Abstract
AIMS
To evaluate tolerability, pharmacokinetics and night-time effects on body sway and critical flicker fusion (CFF) of gaboxadol following bedtime dosing in healthy elderly subjects.
METHODS
Subjects (17 women, seven men) aged 65–75 years received gaboxadol 10 mg, zolpidem 5 mg (active control) or placebo at 22.00 h in a three-period, randomized, double-blind crossover study. They were awakened during the night for evaluation of body sway and CFF. Pharmacokinetics of gaboxadol were assessed during a fourth single-blind treatment period. Adverse events were recorded throughout the study.
RESULTS
The number of subjects with adverse events was 14 for gaboxadol 10 mg, seven for zolpidem and nine for placebo; most were mild or moderate in intensity. Two women discontinued the study following gaboxadol; one vomited and one experienced a severe vasovagal syncope after venepuncture. Mean gaboxadol tmax was 2 h, t½ was 1.7 h, AUC0–∞ was 430 ng·h ml−1 and Cmax was 139 ng ml−1. At 1.5 h and 4 h post dose, zolpidem increased body sway relative to placebo (P < 0.01). Gaboxadol increased body sway at 4 h (P < 0.001) and 8 h (P < 0.05) relative to placebo. At 1.5 h, the time point closest to peak drug concentrations, zolpidem increased body sway compared with gaboxadol (P < 0.01). Gaboxadol and zolpidem had no effects on CFF vs. placebo.
CONCLUSIONS
A bedtime dose of gaboxadol 10 mg was generally well tolerated. Changes in body sway at 1.5 h after bedtime dosing were smaller with gaboxadol 10 mg than with zolpidem 5 mg, whereas changes were similar at 4 h for both treatments and returned to near baseline at 8 h.
Keywords: body sway, critical flicker fusion, elderly, gaboxadol, zolpidem
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Body sway increases in older adults and may lead to an increase in the risk of falling.
The problem of impaired stability in the elderly may be compounded by the use of hypnotics, which have been associated with an increased risk of next-day falls as well as drowsiness.
The potential adverse effects of hypnotic drugs on steadiness may be exacerbated during the night, in the event that an individual needs to get out of bed.
WHAT THIS STUDY ADDS
This study examines the effects of gaboxadol (an investigational treatment for insomnia), zolpidem (a current hypnotic included as an active control) and placebo on body sway and attention/information processing ability following bedtime dosing in elderly subjects who were woken during the night for assessments.
Zolpidem and gaboxadol increased body sway at various time points during the night relative to placebo; at 1.5 h post dose, the time of peak concentrations of both drugs, gaboxadol produced less impairment than zolpidem.
Compared with placebo, neither gaboxadol nor zolpidem impaired attention/information-processing ability as assessed by critical flicker fusion.
Introduction
Poor sleep is a common complaint of the elderly [1, 2] and may involve difficulty falling asleep and staying asleep, as well as difficulty awakening in the morning. These complaints appear related to sleep fragmentation with increased arousals and awakenings, an increased number of stage shifts, difficulty maintaining good quality sleep, and reduced sleep efficiency [3]. In addition to subjective complaints, polysomnography recordings show some evidence of decreased slow wave sleep [3–5]. Other evidence suggests that gender influences age-related changes in sleep, as slow wave sleep may be better preserved in women [6, 7], even though they are more likely to complain of sleep disturbance [8, 9] and to use hypnotics [10]. Early morning awakenings and problems maintaining sleep may be linked to circadian dysfunction, particularly in women [11].
A consequence of poor sleep, especially in the aged, is the possibility of reduced daytime alertness and associated impaired cognition, leading to an increased risk of accidents [12–14]. Further, body sway increases in older adults, and there is some evidence that increased sway leads to an increase in the risk of falling [15]. Body sway increases with increasing drowsiness (and with reduced cortical, EEG alpha amplitude), [16] making it a useful surrogate of impairment and accident risk.
This problem of impaired stability in the elderly may be compounded by the use of hypnotics, which have been associated with an increased risk of falls, increased incidence of hip fractures and increased risk of automobile accidents [17–20]. Elderly subjects may also have various health problems, including nocturia, that result in a need to get up in the night [3], potentially leading to exacerbated drowsiness and stability problems.
In light of the potential effects of age and gender in the use of hypnotics, we carried out a study of the novel hypnotic, gaboxadol, in elderly men and women. In contrast to all GABAA ligands and benzodiazepines currently prescribed for the treatment of sleep disorders, gaboxadol is an agonist that acts directly on the GABA binding site of the GABAA receptor and has no affinity for the benzodiazepine binding site [21]. It has highest functional activity for α4β3δ containing GABAA receptors, which are insensitive to benzodiazepine agonists, probably exist mainly extrasynaptically, and are localized predominantly in the thalamus, dentate gyrus, cerebellum and cortex [21]. Gaboxadol has a peak plasma concentration within approximately 30 min of ingestion and an elimination half-life of 1.5–2 h [22, 23]. Although it is no longer in clinical development for the treatment of insomnia, in the elderly gaboxadol 10 mg reduced the number of awakenings, decreased wake after sleep onset, increased sleep efficiency and increased slow wave sleep [24].
The primary objective of the present study was to determine the safety and tolerability of a single oral dose of gaboxadol 10 mg administered at bedtime in elderly subjects. Based on previous studies in young subjects, it was hypothesized that, as evaluated by an assessment of clinical and laboratory adverse events, a single bedtime dose of gaboxadol 10 mg would be well tolerated in elderly women and men. A secondary objective was to characterize the gaboxadol plasma concentration–time profile in elderly subjects. An exploratory objective was to assess the night-time effects of gaboxadol 10 mg and zolpidem 5 mg administered at bedtime on body sway and critical flicker fusion (CFF), a measure of attention and information-processing ability, in elderly subjects.
Both body sway and CFF have been shown to be sensitive to psychoactive compounds [25–28]. Zolpidem 5 mg was included as a comparator agent. Zolpidem is a benzodiazepine site agonist that is marketed for use in the treatment of occasional or short-term insomnia. The dose of zolpidem selected for use in this study (5 mg) is the label-indicated dose for use in patients >65 years old.
Methods
Design
Gaboxadol protocol 001 was carried out between 13 June and 15 July 2004 at a single site (Human Psychopharmacology Research Unit, University of Surrey). Subjects participated in four treatment periods. The first part was a randomized, double-blind, zolpidem- and placebo-controlled, three-period crossover study for pharmacodynamic evaluation of gaboxadol 10 mg. This was followed by a single-blind pharmacokinetic evaluation of gaboxadol 10 mg in the same subjects.
The protocol was approved by the Quorn Research Review Committee. This study was conducted in conformance with Good Clinical Practice standards and was carried out in accord with ‘The Medicines for Human Use (Clinical Trials) Regulations SI 1031’; written informed consent was obtained from all subjects.
Subjects
Healthy subjects aged ≥65 to ≤80 years were eligible to enrol if they had a self-reported usual bedtime between 20.00 h and midnight, and visual acuity adequate for the study procedures (based on standard eye tests and investigator's judgment). The subjects agreed to avoid exercise that was strenuous or to which they were unaccustomed. They were required to be in good health, as confirmed by their medical history, physical examination, electrocardiogram, and laboratory tests (haematology and clinical biochemistry). Subjects were to have estimated creatinine clearance >70 ml min−1 (or serum creatinine ≤1.2 mg dl−1). During prestudy screening, 11 subjects with estimated creatinine clearance >60 ml min−1 but <70 ml min−1 were considered acceptable to be included by the investigator and the sponsor; estimated creatinine clearance ranged from 60 to 92.2 ml min−1.
Exclusion criteria included the use of hypnotics on more than 22 occasions in the previous year, a history of drug or alcohol abuse, consumption of more than five caffeine-containing beverages or the nicotine equivalent of more than 15 cigarettes a day, and a history of repeated falls or a fracture secondary to a fall within the past 2 years. Subjects were required to be free of any condition that could interfere with their sleep (e.g. apnoea and restless legs syndrome), severe or acute respiratory failure, myasthenia gravis, and muscle spasm. Subjects were required to abstain from prescription and nonprescription drugs and supplements, other than once-daily vitamins, for at least 2 weeks or five half-lives (whichever was longer) before the start of the study and during the study. Certain drugs were permitted if taken regularly in daily doses or occasionally, but not exceeding twice a week: nonsedating drugs used for allergies, drugs for cardiovascular disease (e.g. calcium antagonists, β-blockers), drugs for pain relief (e.g. paracetamol, acetylsalicylic acid, diclofenac) and hormones.
During the study subjects were required to limit their daily consumption of alcoholic beverages to two glasses of wine (118 ml per glass) or two bottles of beer (354 ml per bottle) a day. Alcoholic drinks were not permitted from 48 h before admission to the unit or before prestudy and poststudy visits. Caffeine consumption was limited to the equivalent of five cups a day and nicotine intake was limited to the equivalent of five cigarettes a day. Both caffeine and nicotine were prohibited from 24 h before and during each visit to the unit.
Pharmacodynamic evaluation
In the initial three-period crossover portion of the study, subjects were assigned randomly in a double-blind, double-dummy manner to one of six sequences of treatments, according to a Latin square design for a three-period, three-treatment randomized crossover study. Treatments comprised single oral doses of gaboxadol 10 mg (two 5-mg encapsulated tablets), zolpidem 5 mg (tablet), and placebo separated by wash-out periods of ≥3 days. The double-dummy design was necessary because two gaboxadol 5-mg tablets were used to achieve the 10-mg dose. The gaboxadol and placebo-for-gaboxadol tablets were encapsulated, whereas zolpidem (and matching placebo) was provided as a tablet. On each dosing occasion, patients took two capsules (containing a total of gaboxadol 10 mg or placebo) and a tablet (zolpidem 5 mg or placebo). Treatment periods 1–3 were each approximately 12 h in duration. Treatments were administered at bedtime (22.00 h) following a 4-h fast. A computer-generated random allocation schedule supplied by the study sponsor (Merck Research Laboratories, West Point, PA, USA) was used to assign the order in which subjects received these three treatments.
Body sway was measured using a stabilometric platform (AccuSway™; Advanced Mechanical Technology, Inc., Watertown, MA, USA). In this system, subjects stand on a force platform. Strain gauges under the platform provide input that is used to calculate the centre of pressure (COP); the extent of movement of the COP directly relates to the subject's ability to maintain balance. The AccuSway™ system derives the length of the COP and the area of the 95% confidence ellipse enclosing the COP (A95). The primary measure was A95 [29]; increases in the A95 reflect increased body sway, which indicates impairment. Subjects were tested on the platform in bare feet positioned at hip width and with their vision fixed at a point 50 cm in front at eye level. Foot position was standardized between time points. For each time point, a subject's A95 was measured three times in the ‘eyes open’ condition and three times in the ‘eyes closed’ condition, and the mean of each set of three measurements was used. A representative scatter plot of COP derived from the body sway procedure is shown in Figure 1.
Figure 1.
Representative scatter plot of the centre of pressure (COP) data from an ‘eyes closed’ condition in the body sway test. Scatter plot demonstrates displacement of the COP on the abscissa and ordinate relative to the platform (cm). The plot is overlaid with the 95th percentile ellipse and represents the area of the 95% confidence ellipse enclosing the COP (A95, cm2)
Attention and information-processing ability were assessed by examining ascending and descending CFF, i.e. the perceptual threshold at which a flickering light appears to be steady and vice versa, as determined by the method of limits described as follows [30]. Four light-emitting diodes were arranged in a 1-cm square on a black background and held at foveal fixation at a distance of 1 m. The frequency of light flickering was increased (ascending) or decreased (descending) progressively until the subjects reported that they perceived fusion (ascending) or flicker (descending). A decrease in the frequency at which subjects perceive fusion or flicker reflects central nervous system impairment. The ascending and descending CFF were each determined four times (at each time point) with the mean of these four measurements used. The mean CFF (key end-point) was determined from the mean of ascending and descending CFF combined [31].
Subjects underwent at least two training sessions for the body sway and CFF evaluations during the prestudy phase. During the study, the tests were carried out immediately before subjects ingested study drug and again when they were awakened at 1.5, 4 and 8 h postdose. Blood was drawn for estimations of gaboxadol concentrations before ingestion and 1.5, 4 and 8 h after ingestion to allow exploration of a possible pharmacokinetic–pharmacodynamic relationship. However, no such relationship was apparent on visual inspection of graphical plots of the data, and therefore these data were not evaluated further. Initially, venepuncture was performed prior to pharmacodynamic assessments, but after an episode of vasovagal syncope occurred (see Results) the timing of venepuncture was changed to occur after the pharmacodynamic assessments.
Pharmacokinetic evaluation
Subjects underwent a wash-out period of at least 3 days before they entered the single-blind fourth period. All subjects received a single oral dose of gaboxadol 10 mg at bedtime (22.00 h). Using an in-dwelling catheter, blood samples were collected into sodium heparin-containing polypropylene tubes before the subjects ingested study drug and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12 and 16 h after ingestion for the measurement of gaboxadol concentrations. Urine was collected before subjects ingested study drug and from 0.0 to 4 h, 4 to 8 h, 8 to 12 h, and 12 to 16 h after ingestion. The urine excreted during each period was pooled for storage in separate polyethylene containers at 2–8°C.
Pharmacokinetic parameters estimated for gaboxadol were area under the concentration–time curve (AUC0–∞), maximum plasma concentration (Cmax), time to Cmax (Tmax), apparent terminal half-life (t1/2), urine recovery, and renal clearance (CLR). Calculations were made using the software WinNonlin Enterprise Version 4.1.b. The apparent terminal t1/2 was estimated from the best-fit parameters of a single exponential to the log-linear portion of the plasma concentration–time curve using unweighted linear regression. AUC0–∞ was calculated using the linear up/log down method up to the last measured concentration and the additional area estimated from that concentration and the value of apparent terminal t1/2 estimated for that administration. Cmax and Tmax were obtained by inspection of the concentration–time data. AUC0–∞ and Cmax values were corrected for the assayed potency of the formulation of gaboxadol used in the study. CLR was calculated as the ratio of the amount excreted unchanged in urine through 16 h postdose to AUC0–16h.
Safety evaluation
Subjects were initially screened within 3 weeks of the first administration of study drug, and a similar routine was used in the medical examinations carried out within 5–7 days of the final dose of the study drugs. The medical examinations included estimations of heart rate and blood pressure, respiratory rate and oral temperature, as well as routine laboratory evaluations. During both the pharmacodynamic and pharmacokinetic portions of the study, vital signs were measured at 1 and 0.25 h before and 1.5, 4 and 8 h postdose. Electrocardiograms (ECGs) were also performed at regular intervals. Adverse events were monitored from initial screening to at least 14 days after the last dose of study drug. The investigator judged each adverse experience with respect to intensity (mild, moderate, severe), likelihood of being related to study drug, and seriousness (serious, nonserious).
Analytical methods
Solid-phase extraction was used to isolate gaboxadol from plasma and urine at Merck Research Laboratories. Analysis was conducted by hydrophilic interaction liquid chromatography (Ashipak NH2P-50 2D HPLC column, 150 × 2.0 mm, 5 µm) coupled with mass spectrometric detection (Sciex API 4000 tandem mass spectrometer equipped with a Turbo Ion Spray interface and Analyst software). The internal standard was a stable isotope-labelled analogue of gaboxadol, d4-gaboxadol hydrochloride (Lundbeck, Taastrup, Denmark). The mobile phase was 70/30 v/v% acetonitrile/20 mM ammonium acetate (pH 4.0), and the column flow rate was 0.2 ml min−1. The assay was linear from 0.5 to 20 µg ml−1 for plasma and 0.05 to 20 µg ml−1 for urine. The lower limit of quantification was 0.5 ng ml−1 for plasma and 0.05 µg ml−1 for urine. For the standards in plasma, the intrarun precision (percent coefficient of variation) was ≤4.6% and the accuracy was 98.3–101.1%. For the standards in urine, the intrarun precision was ≤5.6% and the accuracy ranged from 98.6 to 101.2%. For the low, medium and high plasma quality control samples, the interday precision (coefficient of variation of daily mean values) was 2.2–2.9%, and the interday accuracy was 100.5–102.7%. For the low, medium and high urine quality control samples, the interday precision was 0.6–3.1%, and the interday accuracy was 104.5–106.7%.
Statistical methods
Up to 24 subjects, including at least 12 women, were planned to complete the study. Results with P-values ≤0.05 are reported as statistically significant. No multiplicity adjustments were made.
Safety
Adverse experiences were tabulated for each treatment in the pharmacodynamic and pharmacokinetic portions of the study.
Pharmacokinetics
AUC0–∞ and Cmax were potency-adjusted to facilitate comparison of pharmacokinetic data across studies. To assess the pharmacokinetics of gaboxadol in elderly subjects, prior to statistical analysis, AUC0–∞, and Cmax were natural log transformed. An anova with a factor for gender was applied to the gaboxadol AUC0–∞ and Cmax. The geometric mean and 95% confidence intervals (CIs) were calculated for AUC0–∞ and Cmax for both genders using appropriate between-subject standard deviations from the above model. Gender was examined in an exploratory fashion. To assess the effects of gender on the plasma concentration–time profile after a single 10-mg dose of gaboxadol, 95% CIs for the ratio of AUC0–∞ and Cmax geometric means (women vs. men) were calculated. Summary statistics were computed for Tmax (minimum, maximum, median, arithmetic mean and standard deviation) and apparent terminal t1/2 (harmonic and arithmetic means and standard deviation). Arithmetic means were calculated for urine recovery and CLR.
Pharmacodynamics
Assessments of body sway were made using an anova appropriate for a three-period crossover with repeated measures (within a treatment period) design. The anova was applied to the natural log-transformed fold-change from baseline body sway area (A95) values because review of the literature suggested that body sway data are log normally distributed. Baseline was defined as the −0.5 h time point in each period. Fold-change from baseline at time t was defined as (value at time t)/(value at baseline). ‘Eyes open’ and ‘eyes closed’ conditions were analysed separately. The anova for each body sway condition contained between-subject factors for gender (1 d.f.) and subject within gender (21 d.f.), and within-subject factors for period (2 d.f.), treatment (2 d.f.), hour (body sway evaluations performed at 1.5, 4 and 8 h within a treatment period, 2 d.f.), treatment by hour (4 d.f.), treatment by gender (2 d.f.), treatment by gender by hour (6 d.f.) and within-subject error (166 d.f.). The gender by treatment interaction was included in the model because it was statistically significant. The treatment by hour interaction was included in the model to explore pairwise treatment comparisons summed over gender, whereas the treatment by gender by hour interaction was included in the model to explore pairwise treatment comparisons by gender (due to the statistically significant gender by treatment interaction). A test for first-order carryover was also performed at the α = 0.05 level. The analysis was adjusted for carryover where carryover was statistically significant, otherwise it was dropped from the anova model.
To compare gaboxadol 10 mg with placebo, two-sided 95% CIs for the difference (gaboxadol minus placebo) in mean natural log-transformed fold changes from baseline in body sway at 1.5, 4 and 8 h postdose were calculated using the within-subject error from the anova model and referencing a t distribution. These confidence limits were exponentiated to obtain a 95% CI for the geometric mean body sway ratio (gaboxadol/placebo) of fold change from baseline. A similar approach was used to compare gaboxadol with zolpidem, and zolpidem with placebo.
Analyses similar to those described above for body sway were performed on CFF. However, these data were not log transformed, and the gender by treatment interaction was not included in the model because it was not statistically significant.
Results
Twenty-four white subjects were admitted to the study (seven men and 17 women). They were 65–75 years old, with mean body weights of 70.1 kg (range 52.6–87.4 kg) for the women and 90.3 kg (range 80.7–99.7 kg) for the men. Twenty-three subjects completed the body sway assessments and 22 completed the CFF assessments. Two women discontinued following treatment with gaboxadol because of adverse experiences (vomiting and a vasovagal reaction); these women were excluded from the pharmacodynamic analyses but were included in the safety analyses (up to the point that they discontinued).
Safety
Clinical information was gathered on all 24 subjects admitted to the study, including the two subjects who withdrew. There was no evidence from the poststudy evaluations of changes considered to be of clinical significance either in the physical examination or in the vital signs, laboratory data or ECG. There were 58 adverse events in 13 of the 17 women, and 15 adverse events in six of the seven men (Table 1); most of these were classified as mild or moderate. Forty-one of the adverse events that occurred in nine of the 17 women and 10 that occurred in six of seven men were considered by the study physician to be related to treatment. The most frequently occurring events were dizziness and fatigue. For the gaboxadol double-blind treatment, of the five subjects who had dizziness three reported onset approximately 1.5 h after dosing and the other two subjects experienced dizziness at about 4 h post dose. Two women were discontinued from the study. One woman, aged 70 years and weighing 69.8 kg, withdrew because she vomited after ingestion of gaboxadol 10 mg, and this was considered by the study physician to be possibly related to study drug. The other woman, aged 73 years and weighing 64.9 kg, had an event that was classified by the investigator as serious. It occurred with gaboxadol 10 mg and persisted for about 2.5 h. She reported ‘hunger’ around 2 h after ingestion of the drug and, at 4 h (02.00 h), after a difficult venepuncture and during the assessment of body sway, she complained of nausea, became dizzy and lost consciousness. The carotid and femoral pulses could not be detected for about 20 s and there were no respirations. She vomited on regaining consciousness and, subsequently, there were no relevant findings on medical evaluation. Her vital signs were within acceptable limits, the ECG was normal and the laboratory investigations were negative. It was considered by the study physician that the event was a severe vasovagal syncope, and that it was possibly drug related.
Table 1.
Clinical adverse experiences summary
| Double-blind treatment phase | Single-blind | |||
|---|---|---|---|---|
| Number (%) subjects | Gaboxadol 10 mg (n = 24) | Zolpidem 5 mg (n = 23) | Placebo (n = 23) | Gaboxadol 10 mg (n = 22) |
| Subjects with ≥1 adverse experiences | 14 (58.3) | 7 (30.4) | 9 (39.1) | 6 (27.3) |
| Most common adverse experiences* | ||||
| Dizziness | 5 (20.8) | 2 (8.7) | 1 (4.3) | 1 (4.5) |
| Fatigue | 3 (12.5) | 3 (13.0) | 3 (13.0) | 1 (4.5) |
| Headache | 2 (8.3) | 0 (0) | 1 (4.3) | 1 (4.5) |
| Insomnia | 1 (4.2) | 0 (0) | 2 (8.7) | 0 (0) |
| Nausea | 3 (12.5) | 1 (4.3) | 1 (4.3) | 0 (0) |
| Vomiting | 2 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Subjects with ≥1 drug-related adverse experience† | 12 (50.0) | 6 (26.1) | 5 (21.7) | 3 (13.6) |
| Most common drug-related adverse experiences*† | ||||
| Dizziness | 5 (20.8) | 2 (8.7) | 1 (4.3) | 0 (0.0) |
| Fatigue | 3 (12.5) | 3 (13.0) | 3 (13.0) | 1 (4.5) |
| Nausea | 3 (12.5) | 1 (4.3) | 1 (4.3) | 0 (0) |
| Vomiting | 2 (8.3) | 0 (0) | 0 (0) | 0 (0) |
≥5.0% incidence in any one treatment group.
Considered by the investigator to be possibly, probably, or definitely related to study drug. Although a subject may have had an adverse experience more than once or with changing intensity, the subject is counted only once for a specific adverse experience. The same subject may be counted in more than one row. After the first dosing of study drug, adverse experiences that began predose and did not change intensity post dose were counted with the treatment received in the previous period.
Pharmacokinetic end-points
Table 2 gives the pharmacokinetic parameters for gaboxadol. Figure 2 displays the plasma concentrations of gaboxadol over time for men and women and for all subjects combined. These data suggest that the pharmacokinetics of gaboxadol were generally similar between elderly men and women. AUC values were similar for elderly men and women, although Cmax values appeared to be slightly higher for elderly women (∼19%). Tmax values were also similar between elderly men and women. The most notable difference in pharmacokinetics was the slightly shorter apparent terminal half-life for elderly women. Consistent with the renal excretion of gaboxadol in previous studies, an average of 58% of gaboxadol was recovered intact in the urine.
Table 2.
Pharmacokinetic parameters after a single dose of gaboxadol 10 mg in healthy elderly subjects
| Pharmacokinetic variable | All subjects (n = 22) | Women (n = 15) | Men (n = 7) | Women/men | P-value for gender comparison |
|---|---|---|---|---|---|
| AUC0–∞* (ng·h ml−1) | 430 | 440 | 408 | 1.08 | 0.249 |
| (95% CI) | (0.94, 1.23) | ||||
| Cmax* (ng ml−1) | 139 | 147 | 123 | 1.19 | 0.159 |
| (95% CI) | (0.93, 1.53) | ||||
| Tmax† (h) | 2 | 2 | 2 | ||
| (range) | (0.75–3) | (0.75–3) | (0.75–3) | ||
| (arithmetic mean) | 1.8 | 1.8 | 2.0 | ||
| (SD) | (0.7) | (0.6) | (0.8) | ||
| Apparent terminal half-life‡ (h) | 1.7 | 1.5 | 2.1 | ||
| (arithmetic mean) | 1.7 | 1.6 | 2.1 | ||
| (SD) | (0.3) | (0.2) | (0.1) | ||
| Urinary recovery (% of dose) | 58 | 59 | 54 | ||
| (SD) | (6) | (6) | (3) | ||
| CLR (ml min−1) | 227 | 229 | 223 | ||
| (SD) | (42) | (48) | (29) |
AUC0–∞ and Cmax values are geometric means adjusted for assayed potencies of the formulations. Urinary recovery and CLR values are based on arithmetic mean and SD.
Median, min, max, arithmetic mean, and SD for Tmax.
Harmonic mean, arithmetic mean, and SD for apparent terminal half-life. AUC, area under the concentration–time curve; CI, confidence interval; CLR, renal clearance; Cmax, maximum plasma concentration; SD, standard deviation; Tmax, time to Cmax.
Figure 2.
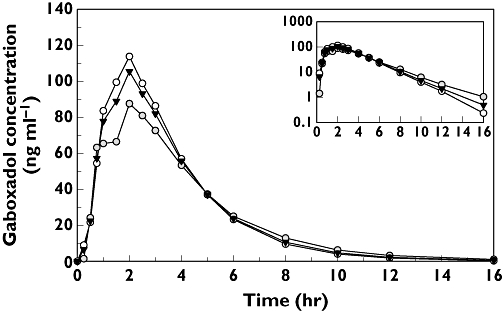
Mean plasma concentrations of gaboxadol 10 mg in healthy elderly men and women at bedtime. The inset shows the data plotted on a logarithmic scale for the ordinate; the abscissa is the same as the main graph (time, in h). Men (n = 7) ( ); Women (n = 15) (○); Overall (n = 22) (▾)
); Women (n = 15) (○); Overall (n = 22) (▾)
Pharmacodynamic end-points
Between-treatment comparisons of body sway for the analysis of the entire sample are given in Table 3 and illustrated in Figure 3. At 1.5 h and 4 h postdose, zolpidem increased body sway (‘eyes open’ and ‘eyes closed’) relative to placebo (P < 0.01), but not at 8 h. At 1.5 h, relative to placebo, gaboxadol had no significant effect on the fold change from baseline in body sway (‘eyes open’ or ‘eyes closed’). Gaboxadol increased body sway (‘eyes open’ condition only) at 4 and 8 h relative to placebo (P < 0.001 and P < 0.05, respectively). In the comparison between gaboxadol and zolpidem at 1.5 h, the time point closest to peak drug concentrations, gaboxadol produced significantly less of an increase in the fold change from baseline in body sway with ‘eyes open’[0.61 (95% CI 0.49, 0.75); P < 0.001] and ‘eyes closed’[0.71 (95% CI 0.57, 0.89); P = 0.003].
Table 3.
Treatment ratios for fold change (from baseline) in body sway area (A95) after administration of gaboxadol 10 mg, zolpidem 5 mg, and placebo, with ‘eyes open’ and ‘eyes closed’, for all subjects and by gender
| Treatment ratio of fold change ‘EYES OPEN’ | Treatment ratio of fold change ‘EYES CLOSED’ | |||||||
|---|---|---|---|---|---|---|---|---|
| Hour | Statistic | Gaboxadol vs. placebo | Zolpidem vs. placebo | Gaboxadol vs. zolpidem | Gaboxadol vs. placebo | Zolpidem vs. placebo | Gaboxadol vs. zolpidem | |
| All subjects (n = 23) | 1.5 | GM | 1.22 | 2.00 | 0.61 | 1.17 | 1.65 | 0.71 |
| 95% CI | (0.98, 1.51) | (1.62, 2.46) | (0.49, 0.75) | (0.93, 1.46) | (1.33, 2.04) | (0.57, 0.89) | ||
| P-value | 0.070 | <0.001 | <0.001 | 0.173 | <0.001 | 0.003 | ||
| 4.0 | GM | 1.59 | 1.78 | 0.90 | 1.21 | 1.38 | 0.88 | |
| 95% CI | (1.29, 1.97) | (1.44, 2.20) | (0.72, 1.11) | (0.98, 1.50) | (1.11, 1.71) | (0.71, 1.09) | ||
| P-value | <0.001 | <0.001 | 0.307 | 0.083 | 0.003 | 0.230 | ||
| 8.0 | GM | 1.27 | 1.23 | 1.03 | 1.15 | 1.09 | 1.06 | |
| 95% CI | (1.03, 1.57) | (1.00, 1.52) | (0.83, 1.27) | (0.93, 1.43) | (0.88, 1.35) | (0.85, 1.31) | ||
| P-value | 0.027 | 0.052 | 0.778 | 0.206 | 0.433 | 0.620 | ||
| Women (n = 16) | 1.5 | GM | 0.96 | 1.91 | 0.50 | 1.33 | 2.21 | 0.60 |
| 95% CI | (0.76, 1.21) | (1.51, 2.41) | (0.40, 0.63) | (1.05, 1.68) | (1.74, 2.80) | (0.48, 0.76) | ||
| P-value | 0.735 | <0.001 | <0.001 | 0.019 | <0.001 | <0.001 | ||
| 4.0 | GM | 1.35 | 1.47 | 0.92 | 1.37 | 1.70 | 0.81 | |
| 95% CI | (1.07, 1.71) | (1.16, 1.85) | (0.73, 1.16) | (1.08, 1.74) | (1.34, 2.15) | (0.64, 1.02) | ||
| P-value | 0.012 | 0.001 | 0.488 | 0.009 | <0.001 | 0.074 | ||
| 8.0 | GM | 1.12 | 1.10 | 1.01 | 1.42 | 1.43 | 0.99 | |
| 95% CI | (0.89, 1.41) | (0.87, 1.39) | (0.80, 1.28) | (1.11, 1.80) | (1.13, 1.81) | (0.78, 1.26) | ||
| P-value | 0.344 | 0.409 | 0.903 | 0.005 | 0.004 | 0.964 | ||
| Men (n = 7) | 1.5 | GM | 1.54 | 2.09 | 0.74 | 1.03 | 1.23 | 0.84 |
| 95% CI | (1.09, 2.18) | (1.48, 2.95) | (0.52, 1.05) | (0.71, 1.49) | (0.87, 1.74) | (0.58, 1.21) | ||
| P-value | 0.015 | <0.001 | 0.088 | 0.885 | 0.248 | 0.341 | ||
| 4.0 | GM | 1.88 | 2.16 | 0.87 | 1.07 | 1.12 | 0.95 | |
| 95% CI | (1.33, 2.66) | (1.53, 3.05) | (0.62, 1.23) | (0.75, 1.52) | (0.79, 1.59) | (0.67, 1.36) | ||
| P-value | <0.001 | <0.001 | 0.432 | 0.708 | 0.517 | 0.787 | ||
| 8.0 | GM | 1.44 | 1.38 | 1.05 | 0.93 | 0.83 | 1.12 | |
| 95% CI | (1.02, 2.04) | (0.98, 1.95) | (0.74, 1.48) | (0.66, 1.33) | (0.59, 1.18) | (0.79, 1.59) | ||
| P-value | 0.038 | 0.068 | 0.792 | 0.694 | 0.299 | 0.522 | ||
GM, geometric mean.
Figure 3.
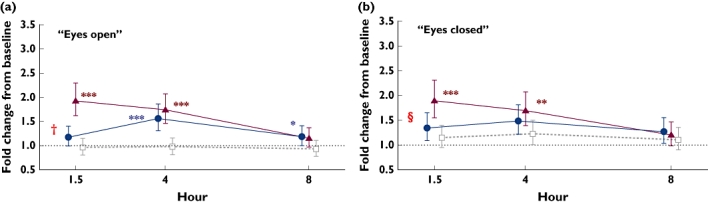
Geometric mean fold change from baseline (95% CI) for body sway area after 10 mg gaboxadol, 5 mg zolpidem, and placebo, and P-values for treatment ratios of fold change from baseline for (a) ‘eyes open’ and (b) ‘eyes closed’. A value >1 indicates increased body sway relative to baseline. ***P < 0.001; **P < 0.01; *P < 0.05 active drug vs. placebo; †P < 0.001 gaboxadol vs. zolpidem; §P < 0.01 gaboxadol vs. zolpidem. Gaboxadol 10 mg (•); Zolpidem 5 mg (▴); Placebo ( )
)
Between-treatment comparisons of body sway for women and men separately are also shown in Table 3. For ‘eyes open’, the effects of the three treatments in the all-subjects-combined analyses were generally similar to the effects in the men-only and the women-only analyses. For ‘eyes closed’, increases in body sway were dependent on the effect in the women for all three time points for both gaboxadol (P < 0.05) and zolpidem (P < 0.01).
The between-treatment differences in change in CFF from baseline over the 8-h period after ingestion of the three treatments are given in Table 4. There were no differences between gaboxadol and placebo, zolpidem and placebo, or gaboxadol and zolpidem at any time point. Subgroup analyses for men only and women only also showed no differences.
Table 4.
Critical flicker fusion – between-treatment differences in change from baseline after administration of gaboxadol 10 mg, zolpidem 5 mg, and placebo (n = 22)
| Between-treatment difference in change from baseline | ||||
|---|---|---|---|---|
| Hour | Statistic | Gaboxadol minus placebo | Zolpidem minus placebo | Gaboxadol minus zolpidem |
| 1.5 | Mean | −0.18 | −0.53 | 0.35 |
| 95% CI | (−0.85, 0.49) | (−1.20, 0.14) | (−0.33, 1.02) | |
| P-value | 0.594 | 0.123 | 0.313 | |
| 4 | Mean | −0.35 | −0.10 | −0.25 |
| 95% CI | (−1.02, 0.32) | (−0.77, 0.57) | (−0.92, 0.42) | |
| P-value | 0.307 | 0.769 | 0.468 | |
| 8 | Mean | −0.34 | −0.05 | −0.28 |
| 95% CI | (−1.01, 0.33) | (−0.72, 0.62) | (−0.96, 0.39) | |
| P-value | 0.325 | 0.881 | 0.405 | |
A negative score indicates a decreased critical flicker fusion (indicative of impairment) relative to baseline.
Discussion
The present study investigated the tolerability, pharmacokinetics and effects on body sway and CFF of gaboxadol 10 mg and zolpidem 5 mg, given at bedtime, in elderly subjects who were repeatedly awakened during the night. The study was directed at understanding the potentially adverse effects that such drugs could have during the night, in the event that the individual would need to get out of bed.
A single dose of gaboxadol 10 mg administered at night-time appeared to be generally well tolerated in healthy elderly subjects. Adverse events that occurred in the study were mostly mild or moderate in intensity. Dizziness and fatigue were the most commonly reported adverse events for both gaboxadol and zolpidem, along with nausea for gaboxadol. Physical and laboratory examinations, including vital signs and ECG, yielded no clinically significant findings. Two women discontinued due to adverse experiences following gaboxadol 10 mg that were considered possibly drug related by the investigator; one discontinued due to an episode of vomiting and the other discontinued due to a severe vasovagal syncope. The latter subject had a difficult venepuncture at the 4-h postdose procedures (at 02.00 h) and subsequently, during the body sway assessments, became nauseous and dizzy, losing and then regaining consciousness. She had no significant findings on laboratory or ECG evaluations, and her blood pressure and heart rate were stable following the event.
Results from this study suggest that the pharmacokinetics of gaboxadol were generally similar between elderly men and elderly women, although Cmax values appeared to be slightly higher, and apparent t1/2 was shorter, for elderly women. Comparison of the present data with that from fasted young subjects in previous studies [22, 23] suggests that there may be small differences in the pharmacokinetics of gaboxadol between young and elderly subjects. AUC and Cmax were up to 40–50% higher in elderly subjects than in young subjects. The tmax also appeared to be longer for elderly subjects than young subjects. However, some of the observed differences may be attributable to differences in the timing of doses relative to meals in different studies, since a high-fat meal has been shown to affect absorption of gaboxadol (Cmax decreased by ∼30% and delayed by ∼0.5 h; unpublished data on file). In the present study, gaboxadol was administered ∼3–4 h after dinner.
With regard to the assessment of body sway, for the ‘eyes open’ evaluation there was increased body sway around 1.5 h after ingestion of zolpidem 5 mg, but not after ingestion of gaboxadol 10 mg. Body sway increased for both gaboxadol 10 mg and zolpidem 5 mg relative to placebo at 4 h, and returned to near baseline levels at 8 h, although a significant difference vs. placebo was seen for gaboxadol at 8 h. For the ‘eyes closed’ evaluation, gaboxadol 10 mg had no effect on body sway at any time, but zolpidem 5 mg increased body sway at 1.5 and 4 h after ingestion. In the comparison between gaboxadol 10 mg and zolpidem 5 mg at 1.5 h, the time point closest to peak drug concentrations, gaboxadol produced significantly less impairment of body sway in both the ‘eyes open’ and ‘eyes closed’ conditions. When observing the gender groups separately, in the eyes closed condition, body sway effects were present in women only and not men for both drugs at all time points. The findings with zolpidem 5 mg in the present study are consistent with previous studies of zolpidem that have shown increases in body sway with both ‘eyes open’ and ‘eyes closed’ up to 8 h after ingestion [28, 32]. The present observations suggest generally reduced effects of gaboxadol 10 mg compared with zolpidem 5 mg on body sway during the night, particularly at 1.5 h after dosing.
These body sway findings should be interpreted within the context of the study design. After the subjects went to bed, they were awakened at 1.5, 4 and 8 h to undergo study procedures; thus, the effects of repeated disruption of sleep should be considered, especially for the later time points. The 8 h time point corresponds to a time point that is often included in studies that assess next-day residual effects of psychoactive compounds after a full night's sleep. In the present context, the 8 h time point should not be viewed as a valid measure of next-day residual effects because subjects were woken regularly during the night.
Neither gaboxadol 10 mg nor zolpidem 5 mg was different from placebo with respect to the change in CFF, and the two drugs were not different from each other. As noted in the Introduction, CFF has been previously shown to be sensitive to psychoactive compounds [25, 26], suggesting that the lack of differences is unlikely to be due to lack of sensitivity of the procedure. However, it should be acknowledged that studies assessing next-day residual effects of zolpidem using CFF have shown variable results, with some studies finding that zolpidem produced impairments [33, 34] and others showing no effects [35, 36]. The CFF method included in this study is not sensitive to pupil diameter, and it is therefore unlikely that variations in pupil size obscured differences between treatments [37].
A relationship between the pharmacokinetic profile of gaboxadol and body sway or CFF could not be established in the present study. Changes in sway with zolpidem 5 mg, at the time point close to that for the peak plasma concentration in the present study, were greater than those seen with gaboxadol 10 mg, although changes in sway with gaboxadol appeared later. Central effects with rapidly absorbed drugs appear quickly, and so the differences between the appearance of effects with zolpidem and gaboxadol cannot be explained on the basis of the pharmacokinetic profile alone, but would appear to depend on the relative activity of the drugs, particularly with respect to the gender of the subjects. Moreover, the blood sampling times were infrequent and perhaps too sparse for appropriate modelling.
In summary, a single oral bedtime dose of gaboxadol 10 mg was generally well tolerated in elderly subjects. Changes in body sway at 1.5 h after bedtime dosing were smaller with gaboxadol 10 mg than with zolpidem 5 mg, and neither drug affected CFF.
Acknowledgments
This study was funded by Merck Research Laboratories. The authors thank Tuli Ahmed and Christopher Lines for assistance in preparing the manuscript, and Kevin Brown and Kristine Cerchio for assistance with study administration. These data were previously presented at the annual meeting of the Associated Professional Sleep Societies, Salt Lake City, UT, USA, 17–22 June 2006.
Competing interests
J.B. has received research funding from Merck Research Laboratories. P.D., R.A., N.C., C.G., N.A., I.F., J.B.McC. and M.G.M. are current or former employees of Merck Research Laboratories and own, or have owned, stock or stock options in Merck.
REFERENCES
- 1.Chiu HF, Leung T, Lam LCW, Wing YK, Chung DWS, Li SW, Chi I, Law WT, Boey KW. Sleep problems in Chinese elderly in Hong Kong. Sleep. 1999;22:717–26. doi: 10.1093/sleep/22.6.717. [DOI] [PubMed] [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons – an epidemiologic study of 3 communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 5.Phillips B, Ancoli-Israel S. Sleep disorders in the elderly. Sleep Med. 2001;2:99–114. doi: 10.1016/s1389-9457(00)00083-6. [DOI] [PubMed] [Google Scholar]
- 6.Allen SR, Seiler WO, Stahelin HB, Spiegel R. 72 hour polygraphic and behavioral recordings of wakefulness and sleep in a hospital geriatric unit – comparison between demented and nondemented patients. Sleep. 1987;10:143–59. doi: 10.1093/sleep/10.2.143. [DOI] [PubMed] [Google Scholar]
- 7.Hoch CC, Dew MA, Reynolds CF, Fu I, Luo R, Alexander R, Agrawal N, Lates C, Ballow C, Blum R. A longitudinal-study of laboratory-based and diary-based sleep measures in healthy old-old and young-old volunteers. Sleep. 1997;20:192–202. [Google Scholar]
- 8.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 9.Hoch CC, Reynolds CF, Kupfer DJ, Berman SR. Stability of EEG sleep and sleep quality in healthy seniors. Sleep. 1988;11:521–7. doi: 10.1093/sleep/11.6.521. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Liu L, Ma D. Sleep habits, insomnia, and hypnotic use among the urban aged population of Shandong, China. Sleep. 2005;28:A117. [Google Scholar]
- 11.Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects – relationships to sleep quality. Sleep. 1989;12:529–36. [PubMed] [Google Scholar]
- 12.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the cardiovascular health study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 13.Leger D, Scheuermaier K, Roger M. The relationship between alertness and sleep in a population of 769 elderly insomniacs with and without treatment with zolpidem. Arch Gerontol Geriatr. 1999;29:165–73. doi: 10.1016/s0167-4943(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernie GR, Gryfe CI, Holliday PJ, Llewellyn A. The relationship of postural sway in standing to the incidence of falls in geriatric subjects. Age Ageing. 1982;11:11–16. doi: 10.1093/ageing/11.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Higuchi S, Motohashi Y. Changes in postural sway during a period of sustained wakefulness in male adults. Occup Med. 2001;51:490–5. doi: 10.1093/occmed/51.8.490. [DOI] [PubMed] [Google Scholar]
- 17.Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48:682–5. doi: 10.1111/j.1532-5415.2000.tb04729.x. [DOI] [PubMed] [Google Scholar]
- 18.Mendelson WB. The use of sedative/hypnotic medication and its correlation with falling down in the hospital. Sleep. 1996;19:698–701. [PubMed] [Google Scholar]
- 19.Schneeweiss S, Wang PS. Claims data studies of sedative-hypnotics and hip fractures in older people: exploring residual confounding using survey information. J Am Geriatr Soc. 2005;53:948–54. doi: 10.1111/j.1532-5415.2005.53303.x. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66:31–41. [PubMed] [Google Scholar]
- 21.Wafford KA, Ebert B. Gaboxadol – a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–6. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Lund J, Helboe T, Mengel H. Absorption, metabolism and excretion profile of gaboxadol in humans. Sleep. 2006;29:A41. [Google Scholar]
- 23.Shadle C, Ramakrishnan R, Gargano C, Fu I, Luo R, Alexander R, Agrawal N, Lates C, Ballow C, Blum R. Assessment of dose proportionality, absolute bioavailability, and tolerability of gaboxadol in healthy young adults. Sleep. 2006;29:A40–1. [Google Scholar]
- 24.Lancel M, Wetter TC, Steiger A, Mathias S. Effect of the GABAA agonist gaboxadol on nocturnal sleep and hormone secretion in healthy elderly subjects. Am J Physiol Endocrinol Metab. 2001;281:E130–7. doi: 10.1152/ajpendo.2001.281.1.E130. [DOI] [PubMed] [Google Scholar]
- 25.Hindmarch I. Psychomotor function and psychoactive drugs. Br J Clin Pharmacol. 1980;10:189–209. doi: 10.1111/j.1365-2125.1980.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hindmarch I. Critical Flicker Fusion Frequency (CFF): the effects of psychotropic compounds. Pharmacopsychiatrica. 1982;15:44–8. [Google Scholar]
- 27.Gandon JM, Allain H. Lack of effect of single and repeated doses of levocetirizine, a new antihistamine drug, on cognitive and psychomotor functions in healthy volunteers. Br J Clin Pharmacol. 2002;54:51–8. doi: 10.1046/j.1365-2125.2002.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allain H, Bentue-Ferrer D, Tarral A, Gandon JM. Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo. Eur J Clin Pharmacol. 2003;59:179–88. doi: 10.1007/s00228-003-0591-5. [DOI] [PubMed] [Google Scholar]
- 29.Norris V, Baisley KJ, Calder N, van Troostenburg A-R, Warrington SJ. Assessment of the AccuSwayPLUS system in measuring the effect of lorazepam on body sway in healthy volunteers. Int J Pharmaceut Med. 2005;19:233–8. [Google Scholar]
- 30.Smith JM, Misiak H. Critical flicker frequency (CFF) and psychotropic drugs in normal human subjects – a review. Psychopharmacology. 1976;47:175–82. doi: 10.1007/BF00735818. [DOI] [PubMed] [Google Scholar]
- 31.Curran S, Wattis JP, Hindmarch I. Concurrent validity of critical flicker fusion in patients with primary degenerative dementia of the Alzheimer type. Int J Geriatr Psychiatry. 1992;7:573–8. [Google Scholar]
- 32.Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav. 1998;59:917–23. doi: 10.1016/s0091-3057(97)00506-6. [DOI] [PubMed] [Google Scholar]
- 33.Danjou P, Paty I, Fruncillo R, Worthington P, Unruh M, Cevallos W, Martin P. A comparison of the residual effects of zaleplon and zolpidem following administration 5 to 2 h before awakening. Br J Clin Pharmacol. 1999;48:367–74. doi: 10.1046/j.1365-2125.1999.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hindmarch I, Patat A, Stanley N, Paty I, Rigney U. Residual effects of zaleplon and zolpidem following middle of the night administration five hours to one hour before awakening. Hum Psychopharmacol. 2001;16:159–67. doi: 10.1002/hup.282. [DOI] [PubMed] [Google Scholar]
- 35.Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol. 1992;43:597–601. doi: 10.1007/BF02284957. [DOI] [PubMed] [Google Scholar]
- 36.Blin O, Micallef J, Audebert C, Legangneux E. A double-blind, placebo- and flurazepam-controlled investigation of the residual psychomotor and cognitive effects of modified release zolpidem in young healthy volunteers. J Clin Psychopharmacol. 2006;26:284–9. doi: 10.1097/01.jcp.0000218985.07425.d9. [DOI] [PubMed] [Google Scholar]
- 37.Fairweather DB, Stanley N, Yoon J, Hindmarch I. The effects of fluoxetine and dothiepin on cognitive function in depressed patients in general practice. Hum Psychopharmacol Clin Exp. 1999;14:325–32. [Google Scholar]


