Abstract
The complement system has an important role in host resistance to systemic candidiasis but regulation of complement activation by C. albicans remains poorly defined. Previous studies have identified a requirement for naturally occurring antimannan IgG antibody in initiation of C3 opsonization of C. albicans through either the classical or alternative pathway. This study characterized antibody-dependent initiation of the alternative pathway using the recombinant human monoclonal antimannan Fab fragment M1 and its full-length IgG1 antibody M1g1. Kinetic analysis of C3b deposition onto C. albicans with flow cytometry demonstrated the ability of M1g1 to restore the activity of either the classical or alternative pathway to the yeast-absorbed normal human serum, but the Fc-free M1 Fab restored only the activity of the alternative pathway. This Fc-independent, antimannan Fab-mediated C3 deposition through the alternative pathway was also observed in a serum-free assay containing the six alternative pathway proteins and in C1q- or C2-depleted serum but not in factor B-depleted serum. M1- or M1g1-dependent alternative pathway initiation of C3b deposition occurred in an asynchronous manner at discrete sites that expanded to cover the entire cell surface over time as revealed with immunofluorescence microscopy, in contrast to a uniform appearance of initial C3 deposition through the classical pathway. Furthermore, antimannan Fab M1 promoted the assembly of the alternative pathway convertase on the cell surface seen as colocalization of C3 and factor B with immunofluorescence microscopy. Thus, human antimannan antibody has a distinct Fc-independent effector function in regulation of C3 deposition to C. albicans.
Keywords: Complement, Candida, Alternative pathway, C3 convertase, Factor B, Antimannan antibody, Fab, Fc-independent
1. Introduction
Candida albicans is an opportunistic yeast-like pathogen and may cause life-threatening hematogenously disseminated candidiasis. A critical role for the complement system has been demonstrated in host resistance to Candida infections. Chemically induced deficiency in C3 (Gelfand et al., 1978), congenital deficiency in C5 (Hector et al., 1982; Lyon et al., 1986), or genetically induced deficiency in C3 (Han et al., 2001) produces a significant increase in susceptibility to candidiasis in experimental animals. In addition, administration of human mannan-binding lectin required for lectin pathway-mediated complement activation enhances the resistance of mice to hematogenously disseminated candidiasis (Lillegard et al., 2006), whereas blockage of initiation of complement activation in mice deficient in mannan binding lectin A/C or in factor B and C2 is associated with reduced resistance to systemic candidiasis (Held et al., 2008). Furthermore, studies with the mouse model of hematogenously disseminated candidiasis showed that protection by a murine antimannan IgM antibody or its IgG3 variant requires an intact complement system (Han et al., 2001). However, regulation of complement activation by C. albicans has not been well understood.
The cell surface of C. albicans is naturally resistant to complement opsonization (Kozel et al., 1996; Zhang et al., 1997; Zhang and Kozel, 1998). Previous studies have established a requirement for antimannan antibody in initiation of C3b deposition onto the cell surface of C. albicans. Absorption of normal human serum with either C. albicans yeast cells (Kozel et al., 1996; Zhang et al., 1997) or immobilized chemically-purified Candida mannan essentially abolished the serum complement activity (Zhang et al., 1997; Zhang et al., 1998). Addition of affinity-purified naturally occurring polyclonal antimannan IgG antibody restored classical pathway activity to the absorbed serum (Zhang et al., 1997). Furthermore, antimannan antibody was found to initiate the alternative pathway under conditions where normal human serum was rendered free of Ca++ with EGTA chelation to inhibit classical pathway initiation or where the alternative pathway was reconstituted from the six alternative pathway proteins (Zhang et al., 1998). The requirement for antimannan antibody in complement activation by C. albicans is further supported by studies that revealed a significant correlation between the amounts of naturally occurring antimannan antibody in individual sera and the ability of the sera to initiate either the classical (Kozel et al., 2004; Zhang et al., 1997) or the alternative pathway (Kozel et al., 2004). Thus, antimannan IgG antibody modulates complement opsonization of C. albicans through both the classical and alternative pathways.
The conventional view is that initiation of the classical complement pathway begins with the attachment of C1q to the Fc region of antibody-antigen complex and thus is antibody dependent. In contrast, initiation of the alternative complement pathway is typically independent of antibody. The influence of antibody on alternative pathway activities has not been well understood. While antimannan antibody is required for alternative pathway activation by C. albicans as described above, some anti-capsular antibodies can suppress alternative pathway-mediated C3 opsonization of encapsulated Cryptococcus neoformans (Kozel et al., 1998). These opposing effects of the adaptive immunity on the alternative pathway of complement activation may influence host innate resistance to fungal infections. Our ability to dissect the molecular mechanisms of antimannan antibody-mediated activation of the alternative pathway by C. albicans has been limited by the polyclonal nature of naturally occurring antimannan antibody.
Our earlier work generated a monoclonal human recombinant antimannan Fab fragment known as M1 and converted it to a full-length IgG1 antibody designated as M1g1 (Zhang et al., 2006). M1g1 was found to activate the mouse complement system and to enhance the resistance of mice to systemic candidiasis (Zhang et al., 2006). M1g1 and M1 are identical in the epitope specificity but differ in the presence of the Fc region of IgG1 (Zhang et al., 2006). They were utilized in the present study to characterize the patterns of antimannan antibody-mediated alternative pathway initiation of C3 opsonization of C. albicans. We found that i) the Fc-free antimannan Fab fragment M1 is unable to activate the classical pathway but is necessary and sufficient for alternative pathway initiation, ii) M1-dependent alternative pathway initiation of C3b deposition occurs in an asynchronous manner at discrete sites that expand rapidly to the entire cell surface, and iii) M1 promotes the formation of the alternative pathway C3 convertase on the cell surface. These results establish an Fc-independent effector function for human antimannan IgG antibody that modulates activities of the complement system.
2. Methods
2.1. Preparation of yeast cells
Yeast cells of C. albicans 3153A were used for all experiments and prepared as described with minor modifications (Zhang et al., 1997). Briefly, yeast cells were passaged daily for three times at 37°C in 3 ml broth containing 2% glucose, 1% peptone, 0.3% yeast extract and then used to initiate a large overnight broth culture. Yeast cells from the large culture were inactivated by one hour treatment with 1% formaldehyde at room temperature, harvested by centrifugation, washed, resuspended in PBS (pH 7.2, 1.9 mM NaH2PO4, 8.1 mM Na2HPO4, 154 mM NaCl), and stored in aliquots at -80°C. No discernible difference between live and formaldehyde-treated cells was observed in C3 activation and binding in a previous study (Kozel et al., 1987) or in the binding of M1g1 or M1 in preliminary experiments of the current study.
2.2. Human recombinant antimannan Fab fragment (M1) and full-length IgG1 antibody (M1g1)
The gene construct of M1g1 contains the DNA sequences for the light chain (VL-CL) and the heavy chain (VH-CH1) of M1 and the DNA sequence for the CH2-CH3 of IgG1 (Zhang et al., 2006). Therefore, M1g1 and M1 are expected to have the same epitope specificity but only M1g1 has the Fc region. The IgG1 subclass specificity was chosen for the recombinant antibody because IgG is the main isotype for naturally occurring antimannan antibody (Jones, 1980; Kozel et al., 2004) and our analysis of normal human sera found IgG1 to be a common subclass. Soluble M1g1 was produced in a Chinese hamster ovarian cell line and affinity-purified (Zhang et al., 2006); the concentration was estimated by absorbance at 280 nm with a molar absorption coefficient adjusted for the amino acid composition of M1g1 (Pace et al., 1995). For this project, the M1 gene construct was modified to include a six-histidine tag at the C-terminus of the heavy chain gene using QuikChange XL Site-Directed Mutagenesis (Stratagene, La Jolla, CA) and subsequently cloned into pET28b (EMD Biosciences, Madison, WI) at the sites of EcoRI and NotI. The light chain gene in pET28b was oriented in frame behind T7 promoter by removal of extraneous N-terminal DNA (Nadkarni et al., 2007) with QuikChange II XL Site-Directed Mutagenesis (Stratagene, La Jolla, CA). The resulting plasmid construct pET28b-M1 was transformed into Rosetta 2(DE3) bacteria for production of soluble M1 with Overnight Express Autoinduction System 1 (EMD Biosciences, Madison, WI). Soluble M1 was collected by centrifugation and concentrated with Pellicon XL tangential flow filtration system (Millipore, Billerica, MA). M1 Fab was captured on an HisTrap FF column with AKTA chromatography (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and eluted with 150 mM NaCl, 20 mM phosphate, 0.5 M imidazole, pH 7.5. Purified M1 was dialyzed against PBS, passed through a 0.45-μm filter, and stored at -20°C. The concentration of purified M1 Fab was determined by absorbance at 280 nm with an adjusted molar absorption coefficient (Pace et al., 1995). The purity and structural integrity of M1 Fab were confirmed with sodium dodecyl sulphate-polyacrylamide gel electrophoresis under reducing and non-reducing conditions and Coomassie Blue staining; the identity was confirmed with Western blot using goat anti-human κ chain antibody (SouthernBiotech, Birmingham, AL). The binding specificity of M1 Fab for the mannan of C. albicans 3153A was verified with ELISA and HRP-conjugated goat anti-human κ antibody (Southern Biotech, Birmingham, AL). The pattern of M1 binding to the cell surface was visualized with FITC-conjugated goat anti-human κ (Southern Biotech, Birmingham, AL) and immunofluorescence microscopy by a digital imaging system described below. His-tagged M1 was found qualitatively and quantitatively similar to non-tagged M1 in binding to C. albicans yeast cells or in the pattern of M1-mediated alternative pathway of complement activation by analysis with immunofluorescence microscopy and flow cytometry.
2.3. Kinetic analysis of C3 deposition onto the cell surface by flow cytometry
Three different sources of complement were utilized to analyze the influence of M1g1 or M1 Fab on the activity of the classical or alternative pathway in initiation of C3b deposition to the surface of C. albicans yeast cells: pooled normal human serum (NHS), an alternative pathway reconstituted from the six alternative pathway proteins, and normal human serum depleted of C1q, C2 or factor B. Complement activity was measured in a Veronal-buffered saline (5 mM sodium Veronal, 142 mM NaCl, pH 7.3) containing 0.1% gelatin (GVB).
NHS was prepared from at least 30 healthy individuals after informed consent, pooled and stored at -80°C. Some experiments used yeast-absorbed serum which was produced by four successive absorptions, each with 5 × 108 C. albicans 3153A yeast cells per ml serum for 30 minutes at 0°C; the absorbed serum was passed through a 0.45-μm filter and used immediately or stored at -80°C. Each reaction mixture was prepared in GVB that contained 40% NHS (untreated or yeast-absorbed) and either 5 mM EGTA and 5 mM MgCl2 to limit complement activation to the alternative pathway or 1.5 mM CaCl2 and 1 mM MgCl2 to permit the activity of both the classical and alternative pathways (Fine et al., 1972). The mixture was warmed to 37°C and supplemented with 2.5, 10 or 40 μg M1 Fab or 10 μg M1g1 per ml of reaction. These amounts of M1 Fab or M1g1 were within the range of naturally occurring antimannan antibody that showed a dose-dependent effect on initiation of complement activation (Zhang et al., 1997). C3 activation was initiated by addition of 1 × 107 C. albicans 3153A yeast per ml of reaction. Yeast cells in a fixed volume were transferred from the reaction at various time points to an ice-cold stop solution containing 10 mM EDTA in PBS. Yeast cells were washed with ice-cold PBS two times and incubated for 1 h at room temperature with yeast-absorbed goat anti-human C3-FITC (Kent Laboratories, Bellingham, WA). Stained cells were washed two times and resuspended in 1% BSA-PBS containing LDS 751 (Invitrogen, Carlsbad, CA) for flow cytometry analysis with Quanta SC MPL at 488 nm excitation (Beckman Coulter, Miami, FL). Yeast cells were identified with LDS 751 in FL3 and single cells were then defined by cell size with Coulter electronic volume technology and side scatter. C3 bound to single yeast cells was quantitatively measured in FL1. Five-thousand single yeast cells were collected for mean fluorescence of FITC-conjugated goat anti-C3 antibody bound to the cell surface.
Six isolated proteins of the alternative pathway (Quidel, Santa Clara, CA) were used for reconstitution of a serum-free C3-activation reaction (Schreiber et al., 1978) in GVB and 1 mM MgCl2 as described (Zhang et al., 1998). Factors B, D, H, and I and C3 were used at 40% of their physiological concentrations and properdin was used at five times as much as 40% of its physiological concentration to compensate for loss of activity during purification and/or storage (Zhang et al., 1998). Yeast cells were incubated in the serum-free mixture alone or in the presence of antimannan Fab M1 and kinetics of C3b deposition was determined as described above.
Human serum depleted of C1q, C2, or factor B (Quidel, Santa Clara, CA) was used to determine the requirement of a specific complement pathway for M1 Fab-mediated C3 activation. Each serum was absorbed with yeast as described above. Yeast cells were incubated in 40% of each serum in GVB and 1 mM MgCl2 alone or in the presence of either antimannan Fab M1 or M1g1 and kinetics of C3b deposition was determined as described above.
2.4. Immunofluorescence microscopic analysis of C3 binding
Yeast cells from some experiments for quantitative analysis of C3 opsonization described above were also examined in VECTASHIELD® (Vector Laboratories, Burlingame, CA) with immunofluorescence microscopy by a digital imaging system as described below.
2.5. Visualization of the alternative pathway C3 convertase on the cell surface
A method for detection of cell-bound factor B was adapted for this purpose (Spitzer et al., 2007). Yeast cells were incubated for 3 min at 37°C in 40% yeast-absorbed NHS in GVB containing 5 mM EGTA and 5 mM MgCl2 and 10 μg of M1 Fab per ml reaction as described above. The reaction was stopped with ice-cold PBS-1% BSA and yeast cells were washed in the same buffer two times at 4°C. The washed yeast cells were then incubated with mouse anti-factor Bb antibody at 1:25 (Quidel, Santa Clara, CA) at 4°C for 1.5 h and washed two times at 4°C, followed by an incubation with both goat anti-mouse antibody conjugated with Alexa-594 at 1:50 (Invitrogen, Carlsbad, CA) and goat anti-human C3 conjugated with FITC at 1:50 (Kent Laboratory, Bellingham, WA) at room temperature for 1 h. Yeast cells without anti-factor Bb antibody yielded no fluorescence following incubation with Alexa-594 conjugated goat anti-mouse antibody. The dual-labeled yeast cells were washed two times and mounted in VECTASHIELD® (Vector Laboratories, Burlingame, CA). Two identical stacks of images along the Z-axis of one cell were acquired with an epifluorescence Olympus BX51 equipped with an F-View imaging sensor (Olympus, Center Valley, PA) and Ludl Automation Controller and programmable filter wheel (Ludl Electronic Products, Hawthorne, NY) under the control of MicroSuite™ software (Olympus, Center Valley, PA). One stack of images was acquired with a red filter set for visualization of yeast-bound factor B and the other with a green filter set for visualization of yeast-bound C3. Each stack of images was then digitally deconvolved with MicroSuite™ software and projected onto a single plane; the red and green images were then merged and overlaid onto a differential interference contrast (DIC) image of the same cell. In addition, individual optical images within each stack were also examined to determine the pattern of distribution of C3 or factor B over the entire cell surface.
3. Results
3.1. Recombinant monoclonal human antimannan M1g1 initiates both the classical and alternative complement pathways
Initial experiments were conducted to determine whether the monoclonal human recombinant antimannan M1g1 (Zhang et al., 2006) is capable of initiating both the classical and alternative pathways, a dual complement-modulating activity that has been shown for naturally occurring polyclonal antimannan antibodies (Zhang et al., 1997; Zhang et al., 1998). Yeast cells were incubated in yeast-absorbed NHS for 1 to 16 min in the absence or presence of 10 μg M1g1 per ml of reaction. Cell-bound C3 fragments were detected with anti-human C3 antibody and either quantified with flow cytometry or visualized with immunofluorescence microscopy. In the absence of M1g1, deposition of C3b to yeast cells was essentially at a background level (Fig. 1). However, C3 opsonization of yeast cells could be restored to the absorbed serum with M1g1 in two ways. When both the classical and alternative pathways were active, M1g1-mediated accumulation of C3 fragments was rapid and it initiated simultaneously at numerous individual sites that had an appearance of uniform distribution and rapidly merged into a confluent binding pattern on the cell surface (Fig. 1, top). This uniform emergence of C3b binding sites was visible as early as 40 seconds following incubation (data not shown). When complement activity was limited to the alternative pathway, initiation of C3b deposition by M1g1 occurred on the cell surface at discrete sites that expanded over time to cover the entire cell surface, seen as a delayed accumulation of C3 fragments in the kinetic analysis (Fig. 1, bottom). However, the capacity of the binding of C3 fragments to the cell surface initiated through either pathway was comparable (Fig. 1). Thus, the monoclonal antimannan antibody M1g1 has a dual role in modulation of complement activation by C. albicans through either the classical or alternative pathway.
FIG. 1.
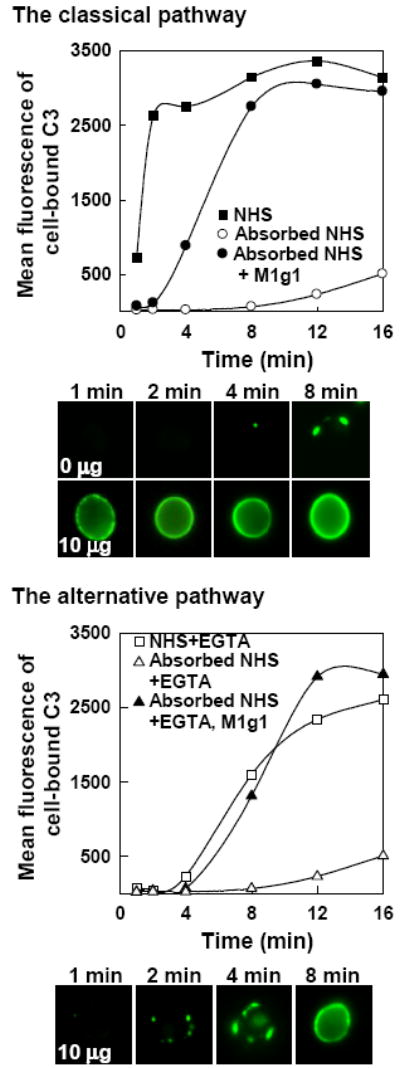
Effect of human monoclonal antimannan antibody M1g1 on the kinetics of C3b deposition onto C. albicans yeast cells through either the classical or alternative pathway. Yeast cells were incubated for 1 to 16 min in NHS, yeast-absorbed NHS, or yeast-absorbed NHS supplemented with 10 μg M1g1 per ml under a condition that permits activity of both the classical and alternative pathways (Top, without EGTA) or under a condition that permits the alternative pathway alone (Bottom, with EGTA). Cell-bound C3 was detected with goat anti-human C3 antibody-FITC and was either quantified with flow cytometry or visualized on the cell surface with fluorescence microscopy. A representative experiment from three is shown.
3.2. Antimannan M1g1-mediated C3 activation through the alternative pathway is Fc-independent
Antibody-mediated initiation of the classical pathway requires the Fc region as a binding site for C1q. To determine whether the Fc region is required for initiation of the alternative pathway by M1g1, we utilized the antimannan M1, the Fc-free recombinant Fab of M1g1 (Zhang et al., 2006). Kinetics of C3b deposition on yeast cells incubated from 2 to 8 min in yeast-absorbed NHS containing 10 μg M1 Fab per ml were compared under two different conditions, one without EGTA that permitted both the classical and alternative pathways and one with EGTA that permitted the alternative pathway only (Fig. 2, top). Under either condition, there was a rapid accumulation of C3 fragments on the cell surface following a short delay and the kinetics were similar to each other (Fig. 2, top). In parallel to the kinetic analysis, the sites for initial C3b deposition were visualized with immunofluorescence microscopy. Yeast cells were incubated for 4 min in EGTA-treated, yeast-absorbed serum in the presence of 2.5, 10, or 40 μg of M1 Fab per ml. Initial deposition of C3b mediated by antimannan Fab M1 through the alternative pathway occurred at discrete sites on the cell surface (Fig. 2, bottom), resembling the pattern seen with M1g1 (Fig. 1, bottom). In addition, more initiation sites emerged with increasing amounts of M1; however, the focal initiation pattern was observed regardless of the concentration of M1 (Fig. 2, bottom). Similar patterns of C3b deposition were observed with M1 Fab in the absence of EGTA when both the classical and the alternative pathways were active (data not shown). In contrast to the patchy binding of initial C3 molecules to the yeast surface, the binding of Fab was uniform over the entire cell surface (Fig. 2, bottom right, Fab binding). Taken together, these observations indicate that antimannan antibody M1g1 employs an Fc-dependent mechanism in activation of the classical pathway and an Fc-independent mechanism in activation of the alternative pathway.
FIG. 2.
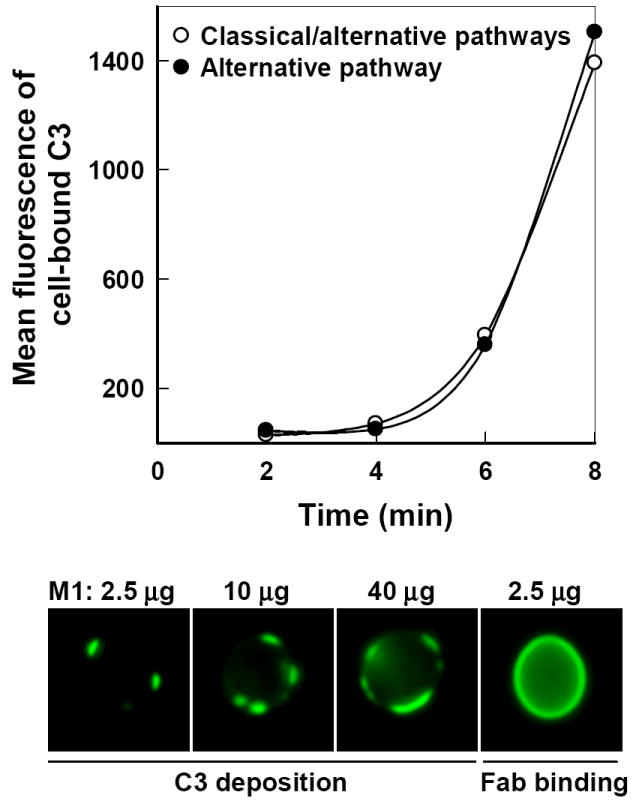
Influence of Fc-free antimannan Fab M1 on the initiation of C3b deposition to C. albicans yeast cells. (Top) Kinetics of C3b deposition in the presence of 10 μg M1 per ml from 2 to 8 min in yeast-absorbed NHS was determined when both the classical and alternative pathways were active (○, without EGTA) or when only the alternative pathway was active (●, with EGTA). Yeast-bound C3 was quantified with FITC-anti-C3 antibody and flow cytometry. (Bottom) Dose effect of M1 on the pattern of C3 deposition through the alternative pathway. Yeast cells were incubated for 4 min in yeast-absorbed NHS containing 2.5, 10, or 40 μg M1/ml in the presence of EGTA. Yeast-bound C3 molecules were detected with FITC-anti-C3 antibody and visualized with fluorescence microscopy. The pattern of M1 binding to yeast cells was visualized with FITC-anti-human κ antibody and fluorescence microscopy and is shown as a comparison (Bottom, far right). A representative experiment from three is shown.
3.3. Antimannan Fab M1 mediates activation of an alternative complement pathway reconstituted from six alternative pathway proteins
As an alternative approach, a serum-free C3-binding assay was prepared from the six alternative pathway proteins (C3, factors B, D, H and I, and properdin) (Zhang et al., 1998) to exclude other mannan-reactive serum components that may activate the classical or the lectin pathway (Roos et al., 2003). In the absence of antimannan Fab M1, no appreciable amounts of C3 were detected on yeast cells incubated with the six proteins alone from 4 to 16 min (Fig. 3), indicating an intrinsic resistance of C. albicans to alternative pathway activation. Addition of antimannan Fab M1 at 40 μg/ml to the serum-free mixture promoted a deposition of C3 fragments to yeast cells (Fig. 3) in a manner similar to that observed with NHS (Fig. 2). The experiment was repeated with 10 μg/ml antimannan Fab M1 and a similar effect was observed (data not shown). In addition, a microscopic analysis of yeast cells from the serum free mixture revealed a patchy deposition of initial C3 molecules on the cell surface (data not shown) as seen with NHS (Fig. 2). These observations indicate that antimannan Fab M1-mediated activation of the alternative pathway is independent of the Fc, C1q-mediated classical pathway, or mannan binding lectin-mediated lectin pathway (Roos et al., 2003).
FIG. 3.
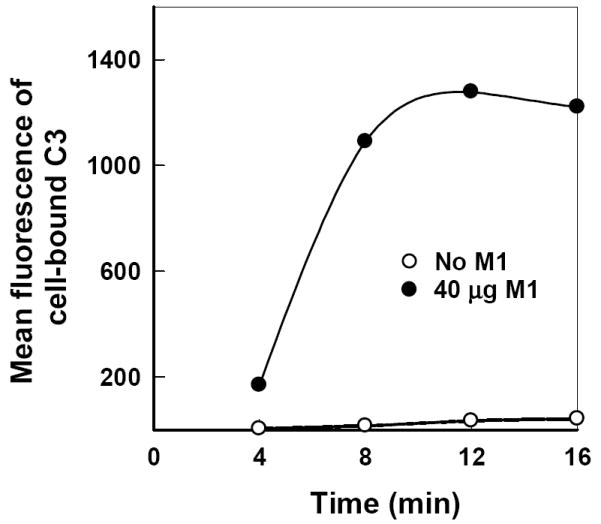
Requirement for antimannan Fab M1 in initiation of C3 binding to C. albicans through an alternative pathway reconstituted from six alternative pathway proteins. Yeast cells were incubated for 4 to 16 min in a serum-free C3 binding medium containing C3, factors B, D, H and I, and properdin in the presence or absence of 40 μg M1 per ml. Cell-bound C3 was quantified by use of FITC-anti-C3 antibody and flow cytometry.
3.4. An intact alternative pathway is required for antimannan Fab M1-mediated complement activation
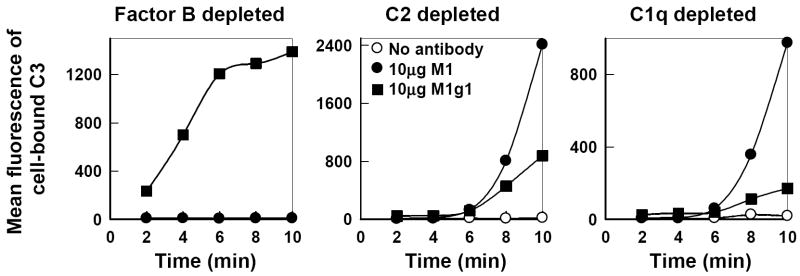
The role of antimannan Fab M1 as an activator of the alternative pathway was further analyzed with yeast-absorbed human sera that were deficient in components of either the classical or alternative pathway. In serum depleted of C1q or C2 where the alternative pathway is intact, a marked accumulation of cell-bound C3 was observed in a kinetic analysis over a period of 2 to 10 min only in the presence of either M1 Fab or M1g1 but not in the absence of the antibody (Fig. 4, right and center) and was verified with immunofluorescence microscopy. This pattern of C3b deposition kinetics resembles that seen with M1g1 (Fig. 1) or M1 (Fig. 2) in EGTA-treated NHS where classical pathway initiation of C3 activation is inhibited. In contrast, in factor B-depleted serum where the classical pathway is intact, only the Fc-containing M1g1 activated C3b deposition onto yeast cells whereas the Fc-free M1 showed no effect (Fig. 4, left). These observations together establish that antimannan Fab M1-mediated complement activation requires an intact alternative pathway.
FIG. 4.
Requirement of an intact alternative pathway for antimannan Fab M1-mediated C3b deposition to C. albicans yeast cells. Kinetics of C3b deposition from 2 to 10 min were determined in yeast-absorbed normal human serum depleted of factor B, C2, or C1q in the absence of antimannan antibody or in the presence of M1 or M1g1 at 10 μg per ml. Cell-bound C3 was quantified by use of FITC-anti-C3 antibody and flow cytometry. A representative experiment from two is shown.
3.5. Antimannan Fab M1 promotes formation of the alternative pathway C3-convertase on the cell surface of C. albicans
Formation of C3 convertase is a hallmark event in initiation of either the classical or alternative pathway, leading to C3 opsonization of microbes. To assess formation of the alternative pathway C3 convertase, association of C3b and Bb, during the initiation of the alternative pathway by M1 Fab, we used immunofluorescence microcopy to detect colocalization of C3b and factor Bb on the yeast cell surface (Fig. 5). Yeast cells were incubated for 3 min in EGTA-treated yeast-absorbed NHS containing antimannan Fab M1 at 10 μg per ml. Cell-bound C3 was detected with green fluorochrome-tagged anti-human C3 antibody (Fig. 5. left, green) and factor B was detected with a combination of mouse anti-human factor B antibody and red fluorochrome-tagged anti-mouse antibody (Fig. 5. middle, red). Distribution of cell-bound C3 or factor B was assessed along the Z-axis of a yeast cell in a stack of 20 optical images. When the green and red images were merged, C3 molecules were found largely in association with factor B molecules, indicating a formation of the alternative pathway C3 convertase, C3bBb (Fig. 5, right, yellow). This association was found in individual optical sections throughout the stack (Fig. 5, Sections 1, 11, 17).
FIG. 5.
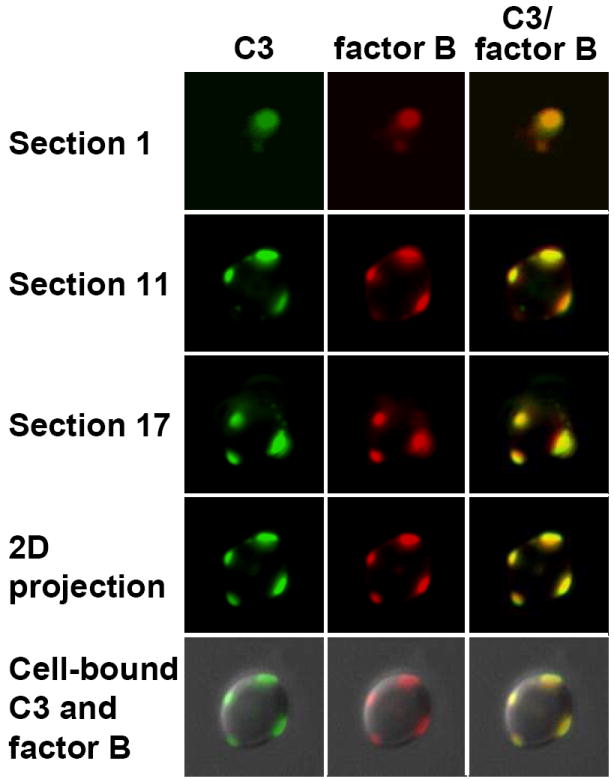
Antimannan Fab M1-mediated formation of the alternative pathway C3 convertase on the cell surface of C. albicans. Yeast cells were incubated for 3 min in EGTA-treated yeast-absorbed normal human serum supplemented with 10 μg antimannan Fab M1 per ml and washed. Cell-bound C3 molecules were detected by green FITC-labeled goat anti-human C3 antibody (left, green) and cell-bound factor B molecules were detected by mouse anti-human factor B antibody and red Alexa 594-labeled goat anti-mouse antibody (middle, red). The green and red images were merged to visualize the distribution of bound C3 and factor B on the cell surface (right, yellow). A stack of 20 green or red images were acquired through the Z-axis of a yeast cell and digitally deconvolved. Binding patterns for C3, factor B, or C3-factor B are shown in three individual optical sections (1st, 11th, and 17th) that span the Z-axis of the cell or as a projected 2D image of the 20 sections either by itself or on a DIC image of the cell.
DISCUSSION
Earlier studies have identified a dual role for naturally occurring polyclonal antimannan antibody in initiation of either the classical or the alternative pathway of complement activation, leading to the deposition of opsonic C3 fragments onto the cell surface of C. albicans (Zhang et al., 1997; Zhang et al., 1998). Antimannan antibody-initiated alternative pathway of complement activation by C. albicans is novel and was characterized here with a matched pair of monoclonal recombinant human antimannan antibodies with the same epitope specificity, the Fc-free Fab fragment M1 and its full length IgG1 antibody M1g1 (Zhang et al., 2006). There are three major findings. First, antimannan Fab fragment M1 alone is necessary and sufficient for C3 activation through the alternative pathway, not the classical pathway (Figs. 2, 3, and 4), indicating an Fc-independent function of antimannan antibody in regulation of complement activation. Second, M1 Fab-mediated C3b deposition through the alternative pathway occurs at discrete focal sites that expand over time to cover the entire cell surface in an asynchronous manner (Fig. 2 bottom), in contrast to a synchronous and uniform emergence of initial C3b bindings mediated by M1g1 through the classical pathway (Fig. 1). Third, antimannan M1 Fab promotes the formation of the alternative pathway C3 convertase (C3bBb) on the cell surface (Fig. 5), thereby initiating the alternative pathway of complement activation. Thus, the antimannan IgG antibody has distinct Fc-independent effector functions in regulation of complement opsonization of C. albicans.
The independence of the Fc region in antibody-mediated alternative pathway initiation has also been observed with several other cell types including bacteria (Joiner et al., 1983; Wachter and Brade, 1989), protozoa (Kipnis et al., 1985), measles virus-infected cells (Ehrnst, 1978; Sissons et al., 1979), and erythrocytes (Albar et al., 1981; Moore, Jr. et al., 1982) and cell-wall particle zymosan (Schenkein and Ruddy, 1981a). However, the ability of the Fab fragment alone to initiate the alternative pathway is variable. For example, rabbit monovalent Fab fragments reactive with measles hemagglutinin induced rabbit alternative pathway-mediated lysis of measles virus-infected cells (Ehrnst, 1978) whereas human bivalent F(ab’)2 fragments against measles hemagglutinin were required for effective lysis of measles-infected cells by human alternative pathway (Sissons et al., 1979). Similarly, human F(ab’)2, not Fab, was able to mediate alternative pathway killing of Escherichia coli even though binding of these two types of fragments to the cell surface was similar (Joiner et al., 1983); in contrast, rabbit Fab fragments were sufficient for activation of guineas pig alternative pathway by Yersinia enterocolitica (Wachter et al., 1989). The difference in the valence requirement for alternative pathway initiation may be in part due to the difference in the experimental systems of interest. It may also suggest that antibody-mediated alternative pathway initiation is a complex process and may involve different mechanisms.
Kinetic analysis showed that M1 Fab promotes a rapid accumulation of C3 fragments on C. albicans cells through the alternative pathway following a short delay (Figs. 2, 3, and 4), a pattern visualized on the cell surface as expansion of focal sites of initially deposited C3 molecules to encompass the entire cell surface (Figs. 2 and 5). More initiation sites were observed under increasing amounts of M1 Fab, but the patchy initiation pattern did not seem to change (Fig. 2). A patchy deposition of C3b initiated through the alternative pathway was first described by Kozel and colleagues on the cell surface of encapsulated C. neoformans yeast in an antibody-independent manner, and the sites for initial C3b deposition appeared to expand to the entire cell surface over time (Kozel et al., 1991). This distinct pattern of alternative pathway initiation was subsequently observed with C. albicans yeast cells in the presence of naturally occurring polyclonal antimannan antibody (Zhang et al., 1998) and is now confirmed with the monoclonal antimannan antibody M1g1 (Fig. 1, bottom) and the Fc-free M1 Fab (Figs. 2 bottom and 5). The latter observation suggests that the Fc region has no discernible effect on the pattern of alternative pathway initiation. In contrast to a synchronous, uniform appearance of initial C3 depositions through the classical pathway seen in C. albicans (Fig. 1), alternative pathway initiations, whether antibody dependent as seen with C. albicans or antibody independent as seen with encapsulated C. neoformans (Kozel et al., 1991), seem to follow a similar pattern of expanding focal sites. Thus, antimannan antibody probably facilitates alternative pathway initiation but does not alter the events during the initiation.
The observation that initially deposited C3 molecules activated through M1 Fab-initiated alternative pathway were largely in association with factor B (Fig. 5) suggests the formation of C3b-Bb complexes. To our knowledge, this is the first documented visual evidence for the formation of the alternative pathway C3 convertase on the cell surface. This result provides a support for the quantitative correlation between antibody-mediated alternative pathway activity and uptake of C3 and factor B by zymosan particles (Schenkein and Ruddy, 1981b). Formation of the alternative pathway C3 convertase, C3bBb, is a critical and hallmark event in complement activation (Pangburn and Muller-Eberhard, 1984). Nascent C3bBb converts inactive fluid phase C3 to metastable C3b that, upon deposition on a surface, has the potential to associate with factor B to form more C3bBb, thereby amplifying complement activation (Pangburn et al., 1984). The colocalization of initial C3b and factor B molecules on discrete surface sites observed in this study thus explains the expansion of foci of initial C3b deposition through the activity of alternative pathway C3 convertase C3bBb.
How antimannan Fab M1 promotes the assembly of the alternative pathway C3 convertase C3bBb and thereby initiates the complement cascade through the alternative pathway is currently unknown. The epitope for both M1 and M1g1 is identical and a uniform binding on the cell surface was observed for both M1 (Fig. 2, bottom far right) and M1g1 (Zhang et al., 2006). However, a uniform appearance of sites was observed for initial C3b deposition mediated by M1g1 through the classical pathway (Fig. 1 top), in contrast to a patchy binding pattern for initial C3 molecules mediated by either M1 in different doses (Fig. 2 bottom) or M1g1 (Fig. 1 bottom) through the alternative pathway. These observations together suggest that, unlike the Fc region as a specific receptor for C1q in classical pathway activation, an antimannan Fab fragment may play a complex role in the Fc-independent antibody-meditated initiation of the alternative pathway. Two models are currently available that explain how antibody-independent activation of the alternative pathway may occur in a sequence of multiple events. In the tick-over model, covalent binding of metastable fluid-phase C3b or C3(H2O) to a surface occurs first, followed by association of initially bound C3b with factor B in formation of the alternative pathway C3 convertase C3bBb with factor D, and stabilization of nascent C3bBb by properdin (Pangburn et al., 1984). In the properdin-directed model, the first event is a non-covalent binding of properdin to a surface, followed by association of initially-bound properdin with fluid phase C3b and factor B and formation of C3bBb with factor D (Spitzer et al., 2007). Consequently, blockage of any event in either model would likely retard alternative pathway initiation, and the multi-event sequence has offered ample opportunities to evolution of mechanisms in microbes for evasion of complement activation (Wurzner, 1999; Zipfel et al., 2007). For example, the cell surface of C. albicans has been shown to bind plasma factor H (Meri et al., 2002), a negative regulator of the alternative pathway. Factor H inhibits the formation of C3bBb by competing with factor B for binding to C3b or degrading C3b with factor I to iC3b, a form inactive for formation of C3bBb (Pangburn et al., 1984). Indeed, the relative binding of factor H to initially deposited C3b has been shown to differ between activators and nonactivators of the alternative pathway (Horstmann et al., 1985; Meri and Pangburn, 1990; Pangburn et al., 1984). The ability to bind factor H by C. albicans may in part explain why the cell surface of C. albicans is naturally resistant to complement activation in the absence of antimannan antibody as observed in this and previous studies (Zhang et al., 1997; Zhang et al., 1998). Thus, binding of antimannan Fab fragments onto C. albicans may produce a surface that promotes the occurrence of one or more events identified in either model and/or inhibits the activities of negative complement regulators, leading to alternative pathway initiation.
The ability of an antimannan Fab to modulate alternative pathway of complement activation may be influenced by the density and topographic distribution of its epitope. In one study, human polyclonal IgG antibodies specific for the polysaccharide or peptidoglycan of group A Streptococcus were found to activate the classical pathway but only anti-polysaccharide antibody activated the alternative pathway (Eisenberg and Schwab, 1986). In another study, both human polyclonal anticapsular antibody and noncapsular antibody against Haemophilus influenzae type b were bactericidal through the classical pathway but only anticapsular antibody was also bactericidal through the alternative pathway (Steele et al., 1984). Since Candida mannan is complex and a number of antigenic determinants have been identified with rabbit and mouse antimannan antibodies (Han et al., 1997; Miyakawa et al., 1986; Suzuki, 1997), it is possible that antimannan antibodies may differ in the ability to initiate the alternative pathway of complement activation.
Kinetic analyses showed a faster initiation of C3 opsonization mediated by M1g1 through the classical pathway than through the alternative pathway (Figs. 1 and 4). Given the potential of initially deposited C3b through the classical pathway to form the alternative pathway C3 convertase with factor B (Lutz et al., 2007), how important is a separate ability of an antimannan antibody to initiate the alternative pathway of complement activation? A direct answer to this question is unavailable. Previous studies have shown a protective role for murine and human antimannan antibodies in the resistance of mice to hematogenously disseminated candidiasis (Han et al., 2000; Han and Cutler, 1995; Zhang et al., 2006) and a requirement for the complement system in murine antimannan antibody-mediated protection (Han et al., 2001). However, little is known about the contribution of different complement pathways in host resistance to systemic candidiasis. Growing evidence has shown differential roles of complement pathways in host resistance to microbial infections (Mehlhop and Diamond, 2006; Mueller-Ortiz et al., 2004; Zhong et al., 1999). Thus, the identification of an Fc-independent effector function for the antimannan antibody in alternative pathway initiation contributes to our understanding of molecular mechanisms underlying antimannan antibody-mediated protection against candidiasis.
Acknowledgments
This work was supported by National Institutes of Health grants AI14209, AI37194, and AI44786 (T.R.K.) and AI52139 and GM63119 (M.X.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albar JP, Juarez C, Vivanco-Martinez F, Bragado R, Ortiz F. Structural requirements of rabbit IgG F(ab’)2 fragment for activation of the complement system through the alternative pathway--I. Disulfide bonds. Mol Immunol. 1981;18:925–934. doi: 10.1016/0161-5890(81)90015-8. [DOI] [PubMed] [Google Scholar]
- Ehrnst A. Separate pathways of C activation by measles virus cytotoxic antibodies: subclass analysis and capacity of F(ab) molecules to activate C via the alternative pathway. J Immunol. 1978;121:1206–1212. [PubMed] [Google Scholar]
- Eisenberg RA, Schwab JH. Arthropathic group A streptococcal cell walls require specific antibody for activation of human complement by both the classical and alternative pathways. Infect Immun. 1986;53:324–330. doi: 10.1128/iai.53.2.324-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine DP, Marney SR, Jr, Colley DG, Sergent JS, Des Prez RM. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972;109:807–809. [PubMed] [Google Scholar]
- Gelfand JA, Hurley DL, Fauci AS, Frank MM. Role of complement in host defense against experimental disseminated candidiasis. J Infect Dis. 1978;138:9–16. doi: 10.1093/infdis/138.1.9. [DOI] [PubMed] [Google Scholar]
- Han Y, Cutler JE. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kanbe T, Cherniak R, Cutler JE. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kozel TR, Zhang MX, MacGill RS, Carroll MC, Cutler JE. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J Immunol. 2001;167:1550–1557. doi: 10.4049/jimmunol.167.3.1550. [DOI] [PubMed] [Google Scholar]
- Han Y, Riesselman MH, Cutler JE. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect Immun. 2000;68:1649–1654. doi: 10.1128/iai.68.3.1649-1654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector RF, Domer JE, Carrow EW. Immune responses to Candida albicans in genetically distinct mice. Infect Immun. 1982;38:1020–1028. doi: 10.1128/iai.38.3.1020-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held K, Thiel S, Loos M, Petry F. Increased susceptibility of complement factor B/C2 double knockout mice and mannan-binding lectin knockout mice to systemic infection with Candida albicans. Mol Immunol. 2008;45:3934–3941. doi: 10.1016/j.molimm.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Horstmann RD, Pangburn MK, Muller-Eberhard HJ. Species specificity of recognition by the alternative pathway of complement. J Immunol. 1985;134:1101–1104. [PubMed] [Google Scholar]
- Joiner KA, Goldman RC, Hammer CH, Leive L, Frank MM. Studies of the mechanism of bacterial resistance to complement-mediated killing. V. IgG and F(ab’)2 mediate killing of E. coli 0111B4 by the alternative complement pathway without increasing C5b-9 deposition. J Immunol. 1983;131:2563–2569. [PubMed] [Google Scholar]
- Jones JM. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect Immun. 1980;30:78–89. doi: 10.1128/iai.30.1.78-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis TL, Krettli AU, Dias dS. Transformation of trypomastigote forms of Trypanosoma cruzi into activators of alternative complement pathway by immune IgG fragments. Scand J Immunol. 1985;22:217–226. doi: 10.1111/j.1365-3083.1985.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Kozel TR, Brown RR, Pfrommer GS. Activation and binding of C3 by Candida albicans. Infect Immun. 1987;55:1890–1894. doi: 10.1128/iai.55.8.1890-1894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, deJong BC, Grinsell MM, MacGill RS, Wall KK. Characterization of anticapsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule. Infect Immun. 1998;66:1538–1546. doi: 10.1128/iai.66.4.1538-1546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, MacGill RS, Percival A, Zhou Q. Biological activities of naturally occurring antibodies reactive with Candida albicans mannan. Infect Immun. 2004;72:209–218. doi: 10.1128/IAI.72.1.209-218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Weinhold LC, Lupan DM. Distinct characteristics of initiation of the classical and alternative complement pathways by Candida albicans. Infect Immun. 1996;64:3360–3368. doi: 10.1128/iai.64.8.3360-3368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Wilson MA, Murphy JW. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991;59:3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard JB, Sim RB, Thorkildson P, Gates MA, Kozel TR. Recognition of Candida albicans by mannan-binding lectin in vitro and in vivo. J Infect Dis. 2006;193:1589–1597. doi: 10.1086/503804. [DOI] [PubMed] [Google Scholar]
- Lutz HU, Fumia S, Schurtenberger C, Alaia V. Opinion paper: Stimulation of complement amplification or activation of the alternative pathway of complement? Mol Immunol. 2007;44:3862–3865. doi: 10.1016/j.molimm.2007.06.146. [DOI] [PubMed] [Google Scholar]
- Lyon FL, Hector RF, Domer JE. Innate and acquired immune responses against Candida albicans in congenic B10. D2 mice with deficiency of the C5 complement component. J Med Vet Mycol. 1986;24:359–367. doi: 10.1080/02681218680000551. [DOI] [PubMed] [Google Scholar]
- Mehlhop E, Diamond MS. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Exp Med. 2006;203:1371–1381. doi: 10.1084/jem.20052388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci U S A. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri T, Hartmann A, Lenk D, Eck R, Wurzner R, Hellwage J, et al. The yeast Candida albicans binds complement regulators factor H and FHL- 1. Infect Immun. 2002;70:5185–5192. doi: 10.1128/IAI.70.9.5185-5192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y, Kagaya K, Fukazawa Y, Soe G. Production and characterization of agglutinating monoclonal antibodies against predominant antigenic factors for Candida albicans. J Clin Microbiol. 1986;23:881–886. doi: 10.1128/jcm.23.5.881-886.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FD, Jr, Austen KF, Fearon DT. Antibody restores human alternative complement pathway activation by mouse erythrocytes rendered functionally deficient by pretreatment with pronase. J Immunol. 1982;128:1302–1306. [PubMed] [Google Scholar]
- Mueller-Ortiz SL, Drouin SM, Wetsel RA. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect Immun. 2004;72:2899–2906. doi: 10.1128/IAI.72.5.2899-2906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni A, Kelley LL, Momany C. Optimization of a mouse recombinant antibody fragment for efficient production from Escherichia coli. Protein Expr Purif. 2007;52:219–229. doi: 10.1016/j.pep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn MK, Muller-Eberhard HJ. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7:163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- Roos A, Bouwman LH, Munoz J, Zuiverloon T, Faber-Krol MC, Fallaux-van den Houten FC, et al. Functional characterization of the lectin pathway of complement in human serum. Mol Immunol. 2003;39:655–668. doi: 10.1016/s0161-5890(02)00254-7. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Ruddy S. The role of immunoglobulins in alternative complement pathway activation by zymosan. I. Human IgG with specificity for Zymosan enhances alternative pathway activation by zymosan. J Immunol. 1981a;126:7–10. [PubMed] [Google Scholar]
- Schenkein HA, Ruddy S. The role of immunoglobulins in alternative pathway activation by zymosan. II. The effect of IgG on the kinetics of the alternative pathway. J Immunol. 1981b;126:11–15. [PubMed] [Google Scholar]
- Schreiber RD, Pangburn MK, Lesavre PH, Muller-Eberhard HJ. Initiation of the alternative pathway of complement: recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc Natl Acad Sci U S A. 1978;75:3948–3952. doi: 10.1073/pnas.75.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons JG, Cooper NR, Oldstone MB. Alternative complement pathway-mediated lysis of measles virus infected cells: induction by IgG antibody bound to individual viral glycoproteins and comparative efficacy of F(ab’)2 and Fab’ fragments. J Immunol. 1979;123:2144–2149. [PubMed] [Google Scholar]
- Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- Steele NP, Munson RS, Jr, Granoff DM, Cummins JE, Levine RP. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984;44:452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr Top Med Mycol. 1997;8:57–70. [PubMed] [Google Scholar]
- Wachter E, Brade V. Influence of surface modulations by enzymes and monoclonal antibodies on alternative complement pathway activation by Yersinia enterocolitica. Infect Immun. 1989;57:1984–1989. doi: 10.1128/iai.57.7.1984-1989.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzner R. Evasion of pathogens by avoiding recognition or eradication by complement, in part via molecular mimicry. Mol Immunol. 1999;36:249–260. doi: 10.1016/s0161-5890(99)00049-8. [DOI] [PubMed] [Google Scholar]
- Zhang MX, Bohlman MC, Itatani C, Burton DR, Parren PW, St Jeor SC, et al. Human recombinant antimannan immunoglobulin G1 antibody confers resistance to hematogenously disseminated candidiasis in mice. Infect Immun. 2006;74:362–369. doi: 10.1128/IAI.74.1.362-369.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MX, Kozel TR. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect Immun. 1998;66:4845–4850. doi: 10.1128/iai.66.10.4845-4850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MX, Lupan DM, Kozel TR. Mannan-specific immunoglobulin G antibodies in normal human serum mediate classical pathway initiation of C3 binding to Candida albicans. Infect Immun. 1997;65:3822–3827. doi: 10.1128/iai.65.9.3822-3827.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Burns T, Chang Q, Carroll M, Pirofski L. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect Immun. 1999;67:4119–4127. doi: 10.1128/iai.67.8.4119-4127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Wurzner R, Skerka C. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol Immunol. 2007;44:3850–3857. doi: 10.1016/j.molimm.2007.06.149. [DOI] [PubMed] [Google Scholar]