Abstract
Background information
During embryonic development, β-catenin is central both to the transcriptional activation of Wnt [wingless-type MMTV (murine-mammary-tumour virus) integration site family] target genes and as a mediator of cell–cell adhesion. Signals that regulate its levels and subcellular localization are critical. One mechanism of Wnt signalling results in stabilization of β-catenin protein, which leads to its translocation into the nucleus, where it interacts with TCF (T-cell factor, HMG box) and activates transcription of target genes. Less well understood are mechanisms of Wnt signalling that do not involve β-catenin stabilization and result in inhibition of β-catenin-mediated transcription.
Results
Here, we show that a member of the Wnt protein family, Wnt4 (Wnt, member 4), regulates the subcellular localization of β-catenin, redirecting it to the cell membrane. Unique among Wnts, this action does not affect the stability of β-catenin but does prohibit its involvement in TCF gene transactivation.
Conclusions
This novel mechanism suggests that Wnt4 acts as a switch between the two modes of β-catenin function, transcriptional activation and cell–cell adhesion.
Keywords: β-catenin, murine-mammary-tumour virus (MMTV), subcellular localization, T-cell factor (TCF), wingless-type MMTV integration site family, member 4 (Wnt4)
Introduction
The importance of Wnt [wingless-type MMTV (murine-mammary-tumour virus) integration site family] signalling in many adult and developmental processes, such as gastrulation, axis formation, cell polarity, organ development and maintenance of stem cell pluripotency, is widely acknowledged (Cadigan and Nusse, 1997; Arce et al., 2006). Wnt signalling is complex; 19 mammalian Wnt genes have been cloned, and more than ten membrane receptors and a plethora of cofactors and regulators are known. Different mechanisms of Wnt signalling have been identified. The best understood of these is the ‘canonical’ pathway, in which β-catenin transduces the Wnt signal to the nucleus (Gordon and Nusse, 2006). In the absence of Wnt signal, β-catenin forms part of a cytosolic protein complex, which includes Axin (axis inhibition protein), APC (adenomatosis polyposis coli), CK1α (casein kinase 1α) and GSK3β (glycogen synthase kinase 3β). CK1α and GSK3β phosphorylate β-catenin at specific serine and threonine residues, targeting it for ubiquitination by the βTrCP (β-transducin repeat containing protein) and subsequent proteasomal degradation. Activation of the canonical Wnt pathway occurs when a Wnt protein binds to its cognate membrane receptor complex, triggering a cascade of intracellular events that lead to the inhibition of GSK3β-mediated β-catenin phosphorylation (Lustig and Behrens, 2003; Brembeck et al., 2006). Thus stabilized (non-phosphorylated) β-catenin accumulates and is translocated into the nucleus, where it interacts with transcription factors, such as TCF (T-cell factor, HMG box), to activate target genes.
In ‘non-canonical’ Wnt signalling, pathways activated by Wnt proteins do not lead to β-catenin stabilization or β-catenin-mediated gene transactivation (Kuhl et al., 2000b; Veeman et al., 2003). There are various reports as to the mechanism by which such pathways are activated. For example, several studies have found that Wnt5A (Wnt, member 5A) signal increases cellular calcium influx, which activates Ca2+-dependent kinases such as CaMKII (Ca2+/calmodulin-dependent protein kinase II) and PKC (protein kinase C) (Slusarski et al., 1997; Sheldahl et al., 1999; Kuhl et al., 2000a). However, a recent report has dismissed the involvement of a Ca2+-mediated pathway, showing that Wnt5A does not alter intracellular concentrations of Ca2+ in HEK-293 cells (human embryonic kidney cells) (Mikels and Nusse, 2006a). In addition to activating its own pathway, Wnt5A can inhibit canonical Wnt signalling, but again, the mechanism remains elusive. It has been proposed that Wnt5A inhibits β-catenin stabilization, resulting in its degradation (Topol et al., 2003), or inhibits TCF-mediated transcription downstream of β-catenin (Ishitani et al., 1999).
Historically, Wnt proteins were classified as either canonical, such as Wnt1 and Wnt3A, or non-canonical, including Wnt4 (Wnt, member 4), Wnt5A and Wnt11 (Moon et al., 1993; Du et al., 1995). The recent characterization of Fzd (frizzled homologue receptor), LRPs (low-density lipoprotein receptor-related protein) and other receptors has challenged this either/or classification of individual Wnt proteins. Evidence suggests that Wnt5A, for example, may activate the canonical pathway or inhibit it, depending on the receptor context (Mikels and Nusse, 2006b). Accordingly, the terms ‘canonical’ and ‘non-canonical’ are used here to indicate molecular mechanisms, not specific Wnt proteins.
In addition to its role in gene transactivation, β-catenin also functions at the cell membrane, where it reversibly links cadherins to α-catenin and to the actin cytoskeleton to form adherens junctions, which are essential for cell–cell adhesion and cell migration (Nelson and Nusse, 2004; Brembeck et al., 2006). One regulator of the switch between β-catenin adhesion and transcriptional functions is the phosphorylation of Tyr142 of β-catenin, which prevents binding of β-catenin to α-catenin, disrupts adherens junction formation and promotes β-catenin transcriptional action (Brembeck et al., 2004).
Wnt4, initially defined as having non-canonical action, is essential for axonal development (Lyuksyutova et al., 2003), proliferation of progenitor cells in the pituitary (Treier et al., 1998) and formation of the zona glomerula in the adrenal cortex (Heikkila et al., 2002). Wnt4 is necessary for initiation of duct formation in the kidney, and Wnt4-null mutant mice die perinatally, probably due to kidney failure (Stark et al., 1994; Kispert et al., 1998). Wnt4 is also involved in gonadogenesis and female mice lacking Wnt4 are masculinized (Vainio et al., 1999). In developing ovaries, Wnt4 prevents migration of endothelial cells from the mesonephros that, in males, participate in formation of the male-specific coelomic blood vessel (Martineau et al., 1997; Brennan et al., 2002; Jeays-Ward et al., 2003; Jordan et al., 2003). Moreover, Wnt4 inhibits synthesis of androgen in the female gonad; testosterone is produced ectopically in Wnt4-null mice and is reduced in the testes of male mice overexpressing Wnt4 (Jordan et al., 2003; Heikkila et al., 2005). Previously, we showed that Wnt4 represses β-catenin-mediated gene transcription by disrupting recruitment of β-catenin at, or near, SF1 (steroidogenic factor 1)/NR5A1 (nuclear receptor subfamily 5, group A, member 1)-binding sites found in multiple steroidogenic gene promoters (Jordan et al., 2003). However, the molecular mechanism by which Wnt4 inhibits the recruitment of β-catenin to a target gene remains unknown.
Here, we show that Wnt4 signals through a novel pathway, which antagonizes canonical Wnt signalling by redirecting β-catenin to the cell membrane in vitro. Using Wnt4-null mice, we also found that, downstream of the Wnt4 signal, β-catenin is strongly associated with the membrane in the developing ovary. Wnt4 signal therefore acts as a switch between the transcriptional and membrane functions of β-catenin.
Results
Wnt4 inhibits canonical Wnt signalling
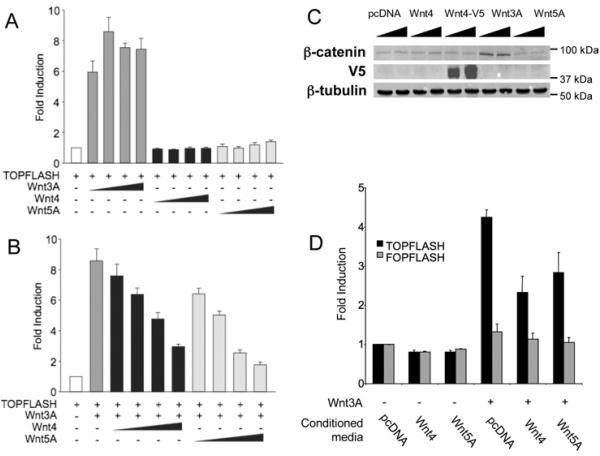
TCF transcriptional activation via canonical Wnt signalling can be assayed in HEK-293T cells [HEK-293 cells expressing the large T-antigen of SV40 (simian virus 40)] using a luciferase-driven reporter, TOPFLASH (Korinek et al., 1997). To investigate Wnt4 function in this context, we first compared its activity with that of Wnt3A, a known activator of the canonical pathway, and with Wnt5A, a known inhibitor (Mikels and Nusse, 2006a). Increasing concentrations of expression plasmids encoding Wnt4, Wnt3A or Wnt5A were co-transfected with the reporter (Figure 1A). Wnt3A expression activated transcription of the reporter 8.6-fold compared with the control, as expected for an activator of the canonical pathway (Figure 1A, dark grey bars). Wnt5A, on the other hand, did not activate the reporter, even at high doses (Figure 1A, light grey bars). Similarly, cells expressing Wnt4 did not show increased transcription of the reporter, indicating that Wnt4 does not activate canonical Wnt signalling in these cells (Figure 1A, black bars). Wnt3A, Wnt4 and Wnt5A did not activate the mutant TOPFLASH reporter, FOPFLASH (results not shown), confirming the specificity of the assay (Korinek et al., 1997).
Figure 1. Wnt4 inhibits the canonical Wnt pathway.
(A) Wnt4 and Wnt5A do not activate the canonical Wnt pathway. Cells were co-transfected with TOPFLASH reporter plasmid (200 ng) and an increasing concentration of Wnt4, Wnt3A or Wnt5A expression plasmid (10, 40, 160 and 640 ng). (B) Wnt4 and Wnt5A inhibit Wnt3A-mediated signalling. Cells were co-transfected with TOPFLASH reporter plasmid (200 ng), Wnt3A expression plasmid (40 ng) to activate the canonical Wnt pathway and an increasing concentration of Wnt4 or Wnt5A expression plasmid (10, 40, 160 and 640 ng). (C) Wnt3A, but not Wnt4 and Wnt5A, affects β-catenin levels in cytoplasmic cell extracts. Cells were co-transfected with 500 ng and 1 μg of pcDNA or expression plasmids for Wnt4, Wnt3A and Wnt5 and β-catenin levels were assayed in Western blot on 10 μg of extract. Wnt4 expression was monitored by using V5-tagged Wnt4 plasmid (Wnt4–V5). Expression of β-tubulin was used as a loading control. (D) Media conditioned with Wnt4 and Wnt5A protein inhibit Wnt3A signalling. Cells were co-transfected with TOPFLASH reporter plasmid (100 ng) and Wnt3A (160 ng) and treated with conditioned media prepared from cells transfected with 1 μg of either pcDNA, Wnt4 or Wnt5A. Activities are expressed as fold activation of TOPFLASH activity in the presence of Wnt expression plasmid over that of empty vector. Results of each experiment are presented as the means±S.E.M. for three independent transfections.
To test whether Wnt4 not only fails to activate the canonical Wnt pathway but also inhibits it, we co-transfected a constant amount of Wnt3A with increasing doses of Wnt4 or Wnt5A (Figure 1B). Activation of the canonical pathway, up to 8.6-fold, was achieved when Wnt3A alone was transfected (Figure 1B, dark grey bar). Wnt5A inhibited this activation in a dose-dependent manner (Figure 1B, light grey bars), and a similar dose-dependent repression was observed with Wnt4 (Figure 1B, black bars). This indicates that Wnt4, like Wnt5A, inhibits the canonical Wnt pathway in HEK-293T cells.
Activation of the canonical Wnt pathway inhibits proteasomal degradation of cytosolic β-catenin, resulting in increased levels of β-catenin (Mikels and Nusse, 2006a). To assess the effect of Wnt4 signal on the levels of β-catenin protein, we prepared cytoplasmic cell extracts from HEK-293T cells transfected with Wnt3A, Wnt4 or Wnt5A and performed Western-blot analyses using an antibody against β-catenin (Figure 1C). As expected, increased levels of β-catenin were observed in cells expressing Wnt3A (Figure 1C, top panel), probably reflecting a stabilization of the protein through inhibition of its phosphorylation by the canonical path (Mikels and Nusse, 2006a). No noticeable change in β-catenin levels was observed in the presence of Wnt4 or Wnt5A when compared with control (Figure 1C, top panel). To demonstrate that Wnt4 protein is produced, V5 epitope-tagged Wnt4 was transfected in HEK-293T cells. Western-blot analysis showed that Wnt4 migrates at 40 kDa, which corresponds to its predicted molecular mass (Figure 1C, middle panel). Wnt4–V5 displayed the same activity as the Wnt4 expression plasmid in the TOPFLASH assay (results not shown). Unlike the action of Wnt3A, Wnt4 and Wnt5A do not appear to increase the cytoplasmic level of β-catenin.
To test the specificity of Wnt4 action, we transfected HEK-293T cells with Wnt3A to activate canonical Wnt signalling and stimulated the cells with conditioned medium from cells transfected with either pcDNA, Wnt4 or Wnt5A (Figure 1D). Both Wnt5A and Wnt4 conditioned media were able to reduce TOPFLASH activity, indicating that active Wnt proteins were produced, secreted and capable of signalling. In summary, Wnt5A and Wnt4 do not activate, but inhibit TCF-mediated transcription, and the mechanism of action maintains a low level of cytoplasmic β-catenin.
Inhibition of canonical Wnt signalling by Wnt4 is independent of CK1α or GSK3β activity
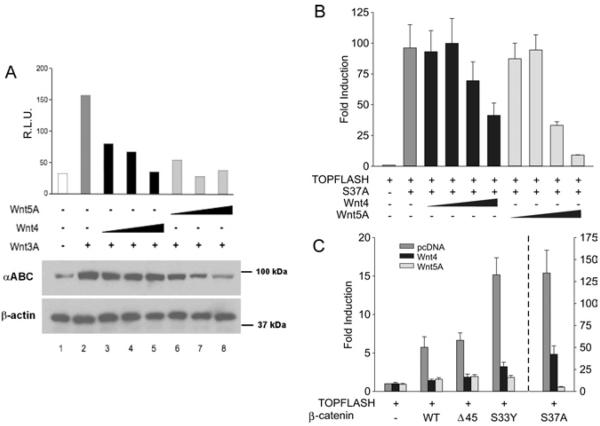
Because stimulation of canonical Wnt signalling leads to stabilization of β-catenin through inhibition of its phosphorylation, we next tested whether Wnt4 signal could modify the level of β-catenin phosphorylation in cells stimulated by a canonical Wnt signal (Figure 2A). To do this, we used an antibody that only recognizes β-catenin that is not phosphorylated at residues Ser37 and Thr41, two of the sites that are phosphorylated by GSK3β in the absence of canonical signal (van Noort et al., 2002). As expected, transfection with Wnt3A alone resulted in increased levels of non-phosphorylated β-catenin protein in total cell extracts (Figure 2A, lane 2), and the expected increase in TOPFLASH reporter activity (Figure 2A, upper panel, dark grey bar). Increasing doses of Wnt5A co-transfected with a constant amount of Wnt3A resulted in a gradual decrease in the amount of non-phosphorylated β-catenin induced by Wnt3A (Figure 2A, lanes 6–8) and a concomitant reduction in TOPFLASH reporter activity (Figure 2A, upper panel, light grey bars), indicating inhibition of the canonical Wnt pathway as previously observed (Topol et al., 2003). Contrary to the action of Wnt5A, increasing doses of Wnt4 did not change the level of β-catenin Ser37/Thr41 phosphorylation (Figure 2A, lanes 3–5), although TOPFLASH reporter activity was again inhibited (Figure 2A, upper panel, black bars). These results suggest that Wnt4 uses a different mechanism than Wnt5A to inhibit the canonical Wnt pathway.
Figure 2. Wnt4 inhibits canonical Wnt signalling independently of GSK3β activity.
(A) Wnt4 does not change the phosphorylation status of β-catenin. Cells were co-transfected with TOPFLASH reporter plasmid (200 ng), Wnt3A expression plasmid (40 ng) and an increasing concentration of Wnt4 or Wnt5A expression plasmid (160, 640 and 1280 ng). Lower panels: 10 μg of the cell extracts was used for Western-blot detection of stabilized (ABC) β-catenin. Actin detection was used as a loading control. Upper panel: the remaining extract from each transfection was used to measure luciferase activity normalized to β-galactosidase activity (RLU, relative luciferase units). (B) Cells were co-transfected with TOPFLASH reporter plasmid (200 ng), S37A β-catenin mutant expression plasmid (10 ng) and an increasing concentration of Wnt4 or Wnt5A expression plasmid (10, 40, 160 and 640 ng). (C) Cells were co-transfected with TOPFLASH reporter plasmid (200 ng); pcDNA 3.1 control vector, or mutant (S33Y, Δ45 or S37A) or wild-type (WT) β-catenin expression plasmid (10 ng); and Wnt4 or Wnt5A expression plasmid (1280 ng). Activities are expressed as fold activation of TOPFLASH activity in the presence of S37A β-catenin mutant expression plasmid over that of empty vector. Results of each experiment are presented as the means±S.E.M. for three independent transfections.
Since Wnt4 action did not decrease the levels of Ser37/Thr41-phosphorylated β-catenin produced by canonical signalling, we tested whether its mechanism involves inhibition of GSK3β or CK1α. Mutant forms of β-catenin have been identified in cancer patients, where β-catenin is stabilized through mutations at serine/threonine residues, such as Ser37, Ser33 and Thr41, normally phosphorylated by GSK3β, or Ser45, normally phosphorylated by CK1α, leading to ectopic activation of canonical Wnt signalling target genes (Rubinfeld et al., 1997; Liu et al., 2002). Co-transfection of a Ser37 β-catenin mutant (S37A) and the TOPFLASH reporter into HEK-293T cells led to strong transcriptional activation, over 90-fold, of the reporter (Figure 2B, dark grey bar). Increasing the concentrations of either Wnt4 or Wnt5A led to a reduction in S37A-mediated activation (Figure 2B, black bars and light grey bars respectively). Therefore inhibition of canonical Wnt signalling by Wnt4 and Wnt5A does not involve modulation of GSK3β kinase activity, since this enzyme cannot phosphorylate the S37A mutant. To confirm these observations, we co-transfected two other naturally occurring phosphorylation mutants of β-catenin with TOPFLASH: Δ45, which cannot be phosphorylated by CK1α, and S33Y, which cannot be phosphorylated by GSK3β (Figure 2C). Each of these mutant β-catenin proteins showed an increase in luciferase activity when compared with cells that were transfected with TOPFLASH alone (Figure 2C, dark grey bars). When Wnt4 or Wnt5A was co-transfected with the mutant β-catenin plasmids, we observed a strong reduction in luciferase activity, indicating an inhibition of the canonical Wnt pathway (Figure 2C, dark bars and light grey bars respectively). Together, these results indicate that Wnt4 inhibits canonical Wnt signalling by a mechanism that is independent of the serine/threonine phosphorylation events triggered by GSK3β or CK1α.
Wnt4 relocates β-catenin to the cell membrane
β-Catenin has been localized to three cell compartments: the cytoplasm, the nucleus and the cell membrane (Nelson and Nusse, 2004; Brembeck et al., 2006). We examined the subcellular localization of β-catenin downstream of Wnt signal by stably transfecting HEK-293 cells with pcDNA, Wnt4 or Wnt3A. The use of stably transfected cell lines allows a more robust and homogeneous signal compared with the variability often observed in transient transfection. We prepared cytoplasmic and membrane extracts from these clones and analysed β-catenin levels in the two compartments (Figure 3A). Purity of the fractions was estimated by measuring the amount of Hspa5 (heat-shock 70 kDa protein 5) in the membrane fraction (Figure 3A, upper panel, Hspa5) and β-tubulin in the cytosol (Figure 3A, upper panel, β-tubulin). Cytosolic β-catenin was barely detectable in the control cell line and was strongly increased by Wnt3A overexpression, while a very modest but significant increase was observed by overexpression of Wnt4 construct (Figure 3A, upper panel, Cytosol, and the graphical representation). Membrane-bound β-catenin was increased in both Wnt4- and Wnt3A-expressing cells when compared with the control cell line (Figure 3A, upper panel, Membrane, and the graphical representation). These results suggest that the increased membrane levels of β-catenin after Wnt3A stimulation may be a direct consequence of increased cytosolic levels of β-catenin, whereas the slight increase in cytosolic β-catenin levels upon Wnt4 stimulation cannot explain the strong increase in β-catenin levels at the cytoplasmic membrane. Altogether, this indicates that Wnt4 action specifically triggers β-catenin relocalization to the cytoplasmic membrane.
Figure 3. Wnt4 redirects β-catenin to the cell membrane in vitro and in vivo.
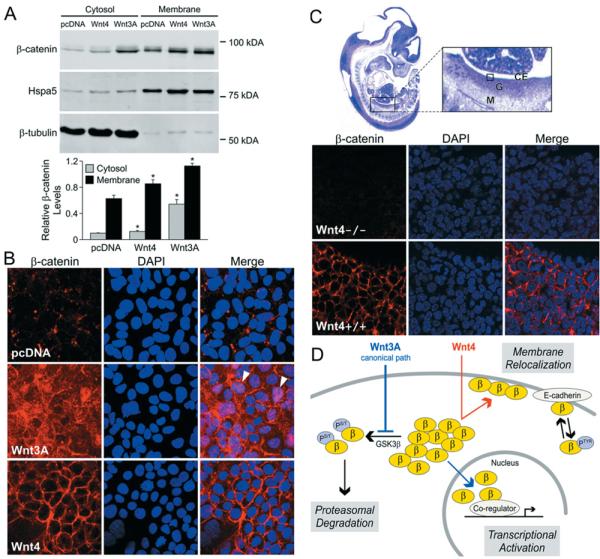
(A) Wnt4 increases β-catenin levels at the cytoplasmic membrane. Immunoblotting of β-catenin in subcellular fractions prepared from stably transfected cell lines. Hspa5, marker of membrane fraction, and β-tubulin, marker of cytosolic fraction, were used as purity and loading controls. Densitometric analysis is shown below and corresponds to three independent experiments. Relative β-catenin levels correspond to the ratio between β-catenin protein levels and the protein level for the matching fraction (Hspa5 for the membrane fraction or β-tubulin for the cytosolic fraction). Error bars represent the S.E.M. values. Two-tailed t test of paired sample means was performed between the control cell line (pcDNA) and Wnt-expressing cell line (Wnt4 or Wnt3A). *P < 0.05. (B) Immunofluorescence assay of β-catenin localization in cells stably transfected with empty vector pcDNA, Wnt3A or Wnt4. Arrowheads indicate nuclear staining; DAPI (4′,6-diamidino-2-phenylindole), nuclear stain. (C) Immunofluorescence detection shows differential localization of β-catenin in Wnt4−/− compared with wild-type (Wnt4+/+) 12.5 dpc mouse ovaries. The boxed area within the enlarged image of the haematoxylin/eosin-stained full embryo section indicates the region depicted in the lower panels. Abbreviations: CE, coelomic epithelium of gonad; G, gonad; M, mesonephros. (D) Model depicting proposed mechanism of non-canonical Wnt4 action compared with the known mechanism of Wnt3A. Abbreviations: β, β-catenin; PS/T, serine/threonine phosphorylation; PTyr, tyrosine phosphorylation.
To explore this possibility further, we examined β-catenin subcellular localization in stably transfected HEK-293 cells by using immunofluorescence (Figure 3B). In the control cell line, β-catenin was scarcely detectable in the cytoplasm, with a few punctate structures observed at the cell membrane (Figure 3B, top panel). Cells expressing Wnt3A showed a much higher level of overall expression of β-catenin, with considerable staining observed in the cytoplasm, cell membrane and nucleus (Figure 3B, arrowheads), indicative of active canonical Wnt signalling (Figure 3B, middle panel) (Staal et al., 2002). Cells expressing Wnt4 showed a radically different pattern, with most of the β-catenin staining localized exclusively to the cell membrane, while cytoplasmic and nuclear β-catenins were absent (Figure 3B, bottom panel). Altogether, these results demonstrate that Wnt4 signalling triggers relocalization of β-catenin to the cell membrane in HEK-293 cells, thereby preventing both its degradation and its involvement in gene transactivation in the nucleus.
To investigate a possible developmental effect of Wnt4 on β-catenin in vivo, we performed immunofluorescence in mouse embryos at 12.5 dpc (days post coitum) (Figure 3C). Previous studies have shown that Wnt4 mRNA is expressed in the ovary at this developmental stage and that ovarian development in Wnt4−/− embryos is altered towards a male-like phenotype (Vainio et al., 1999). In cell layers just beneath the coelomic epithelium of developing wild-type ovaries, we observed strong β-catenin expression localized at the cell membrane. A comparable region of the ovaries of Wnt4−/− XX animals showed little or no β-catenin in all cell compartments (Figure 3C, compare the upper and lower panels). Because Wnt4−/− XX gonads are masculinized, male genes that are not expressed in the wild-type ovary become activated in the Wnt4−/− XX gonad. Such is the case for Sox9 (Kim et al., 2006b), which has been shown to stimulate the cytosolic degradation of β-catenin in chondrocytes (Akiyama et al., 2004). This suggests that the loss of β-catenin at the membrane in Wnt4−/− XX gonads is probably due to the loss of Wnt4. This finding is consistent with in vitro results above and suggests that there is a downstream effect of Wnt4 on β-catenin in vivo that results in its association with the cell membrane.
Discussion
β-Catenin is critical to the canonical Wnt signalling mechanism. Activation of the canonical pathway by Wnt3A enhances β-catenin-dependent gene transactivation by preventing degradation of β-catenin (Lustig and Behrens, 2003). Here, we show that Wnt4 can direct the function of β-catenin by a novel mechanism that inhibits β-catenin-dependent gene transactivation by relocating β-catenin to the cell membrane.
Our in vitro results indicate that, like Wnt5A, Wnt4 not only fails to activate canonical Wnt signalling, but also inhibits the action of that pathway in HEK-293T cells. Wnt4 signal does not decrease the level of active, non-phosphorylated β-catenin, yet it interferes with β-catenin-mediated gene transactivation. In addition, while the canonical Wnt mechanism inhibits phosphorylation of β-catenin by GSK3β, we found that inhibition of canonical signalling by Wnt4 is independent of GSK3β action. Rather, using several approaches, we showed that the Wnt4 signal inhibits canonical signalling by triggering relocalization of β-catenin to the cell membrane, thus removing it from participation in gene transactivation. Surprisingly, β-catenin relocalization to the cell membrane is not associated with a strong increase in cytoplasmic β-catenin. It is possible that Wnt4 action directly modifies the protein properties of β-catenin, through inhibition of tyrosine phosphorylation for instance, thereby flagging it for direct membrane relocalization without cytoplasmic pooling.
The dual functions of β-catenin, and appropriate switching between the two, are critical to normal development, and as such have been the focus of many recent studies (Nelson and Nusse, 2004; Brembeck et al., 2006). In the nucleus, β-catenin takes part in gene transactivation. At the cell membrane, formation of the β-catenin–E-cadherin–α-catenin complex in adherens junctions results in increased cadherin-mediated cell–cell adhesion and decreased cell migration. Conversely, loss of cadherin-mediated cell–cell adhesion correlates with increased β-catenin-mediated gene transactivation. Tyrosine phosphorylation plays a role in regulating this switch. Phosphorylation of Tyr142, for instance, induces binding of B-cell CLL/lymphoma 9-like to β-catenin and inhibits β-catenin–α-catenin interaction (Brembeck et al., 2004), while phosphorylation of Tyr654 results in loss of β-catenin/E-cadherin binding (Brembeck et al., 2006). Both events lead to nuclear translocation of β-catenin and subsequent target gene activation (Brembeck et al., 2004). Here, we show that Wnt4 signal can act in the opposite way in HEK-293T cells, resulting in both a decrease in β-catenin-mediated gene transcription and an increase in β-catenin at the cell membrane. Although further investigation is needed to reveal the specific mechanism involved (possibly, control of the β-catenin tyrosine phosphorylation status or regulation of B-cell CLL/lymphoma 9-like activity), Wnt4 can operate as a switch between the two modes of β-catenin function. In Figure 3(D), we present a model for β-catenin-dependent Wnt4 action in comparison with canonical Wnt signalling.
Several studies have described non-canonical mechanisms for Wnt4 that do not utilize β-catenin in gene transactivation. Two groups have found that Wnt4 activates MAPK8 (mitogen-activated protein kinase 8) (Cai et al., 2002; Maurus et al., 2005), which results in phosphorylation of paired box 2 and subsequent paired box 2-dependent gene transactivation (Cai et al., 2002). A second β-catenin-independent mechanism has been inferred from assays that show a strong interaction between Wnt4 and the Frizzled-6 receptor (Lyons et al., 2004). Frizzled-6 mediates activation of the calcium-sensitive enzymes PKC and CaMK (Sheldahl et al., 1999; Kuhl et al., 2000b). Binding to Frizzled-6 and activation of MAPK8 may represent early cellular events in Wnt4 signalling that subsequently lead to translocation of β-catenin to the cell membrane.
Contrary to expectations from early functional studies of Wnt family members (Moon et al., 1993; Du et al., 1995), Wnt4 activation of the canonical pathway has also been reported. β-Catenin-mediated gene transactivation using the canonical mechanism was seen in response to Wnt4 signal in three different kidney cell lines (Surendran and Simon, 2003; Terada et al., 2003; Lyons et al., 2004) and in renal tissue following injury (Surendran and Simon, 2003; Terada et al., 2003).
The fact that Wnt4 can elicit different responses in different cell types reflects a general trend in our understanding of Wnt signalling: the response to a given stimulus depends not only on which Wnt is present, but also on which cognate receptor is expressed on the cell (Perez-Moreno and Fuchs, 2006). For example, while injection of Wnt5A alone into Xenopus embryos results in a phenotype associated with a non-canonical Wnt stimulus, co-injection of Wnt5A and the Frizzled-5 receptor triggers a phenotype that typically follows a canonical Wnt signal (He et al., 1997). It is likely, in fact, that one Wnt protein can signal more than one type of response in a cell if multiple types of receptors are present (Lyons et al., 2004). Adding still more layers of complexity, cofactors and secreted antagonists of Wnt signalling are likely to affect both canonical and non-canonical actions (Hecht and Kemler, 2000; Kawano and Kypta, 2003).
We previously demonstrated that Wnt4 action inhibits β-catenin/SF1 co-regulation of StAR (steroidogenic acute regulatory protein), a gene critical to steroidogenesis, by attenuating recruitment of β-catenin to the StAR promoter, but the mechanism involved remained unclear (Jordan et al., 2003). The present study suggests that this attenuation may be due to relocation of β-catenin to the cell membrane following Wnt4 signal. We also performed a micro-array analysis of gene expression in Wnt4-expressing HEK-293T cells compared with controls (results not shown). Consistent with Wnt4 repression of canonical Wnt signalling, our results showed a 2-fold decrease in expression of v-myc myelocytomatosis viral oncogene homologue (Myc) and cyclin D1, two genes previously shown to be up-regulated by canonical Wnt signalling (He et al., 1998; Shtutman et al., 1999; Tetsu and McCormick, 1999).
In vivo, Wnt4 signal is required for development of the ovary. It is expressed in the bipotential gonad of both males and females, but is down-regulated in the testis at 11.5 dpc (Vainio et al., 1999). We examined the effect of Wnt4 on β-catenin during gonadogenesis, using a Wnt4 mutant mouse model. At 12.5 dpc, strong membrane-associated β-catenin staining was observed in the coelomic region of wild-type ovaries but was absent from that region of Wnt4−/− mutant ovaries. Two of the known roles of Wnt4 in ovarian development are inhibition of male-like cell migration (Martineau et al., 1997; Brennan et al., 2002; Jeays-Ward et al., 2003) and repression of steroidogenesis (Jordan et al., 2003; Heikkila et al., 2005). Together, our results provide mechanistic insight into each of these functions of Wnt4. In the developing ovary, relocalization of β-catenin to the cell membrane could both increase cell–cell adhesion and thereby inhibit male-like cell migration, while, at the same time, prevent involvement of β-catenin in transcription of genes, such as StAR, that are necessary for steroidogenesis.
The recent identification of RSpo1 (R-spondin1) as a female sex-determining gene in humans (Parma et al., 2006) suggests that nuclearization of β-catenin is critical to this process. R-spondins have been shown to activate β-catenin/TCF-dependent gene transactivation (Kazanskaya et al., 2004; Kim et al., 2005, 2006a; Nam et al., 2006), and do so by enhancing canonical Wnt signalling (Kazanskaya et al., 2004; Binnerts et al., 2007). Yet we observe strong membrane localization of β-catenin during development in wild-type ovaries. To reconcile these observations, we note that RSpo1 has recently been shown to activate β-catenin-dependent transactivation independent of Wnt signalling (Kim et al., 2005, 2006a; Nam et al., 2006) and speculate that this may occur in the ovary, with the resulting nuclear β-catenin below our method of detection. Wnt4 could then act independently to relocalize additional β-catenin to the cell membrane. Alternatively, RSpo1 may synergize with Wnt4 to localize β-catenin at the membrane using a mechanism that does not involve nuclear β-catenin and gene transactivation.
Fine modulation of the dual roles of β-catenin in gene transactivation and in cell–cell adhesion is crucial to normal developmental processes, and mechanisms that switch β-catenin from one mode to the other are central to this modulation. In the present study, we have presented evidence for a novel mechanism of Wnt4 action that acts as such a switch (see model, Figure 3D). Wnt4 signal inhibits canonical Wnt transcriptional activity by relocalizing β-catenin to the cell membrane.
Materials and methods
Plasmids
Plasmids expressing human Wnt4 and S37A mutant human β-catenin have previously been described (Jordan et al., 2003). The TCF reporter plasmid kit with TOPFLASH and FOPFLASH plasmids was purchased from Upstate Cell Signaling Solutions. Expression vectors encoding the wild-type human β-catenin, S33Y mutant human β-catenin and Δ45 mutant human β-catenin were provided by Hans Clevers (Netherlands Institute for Developmental Biology, Utrecht, The Netherlands). Plasmids expressing Wnt3A and GFP (green fluorescent protein)–Wnt5A were provided by Yingzi Yang [NIH (National Institutes of Health), Bethesda, MD, U.S.A.]. The V5-tagged Wnt4 expression plasmid was obtained by PCR amplification of the coding sequence of human Wnt4 and insertion into the pcDNA31/V5-His plasmid (Invitrogen). All constructs were verified by DNA sequencing.
Antibodies
Mouse monoclonal antibody against β-catenin (E5, sc-7963) and fluorochrome-coupled secondary antibodies were purchased from Santa Cruz Biotechnology. Mouse monoclonal antibody against stabilized β-catenin (αABC, 8E7, 05-665) was purchased from Upstate Biotechnology. Mouse monoclonal antibody against V5 tag was obtained from Invitrogen. Hspa5 mouse primary antibody was purchased from BD Transduction Laboratories. Mouse monoclonal antibody against β-tubulin was purchased from Chemicon. IR rabbit anti-mouse secondary antibody Dye800 was purchased from Rockland Immunochemicals.
Cell culture and transfections
HEK-293T cells (A.T.C.C., CRL-11268) and HEK-293 cells (A.T.C.C., CRL-1573) were routinely cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) fetal calf serum, in a 5% CO2 humidified atmosphere. For transient transfections, HEK-293T cells were plated on to 12-well plates, grown to 70–80% confluence and transfected with the indicated plasmids using Lipofectamine™ 2000 according to the manufacturer's instructions (Invitrogen). Transfections were performed with 200 ng of either TOPFLASH or FOPFLASH reporter plasmids, the indicated expression plasmid and 5 ng of CMV (cytomegalovirus)-β-galactosidase as an internal control. The total amount of DNA was kept constant at 1.6 μg per well by using empty pcDNA3.1 expression vector. After 36 h, luciferase activity was measured according to the Luciferase Assay System (Promega) and data were normalized to the β-galactosidase activity. For stable transfections, HEK-293 cells were plated on to 10 cm plates, grown to 70–80% confluence and transfected with 4 μg of the human Wnt4 or Wnt3A expression plasmids or mock-transfected with 4 μg of the empty pcDNA3.1 vector. Stably transfected clonal cell lines were selected using Geneticin (also known as G418; Invitrogen; 400 μg/ml). For conditioned media, cells were transfected with 1 μg of pcDNA, Wnt4 or Wnt5A, grown to near confluence. The media were then removed and used to replace the media from untransfected or Wnt3A-transfected HEK-293T cells grown to near confluence in separate flasks. These cells were grown for 6 h in the conditioned media and then harvested.
Western blots and subcellular fractionation
HEK-293T cells were seeded on to 6-well plates at 0.3 × 106 cells per well and co-transfected with the indicated plasmids by using the Lipofectamine™ 2000 reagent. At 36 h after transfection, proteins were solubilized, and protein concentration was determined by Bradford assay (Bio-Rad Protein Assay kit; Bio-Rad). Subcellular fractionation was performed using the Qproteome cell compartment kit according to the manufacturer's instructions (Qiagen). Cell lysates were incubated for 5 min at 95°C and resolved by SDS/PAGE. Proteins were transferred to a PVDF membrane (Immun-Blot PVDF membrane; Bio-Rad), incubated with primary antibodies, washed and incubated with rabbit anti-mouse Dye800 secondary antibody. Membranes were scanned on the Odyssey Infrared Imaging System (LiCor). Densitometric analysis was performed by using the ImageJ software package (http://rsb.info.nih.gov/ij/).
Immunofluorescence
Stable cell lines expressing Wnt4, Wnt3A or empty vector were grown on coverslips, fixed with 4% (w/v) paraformaldehyde, permeabilized in TBS (Tris-buffered saline) with 0.2% Triton X-100 and incubated with antibody against β-catenin (1:500) in TNB blocking buffer [TBS plus 0.5% blocking reagent (PerkinElmer)] overnight at 4°C. Slides were then washed three times with TBS and incubated overnight at 4°C with a 1:100 dilution of Cy3-conjugated anti-mouse IgG secondary antibody (Jackson ImmunoResearch) in TNB. Coverslips were mounted with Vectashield Plus DAPI (Vector Laboratories), and cells were visualized on a Leica (Heidelberg, Germany) TCS-SP MP confocal and multiphoton inverted microscope.
Wnt4+/− mice from The Jackson Laboratory were time mated, with the noon on the day of the vaginal plug designated as E0.5 (embryonic day 0.5). Embryos were genotyped, fixed overnight at 4°C in 4% paraformaldehyde, paraffin-embedded, sectioned at 6 μm and mounted on SuperFrost Plus slides (Fisher). De-paraffinized and rehydrated sections were placed in boiling Antigen Unmasking Solution (Vector Laboratories) for 15 min and then treated for endogenous peroxidases. Antibody against β-catenin (1:6000) was used with the M.O.M. Basic kit (Vector Laboratories), and the signal was amplified further with the TSA Fluorescein System (PerkinElmer Life Sciences) following the manufacturer's protocols. Coverslips were mounted on to slides with Vectashield Plus DAPI (Vector Laboratories). Confocal images were taken on a Leica TCS-SP MP confocal and multiphoton inverted microscope.
Acknowledgements
This work was supported by NIH grant RO1 HD 44513 to E.V. and by NHMRC (National Health and Medical Research Council; Australia) grant 334314 to V.R.H.
Abbreviations used
- CaMK
Ca2+/calmodulin-dependent protein kinase
- CK1α
casein kinase 1α
- dpc
days post coitum
- GSK3β
glycogen synthase kinase 3β
- HEK-293 cell
human embryonic kidney cell
- HEK-293T cells
HEK-293 cells expressing the large T-antigen of SV40 (simian virus 40)
- Hspa5
heat-shock 70 kDa protein 5
- MAPK8
mitogen-activated protein kinase 8
- MMTV
murine-mammary-tumour virus
- PKC
protein kinase C
- SF1
steroidogenic factor 1
- RSpo1
R-spondin 1
- StAR
steroidogenic acute regulatory protein
- TBS
Tris-buffered saline
- TCF
T-cell factor, HMG box
- Wnt
wingless-type MMTV integration site family
- Wnt4
Wnt, member 4
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, III, Dixon M, et al. R-spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. U.S.A. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Brennan J, Karl J, Capel B. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev. Biol. 2002;244:418–428. doi: 10.1006/dbio.2002.0578. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Cai Y, Lechner MS, Nihalani D, Prindle MJ, Holzman LB, Dressler GR. Phosphorylation of Pax2 by the c-Jun N-terminal kinase and enhanced Pax2-dependent transcription activation. J. Biol. Chem. 2002;277:1217–1222. doi: 10.1074/jbc.M109663200. [DOI] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol. 1995;15:2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hecht A, Kemler R. Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Rep. 2000;1:24–28. doi: 10.1093/embo-reports/kvd012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Heikkila M, Prunskaite R, Naillat F, Itaranta P, Vuoristo J, Leppaluoto J, Peltoketo H, Vainio S. The partial female to male sex reversal in Wnt-4-deficient females involves induced expression of testosterone biosynthetic genes and testosterone production, and depends on androgen action. Endocrinology. 2005;146:4016–4023. doi: 10.1210/en.2005-0463. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1–NLK–MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Shen JH, Olaso R, Ingraham HA, Vilain E. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/beta-catenin synergy. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10866–10871. doi: 10.1073/pnas.1834480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-spondin proteins: a novel link to beta-catenin activation. Cell Cycle. 2006a;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006b;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin–Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 2000a;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000b;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J. Cancer Res. Clin. Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, Barth AM, McCrea PD. Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp. Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior–posterior guidance of commissural axons by Wnt–frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr. Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Maurus D, Heligon C, Burger-Schwarzler A, Brandli AW, Kuhl M. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J. 2005;24:1181–1191. doi: 10.1038/sj.emboj.7600603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin–TCF signaling depending on receptor context. PLoS Biol. 2006a;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006b;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev. Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Noort Mv M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Surendran K, Simon TC. CNP gene expression is activated by Wnt signaling and correlates with Wnt4 expression during renal injury. Am. J. Physiol. Renal. Physiol. 2003;284:F653–F662. doi: 10.1152/ajprenal.00343.2002. [DOI] [PubMed] [Google Scholar]
- Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J. Am. Soc. Nephrol. 2003;14:1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J. Biol. Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]