Voriconazole has become the drug of first choice to treat invasive fungal infections (IFI) [1]. Standard intravenous dosing of voriconazole consists of an infusion of a dose of 6 mg kg–1 b.i.d. on day 1 followed by a maintenance dose of 4 mg kg–1. Voriconazole is metabolized by the liver, by CYP2C19, CYP2C9 and CYP3A4 enzymes, with renal excretion of the metabolites. The elimination half-life of voriconazole is approximately 6 h. As the capacity of the CYP isoenzymes is limited, the metabolism of voriconazole can be saturated [2]. The observed large inter- and intrapatient variation of its pharmacokinetics [3], the possible relationship between therapeutic failure and low serum levels [3–5], and the relation of high serum levels with adverse effects [6, 7] have resulted in the advice to monitor serum levels. How to adjust the dosage to improve therapy is unfortunately not described.
We report our experience with therapeutic monitoring of voriconazole in 20 patients with haematological malignancies receiving voriconazole for the treatment of proven or suspected IFI. In most of these patients multiple samples (trough levels) were drawn for pharmacokinetic evaluation of voriconazole concentrations over time. It has been recommended that trough levels should be 1–2 mg l–1 and not exceed 5 mg l–1[8]. In 11 of the 20 patients (55%), a mean trough concentration of 2.7 mg l–1 (range 1.6–4.1 mg l–1) was observed, i.e. within the desired range. In three of the 20 patients (15%) a mean trough concentration below the therapeutic range was observed (mean 0.6 mg l–1, range 0.2–0.9 mg l–1). In six of the 20 patients (30%), however, standard intravenous dosing led to trough concentrations 5 mg l–1 (mean 7.2 mg l–1; range 5.4–8.8 mg l–1), which is the presumed toxic range. High voriconazole concentrations occurred within 2–3 days after the start of therapy. As voriconazole is mainly metabolized by the liver, we focused our evaluation on the possibility of voriconazole metabolism influencing factors such as CYP isoenzymes and concomitant medication. The hepatic function in relation to medication, chemotherapy and parenteral nutrition were evaluated from 1 week before the start of until 2 weeks after stopping voriconazole therapy. In five of the six patients with a trough level 5 mg l–1, mild pre-existing liver dysfunction was present at the start of voriconazole. Mild hepatic dysfunction was characterized by a bilirubin level of five times > upper level of normal (ULN) and at least two of the following: alkaline phosphatase (ALP), aspartate aminotransferase (ASAT), alanine transaminase (ALAT), gamma glutamyl transpeptidase, with a level of three times > ULN. In these cases, the high voriconazole level seemed to result from, rather than being the cause of, the liver dysfunction. Only in one patient was acute deterioration of hepatic liver tests observed, probably caused by voriconazole since the Naranjo score was 8 (where 5–8 is probable, and 9 is definite) [9]. The hepatic dysfunction displayed a characteristic pattern of a strong rise in ASAT/ALAT level combined with a smaller and enduring increase in ALP level, pointing to acute drug-induced liver toxicity [10]. In five of these six patients, CYP2C19 and CYP2C9 isoenzymes were determined, but in only one patient was an intermediate CYP2C19 isoenzyme for reduced metabolism detected. In none of the six patients could any drug–drug interaction be noted between co-medication and voriconazole. In patients presenting with a trough level of 5 mg l–1, voriconazole was stopped. Strikingly, an exponentially increased voriconazole elimination half-life of 77.6 h (range 46.8–99.4 h) was calculated [11] in patients presenting with a voriconazole trough level of 5 mg l–1, compared with the commonly observed half-life of 6–8 h [12]. Our results show that voriconazole could be stopped for at least two consecutive days. Meanwhile, serum monitoring continued, and voriconazole levels slowly decreased to levels within the therapeutic window (Figure 1). Voriconazole was then restarted successfully using a lower dosage and monitoring continued in those patients who needed continued therapy. Eventually the dosage had to be increased stepwise in two patients to maintain levels within the therapeutic window. The nonlinear pharmacokinetics of voriconazole combined with decreased hepatic function is probably the most logical explanation of these high serum voriconazole levels and increased elimination half-lives. It seems logical that voriconazole metabolism is reduced above a certain serum threshold level (limited capacity of hepatic clearance), compatible with a saturation mechanism for enzymatic degradation [13]. In our experience patients presenting with multiple liver enzyme values of three to five times ULN are ‘at risk’ of developing high voriconazole trough levels. The observations suggest that routine monitoring of voriconazole levels in patients with haematological malignancies after 3 days of therapy is necessary to prevent voriconazole adverse effects caused by toxic levels.
Figure 1.
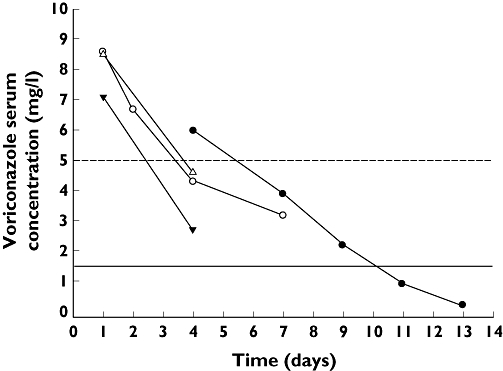
Voriconazole concentration over time after stopping of voriconazole. Dosage of voriconazole at time of stopping is displayed. Case 1 was a 55-year-old man with diffuse large B-cell lymphoma who received empiric stepping-up after not responding to antibacterial drugs for febrile neutropenia and recovered uneventfully. Case 2 was a 61-year-old woman with relapsed acute myeloid leukaemia (AML) who was suspected of having fungal pneumonia during a consolidation course. Case 3 was a 55-year-old woman with AML who developed febrile neutropenia and pulmonary infiltrates on X-rays and a positive galactomannan. Case 4 was a 42-year-old man who developed fungal pneumonia during aplasia, initially responding to antifungal therapy, but who succumbed later due to pulmonary bleeding. case 1 (4 mg/kg b.i.d. IV) ( ); case 2 (4 mg/kg b.i.d. IV) (
); case 2 (4 mg/kg b.i.d. IV) ( ); case 3 (3 mg/kg b.i.d. IV) (
); case 3 (3 mg/kg b.i.d. IV) ( ); case 4 (3 mg/kg b.i.d. IV) (
); case 4 (3 mg/kg b.i.d. IV) ( ); uppel level (
); uppel level ( ); lower level (
); lower level ( )
)
Acknowledgments
The authors thank Pfizer (USA) for kindly providing the purified voriconazole powder.
REFERENCES
- 1.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 2.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 3.Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50:1570–2. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34:563–71. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 5.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–11. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 6.Imhof A, Schaer DJ, Schanz U, Schwarz U. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med Wkly. 2006;136:739–42. doi: 10.4414/smw.2006.11547. [DOI] [PubMed] [Google Scholar]
- 7.Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006;46:235–43. doi: 10.1177/0091270005283837. [DOI] [PubMed] [Google Scholar]
- 8.Bruggemann RJ, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NM, Mouton JW, Verweij PE, Burger DM. Therapeutic drug monitoring of voriconazole. Ther Drug Monit. 2008;30:403–11. doi: 10.1097/FTD.0b013e31817b1a95. [DOI] [PubMed] [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 10.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050–68. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proost JH, Meijer DK. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med. 1992;22:155–63. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- 12.Purkins L, Wood N, Greenhalgh K, Eve MD, Oliver SD, Nichols D. The pharmacokinetics and safety of intravenous voriconazole—a novel wide-spectrum antifungal agent. Br J Clin Pharmacol. 2003;56(Suppl)(1):2–9. doi: 10.1046/j.1365-2125.2003.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649–63. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]


