitive conclusions about the effectiveness of this intervention approach. More well-controlled, randomized clinical trials have been called for (Bize et al., 2007; Kaminsky & Marcy, 2004; Kotz, van Schayck, Huibers, & Wesseling, 2007; McClure, 2001). In particular, additional studies examining the effectiveness of spirometric testing are needed (Wilt et al., 2007).
The present randomized trial examined the impact of a personalized risk assessment that highlighted smokers’ lung functioning, CO exposure, and personal health history in an effort to enhance motivation to quit smoking. We hypothesized that smokers who received this personalized feedback would exhibit an increase in their motivation to quit relative to smokers advised to quit but only informed of the generic health risks of smoking. This was not the case. Immediately posttreatment, persons in the experimental group reported more significant change in their motivation to quit and felt that they were more likely to try to quit smoking, but group differences were not significant at 1 month on any of the motivational indices. Slightly more experimental subjects did make a quit attempt (22.5% vs. 19%), but they also were less likely to have enrolled in the cessation treatment program (11.6% vs. 15.2%). In short, we found no evidence of a robust or sustained change in motivation to quit resulting from the personalized health risk assessment. This finding is consistent with the conclusions of a trial in which college smokers were informed of their lung age, assessed via spirometry test, and respiratory symptom feedback (Lipkus & Prokhorov, 2007). In that study, lung age and respiratory feedback did not translate into appreciable changes in motivation to quit. The results also are consistent with the balance of data available regarding the use of spirometry to promote smoking cessation. In a review of randomized trials, Wilt et al. (2007) concluded that despite growing enthusiasm for using spirometric results to motivate smoking cessation, the current literature base does not support the use of such testing for this purpose.
In retrospect, our findings are not completely surprising. We hypothesized a priori that any effects on motivation would be mediated in part through smokers’ perceived disease risk. Our goal was to increase smokers’ perceived susceptibility to smoking-related disease, particularly lung disease. Not only did groups not differ in their ratings of perceived disease risk at postintervention, but we did not observe a significant change in either perceived risk rating from baseline to follow-up. In fact, among experimental participants, mean ratings declined very slightly postintervention (≤.05 for each scale).
It is not clear why the counseling did not have its intended effect on smokers’ perceived disease risk. The information was presented in writing and highlighted during the counseling. All materials were written at an eighth-grade or lower reading level, and participants were reasonably educated, so comprehension should not have been a problem. The intervention was monitored to ensure adequate treatment fidelity, and we found no evidence that the counseling caused undue emotional distress or anxiety that would have interfered with participants’ ability to process the information they received. Most likely, perceived risk was not affected because most experimental participants (63%) had no demonstrable evidence of lung impairment. Even though this group still had high levels of CO exposure, and the high likelihood of future lung disease with continued smoking was highlighted for this group, lack of any demonstrable current impairment could have undermined the impact of the counseling. This would account for the slight decline in perceived risk scores posttreatment. This supposition also is supported by the post-hoc analyses. Across both measures of perceived disease risk, ratings went down from baseline to postintervention among those with no impairment and increased among those with evidence of lung impairment. Change in posttreatment motivation to quit also was greater among the group with impairment (adjusted p = .05), and 16% of this group enrolled in the free treatment program by 1 month compared with 9% of those with no impairment. This difference was not statistically significant but could have clinical relevance if it translates into a higher abstinence rate over the long term.
Several caveats to these findings should be noted. First, our intervention was designed to be a standardized, brief motivational intervention. It was not targeted specifically to people with known health conditions or lung impairment, and it was not conducted in the context of a medical appointment. We also combined multiple forms of biomedical risk assessment. As such, our results may not generalize to the use of spirometry alone, the use of biomedical assessments conducted in the context of a medical encounter or other settings, or with persons with existing smoking-related diseases. Second, although we tried to present individuals’ risk information verbally, graphically, and in writing in a way that participants could comprehend, there may be better ways to present this type of feedback, particularly to persons who do not have demonstrable lung impairment. At present, there are no accepted best practices for how to present health risk information (Lipkus, 2007), but ongoing risk communications research could inform these decisions in the future. Third, the results presented here should be considered preliminary. The true measure of this intervention will be its impact on smoking cessation assessed over the long term. One-year outcome data are currently being collected and will be presented in a future report. Until then, we cannot comment on the effectiveness of this intervention as a means of promoting smoking cessation, but we can conclude that the intervention did not significantly enhance motivation to quit over the first month posttreatment. Furthermore, our results suggest the need for caution when using biomedical risk assessments to promote motivation for tobacco cessation, particularly among smokers with no demonstrable health impairment. In this case, a lack of disease evidence may undermine the intervention intent and inadvertently support continued smoking. We will watch closely for this effect in our long-term outcomes. In the meantime, these findings add to the small, but growing, cadre of randomized trials assessing the impact of biomedical risk assessments on motivation to quit smoking.
Funding
National Cancer Institute (CA100341) and Group Health.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Richard Hert, M. D., Ralph Stumbo, Rick Bloss, Zoe Bermet, Mary Shea, Lisa Shulman, Emily Westbrook, Mona Deprey, Free & Clear, Inc., the Washington State Quitline, and the staff of the Center for Health Studies’ Survey Research Program for their help with this work.
References
- Audrain J, Boyd NR, Roth J, Main D, Caporaso NF, Lerman C. Genetic susceptibility testing in smoking-cessation treatment: One-year outcomes of a randomized trial. Addictive Behavior. 1997;22:741–751. doi: 10.1016/s0306-4603(97)00060-9. [DOI] [PubMed] [Google Scholar]
- Bednarek M, Goerecka D, Wielgomas J, Czajkowska-Malinowska M, Regula J, Mieszko-Filipczyk G, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869–873. doi: 10.1136/thx.2006.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bize R, Burnand B, Mueller Y, Cornuz J. Effectiveness of biomedical risk assessment as an aid for smoking cessation: A systematic review. Tobacco Control. 2007;16:151–156. doi: 10.1136/tc.2006.017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Martinet Y. Detecting airflow obstruction in smoking cessation trials: A rationale for routine spirometry. Chest. 2005;128:1252–1257. doi: 10.1378/chest.128.3.1252. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: The example of vaccination. Health Psychology. 2007;26:136–145. doi: 10.1037/0278-6133.26.2.136. [DOI] [PubMed] [Google Scholar]
- Eraker SA, Kirscht JP, Becker MH. Understanding and improving patient compliance. Annals of Internal Medicine. 1984;100:258–268. doi: 10.7326/0003-4819-100-2-258. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear, Nose, & Throat Journal. 1990;69:763–765. [PubMed] [Google Scholar]
- Ferguson GT, Enright PL, Buist AS, Higgins MV. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–1161. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. British Medical Journal. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: Examples from the child-clinical and pediatric psychology literatures. Journal of Consulting and Clinical Psychology. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: A decade later. Health Education Quarterly. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Kaminsky DA, Marcy TW. COPD and smoking cessation motivation. Chest. 2004;125:1958. doi: 10.1378/chest.125.5.1958. [DOI] [PubMed] [Google Scholar]
- Kotz D, van Schayck CP, Huibers MJH, Wesseling GJ. Assessing the efficacy of spirometry for smoking cessation. Thorax. 2007;62:742. [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans TR, et al. Incorporating biomarkers of exposure to genetic susceptibility into smoking cessation treatment: Effects on smoking-related cognitions, emotions, and behavior change. Health Psychology. 1997;16:87–99. doi: 10.1037//0278-6133.16.1.87. [DOI] [PubMed] [Google Scholar]
- Lerman C, Orleans CT, Engstrom PF. Biological markers in smoking cessation treatment. Seminars in Oncology. 1993;20:359–367. [PubMed] [Google Scholar]
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: Suggested best practices and future recommendations. Medical Decision Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Prokhorov AV. The effects of providing lung age and respiratory symptoms feedback on community college smokers' perceived smoking-related health risks, worries, and desire to quit. Addictive Behaviors. 2007;32:516–532. doi: 10.1016/j.addbeh.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Lerman C. Genetic risk and behavioural change. British Medical Journal. 2001;322:1056–1059. doi: 10.1136/bmj.322.7293.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB. Are biomarkers a useful aid in smoking cessation: A review and analysis of the literature. Behavioral Medicine. 2001;27:37–47. doi: 10.1080/08964280109595770. [DOI] [PubMed] [Google Scholar]
- McClure JB, Richards J, Westbrook EO, Pabiniak C, Ludman E. Use of a commercial mailing list to recruit smokers: A cautionary tale. American Journal of Preventive Medicine. 2007;33:436–437. doi: 10.1016/j.amepre.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Preventive Medicine. 1985;14:655–662. doi: 10.1016/0091-7435(85)90085-4. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Clearing the air: Quit smoking today. Rockville, MD: Author; 2003. (NIH Publication No. 03-1647) [Google Scholar]
- Orleans CT, Schoenback VJ, Wagner EH, Quade D, Salmon MA, Pearson DC, et al. Self-help quit smoking interventions: Effects of self-help materials, social support instructions, and telephone counseling. Journal of Consulting and Clinical Psychology. 1991;59:439–448. doi: 10.1037//0022-006x.59.3.439. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF, Rossi JS, Goldstein MG, Marcus BH, Rakowski W, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychology. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- Rimer BK, Orleans CT. Does tailoring matter? The impact of a tailored guide on ratings and short term smoking-related outcomes for older smokers. Health Education Quarterly. 1994;271:601–607. doi: 10.1093/her/9.1.69. [DOI] [PubMed] [Google Scholar]
- Rogers RW. Cognitive and psychological factors in fear appeals and attitude change: A revised theory of protection motivation. In: Cacioppo J, Petty R, editors. Social psychophysiology. New York: Guilford Press; 1983. pp. 153–176. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Testing four competing theories of health-protective behavior. Health Psychology. 1993;12:324–333. doi: 10.1037//0278-6133.12.4.324. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, Niewoehner D, Kane RL, MacDonald R, Joseph AM. Spirometry as a motivational tool to improve smoking cessation rates: A systematic review of the literature. Nicotine & Tobacco Research. 2007;9:21–32. doi: 10.1080/14622200601078509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.