Abstract
AIMS
To assess the prevalence of co-medication of statins and CYP3A4 inhibitors before and after introduction of a new Norwegian reimbursement policy, which states that all patients should be prescribed simvastatin as first-line lipid-lowering therapy.
METHODS
Data from patients receiving simvastatin, lovastatin, pravastatin, fluvastatin or atorvastatin in 2004 and 2006, including co-medication of potent CYP3A4 inhibitors, were retrieved from the Norwegian Prescription Database covering the total population of Norway. Key measurements were prevalence of continuous statin use (two or more prescriptions on one statin) and proportions of different statin types among all patients and those co-medicated with CYP3A4 inhibitors.
RESULTS
In 2004, 5.9% (n = 272 342) of the Norwegian population received two or more prescriptions on one statin compared with 7.0% (n = 324 267) in 2006. The relative number of simvastatin users increased from 39.7% (n = 112 122) in 2004 to 63.1% (n = 226 672) in 2006. A parallel increase was observed within the subpopulation co-medicated with statins and CYP3A4 inhibitors, i.e. from 42.9% (n = 7706) in 2004 to 63.6% (n = 13 367) in 2006. For all other statins the number of overall users decreased to a similar extent to those co-medicated with CYP3A4 inhibitors.
CONCLUSIONS
In both 2004 and 2006, the choice of statin type did not depend on whether the patient used a CYP3A4 inhibitor or not. Considering the pronounced interaction potential of simvastatin with CYP3A4 inhibitors, a negative influence of the new policy on overall statin safety seems likely.
Keywords: CYP3A4, drug interactions, HMG-CoA reductase inhibitors, reimbursement, simvastatin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
HMG-CoA reductase inhibitors (statins) are frequently used drugs in the treatment of dyslipidaemia.
Co-medication with interacting drugs increases the risk of statin-induced muscular side-effects.
Simvastatin exhibits particularly high interaction potential due to substantial metabolism via cytochrome P450 3A4 (CYP3A4).
WHAT THIS STUDY ADDS
In June 2005, a new reimbursement policy was introduced by the Norwegian Medicines Agency stating that simvastatin should be prescribed as first-line lipid-lowering therapy.
Following introduction of the new policy, the number of patients co-medicated with simvastatin and CYP3A4 inhibitors almost doubled.
A potential consequence is increased incidence of muscular side-effects in the statin-treated population.
Introduction
HMG-CoA reductase inhibitors (statins) are a well-established treatment for improving lipid profile in dyslipidaemic patients. Several studies have shown the benefits of statins on cardiovascular morbidity and mortality [1–3]. The statins are generally well tolerated, but in 2001 cerivastatin was withdrawn from the market worldwide because of an unacceptably high frequency of serious myopathy (creatine kinase >10 times the upper limit of normal) and rhabdomyolysis [4]. Although rare, myopathy and rhabdomyolysis remain a serious concern with other statins also [5].
In large clinical trials statin-induced myopathy and rhabdomyolysis have been reported in 0.1–0.5 and 0.02–0.2% of patients, respectively [6–9]. The mode of action for muscle toxicity is not known, but risk factors for this adverse reaction include high statin dose/serum concentration, female sex, age and drug–drug interactions [10–12]. The statins have different drug–drug interaction potentials due to different pharmacokinetic properties. Simvastatin, lovastatin and atorvastatin are all metabolized by cytochrome P450 3A4 (CYP3A4), whereas pravastatin and fluvastatin mainly have other eliminating pathways [13]. Many agents are inhibitors or inducers of CYP3A4, and the general interaction potential of simvastatin, lovastatin and atorvastatin is therefore higher than for other statins [8, 10]. Of the CYP3A4 statins, simvastatin and lovastatin are more sensitive than atorvastatin in terms of relative increase in serum concentration during co-administration of CYP3A4 inhibitors. Compared with simvastatin and lovastatin, where 20–30-fold increases have been reported in combination with potent CYP3A4 inhibitors, only a threefold increase has been observed for atorvastatin [14–16]. Although an increased risk of adverse events is associated with co-administration of CYP3A4 inhibitors for all three agents, the risk is likely to be greatest for simvastatin and lovastatin.
A European study from 2003 indicated that Norway had the highest statin consumption per inhabitant in Europe [17]. Due to the substantial costs related to statin use, a new reimbursement policy was introduced by the Norwegian Medicines Agency in June 2005 [18], stating that all patients requiring statins should be prescribed simvastatin as first-line therapy. The arguments for the new policy were lower price of simvastatin after patent expiry and good clinical documentation of its effectiveness. However, simvastatin has a pronounced drug–drug interaction potential with CYP3A4 inhibitors [10, 14, 15, 19], and the presence of interactions was pointed out as a valid medical cause for prescribing other statins than simvastatin [18]. The aim of this study was to assess the co-medication prevalence of statins and CYP3A4 inhibitors in an outpatient population in Norway before and after introduction of the new reimbursement policy, which stated that simvastatin should be used as first-line therapy.
Methods
Prescription database
Data were drawn from the Norwegian Prescription Database (NorPD), which covers all prescriptions dispensed at Norwegian pharmacies, reimbursed or not. Since January 2004 Norwegian pharmacies have been obliged by law to submit prescription data on a monthly basis to NorPD at the Norwegian Institute of Public Health. The variables used in the present study were: patient's unique encrypted identifying number, sex, age, residence county, prescribers’ unique identifiers (encrypted), date of dispensing and information on drugs dispensed [number of packages, tablet strength, Anatomical Therapeutic Chemical (ATC) code]. This information was withdrawn from patients receiving statins in 2004 and 2006. The following statins were marketed in Norway in 2004 and 2006: simvastatin, lovastatin, pravastatin, fluvastatin and atorvastatin.
Study population
The study population comprised patients who had at least one statin prescription (ATC group C10AA) dispensed at Norwegian pharmacies during 2004 (before new reimbursement policy) or 2006 (after reimbursement policy). Only individuals defined as continuous statin users were included in the analyses. A continuous statin user was defined as an individual who received two or more prescriptions of the same statin type during 2004 or 2006. The time period for continuous statin use in each calendar year, and possible co-medication of CYP3A4 inhibitor, was defined by the first and last dispensing date of the statin. If a CYP3A4 inhibitor was dispensed at the first statin dispensing date or in between the first and last statin dispensing dates, possible co-medication was registered. A match was registered as probable concomitant use. In Norway >90% of statin prescriptions are reimbursed by the National Health Insurance, and patients usually receive statin treatment for 3 months (i.e. 100 tablets) at a time.
The term co-medication was used to describe possibly concomitant drug use according to the definition of Tobi et al.[20]. The drugs could be prescribed from one or more physician and dispensed at the same or a different date (but within the time period for statin use).
CYP3A4 inhibitors were divided into short-term and long-term inhibitors according to their indication for use. Short-term CYP3A4 inhibitors were drugs usually prescribed for shorter time periods (i.e. cure treatment), and long-term inhibitors were drugs usually prescribed for longer time periods (i.e. life-time treatment). Erythromycin, clarithromycin, fluconazole, itraconazole and ketoconazole were defined as short-term CYP3A4 inhibitors, but it is important to point out that treatment course could differ between the various inhibitors and within the same inhibitor (days to months). Amiodarone, diltiazem, verapamil, saquinavir, indinavir, ritonavir, nelfinavir, lopinavir, fosamprenavir, atazanavir and tipranavir were defined as long-term CYP3A4 inhibitors.
Analysis
One year prevalence for continuous statin use was based on the entire statin-treated population in 2004 and 2006. Continuous statin users were grouped according to type of statin used (i.e. five groups). If type of statin was switched within a study year, the patient could contribute to more than one group. Patients co-medicated with CYP3A4 inhibitors and statins were identified among continuous statin users. Continuous statin users were divided into two populations: those co-medicated with CYP3A4 inhibitors and those not co-medicated with CYP3A4 inhibitors. Each population was analysed considering distribution in age, sex and statin dose. Differentiating on type of CYP3A4 inhibitor dispensed in the time period for statin use, short- and long-term co-medicated statin users were identified. Some patients used both short-term and long-term CYP3A4 inhibitors and these patients contributed exposure in both groups. The average statin dose was based on the tablet strength of the last statin prescription dispensed, and the proportion of co-medicated drugs prescribed from the same physician was identified using the physicians’ unique identifiers.
The NorPD covers all prescriptions nationwide and represents the entire Norwegian statin population. Thus, 95% confidence intervals were not presented for the prevalences.
Results
One-year prevalence for continuous statin use was 5.9% (n = 272 342) in 2004 and 7.0% (n = 324 267) in 2006, representing ∼90% of the Norwegian statin population (Figure 1). Among continuous statin users, 6.3% (n2004= 17 079 and n2006= 20 418) were co-medicated with CYP3A4 inhibitors in both 2004 and 2006 (Figure 1). A 19.5% increase in the number of statin-treated patients exposed to CYP3A4 inhibitors was observed from 2004 to 2006. As regards short-term co-medication of CYP3A4 inhibitors, the number of patients increased from 8238 in 2004 to 10 848 in 2006 (Figure 1), i.e. a 31.7% increase. In comparison, the number of patients co-medicated with long-term CYP3A4 inhibitors increased by 4.6%, from 9533 patients in 2004 to 9968 patients in 2006 (Figure 1).
Figure 1.
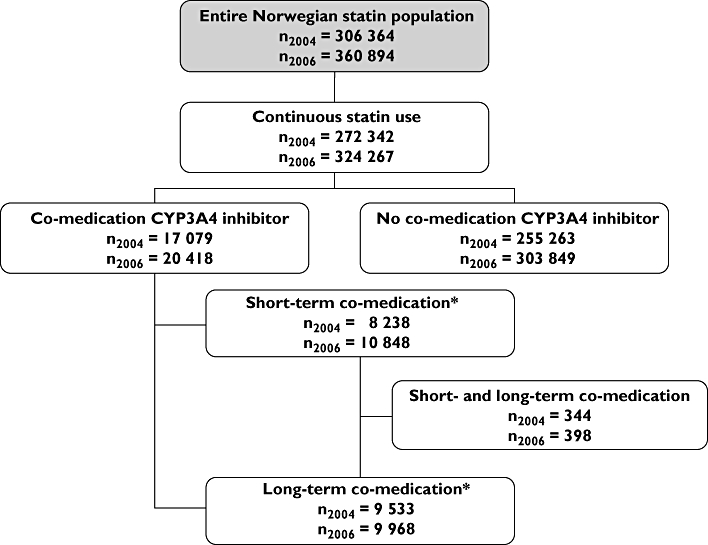
Flowchart of unique statin users included in the study. The Norwegian Prescription Database 2004/2006. *Some patients will be exposed to both short-term and long-term CYP3A4 inhibition during their period of statin use and will therefore be identified as both short-term and long-term co-medicated patients. The sum of, for example, n2004 short-term co-medication and n2004 long-term co-medication will be bigger than the actual sum of individuals overall exposed to CYP3A4 inhibitors in 2004
Table 1 shows the number of overall continuous statin users and continuous statin users exposed to CYP3A4 inhibitors for each statin in 2004 and 2006. In 2004, the number of continuous simvastatin and atorvastatin users was approximately the same (nsimvastatin= 112 122, natorvastatin= 120 051). By 2006, simvastatin had more than doubled its number of continuous users (nsimvastatin= 226 672). The relative number of simvastatin users increased from 39.7% (n = 112 122) in 2004 to 63.1% (n = 226 672) in 2006. A parallel increase was observed within the subpopulation co-medicated with statins and CYP3A4 inhibitors, i.e. from 42.9% (n = 7706) in 2004 to 63.6% (n = 13 367) in 2006. For all other statins, the number of overall users decreased to a similar extent to those co-medicated CYP3A4 inhibitors.
Table 1.
Number and proportion of unique continuous statin users and unique continuous statin users exposed to one or several CYP3A4 inhibitors, by year and change
| Statin types | Continuous statin use | Co-medicated with CYP3A4 inhibitors | ||||
|---|---|---|---|---|---|---|
| 2004 | 2006 | Change (between proportions) | 2004 | 2006 | Change (between proportions) | |
| n (%) | n (%) | % | n (%) | n (%) | % | |
| Simvastatin | 112 122 (39.7) | 226 672 (63.1) | 23.4 | 7706 (42.9) | 13 367 (63.6) | 20.7 |
| Lovastatin | 2808 (1.0) | 2017 (0.6) | −0.4 | 285 (1.6) | 186 (0.9) | −0.7 |
| Pravastatin | 39 400 (14.0) | 26 425 (7.4) | −6.6 | 2587 (14.4) | 1776 (8.5) | −5.9 |
| Fluvastatin | 8003 (2.8) | 6627 (1.9) | −1.0 | 402 (2.2) | 370 (1.8) | −0.5 |
| Atorvastatin | 120 051 (42.5) | 97 343 (27.1) | −15.4 | 6997 (38.9) | 5314 (25.3) | −13.6 |
| Total* | 282 384 (100) | 359 084 (100) | 17 977 (100) | 21 013 (100) | ||
According to our definition of a continuous statin user, some patients will be able to use more than one statin type during one calendar year. Some patients may therefore be identified as both simvastatin and lovastatin users in Table 1, consequently counted in both places. The summarized number of continuous statin users in each in Table 1 will therefore be higher than in Figure 1 (which shows the number of unique statin users in each group). The Norwegian Prescription Database 2004/2006.
In 2004, the most frequent combination among short-term inhibitors was simvastatin/erythromycin and atorvastatin/erythromycin, with 1823 and 1867 co-medicated patients, respectively (Table 2). Clearly dominating in 2006 was the combination simvastatin/erythromycin, with 4078 co-medicated patients. Among the long-term inhibitors, the combination simvastatin/verapamil was most frequent in both 2004 (n = 2027, Table 2) and 2006 (n = 3191, Table 3).
Table 2.
Number and proportion of continuous statin users exposed to different short-term CYP3A4 inhibitors, by year, statin types and short-term CYP3A4 inhibitors
| Statin types | Erythromycin | Clarithromycin | Azole antimycotics | |||
|---|---|---|---|---|---|---|
| 2004 | 2006 | 2004 | 2006 | 2004 | 2006 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Simvastatin | 1823 (40.3) | 4078 (62.5) | 1207 (40.7) | 2190 (63.9) | 385 (40.6) | 770 (63.2) |
| Lovastatin | 57 (1.3) | 49 (0.6) | 31 (1.1) | 18 (0.5) | 8 (0.8) | 8 (0.7) |
| Pravastatin | 682 (15.1) | 525 (8.0) | 402 (13.6) | 265 (7.7) | 132 (13.9) | 103 (8.5) |
| Fluvastatin | 97 (2.1) | 103 (1.6) | 75 (2.5) | 51 (1.5) | 23 (2.4) | 27 (2.2) |
| Atorvastatin | 1867 (41.3) | 1772 (27.1) | 1249 (42.1) | 902 (26.3) | 401 (42.3) | 311 (25.5) |
| Total* | 4526 (100) | 6527 (100) | 2964 (100) | 3426 (100) | 949 (100) | 1219 (100) |
According to our definition of a continuous statin user, some patients will be able to use more than one statin type or one short-term CYP3A4 inhibitor during one calendar year. Some patients may therefore be identified in more than one place in Table 2, consequently counted in more than once in the table. The total number of continuous statin users exposed to CYP3A4 inhibitors in Table 2 will therefore be higher than in Figure 1 (which shows the number of unique statin users in each group). The Norwegian Prescription Database 2004/2006.
Table 3.
Number and proportion of continuous statin users exposed to different long-term CYP3A4 inhibitors, by year, statin types and long-term CYP3A4 inhibitors
| Statin type | Amiodarone | Diltiazem | Verapamil | HIV protease inhibitors | ||||
|---|---|---|---|---|---|---|---|---|
| 2004 | 2006 | 2004 | 2006 | 2004 | 2006 | 2004 | 2006 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Simvastatin | 570 (43.9) | 1143 (64.2) | 1815 (46.7) | 2219 (63.1) | 2027 (43.8) | 3191 (64.9) | 1 (2.4) | 10 (20.4) |
| Lovastatin | 21 (1.6) | 14 (0.8) | 87 (2.2) | 48 (1.4) | 83 (1.8) | 53 (1.1) | 0 (–) | 0 (–) |
| Pravastatin | 242 (18.7) | 168 (9.4) | 571 (14.7) | 355 (10.1) | 610 (13.2) | 397 (8.1) | 29 (69.0) | 26 (53.1) |
| Fluvastatin | 19 (1.5) | 16 (0.9) | 94 (2.4) | 77 (2.4) | 100 (2.2) | 100 (2.0) | 0 (–) | 0 (–) |
| Atorvastatin | 445 (34.3) | 438 (24.6) | 1323 (34.0) | 819 (23.3) | 1809 (39.1) | 1178 (23.9) | 12 (28.6) | 13 (26.5) |
| Total* | 1297 (100) | 1779 (100) | 3890 (100) | 3518 (100) | 4629 (100) | 4919 (100) | 42 (100) | 49 (100) |
According to our definition of a continuous statin user, some patients will be able to use more than one statin type or one long-term CYP3A4 inhibitor during one calendar year. Some patients may therefore be identified in more than one place in Table 3, consequently counted in more than once in the table. The total number of continuous statin users exposed to CYP3A4 inhibitors in Table 3 will therefore be higher than in Figure 1 (which shows the number of unique statin users in each group). The Norwegian Prescription Database 2004/2006.
Among the co-medicated patients, younger women (<65 years) were more frequently exposed to short-term CYP3A4 inhibition than men (Figure 2). Men exposed to short-term CYP3A4 inhibition did not show variation among different age groups (Figure 2). Exposure to long-term CYP3A4 inhibition increased with age, and was most prominent in women in the age group 80+ (Figure 2).
Figure 2.
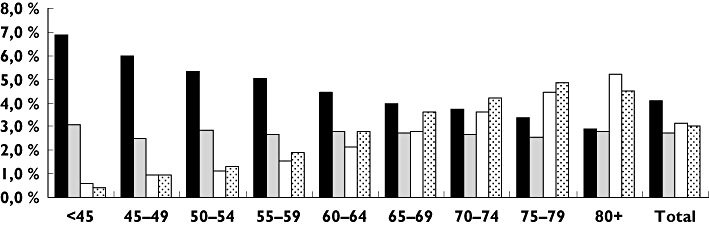
Proportion of unique continuous statin users exposed to short-term and long-term CYP3A4 inhibition in 2006 (Figure 1: nshort= 10 848, nlong= 9968), by sex and age. The Norwegian Prescription Database 2004/2006. WomenShortC-M ( ); MenShortC-M (
); MenShortC-M ( ); WomenLongC-M (□); MenLongC-M (
); WomenLongC-M (□); MenLongC-M ( ). C-M: co-medicated
). C-M: co-medicated
In Figure 3, the average prescribed daily dose of the most frequently used statins (simvastatin, atorvastatin and pravastatin) are shown in patients with and without co-medication of long-term CYP3A4 inhibitors. In both 2004 and 2006, the average daily doses were approximately the same for all three statins with or without co-medication of long-term CYP3A4 inhibitors (Figure 3). Whereas the average pravastatin dose was similar in 2004 and 2006 among those not exposed to long-term co-medication (32 mg), the average simvastatin and atorvastatin dose increased by approximately 16 and 33–35%, respectively, as shown in Figure 3.
Figure 3.
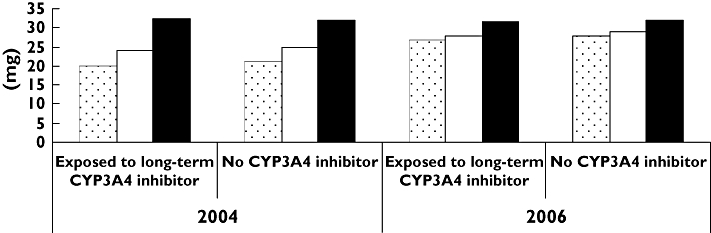
Average statin dose dispensed at a pharmacy, by year and exposure/non-exposure. The Norwegian Prescription Database 2004/2006. Atorvastatin ( ); Simvastatin (□); Pravastatin (
); Simvastatin (□); Pravastatin ( )
)
Co-medication with short-term CYP3A4 inhibitors was prescribed by different physicians to 53.5% (n = 4406) of patients in 2004 and 45.4% (n = 4921) in 2006. Among those co-medicated with long-term CYP3A4 inhibitors, 22.5% (n = 2148) received prescriptions for statins and inhibitors from different physicians in 2004 compared with 20.2% (n = 2014) in 2006.
Discussion
In this Norwegian study, which included ∼300 000 continuous statin users each year, about 6% were co-medicated with one or more CYP3A4 inhibitors in 2004 and 2006. The prescription of the five statins studied was more or less random within the subpopulation using CYP3A4 inhibitors in both study years, as indicated by the parallel proportions of overall use and use among co-medicated patients for each statin. After introduction of the new reimbursement policy for lipid-lowering treatment, the proportion of simvastatin users among patients co-medicated with CYP3A4 inhibitors increased from 39.7 to 63.1%, whereas the proportions of atorvastatin and pravastatin users decreased from 38.9 to 25.3% and from 14.4 to 8.5%, respectively. As the interaction potential of simvastatin with CYP3A4 inhibitors is greater than for atorvastatin and pravastatin, it is likely that the new policy has influenced statin safety negatively.
The Norwegian government's statin costs was reduced from €120 million the year before the new reimbursement policy to €95 million the year after, and is thus considered an economic success [21]. However, the present study has shown that the new policy may have affected the safety of statin treatment as well. Before the new reimbursement policy atorvastatin was the most prescribed statin, followed by simvastatin. Thus, many of the new simvastatin users in 2006 were switched from atorvastatin [21]. Both simvastatin and atorvastatin are subjected to metabolism via CYP3A4, but the interaction potential with CYP3A4 inhibitors is greater for simvastatin than for atorvastatin. Whereas 20–30-fold increases in systemic exposure of simvastatin have been reported in combination with potent CYP3A4 inhibitors, only a threefold increase has been observed for atorvastatin [14–16]. Thus, it is likely that patients co-medicated with CYP3A4 inhibitors are at higher risk of developing muscular side-effects with simvastatin than with atorvastatin. This is supported by a recent case report where myopathy was observed following switch from atorvastatin to simvastatin (equipotent doses) in a patient co-medicated with diltiazem [22]. Moreover, in a study of cases of rhabdomyolysis reported in Australia, CYP3A4 inhibitors were more often involved in events with simvastatin (42%) than with atorvastatin (25%) [10].
Among the statins, pravastatin appears to have the lowest interaction potential with CYP3A4 inhibitors. Where there is need of co-medication of statins and CYP3A4 inhibitors, pravastatin should therefore be the preferred statin [14, 23, 24]. However, the reduction in use of pravastatin from 2004 to 2006 was similar in patients both exposed and not exposed to CYP3A4 inhibitors. This shows that pravastatin is not preferred in co-medicated patients by Norwegian physicians. The Norwegian Medicines Agency has pointed out that the presence of drug–statin interactions is a valid medical cause for prescribing other statins than simvastatin [18]. In Ireland, however, pravastatin is the most frequently prescribed statin, and Heerey et al. have shown that Irish prescribers prefer pravastatin when a CYP3A4 inhibitor is present [17, 23, 25]. This indicates greater attention to drug–statin interactions in Ireland compared with Norway. Introducing new reimbursement policies requires attention to safety issues and more effective strategies should be implemented to prevent them.
This study has provided detailed information about all prescriptions dispensed to individuals outside institutions in Norway. The NorPD contains information that makes it possible to follow each individual over time in order to study concomitant drug use. Use of the nationwide NorPD eliminates the possibility of selection and recall bias. Several other countries have healthcare databases containing information on drug prescriptions [20, 26–28]. However, many of these are based on insurance plans that cover only parts of the population, which may introduce selection bias. Patients receiving statins may change statin type during 1 year, which could overestimate positive findings of co-medication. However, focusing on co-medication within statin types this was not considered a problem, and approximately 1% of the continuous statin users switched between statin types within a year (both 2004 and 2006). One weakness using prescription databases is that we do not know if patients temporarily discontinue their statin use while being treated with, for example, antibiotics.
Concomitant use of CYP3A4 inhibitors and simvastatin is associated with an increased risk of developing myopathy and rhabdomyolysis [8, 10, 15, 29, 30]. In 2006 the most frequent combination overall was simvastatin/erythromycin, and the number of patients exposed to this combination more than doubled from 2004 to 2006. This drug combination is contraindicated because of CYP3A4 inhibition and its strong correlation with statin-induced muscle toxicity [14, 31]. From our database, NorPD, we do not know whether the statin was temporarily discontinued during the macrolide treatment. However, short-term CYP3A4 inhibitors such as erythromycin may often be prescribed in an acute situation, e.g. emergency rooms. The prescribing physician may not be aware of the patient's statin use, and different prescribing physicians can complicate proper management of the drug–statin interaction. In both 2004 and 2006, about 50% of those exposed to short-term CYP3A4 inhibition received statin and short-term CYP3A4 inhibitor from different physicians. In cases involving different physicians, we consider it less likely that the contraindicated use of simvastatin with erythromycin/clarithromycin had been managed.
With respect to long-term CYP3A4 inhibition, the most frequent combination was simvastatin/verapamil in both 2004 and 2006. Simvastatin in combination with long-term CYP3A4 inhibitors is not contraindicated, but according to the simvastatin Summary of Product Characteristics (SPC), the daily dose should not exceed 20 mg in combination with verapamil and amiodarone (40 mg with diltiazem) [31]. Simvastatin doses in this study were similar in patients both exposed and not exposed to long-term CYP3A4 inhibitors. Moreover, the average simvastatin dose in patients exposed to long-term CYP3A4 inhibition actually increased from 25 mg in 2004 to 28 mg in 2006. This is thought provoking with respect to the risk for simvastatin-induced adverse events and indicates that the simvastatin dosing precautions in combination with CYP3A4 inhibitors are not followed by Norwegian physicians.
Women and elderly patients are generally at higher risk for statin-induced muscle toxicity [10, 11]. We observed a higher proportion of short-term CYP3A4 inhibitors in combination with statin treatment in women <65 years old than in men. This can be explained by the use of single-dose fluconazole in premenopausal women, which is unlikely to represent a clinically relevant drug–statin interaction. Also, considerably fewer younger women than men received a prescription for statins. The proportion of patients exposed to long-term CYP3A4 inhibition increased with age in both men and women. In Switzerland, Egger et al. also found that prevalence of clinically relevant drug–drug interactions among statin users significantly increased with advancing age [32]. Statins are usually a life-long treatment, and elderly patients are at special risk for drug–drug interactions because of polymorbidity and consequent prescribing of multiple drugs [9, 33]. This study has shown that the highest proportion among those exposed to long-term CYP3A4 inhibition are women in the 80+ age group.
Only CYP3A4 inhibitors were included in the study, but it should be mentioned that interactions with other drugs are also important with respect to statin safety [15]. Examples include gemfibrozil and ciclosporin, which interact with statins via inhibition of CYP2C8, glucuronidation, P-glycoprotein and/or organic anion transporting polypeptide [15]. In Norway, gemfibrozil is not registered for use, whereas pravastatin or fluvastatin are considered as first-line statins in transplant patients.
In summary, the proportion of simvastatin users among those co-medicated with a CYP3A4 inhibitor increased in parallel with the overall increase in simvastatin use from 2004 to 2006. The new reimbursement policy for lipid-lowering treatment introduced in 2005 aimed to increase simvastatin prescribing and has substantially reduced Norwegian statin costs. At the same time, the risk of adverse effects has increased among many statin-treated patients due to the pronounced interaction potential of simvastatin with CYP3A4 inhibitors. This study has shown that the choice of statin type did not depend on whether the patient used a CYP3A4 inhibitor or not. In conclusion, warnings and contraindications in SPCs are insufficient to prevent undesirable statin interactions in Norway.
REFERENCES
- 1.Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyorala K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) 1994. Atheroscler Suppl. 2004;5:81–7. doi: 10.1016/j.atherosclerosissup.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–67. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 3.Larosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 4.Maggini M, Raschetti R, Traversa G, Bianchi C, Caffari B, Da Cas R, Panei P. The cerivastatin withdrawal crisis: a ‘post-mortem’ analysis. Health Policy. 2004;69:151–7. doi: 10.1016/j.healthpol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–90. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 6.Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 7.Mukhtar RY, Reckless JP. Statin-induced myositis: a commonly encountered or rare side effect? Curr Opin Lipidol. 2005;16:640–7. doi: 10.1097/01.mol.0000188414.90528.71. [DOI] [PubMed] [Google Scholar]
- 8.Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36:288–95. doi: 10.1345/aph.1A289. [DOI] [PubMed] [Google Scholar]
- 9.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke. 2002;33:2337–41. doi: 10.1161/01.str.0000034125.94759.41. [DOI] [PubMed] [Google Scholar]
- 10.Ronaldson KJ, O'Shea JM, Boyd IW. Risk factors for rhabdomyolysis with simvastatin and atorvastatin. Drug Saf. 2006;29:1061–7. doi: 10.2165/00002018-200629110-00005. [DOI] [PubMed] [Google Scholar]
- 11.Schech S, Graham D, Staffa J, Andrade SE, La Grenade L, Burgess M, Blough D, Stergachis A, Chan KA, Platt R, Shatin D. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007;16:352–8. doi: 10.1002/pds.1287. [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne CM, Corsini A, Davidson MH, Holdaas H, Jacobson TA, Leitersdorf E, Marz W, Reckless JP, Stein EA. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163:553–64. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- 13.Molden E. Variability in cytochrome P450-mediated metabolism of cardiovascular drugs: clinical implications and practical attempts to avoid potential problems. Heart Drug. 2004;4:55–79. [Google Scholar]
- 14.Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140–6. doi: 10.1016/j.amjcard.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 15.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Silva M, Matthews ML, Jarvis C, Nolan NM, Belliveau P, Malloy M, Gandhi P. Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clin Ther. 2007;29:253–60. doi: 10.1016/j.clinthera.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Walley T, Folino-Gallo P, Stephens P, Van Ganse E. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br J Clin Pharmacol. 2005;60:543–51. doi: 10.1111/j.1365-2125.2005.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Refusjon: Statiner-Nye Vilkår For Refusjon (in English: Statins-New Reimbursement Regulations) Oslo: Norwegian Medicines Agency; 13-4-2005. Ref Type: Electronic Citation. [Google Scholar]
- 19.Kantola T, Kivisto KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998;64:177–82. doi: 10.1016/S0009-9236(98)90151-5. [DOI] [PubMed] [Google Scholar]
- 20.Tobi H, Faber A, Van Den Berg PB, Drane JW, De Jong-Van Den Berg LT. Studying co-medication patterns: the impact of definitions. Pharmacoepidemiol Drug Saf. 2007;16:405–11. doi: 10.1002/pds.1304. [DOI] [PubMed] [Google Scholar]
- 21.Sakshaug S, Furu K, Karlstad O, Ronning M, Skurtveit S. Switching statins in Norway after new reimbursement policy: a nationwide prescription study. Br J Clin Pharmacol. 2007;64:476–81. doi: 10.1111/j.1365-2125.2007.02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molden E, Westergren T. Interaction risk with statin switch. Tidsskr Nor Laegeforen. 2007;127:428–31. [PubMed] [Google Scholar]
- 23.Heerey A, Barry M, Ryan M, Kelly A. The potential for drug interactions with statin therapy in Ireland. Ir J Med Sci. 2000;169:176–9. doi: 10.1007/BF03167690. [DOI] [PubMed] [Google Scholar]
- 24.Einarson TR, Metge CJ, Iskedjian M, Mukherjee J. An examination of the effect of cytochrome p450 drug interactions of hydroxymethylglutaryl-coenzyme A reductase inhibitors on health care utilization: a Canadian population-based study. Clin Ther. 2002;24:2126–36. doi: 10.1016/s0149-2918(02)80102-3. [DOI] [PubMed] [Google Scholar]
- 25.Teeling M, Bennett K, Feely J. The influence of guidelines on the use of statins: analysis of prescribing trends 1998–2002. Br J Clin Pharmacol. 2005;59:227–32. doi: 10.1111/j.1365-2125.2004.02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furu K. Establishment of the nationwide Norwegian Prescription Database (NorPD) – New opportunities for research in pharmacoepidemiology in Norway. Norsk Epidemiologi. 2008;18:129–36. [Google Scholar]
- 27.Wettermark B, Hammar N, Michaelfored C, Leimanis A, Otterblad OP, Bergman U, Persson I, Sundstrom A, Westerholm B, Rosen M. The new Swedish prescribed drug register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–35. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 28.Williams D, Bennett K, Feely J. The application of prescribing indicators to a primary care prescription database in Ireland. Eur J Clin Pharmacol. 2005;61:127–33. doi: 10.1007/s00228-004-0876-3. [DOI] [PubMed] [Google Scholar]
- 29.Bottorff MB. Statin safety and drug interactions: clinical implications. [Review. Am J Cardiol. 2006;97:27C–31C. doi: 10.1016/j.amjcard.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Cziraky MJ, Willey VJ, Mckenney JM, Kamat SA, Fisher MD, Guyton JR, Jacobson TA, Davidson MH. Statin safety: an assessment using an administrative claims database. Am J Cardiol. 2006;97:61C–8C. doi: 10.1016/j.amjcard.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Summary of product characteristics zocor. [last accessed 14 November 2006]. Available at http://www.legemiddelverket.no/spc/godkjente/zocor-no-spc-godkj_mai03.doc Ref Type: Electronic Citation.
- 32.Egger SS, Ratz Bravo AE, Hess L, Schlienger RG, Krahenbuhl S. Age-related differences in the prevalence of potential drug–drug interactions in ambulatory dyslipidaemic patients treated with statins. Drugs Aging. 2007;24:429–40. doi: 10.2165/00002512-200724050-00006. [DOI] [PubMed] [Google Scholar]
- 33.Raffel OC, White HD. Drug insight: statin use in the elderly. Nat Clin Pract Cardiovasc Med. 2006;3:318–28. doi: 10.1038/ncpcardio0558. [DOI] [PubMed] [Google Scholar]


