Abstract
AIMS
Despite evidence of an increase in the incidence of both type 1 and type 2 diabetes in youths, there are few data on the prevalence of either type in children and adolescents. The aim of this study was to investigate the prevalence of childhood diabetes over an 8-year period in the UK.
METHODS
This was a retrospective cohort study that covered 8 years (January 1998 to December 2005) of UK IMS Disease Analyzer (IMS DA) data. The cohort comprised all children and adolescents aged 0–18 years who received at least one antidiabetic drug prescription during the study period. The prevalence of antidiabetic drug prescribing was used as a proxy for diabetes itself.
RESULTS
Data were available on 505 754 children aged 0–18 years and a total of 37 225 antidiabetic prescriptions were issued. Insulin use increased significantly from 1.08 per 1000 children [95% confidence interval (CI) 0.96, 1.20] in 1998 to 1.98 (95% CI 1.80, 2.10) in 2005 (P < 0.001), more markedly in those aged 12 and 18 years. The use of oral antidiabetic drugs for diabetes treatment rose significantly from 0.006 per 1000 children in 1998 (95% CI 0.0043, 0.017) to 0.05 (95% CI 0.025, 0.080) (P < 0.001) in 2005.
CONCLUSIONS
This study indicates a significant increase in prevalence on both type 1 and type 2 diabetes treatment in children and adolescents in the UK. Thus, this supporting evidence from other sources that the prevalence of childhood diabetes is rising rapidly. Further epidemiological studies are required to investigate the aetiology and risk factors.
Keywords: antidiabetic drugs, diabetes, epidemiology, paediatric, prevalence
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Increasing antidiabetic drugs use in youths has been reported in the USA, however there is a lack of epidemiological evidence in the UK.
There is an increase in the prevalence of both type 1 and 2 diabetes, but precise estimates are difficult to obtain and as such are uninformative for future health services planning.
WHAT THIS STUDY ADDS
The prevalence of children receiving insulin and oral antidiabetic drugs has increased twofold and eightfold, respectively, between 1998 and 2005.
The data reflect the prevalence of both type 1 and type 2 diabetes rapidly increase in recent years.
The prevalence of antidiabetic drug use increases with increasing age, especially among those aged 12–18 years.
Consideration needs to be given to the funding and design of future services for children and particularly adolescents with diabetes to take account of these epidemiological findings.
Introduction
Diabetes is one of the most common chronic conditions of childhood and there has been a marked increase in incidence of both type 1 and type 2 diabetes in children and adolescents over the past 20 years [1–3]. In the USA, more than 13 000 children are diagnosed with type 1 diabetes each year [4]. The incidence of type 1 diabetes in the USA is about seven per 100 000 per year in children aged ≤4 years, 15 per 100 000 per year in children aged 5–9 years, and about 22 per 100 000 per year in those aged 10–14 years [5]. In 2007, the incidence rate of both type 1 and type 2 diabetes was 24.3 per 100 000 person-years in young people aged <20 years in the USA [6]. Approximately 75% of all newly diagnosed cases of type 1 diabetes occur in individuals younger than 18 years old. In the UK, it was reported that the incidence of type 1 diabetes in children aged ≤14 years ranged from 15 to 26 per 100 000 per year during the 1990s, with an estimated annual increase in incidence of 4% per year. Also, the UK is one of the countries with the highest incidence rate of type 1 diabetes in children, ranking fifth out of 57 countries [7]. Previous studies have estimated the incidence of diabetes in young people using diabetes registries to monitor new cases. There is sufficient evidence to demonstrate that the incidence of children with diabetes is rising [1–3, 8].
However, studies investigating the prevalence of diabetes in children and adolescents are lacking. This information is particularly important from a health policy point of view as it will enable the monitoring of disease burden, which is directly related to the planning of healthcare resources. In 2005, the US National Institute of Health estimated that the prevalence of diabetes in people aged ≤ 20 years was 0.22%; however, the authors suggested that nationally representative data that would be needed to monitor diabetes trends by type are not available to date [9]. As a result, a 5-year project was set up and the findings will be reported in the near future. In the UK, it was estimated that 20 000 children and adolescents have diabetes [8]. However, similar to the USA, national representative data for diabetes trends are lacking.
The prescribing of insulin is an excellent proxy for estimating the prevalence of type 1 diabetes in the UK as it is mainly prescribed for type 1 diabetes and is not normal practice to manage type 2 diabetes in children with insulin. Previous studies have applied this approach to estimate the prevalence of diabetes in the USA and Scandinavia [10–13]. The treatment of type 2 diabetes is more complex. For example, metformin is also being used for the treatment of polycystic ovarian syndrome (PCOS), obesity and for impaired glucose tolerance. In addition, type 2 diabetes may persist unrecognized in obese adolescents or be managed by diet alone. Therefore, the approach of using oral antidiabetic drugs as a means to estimate the prevalence of type 2 diabetes needs to be cautious. In the UK, children and adolescents with diabetes are almost entirely managed in hospital, whereas the prescribing of insulin and oral antidiabetic drugs is mostly undertaken in general practice. Therefore, the national general practice databases are appropriate to investigate the prevalence of childhood diabetes by using antidiabetic drug prescribing as a proxy for diabetes itself.
Methods
Data source
A retrospective cohort analysis was conducted using the IMS Disease Analyzer (IMS DA) database. This database contains approximately 2 million anonymous patient records and more than 95 million prescriptions from about 125 general practices with >500 general practitioners (GPs) [14]. Information held on the database includes patient demographics, indications for treatment and prescription details. Prescribed drugs are coded based on the Anatomical Therapeutic Chemical (ATC) classification issued by the European Pharmaceutical Market Research Association [15], and medical diagnoses are coded to Read code (a UK medical diagnostic code) that can be linked to the International Classification of Disease (ICD) version 10 codes [16]. The database is subject to internal validation and quality checks and is consistent with other UK prescription counts [17]. The database has also been shown to be of high quality and is widely used in drug utilization studies [18–20].
The study consisted of children and adolescents aged 0–18 years registered with a GP who contributed data to the IMS DA between 1 January 1998 and 31 December 2005. All subjects needed to have a minimum of six months valid data in the database. Subjects with at least one prescription for antidiabetic drugs were classified as cases. Antidiabetic drugs were classified based on the ATC therapeutic level A10 (drugs used in diabetes) and stratified according to the subsequent levels: A10B (blood glucose-lowering drugs comprised oral antidiabetic drugs); A10C (human insulin and analogues); and A10D (animal insulin) (Table 1). The age classification of the International Conference of Harmonization was modified in this analysis and the age bands were stratified as follows: 0–1, 2–5, 6–11 and 12–18 years [21]. Prevalence was calculated as the total number of children with at least one prescription of antidiabetic drugs during each year of investigation divided by the total number of children registered on the database in the same year, stratified by age and gender.
Table 1.
Number of children and adolescents with antidiabetic drugs and total number of patients in the IMS DA by calendar year
| Year | Insulin Boys | Oral antidiabetic drugs | Total cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Total | Boys | Girls | Total | Boys | Girls | Total | |
| 1998 | 166 | 174 | 340 | 3 | 4 | 7 | 157 685 | 151 722 | 309 407 |
| 1999 | 182 | 206 | 388 | 3 | 4 | 7 | 167 218 | 162 202 | 329 420 |
| 2000 | 206 | 238 | 444 | 4 | 12 | 16 | 175 722 | 171 545 | 347 267 |
| 2001 | 220 | 235 | 455 | 9 | 15 | 24 | 184 573 | 181 133 | 365 706 |
| 2002 | 238 | 237 | 475 | 6 | 16 | 22 | 193 121 | 190 220 | 383 341 |
| 2003 | 265 | 237 | 502 | 11 | 26 | 37 | 201 889 | 199 346 | 401 235 |
| 2004 | 277 | 248 | 525 | 9 | 40 | 49 | 200 041 | 197 908 | 397 949 |
| 2005 | 271 | 255 | 526 | 10 | 33 | 43 | 192 234 | 189 802 | 382 036 |
As the oral antidiabetic drug metformin is increasingly used for the treatment of PCOS (ICD10 E282) amongst female adolescents in clinical practice [22], subgroup analysis was carried out to estimate the prevalence of oral antidiabetic drugs prescribed only for the treatment of diabetes. This subgroup analysis included only subjects who had at least one prescription for oral antidiabetic (A10B) linked with an indication of diabetes. The ICD 10 codes for diabetes in the IMS DA were E10.9 (insulin-dependent diabetes mellitus without complication), E11.9 (non-insulin dependent diabetes mellitus without complication), E14.2 [unspecific diabetes mellitus (DM) with renal complication], E14.6 (unspecific DM with specific complication) and E14.9 (unspecific DM without complication).
Validation of IMS DA data
An external validation was carried out to verify IMS DA data using the General Practice Research Database (GPRD). The GPRD contains anonymous patient records from general practice and covers approximately 5% of the UK population [23]. The demographic distribution of the GPRD is broadly representative of that of the UK. The GPRD has been collecting patient records continuously since 1987. Information held on the GPRD is very similar to IMS DA but contains data from difference general practices. Similar to IMS DA, the quality of the information of GPRD has been validated in a number of studies, and the completeness of medical recording is held to be high [18], and it has been used to investigate child health issues [23–26].
The GPs contributing data to either IMS DA and GPRD on a voluntary basis with a small incentive reward. Ethical approval was granted from the IMS DA and GPRD Scientific and Ethical Advisory Group.
Statistical analysis
Annual, sex- and age-specific prevalence of antidiabetic drug prescribing was calculated using Poisson distribution with a 95% confidence interval (CI). A χ2 test (Cochran–Armitage test for trend) was used to examine the yearly trend of antidiabetic drug prescribing. Analyses were carried out using Stata version 9.1 (Statistical Software, Release 9.1; College Station, TX, USA).
Results
The IMS DA study population included 505 754 children and adolescents aged between 0 and 18 years during the study period (Table 1). Among these, 37 225 antidiabetic prescriptions were issued to 1098 study subjects. Insulin accounted for the majority of antidiabetic prescriptions (98.1%) (Table 2). The age-specific prevalence of all antidiabetic drugs (including insulin) between 1998 and 2005 is shown in Figure 1. There was a linear increase in prevalence with increasing age from 0.06 per 1000 children at the age of 1 year to 3.07 per 1000 at 18 years.
Table 2.
Number of antidiabetic prescriptions in children and adolescents aged 0–18, stratified by ATC (Anatomical Therapeutic Chemical) classification
| ATC codes | Drug name | No. of prescriptions | % |
|---|---|---|---|
| Insulin | |||
| A10C1 | Human insulin analogue fast acting | 8 920 | 24.4 |
| A10C2 | Human insulin analogue intermediate acting | 4 281 | 11.7 |
| A10C3 | Human insulin analogue intermediate + fast acting | 20 127 | 55.1 |
| A10C4 | Human insulin analogue intermediate + long acting | 319 | 0.87 |
| A10C5 | Human insulin analogue long acting | 2 735 | 7.5 |
| A10C9 | Other human insulin | 1 | 0.003 |
| A10D0 | Animal insulin | 129 | 0.35 |
| Total | 36 512 | 100 | |
| Oral antidiabetic drugs | |||
| A10B1 | Sulphonylureas | ||
| Gliclazide | 35 | 4.9 | |
| Glipizide | 3 | 0.4 | |
| Glibenclamide | 21 | 2.9 | |
| Chlorpropamide | 1 | 0.1 | |
| A10B2 | Biguanides | ||
| Metformin | 613 | 86.0 | |
| A10B4 | Glitazones | ||
| Rosiglitazone | 3 | 0.4 | |
| A10B9 | Other oral antidiabetics | ||
| A10B9 | Nateglinide | 19 | 2.7 |
| Repaglinide | 18 | 2.5 | |
| Total | 713 | 100 | |
Figure 1.
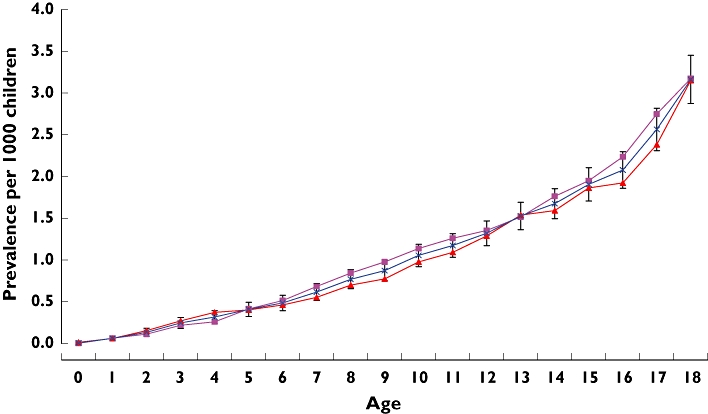
Overall prevalence of antidiabetic drug use in children and adolescents by age on IMS DA, 1998–2005 inclusive. girls ( ); boys (
); boys ( ); overall (
); overall ( )
)
Figure 2 shows the prevalence of insulin and oral antidiabetic drug use during the period covered. Insulin use rose significantly from 1.08 per 1000 children (95% CI 0.96, 1.20) in 1998 to 1.98 per 1000 (95% CI 1.80, 2.10) in 2005 (P < 0.001). Similarly, the use of oral antidiabetic drugs significantly increased during the study period from 0.02 per 1000 children (95% CI 0.006, 0.04) to 0.16 per 1000 (95% CI 0.11, 0.21) (P < 0.001). Figure 3 shows the age-specific prevalence of insulin use. The use of insulin was significantly increased at age 6–18 years. Among the group of oral antidiabetic drugs a total number of 713 prescriptions were issued to 128 children during the study period. Metformin (n = 613 prescriptions, 86%) was the most commonly prescribed oral antidiabetic drug (Table 1). Of 103 children who received metformin, 23 (22.3%) were recorded as being prescribed this drug for the treatment of PCOS. The prevalence of oral antidiabetic drugs used solely for the treatment of diabetes increased significantly from 0.006 per 1000 children in 1998 (95% CI 0.0043, 0.017) to 0.05 per 1000 children in 2005 (95% CI 0.025, 0.080) (P < 0.001). Only 26 patients from the study cohort received both insulin and oral antidiabetic drugs; therefore the increase in insulin is unlikely to be caused by the increase in type 2 diabetes.
Figure 2.
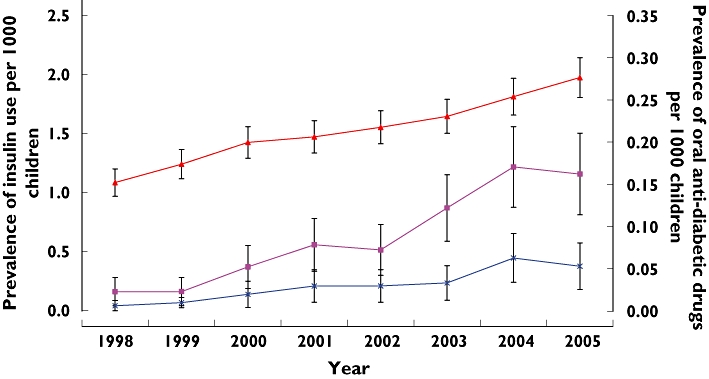
Prevalence of insulin, oral antidiabetic drugs and oral antidiabetic drugs with a diabetes indication amongst children and adolescents aged 0–18 (with 95% CIs). insulin* ( ); oral antidiabetic drugs* (
); oral antidiabetic drugs* ( ); oral antidiabetic drugs with diabetes indication* (
); oral antidiabetic drugs with diabetes indication* ( ); *a significant trend for increasing use (p < 0.001)
); *a significant trend for increasing use (p < 0.001)
Figure 3.
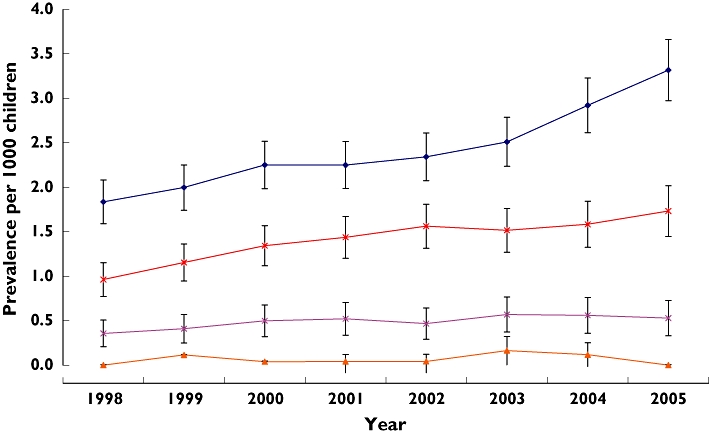
Prevalence of insulin use by age groups between 1998 and 2005 in the IMS DA (with 95% CIs). 0–1 years ( ); 2–5 years (
); 2–5 years ( ); 6–11 years* (
); 6–11 years* ( ); 12–18 years* (
); 12–18 years* ( ); *a significant trend for increasing use (p < 0.001)
); *a significant trend for increasing use (p < 0.001)
As insulin accounted for the majority of antidiabetic prescriptions, we validated its prevalence using the GPRD, where a total of 3466 subjects aged 0–18 years received insulin between 1998 and 2005. The data also show a steady increase amongst subjects aged 6–11 and 12–18 years, with a significant rise from 1.26 per 1000 children (95% CI 1.16, 1.50) to 1.83 per 1000 children (95% CI 1.75, 2.11) in the 6–11-year-old group and 2.52 per 1000 (95% CI 2.40, 2.88) to 3.43 per 1000 children (95% CI 3.39, 3.83) in the 12–18-year-old group (P < 0.001) (Figure 4).
Figure 4.
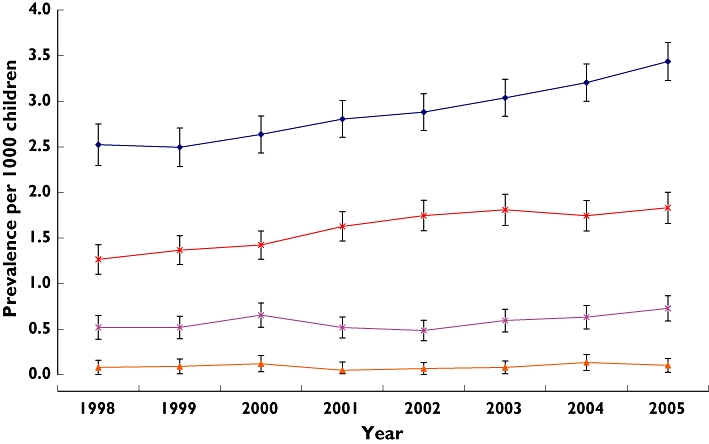
Prevalence of insulin use by age groups between 1998 and 2005 in the General Practice Research Database (GPRD) (with 95% CIs). 0–1 years ( ); 2–5 years (
); 2–5 years ( ); 6–11 years* (
); 6–11 years* ( ); 12–18 years* (
); 12–18 years* ( ); *a significant trend for increasing use (p < 0.001)
); *a significant trend for increasing use (p < 0.001)
Discussion
The main finding of this study is that the use of insulin and oral antidiabetic drugs in children and adolescents has steadily increased in the UK. The overall prevalence of insulin use has almost doubled in children and adolescents, rising from 1.08 to 1.98 per 1000 children between 1998 and 2005. The prevalence of oral antidiabetic drug prescribing shows almost an eightfold increase. This suggests that the prevalence of both type 1 and type 2 diabetes is increasing rapidly, particularly among adolescents.
To our knowledge, this is the first large paediatric study in the UK. The strengths of this study are that, first, insulin prescribing is an excellent proxy to estimate the prevalence of type 1 diabetes, as diagnosed patients are rarely left untreated. Second, compared with questionnaire studies, the study is population based and does not rely on responses from clinicians and, as such, is less prone to under-reporting. Third, the study consists of a cohort of over half a million children, allowing us to provide a good estimate of the prevalence of treated type 1 and type 2 diabetes. Fourth, vigorous steps have been taken to conduct external validation of IMS DA data using the GPRD. These two databases cover approximately 8% of the UK population. The analyses from both databases demonstrated similar results that support the veracity of the findings. Therefore, these findings can be generalized to UK practice.
The major limitation of the study is that we were unable to identify patients with type 2 diabetes treated with lifestyle modifications such as diet alone [27], and asymptomatic undiagnosed type 2 diabetes patients. As a result, our study is likely to have underestimated the prevalence of type 2 diabetes. In addition, neither database contains ethnicity information, so it is not possible to investigate whether the increase in diabetes treatment is associated with changes in UK demographic structure, such as the increase in the young non-White population, which is more predisposed to diabetes [28–30].
This study has demonstrated a steady increase in the prescribing of antidiabetic drugs in children and adolescents. This is in line with a study using prescription claims data in the USA, which has shown an increase in the prevalence of antidiabetic drug prescribing from 1.85 per 1000 children (aged 5–19 years) to 2.66 per 1000 between 2002 and 2005 [10]. Their study also revealed that the prevalence of antidiabetic drug prescribing among those aged 15–19 years was three times the rate seen in children aged 5–9 years, and 1.5 times that of children aged 10–14 years, which is the same trend as seen in our study.
In 1997, the American Diabetes Association (ADA) revised the diabetes diagnostic criteria [31]. This could have affected the antidiabetic drug prescribing. However the evidence suggested the revised ADA diagnostic criteria caused an underestimate in adult studies [32–33]. Thus, it is unlikely that the increase of antidiabetic drug prescribing was due to the revision of diagnostic criteria. In April 2004, the National Quality and Outcome Framework (QOF) was introduced as part of the new General Medical Service to improve quality of services to patients through GPs. However, the increased antidiabetic drug prescribing was observed before 2004. Thus, this cannot be the cause of the increased prescribing [34].
Recently, attention and debate has focused on the rise in type 2 diabetes in children due to obesity; therefore, it is not surprising that we have identified an increasing prevalence of children receiving oral antidiabetic drugs for diabetes treatment. However, the magnitude of increase was unexpected. Metformin was the most commonly prescribed oral antidiabetic drug in our study cohort (86%). After scrutinizing the medical records of those subjects who received metformin, it became apparent that 22.3% had the drug prescribed for PCOS. Although the absolute number and prevalence of patients in our study cohort treated with oral antidiabetic drugs for type 2 diabetes was low, it still indicates that there has been a significant increase over the past 8 years.
A recent paper published by the British Paediatric Surveillance Unit has shown that the incidence of non-type 1 diabetes was 1.3 per 100 000 per year (children aged <17 years) in 2004–2005. However, there were no previous incidence data to demonstrate the magnitude of increase. This study, which is probably the first of its kind in the UK, systematically demonstrates the extent of the increase in the prevalence in recent years from 0.006 per 1000 children in 1998 (95% CI 0.0043, 0.017) to 0.05 per 1000 children in 2005 (95% CI 0.025, 0.080) [35].
We acknowledge that the actual prevalence of type 2 diabetes will be underestimated because of the undiagnosed asymptomatic cases and those controlled with diet alone. When comparing the prevalence of treated type 2 diabetes in our study with the only previous prevalence study of childhood type 2 diabetes in the UK [36], our prevalence (1.9 per 100 000 children in 2000) is almost 10 times higher than the previously reported prevalence of 0.21 per 100 000 children (<16 years) in 2000. The reason for this difference may be because approximately 25% of paediatricians in the previous study did not respond to the questionnaire, leading to underestimation of the prevalence. Furthermore, our study covers children and adolescents up to 18 years of age, and adolescents aged between 16 and 18 years are more likely to develop type 2 diabetes.
The most unexpected finding was the steady increase in the prevalence of insulin use, especially in adolescents. One possible explanation is that type 2 diabetes patients received combination treatment, i.e. insulin and oral antidiabetic drugs. However, in our study there were only 26 patients receiving both insulin and oral antidiabetics. Due to this small number, it is very unlikely that the increase in insulin use is due to an increase in type 2 diabetes. This also demonstrates that few patients received combined treatment (insulin and oral antidiabetic drugs). This is in line with the UK National Institute for Clinical Excellence guideline, which suggests that children and young people with type 1 diabetes should not receive acarbose or sulphonylureas (e.g. glibenclamide, gliclazide, glipizide, tolazamide or glyburide) combined with insulin, as these drugs may increase the risk of hypoglycaemia [37].
A systematic review of type 1 diabetes incidence trends during the period 1960–1996 has shown a significant rise in incidence of childhood type 1 diabetes. The average annual increase was 3.0% (95% CI 2.60, 3.30) [2]. Similarly, a large European survey between 1989 and 1998 showed an annual increase of 3.2% (95% CI 2.70, 3.70) [38]. A US study demonstrated that the incidence of type 1 diabetes in children <18 years old in Colorado was 14.8 per 100 000 person-years in 1978–1988 and 23.9 in 2002–2004 [39]. A study from Australia has reported similar findings [40]. Taking all the above information into consideration, the incidence of type 1 diabetes has continuously risen up to the present time. Hence, there is compelling evidence that the increased insulin prescribing in our study reflects an increase in the incidence and prevalence of type 1 diabetes in children and adolescents. In addition, the study shows a steady increase in the prevalence of type 1 diabetes particularly amongst patients aged 12–18 years. This is in line with a US study in which the prevalence of diabetes was estimated on the basis of prescription claim data. Their results showed that the prevalence of diabetes in adolescents aged 15–19 years was three times higher than the rate amongst children aged 5–9 years [10]. In 2007, a study presented at the Diabetes UK conference showed that the incidence of type 1 diabetes in children aged <15 years almost doubled between 1985 and 2004 in the Oxfordshire region and that the largest rise was amongst children <5 years old [41]. The authors suggested that this might be because the peak age of diabetes diagnosis is becoming younger in the UK. It should be noted that this study did not investigate children aged <15 years, which may explain why a steep rise in adolescents was not identified as shown in our study.
Conclusions
Our results show a marked rise in the use of insulin and oral antidiabetic drugs in children and adolescents in the UK over an 8-year period. The overall prevalence of insulin use almost doubled in children and adolescents, rising from 1.08 to 1.98 per 1000 children between 1998 and 2005. The prevalence of oral antidiabetic drugs shows an almost eightfold increase. This suggests that the prevalence of both type 1 and type 2 diabetes is increasing rapidly, particularly amongst adolescents. As the actual causes for this increased trend remain unclear, further large-scale epidemiological studies are urgently required. Furthermore, most previous studies have included only children up to age 15 or 16 years. We recommend that further studies should include the older adolescent, as this can provide more in-depth understanding of diabetes management in this group.
Competing interests
None declared
REFERENCES
- 1.Fagot-Campagna A. Emergence of type 2 diabetes mellitus in children: epidemiological evidence. J Pediatr Endocrinol Metab. 2000;13(Suppl. 6):1395–402. doi: 10.1515/jpem-2000-s613. [DOI] [PubMed] [Google Scholar]
- 2.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–61. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman FR. Type 2 diabetes mellitus in children and youth: a new epidemic. J Pediatr Endocrinol Metab. 2002;15(Suppl. 2):737–44. doi: 10.1515/JPEM.2002.15.s2.737. [DOI] [PubMed] [Google Scholar]
- 4.Libman IM, Songer T, LaPorte R. How many people in the U.S. have IDDM? Diabetes Care. 1993;16:841–2. doi: 10.2337/diacare.16.5.841. [DOI] [PubMed] [Google Scholar]
- 5.Karvonen M, Viik-Kajander M, Moltchanova E, Libman IM, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (Diamond) Project Group. Diabetes Care. 2000;23:1516–26. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 6.Writing Group for the SEARCH for Diabetes in Youth Study Group. Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 7.The DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 8.Royal College of Nursing Diabetes. Good Practice. [8 May 2008]; Available at http://www.rcn.org.uk/development/practice/diabetes/good_practice/children.
- 9.National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005. Bethesda, MD: Department of Health and Human Services, National Institute of Health; 2005. [Google Scholar]
- 10.Cox E, Mager D, Behm A, Miller S. 2002–2005 Trends in the Prevalence of Anti-diabetic Drug Therapy in Children Age 5 Years to 19 Years. 2006. pp. 1–4. St Louis, MO: Express Scripts, Inc.
- 11.Forrest RD. Diabetes mellitus in north Sweden: prevalence assessed from prescriptions for anti-diabetic agents. J Intern Med. 1990;228:267–73. doi: 10.1111/j.1365-2796.1990.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Staff AC, Baksaas I. An estimation of the prevalence of diabetes mellitus in Norway. A prescription registration study. Scand J Prim Health Care. 1988;6:233–7. doi: 10.3109/02813438809009323. [DOI] [PubMed] [Google Scholar]
- 13.Stovring H, Andersen M, Beck-Nielsen H, Green A, Vach W. Rising prevalence of diabetes: evidence from a Danish pharmaco-epidemiological database. Lancet. 2003;362:537–8. doi: 10.1016/S0140-6736(03)14116-5. [DOI] [PubMed] [Google Scholar]
- 14.Strom BL. Other approaches to pharmacoepidemiology studies. In: Strom BL, Kimmel SE, editors. Textbook of Pharmacoepidemiology. Philadelphia, PA: John Wiley & Sons, Ltd; 2006. pp. 224–5. [Google Scholar]
- 15.European Pharmaceutical Market Research Association. ATC – Anatomical Classification. [8 May 2008]; Available at http://www.ephmra.org/main.asp?page=1290.
- 16.International Statistical Classification of Diseases and Health Problems, 10th revision. Geneva: World Health Organisation; [8 May 2008]. Available at http://www.who.int/classifications/icd/en/ [Google Scholar]
- 17.Wong IC, Murray ML. The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol. 2005;59:750–5. doi: 10.1111/j.1365-2125.2005.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langman M, Kahler KH, Kong SX, Zhang Q, Finch E, Bentkover JD, Stewart EJ. Drug switching patterns among patients taking non-steroidal anti-inflammatory drugs: a retrospective cohort study of a general practitioners database in the United Kingdom. Pharmacoepidemiol Drug Saf. 2001;10:517–24. doi: 10.1002/pds.653. [DOI] [PubMed] [Google Scholar]
- 19.Murray ML, Thompson M, Santosh PJ, Wong IC. Effects of the committee on safety of medicines advice on antidepressant prescribing to children and adolescents in the UK. Drug Saf. 2005;28:1151–7. doi: 10.2165/00002018-200528120-00009. [DOI] [PubMed] [Google Scholar]
- 20.Lawrenson R, Williams T, Farmer R. Clinical information for research; the use of general practice databases. J Public Health Med. 1999;21:299–304. doi: 10.1093/pubmed/21.3.299. [DOI] [PubMed] [Google Scholar]
- 21.The European Medicines Agency (EMEA) Clinical Investigation of Medicinal Products in the Paediatric Population. [8 May 2008]. Available at http://www.emea.europa.eu/pdfs/human/ich/271199en.pdf.
- 22.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 23.Murray ML, de Vries CS, Wong ICK. A drug utilisation study of antidepressants in children and adolescents using the General Practice Research Database. Arch Dis Child. 2004;89:1098–102. doi: 10.1136/adc.2004.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackers R, Murray ML, Besag FMC, Wong ICK. Prioritising children's medicines for research: a pharmacoepidemiological study on antiepileptic drugs. Br J Clin Pharmacol. 2007;63:689–97. doi: 10.1111/j.1365-2125.2006.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rani F, Murray M, Byrne P, Wong ICK. Epidemiological features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics. 2008;121:1002–9. doi: 10.1542/peds.2007-2008. [DOI] [PubMed] [Google Scholar]
- 26.Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong ICK. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study using the UK General Practice Research Database. Pediatrics. 2008 doi: 10.1542/peds.2007-3349. in press. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbloom AL. Increasing incidence of type 2 diabetes in children and adolescents: treatment considerations. Paediatr Drug. 2002;4:209–21. doi: 10.2165/00128072-200204040-00001. [DOI] [PubMed] [Google Scholar]
- 28.Coleman D, Scherbov S. Immigration and ethnic change in low-fertility countries – towards a new demographic transition? Popul Dev Rev. 2006;32:401–46. [Google Scholar]
- 29.Lee JM, Herman WH, McPheeters ML, Gurney JG. An epidemiologic profile of children with diabetes in the U.S. Diabetes Care. 2006;29:420–1. doi: 10.2337/diacare.29.02.06.dc05-2182. [DOI] [PubMed] [Google Scholar]
- 30.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Evidence for heterogeneous pathogenesis of insulin-treated diabetes in black and white children. Diabetes Care. 2003;26:2876–82. doi: 10.2337/diacare.26.10.2876. [DOI] [PubMed] [Google Scholar]
- 31.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 32.Ko GT, Chan JC, Woo J, Cockram CS. Use of the 1997 American Diabetes Association diagnostic criteria for diabetes in a Hong Kong Chinese population. Diabetes Care. 1998;21:2094–7. doi: 10.2337/diacare.21.12.2094. [DOI] [PubMed] [Google Scholar]
- 33.Costa B, Franch J, Martín F, Morató J, Donado A, Basora J, Daniel J. Impact of the American Diabetes Association diagnosis criteria on high-risk Spanish population. Diabetes Res Clin Pract. 1999;46:75–81. doi: 10.1016/s0168-8227(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 34.The Quality and Outcomes Framework (QOF) QOF guidance. [3 September 2008]; Available at http://www.dh.gov.uk/en/Healthcare/Primarycare/Primarycarecontracting/QOF/index.htm.
- 35.Haines L, Wan KC, Lynn R, Barrett TG, Shield JP. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30:1097–101. doi: 10.2337/dc06-1813. [DOI] [PubMed] [Google Scholar]
- 36.Ehtisham S, Hattersley AT, Dunger DB, Barrett TG. First UK survey of paediatric type 2 diabetes and MODY. Arch Dis Child. 2004;89:526–9. doi: 10.1136/adc.2003.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute for Clinical Excellence. Type 1 diabetes: diagnosis and management of type 1 diabetes in children, young people and adults. [9 September 2008]; Available at http://www.nice.org.uk/nicemedia/pdf/CG015NICEguideline.pdf.
- 38.Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001;44(Suppl. 3):B3–8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 39.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, Rewers M, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–9. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 40.O’Connell MA, Donath S, Cameron FJ. Major increase in Type 1 diabetes—no support for the Accelerator Hypothesis. Diabet Med. 2007;24:920–3. doi: 10.1111/j.1464-5491.2007.02203.x. [DOI] [PubMed] [Google Scholar]
- 41.Diabetes UK. Type 1 rises in under-fives. [8 May 2008]; Available at http://www.diabetes.org.uk/About_us/News_Landing_Page/Type-1-rises-in-under-fives/


