Abstract
Background
Certain autoimmune and infectious conditions are associated with increased risks of subtypes of non-Hodgkin’s lymphomas (NHL). A few prior studies suggest that chronic inflammation may particularly elevate risk for the distinct NHL subtype Waldenström’s macroglobulinemia (WM). We assessed WM risk in relation to a wide range of chronic immune stimulatory conditions among 4 million U.S. veterans.
Methods
We identified 361 WM cases with up to 27 years of follow-up. Using time-dependent Poisson regression we estimated rate ratios (RR) and 95% confidence intervals (CI) for WM risk in relation to history of autoimmune diseases that typically have autoantibodies (with systematic or organ involvement) or do not have autoantibodies, infections, and allergies. All models were adjusted for attained age, calendar-year, race, number of hospital visits, and latency between study entry and exit.
Results
The age-standardized incidence of WM was 0.34/100,000 person-years. WM risk was elevated among individuals with any prior autoimmune condition (RR, 2.2; 95% CI, 1.7–3.0), autoantibodies with systemic involvement (RR, 2.50; 95% CI, 1.55–4.02), autoantibodies with organ involvement (RR, 2.30; 95% CI, 1.57–3.37). Risks for WM were also increased with hepatitis (RR, 3.39; 95% CI, 1.38–8.30), human immunodeficiency virus (HIV) (RR, 12.05; 95% CI, 2.83–51.46), and rickettsiosis (RR, 3.35; 95% CI, 1.38–8.14)
Conclusions
In the largest investigation of WM risk factors to date, we found 2- to 3-fold elevated risk of WM among persons with a personal history of autoimmune diseases with autoantibodies and notably elevated risks for hepatitis, HIV, and rickettsiosis. Our findings provide novel insights into the as yet unknown etiology of WM.
INTRODUCTION
Waldenström’s macroglobulinemia (WM) is a distinct B-cell subtype of non-Hodgkin’s lymphoma (NHL) characterized by lymphoplasmacytic infiltration of the bone marrow and a monoclonal immunoglobulin M (IgM) protein.1;2 Patients often present initially with non-specific symptoms. The most common symptoms are fatigue and malaise attributable to anemia. Other findings may include hepatomegaly (20% of patients), splenomegaly (15%), lymphadenopathy (15–20%), hyperviscosity (15%), and peripheral neuropathy (20%).3 Typically, clinical symptoms are attributable to the extent of tumor infiltration and to elevated monoclonal IgM levels.4
Based on data from the Surveillance, Epidemiology, and End Results Program (SEER), WM is a rare malignancy, affecting 3 to 4 people out of every one million in the United States (US) annually.5;6 In SEER, whites appear to have a higher incidence rate than blacks, and risk increases with age.5;6 Individuals with a history of the precursor condition monoclonal gammopathy of undetermined significance (MGUS) of IgM class have an elevated risk of developing WM, other types of lymphomas, or related disorders.7 Based on data from the Mayo Clinic, risk for progression from MGUS to WM is associated with the initial monoclonal protein concentration.8 Reports of multiply affected families from case series and smaller single center studies suggest that genetic factors may contribute.9–16 While the first genome wide linkage analysis based on 11 high-risk WM families was recently conducted, the genetic determinants of WM susceptibility remain to be defined.17
Small case studies of WM patients have found Ig gene mutations suggesting a role for repeated antigenic stimulation in the etiology of WM.18;19 Indeed, a recent study on hepatitis C virus (HCV) infection among US military veterans found an almost 3-fold increased risk of WM in HCV-infected compared to uninfected individuals.20 The study also found a slight increase in MGUS incidence, which may suggest that HCV infection drives progression from MGUS to WM through chronic immune stimulation.20 In addition, lymphoma incidence increases notably with age for most subtypes.6 This pattern suggests that chronic inflammation, which is associated with older age and cancer,21;22 may be a potential risk factor.6 Thus, it may be important to evaluate a broad range of infectious diseases that could increase risk of WM through chronic inflammation and immune stimulation. Finally, autoimmune disease is another important source of chronic immune stimulation. Although autoimmune disease is associated with lymphomas overall,23–25 the association between autoimmune disease and the WM subtype has not been well examined. To our knowledge, only one previous small case control study of 65 cases has evaluated WM risk in relation to autoimmunity.26 Analysis of all conditions combined found no association with WM risk. However, because of the restricted sample size, autoimmune conditions could not be analyzed individually.
To address these gaps and further explore underlying pathologic mechanisms, we conducted a study of WM risk in a cohort of 4 million adult male military veterans admitted to US Veterans Affairs (VA) hospitals. This study is the largest study to date to evaluate a wide range of chronic immune stimulatory conditions in relation to WM risk.
MATERIALS AND METHODS
Patients, outcome, and exposures
The study population came from a pool of approximately 30 million US veterans entitled to VA hospital admission during the study period.27 The cohort was identified from the VA database of inpatient records from 142 US VA hospitals between July 1, 1969, and September 30, 1996. Eligible patients included male white or black US veterans age 18 or older with at least one hospitalization during the study period. They also had to be cancer-free during the first year of follow-up and had to survive at least one year after the initial visit. Other ethnic/racial groups and females were excluded given their limited representation. The NIH Office of Human Subjects Research granted exemption from IRB review and waived informed consent because the study was restricted to existing data that were stripped of personal identifiers.
The 8th and 9th revisions of the International Classification of Diseases (ICD8-A, ICD9-CM) were used to code diagnoses for WM (code=275.5 or 273.3, respectively), as well as for specific autoimmune, infectious, and allergic conditions. In accord with prior studies,23 autoimmune conditions were categorized according to those that generally have detectable autoantibodies and those that do not. Bacterial, viral, parasitic, and total infections were analyzed as combined categories. Results for individual immune stimulatory conditions are presented only if three or more people with the condition developed WM.
Statistical analysis
For all subjects, person-time began one year after the index hospital discharge and continued until the diagnosis of first malignancy (WM), death, or the end of the observation period (September 30, 1996), whichever came first. Dates of death were ascertained from record linkage to Social Security Administration mortality files. The length of time from diagnosis of a chronic immune stimulatory condition (autoimmunity, infections, and allergies) to the development of WM was calculated by subtracting the date of the first hospital discharge diagnosis for an immune stimulatory condition from the date of the first hospital discharge diagnosis of WM.
Incidence rates for WM were age-standardized to the 2000 US population distribution (available at: http://factfinder.census.gov/). Rate ratios (RR) and 95% confidence intervals for the association between chronic immune stimulatory conditions and risk of WM were calculated using time-dependent Poisson regression 28 (AMFIT module in Epicure version 1.4) adjusting for attained age (<40, 40–49, 50–59, 60–69, 70–79, 80+ years), calendar-year (1969–1974, 1975–1979, 1980–1984, 1985–1989, 1990–1996), race (black versus white), number of hospital visits (1–2, 3–4, 5+ times), and latency between study entry and exit (2–3, 4–5, 6–9, 10–14, 15+ years). All P values and confidence intervals were two-sided.
To evaluate the potential for reverse causality (i.e., undetected WM causing the immune-related conditions), models were stratified by latency (time in the cohort) ≤5 years and >5 years for conditions with more than 10 exposed cases and at least three in each stratum (≤5 and >5 years). Modification of the RRs for these conditions and WM by latency was formally evaluated using the likelihood ratio test (LRT) for multiplicative interaction. For conditions of particular interest with fewer than 10 exposed cases, we described the time between exposure and WM for the exposed cases. We evaluated models stratified by median calendar time (<1985 and ≥1985) using the strategy described above (>10 exposed cases, >2 in each stratum) to assess whether the estimates were stable over time, and the results were very similar before and after 1985. Because the incidence of WM varies by race,5;6 we also stratified models by race as per above.
RESULTS
There were a total of 361 WM patients in this cohort of hospitalized veterans with a mean follow-up of 12 years (Table 1). The age-standardized incidence rates for WM was 0.34/100,000 person-years. There was some evidence that the rate was lower in blacks (0.26/100,000 person-years) than whites (0.37/100,000 person-years), corresponding to an RR of 0.74 (95% CI, 0.54–1.01).
TABLE 1.
Characteristics of the study cohort (US Veterans Affairs): White and black male veterans with at least one hospital admission between July 1, 1969 and September 30, 1996, who were followed more than one year.a
| Characteristics | Whites |
Blacks |
||
|---|---|---|---|---|
| Non-WM | WM | Non-WM | WM | |
| No. of persons | 3,668,667 | 316 | 832,250 | 45 |
| Mean age at study entrya | 51.1 | 61.0 | 47.7 | 53.2 |
| Years of follow-up (mean)b | 11.7 | 7.4 | 11.9 | 7.5 |
| Person-years at riskb | 42,759,826 | 2,352 | 9,888,663 | 338 |
| Median number of hospital visits | 3 | 4c | 3 | 4c |
Age at first discharge record for inpatient hospitalization at Veterans Affairs hospitals between July 1, 1969 and September 30, 1996.
Follow-up started one year after the first hospital visit.
Includes visits up to the exit date.
Abbreviations: WM=Waldenström’s macroglobulinemia.
Autoimmune disease was associated with a 2- to 3-fold increase in risk of subsequent WM (Table 2). The RR for autoimmune disease overall was 2.23 (95% CI, 1.68–2.97). Much of this increase seemed to be due to autoimmune diseases that generally have detectable autoantibodies (RR, 2.50; 95% CI, 1.55–4.02 for systemic involvement and 2.30; 95% CI, 1.57–3.37 for organ involvement). However, Crohn’s disease, which does not have detectable autoantibodies, seemed to be very strongly associated with WM (RR, 6.68; 95% CI, 2.76–16.20), although the estimate was imprecise since there were only five exposed cases. The relation between WM and immune thrombocytopenic purpura (ITP) was also strong (RR, 6.88; 95% CI, 2.84–16.64), and Sjögren’s syndrome produced the most prominent association with WM (RR, 13.59; 95% CI, 4.36–42.41), although based on small numbers.
TABLE 2.
Risk of Waldenström’s macroglobulinemia in relation to prior personal history of selected autoimmune diseases.
| Category/group | No. Exposed |
RR (95%CI) | |
|---|---|---|---|
| Non-WM | WM | ||
| Autoantibodies detectable | |||
| Systemic involvement, totala | 66,290 | 18 | 2.50 (1.55–4.02) |
| Systemic involvement, by latencyb | |||
| ≤5 years | 15,459 | 8 | 3.60 (1.68–7.74) |
| >5 years | 50,831 | 10 | 2.08 (1.14–3.82) |
| Rheumatoid arthritis, total | 59,463 | 14 | 2.09 (1.22–3.57) |
| Rheumatoid arthritis, by latencyb | |||
| ≤5 years | 13,642 | 7 | 3.32 (1.46–7.55) |
| >5 years | 45,821 | 7 | 1.63 (0.80–3.30) |
| Sjögren’s syndrome | 1,887 | 3 | 13.59 (4.36–42.41) |
| Organ involvement, totala | 137,376 | 30 | 2.30 (1.57–3.37) |
| Organ involvement, by latencyb | |||
| ≤5 years | 37,326 | 14 | 3.89 (2.19–6.91) |
| >5 years | 100,050 | 16 | 1.66 (1.00–2.77) |
| Chronic rheumatic heart disease, totala | 64,969 | 13 | 1.94 (1.12–3.39) |
| Chronic rheumatic heart disease, by latencyb | 13 | ||
| ≤5 years | 18,710 | 4 | 2.18 (0.80–5.91) |
| >5 years | 46,259 | 9 | 1.86 (0.95–3.62) |
| Immune thrombocytopenic purpura | 9,187 | 5 | 6.88 (2.84–16.64) |
| Multiple sclerosis | 19,365 | 3 | 1.92 (0.61–5.99) |
| Pernicious anemia | 9,241 | 4 | 2.32 (0.74–7.20) |
| Autoantibodies not detectable, totala | 106,627 | 15 | 1.38 (0.80–2.35) |
| Autoantibodies not detectable, by latencyb | |||
| ≤5 years | 20,389 | 5 | 2.02 (0.82–4.95) |
| >5 years | 86,238 | 10 | 1.17 (0.60–2.27) |
| Crohn’s disease | 9,339 | 5 | 6.68 (2.76–16.20) |
| Psoriasis | 40,664 | 4 | 0.97 (0.36–2.31) |
Includes individual conditions for which fewer than three people with the exposure developed WM. See Appendix 1.
Time between first inpatient discharge listing the defined condition and subsequent WM. Likelihood ratio test P values for interaction with latency were 0.28 for systemic involvement, 0.21 for rheumatoid arthritis, 0.04 for organ involvement, >0.50 for chronic rheumatic heart disease, and 0.35 for autoantibodies not detectable.
Abbreviations: WM=Waldenström’s macroglobulinemia; RR=rate ratio; CI=confidence interval. All analyses were adjusted for age, calendar time, race, latency, and number of hospital visits.
The RRs for WM associated with autoimmune conditions tended to be higher for latency ≤5 years compared to >5 years (Table 2). For example, the RR for total autoimmune disease was 4.01 (95% CI, 2.06–6.18) for ≤5 years versus 1.61 (95% CI, 1.11–2.34) for >5 years (LRT P=0.002). Numbers were too small to evaluate Sjögren’s syndrome, ITP, and Crohn’s disease in stratified latency models, but the mean time between exposure and WM was fairly short for both Sjögren’s syndrome and ITP: 2.2 years (median, 0.9; range, 0.3–5.4) and 2.6 years (median, 2.1; range, 0.8–4.2), respectively. However, the mean time between exposure and WM was 9.2 years for Crohn’s disease (median, 9.9; range, 0.7–18.1).
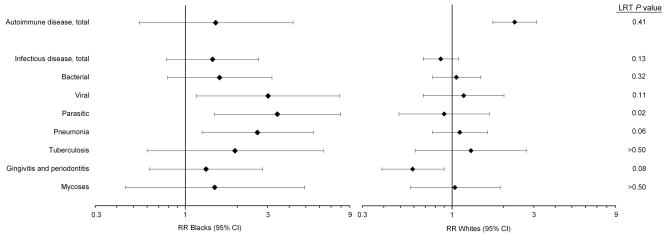
The RR for autoimmune disease and WM among blacks was lower than that among whites (Figure 1), but there was no indication of multiplicative interaction between race and autoimmune disease (LRT P=0.41). Only four blacks had both autoimmune disease and WM.
FIGURE 1. Forest plots of the RRs for each of the specific conditions (left) and subsequent Waldenström’s macroglobulinemia among blacks and whites. The LRT provides a formal evaluation of the difference in RRs by race.
Abbreviations: CI=confidence interval; LRT=likelihood ratio test; RR=rate ratio. All analyses were adjusted for age, calendar time, race, latency, and number of hospital visits.
Infectious diseases generally did not appear to be associated with increased risk of WM, with a few exceptions: hepatitis (RR, 3.39; 95% CI, 1.38–8.30), human immunodeficiency virus (HIV) (RR, 12.05; 95% CI, 2.83–51.46), and rickettsiosis (RR, 3.35; 95% CI, 1.38–8.14) (Table 3). There was also some evidence of a positive association between herpes zoster and WM (RR, 2.30; 95% CI, 0.95–5.58). There were no apparent associations with grouped bacterial (RR, 1.13; 95% CI, 0.87–1.51), viral (RR, 1.39; 95% CI, 0.87–2.22), or parasitic (RR, 1.24; 95% CI, 0.77–2.02) infections, or with infections overall (RR, 0.92; 95% CI, 0.74–1.15).
TABLE 3.
Risk of Waldenström’s macroglobulinemia in relation to prior personal history of selected infectious diseases.
| Category/group | No. Exposed |
RR (95%CI) | |
|---|---|---|---|
| Non-WM | WM | ||
| Upper airway | |||
| Acute bronchitis/bronchiolitis | 89,592 | 10 | 0.86 (0.43–1.74) |
| Chronic sinusitis, total | 74,924 | 11 | 1.49 (0.79–2.80) |
| Chronic sinusitis, by latencya | |||
| ≤5 years | 13,497 | 4 | 2.22 (0.70–7.00) |
| >5 years | 61,427 | 7 | 1.30 (0.61–2.77) |
| Nasopharyngitis/pharyngitis | 54,541 | 7 | 1.23 (0.50–2.98) |
| Lower airway | |||
| Pneumonia, total | 362,543 | 47 | 1.27 (0.92–1.77) |
| Pneumonia, by latencya | |||
| ≤5 years | 268,002 | 17 | 1.35 (0.74–2.46) |
| >5 years | 82,357 | 30 | 1.24 (0.84–1.85) |
| Tuberculosis, total | 82,357 | 11 | 1.43 (0.78–2.69) |
| Tuberculosis, by latencya | |||
| ≤5 years | 19,333 | 4 | 2.61 (0.96–7.11) |
| >5 years | 63,024 | 7 | 1.09 (0.49–2.47) |
| Gastro–hepatic | |||
| Cholangitis/Cholecystitis | 32,922 | 4 | 0.98 (0.37–2.64) |
| Hepatitis virus, total | 45,967 | 6 | 3.39 (1.38–8.30) |
| Hepatitis B virus | 14,727 | 3 | 5.56 (1.76–17.54) |
| Intestinal | 70,599 | 7 | 0.90 (0.40–2.02) |
| Genital | |||
| Syphilis | 49,309 | 5 | 1.15 (0.47–2.81) |
| Reproductive | |||
| Orchitis and epididymitis | 48,968 | 5 | 0.91 (0.37–2.19) |
| Urinary | |||
| Cystitis | 39,289 | 6 | 1.21 (0.54–2.73) |
| Pyelonephritis | 31,808 | 3 | 0.91 (0.29–2.83) |
| Cardiovascular | |||
| Endocarditis, total | 104,732 | 14 | 1.08 (0.59–1.98) |
| Endocarditis, by latencya | |||
| ≤5 years | 25,707 | 5 | 1.29 (0.47–3.52) |
| >5 years | 79,025 | 9 | 0.99 (0.46–2.11) |
| Systemic | |||
| HIV | 14,736 | 3 | 12.05 (2.83–51.46) |
| Septicemia | 72,798 | 8 | 1.19 (0.56–2.51) |
| Other | |||
| Gingivitis and periodontitis, total | 479,190 | 37 | 0.69 (0.48–0.99) |
| Gingivitis and periodontitis, by latencya | |||
| ≤5 years | 72,949 | 8 | 0.77 (0.36–1.65) |
| >5 years | 406,241 | 29 | 0.67 (0.45–1.01) |
| Herpes zoster | 18,992 | 5 | 2.30 (0.95–5.58) |
| Mycoses | 175,519 | 16 | 1.10 (0.65–1.90) |
| Rickettsiosis | 22,068 | 5 | 3.35 (1.38–8.14) |
| Osteomyelitis | 57,757 | 7 | 1.13 (0.50–2.55) |
| Skin and soft tissue infections, total | 297,226 | 27 | 0.97 (0.64–1.46) |
| Skin and soft tissue infections, by latencya | |||
| ≤5 years | 53,122 | 10 | 1.43 (0.69–2.93) |
| >5 years | 244,104 | 17 | 1.84 (0.51–1.38) |
Time between first inpatient discharge listing the defined condition and subsequent WM. Likelihood ratio test P values for interaction with latency were 0.47 for chronic sinusitis, >0.50 for pneumonia, 0.20 for tuberculosis, >0.50 for endocarditis, >0.50 for gingivitis and periodontitis, and 0.25 for skin and soft tissue infections.
Abbreviations: WM=Waldenström’s macroglobulinemia; RR=rate ratio; CI=confidence interval. All analyses were adjusted for age, calendar time, race, latency, and number of hospital visits.
In contrast to the latency pattern for autoimmune conditions, the RRs for infectious conditions were fairly similar for latency ≤5 years compared to >5 years (Table 3). The RRs appeared to be somewhat elevated in the ≤5 year group for chronic sinusitis and tuberculosis, although there was little evidence of multiplicative interaction (LRT P=0.47 and 0.20, respectively). The mean time between exposure and WM was 3.4 years for hepatitis (median, 2.9; range, 0.1–7.9), 9.8 years for gingivitis/periodontitis (median, 6.8; range, 0.1–24.1), 1.6 years for HIV (median, 1.1; range, 1.1–2.7), 4.2 years for rickettsiosis (median, 1.4; range, 0.7–15.4), and 4.7 years for herpes zoster (median, 4.7; range, 1.5–7.4).
For infectious diseases, the RRs from models restricted to blacks were higher than those from models restricted to whites (Figure 1). There was some evidence of multiplicative interaction between race and parasitic infections (LRT P=0.02), pneumonia (LRT P=0.06), and gingivitis/periodontitis (LRT P=0.08), although the estimates were imprecise. Blacks had a higher percentage of the infectious conditions in Figure 1 than whites (Table 4). However, the percentage of infections was generally only 1–2% higher among black versus white non-WM patients but approximately 3–14% higher among black versus white WM patients (Table 4).
TABLE 4.
Number and percentage of selected infectious conditions among black and white Waldenström’s macroglobulinemia and non-Waldenström’s macroglobulinemia patients.
| WM cases |
non-WM cases |
|||||||
|---|---|---|---|---|---|---|---|---|
| Blacks |
Whites |
Blacks |
Whites |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Bacterial | 11 | 24.4 | 53 | 16.8 | 142023 | 17.1 | 438156 | 11.9 |
| Viral | 6 | 13.3 | 16 | 5.1 | 40825 | 4.9 | 143894 | 3.9 |
| Parasitic | 7 | 15.6 | 13 | 4.1 | 52930 | 6.4 | 150971 | 4.1 |
| Lower airways | ||||||||
| Pneumonia | 10 | 22.2 | 37 | 11.7 | 72617 | 8.7 | 289926 | 7.9 |
| Tuberculosis | 3 | 6.7 | 8 | 2.5 | 26760 | 3.2 | 55597 | 1.5 |
| Other | ||||||||
| Gingivitis and periodontitis | 10 | 22.2 | 27 | 8.5 | 103739 | 12.5 | 375451 | 10.2 |
| Mycoses | 3 | 6.7 | 13 | 4.1 | 46643 | 5.6 | 128876 | 3.5 |
Abbreviations: WM=Waldenström’s macroglobulinemia
Twenty-two WM patients had allergies, including 10 with asthma, 5 with dermatitis, and 2 with urticaria. Overall allergy was not associated with WM (RR, 1.03; 95% CI, 0.67–1.60). RRs were 0.53 (95% CI, 0.22–1.29) for dermatitis and 1.28 (95% CI, 0.68–2.40) for asthma. The RRs for allergy by latency were the same (LRT P>0.50). Data were too sparse to evaluate allergies by race.
In a sub-analyses, we found that 19 (5%) of the 361 WM patients had a prior reported MGUS diagnosis. There is likely a very high degree of underascertainment of MGUS in our database because MGUS is generally an asymptomatic condition. Also, our patient population was never systematically screened for MGUS.
COMMENT
In the first large, systematic assessment of immune-related risk factors in relation to risk of developing WM, we found that autoimmune conditions such as rheumatoid arthritis, Sjögren’s syndrome, and Crohn’s disease were positively associated with WM, consistent with prior studies of NHL overall.24;25 Infections generally did not appear to be associated with WM risk, with a few exceptions, and allergies were not associated, although power was limited.
To our knowledge, the only previous study of chronic immune stimulatory conditions and WM is a case-control study of 65 WM patients and 213 hospital controls that could not evaluate specific, individual immune-related conditions due to the small number of cases.26 We found that the risk of developing WM was elevated 2- to 3-fold for autoimmune disorders with detectable autoantibodies but not for those without detectable autoantibodies. Thus, associations with WM may vary by category of autoimmune disease. We also found that specific autoimmune diseases (Sjögren’s syndrome, ITP, and Crohn’s disease) were the most strongly associated with WM risk, although these estimates were imprecise.
Based on small numbers, we found a prominent 14-fold risk for WM among persons with Sjögren’s syndrome. Previous studies have consistently shown increased risk of B-cell NHL among patients with Sjögren’s syndrome.25 Anatomically, these lymphomas are typically of indolent NHL subtypes, often of the mucosa associated lymphoid tissue (MALT) type.25 Recently, there has been some evidence that Sjögren’s syndrome patients might also have elevated risk of other lymphomas, such as diffuse large B-cell and anaplastic large T-cell lymphomas.29 To our knowledge, this study is the first to report an association between Sjögren’s syndrome and WM.
Both our study and the previous study by Linet et al. found no association between infectious diseases overall and WM.26 However, in the current study we were able to evaluate specific, individual infections and found that some (e.g., hepatitis, HIV, and rickettsiosis) were associated with increased risk of WM. Our study expands on the previous analysis of HCV and risk of WM by demonstrating that hepatitis B was also associated with WM, supporting the hypothesis that infection with hepatitis viruses may cause chronic immune stimulation leading to the development of WM.20 To our knowledge, we are the first to find that HIV was associated with increased WM risk. This observation is consistent with the large body of literature showing HIV to be associated with highly elevated risks of B-cell NHLs.30 The association with rickettsiosis is unexpected, especially since rickettsiosis is rare. Patients with rickettsial pathogens can show symptoms of lymphadenopathy,31 which may indicate activation of the immune system leading to chronic immune stimulation. Alternatively, hospitalization due to rickettsiosis may suggest the presence of an underlying immune deficit, leading to the diagnosis of WM.
This study is also the first to evaluate effect of race on the association between chronic immune stimulatory conditions and WM. The risk of WM associated with infections was consistently higher in blacks than in whites, which is surprising given that the incidence of WM is lower in blacks than in whites. If true, some other risk factor must be driving the risk in whites. It is possible that the pattern of elevated RRs for infections in blacks might reflect racial differences in immune responses to infectious agents.32–34 However, given the number of exposures evaluated, these findings have to be interpreted with caution since they could be due to chance.
To evaluate the possibility that WM may have been present prior to diagnosis of the chronic immune stimulatory conditions and may have actually caused these conditions (i.e., reverse causality), we stratified by latency ≤5 years and >5 years. For autoimmune disease, the increase in risk for WM was evident mainly in the 5 years prior to WM, and some conditions were diagnosed less than 1 year prior to WM diagnosis. These patterns suggest that in some cases the autoimmune disease could be a manifestation of underlying undetected WM, especially considering that WM often has a long latency period.35 ITP in particular is a recognized complication of WM.36;37 However, because the risk of WM among patients with autoimmune diseases remained elevated after 5 years, similar to the results from a population-based study of NHL in Denmark and Sweden,24 we feel that these findings are not entirely due to undetected WM. The clinical work-up for the suspicion of an autoimmune disease typically includes a complete blood count and may include protein electrophoresis, which should lead to WM diagnosis at or shortly after onset of the autoimmune disease. While some autoimmune diseases did have a relatively short median time to WM diagnosis (e.g., ITP and Sjögren’s syndrome), others did not (e.g., Crohn’s disease). Unfortunately, we do not have access to individual detailed clinical data that would allow us to evaluate the role of prior diagnosis of monoclonal gammopathy of undetermined significance or undetected early stage WM directly. Thus, future epidemiologic studies must verify our findings through medical-record validation of clinical, diagnostic, prognostic, and treatment data.
For infections, the similarity in RRs ≤5 years and >5 years argues against reverse causality for this group of chronic immune stimulatory conditions. While the data for many individual infectious conditions were too sparse to stratify by latency, the relatively short medians for some conditions suggest that interpretation requires caution. For example, the median time to WM diagnosis was approximately 1 year for both rickettsiosis and HIV, which could reflect detection bias (i.e., rickettsiosis or HIV detected due to workup for WM). On the other hand, the short time to WM diagnosis for HIV may reflect the fact that a separate ICD code for HIV was not added until 1987, resulting in a shorter amount of time available for the diagnosis of both HIV and WM.
Chronic immune stimulatory conditions may contribute to lymphomagenesis through persistent activation of lymphocytes leading to reduced antigen response, increased mutation rates, and downregulated T-cell response, potentially disrupting immune response to pathogens and malignant cells.38;39 While further studies are needed, our findings support the hypothesis that chronic immune stimulation may contribute to the development of WM.
The study has some limitations. First, these results may not be generalizable to the US population. It is, however, encouraging that the age-adjusted rates of WM in this cohort are similar to those found for males in SEER overall (0.35/100,000 person-years in the current study versus 0.35/100,000 person-years in SEER),5 in whites (0.37/100,000 person-years in the current study versus 0.36–0.56/100,000 person-years in SEER),5;6 and in blacks (0.26/100,000 person-years in the current study versus 0.17–0.25/100,000 in SEER).5;6 Because the data are based on hospital claims, it is possible that some conditions might have developed prior to the patient entering the VA system. The definition of WM is limited by the fact that the ICD-8-A code for WM was not entirely specific but also included macroglobulinemia (i.e., MGUS type IgM), by the lack of review for WM cases and immune-mediated conditions since demographic, clinical, treatment, laboratory, and biomarker information were unavailable, and by revisions in the definition of WM over time (e.g., it was not reportable as a malignancy in the US until 1988 40). However, as mentioned above, the risk estimates were very similar when stratified by calendar-period, suggesting that these limitations have not introduced any major bias. Since we did not have treatment data, we could not evaluate potential confounding by immunosuppressive therapy, which is used to treat severe autoimmune disease and is also associated with development of lymphoma.41 Because the clinical work-up of immune-related diseases might include protein electrophoresis, it is possible that some of our results for autoimmune conditions are overestimated due to surveillance bias: patients with autoimmune disease may be more likely to have WM diagnosed than patients without autoimmune disease. Also, one has to keep in mind that all patients (WM as well as non-WM patients) in this cohort were hospitalized, which presumably led to more clinical work-up than in the general population. For this reason, we did not use external controls but instead applied a cohort study design allowing us to use internal controls. Although this cohort included over 4 million patients, the rarity of WM and of some of the chronic immune stimulatory conditions evaluated limited analyses and led to imprecision in some estimates. Multiple comparisons may result in chance findings. Further, exposures that have been discovered recently (e.g., HCV in 1989, HIV in 1983) and less severe autoimmune and infectious conditions that do not lead to hospitalization may not be well represented.
Strengths of our study include its extensive follow-up in a patient population with access to standardized medical care that does not depend on socioeconomic status. Further, there is no recall bias because the exposure information is obtained from medical records. Because of the size of this population, we were able to evaluate the incidence of WM in blacks and to provide the first analysis of risk factors for WM in blacks.
In summary, we found that WM is associated with autoimmune disease, especially those with detectable autoantibodies, in the largest study of chronic immune stimulatory conditions and WM to date. Patients with specific infectious diseases, such as hepatitis, HIV, and rickettsiosis, also appeared to be at increased of WM. These results suggest that chronic immune stimulation plays an important role in the etiology and pathogenesis of WM. Better characterization of mechanisms mediating clonal proliferation and survival will ultimately enhance our understanding of WM pathophysiology, provide clues to etiology, and allow identification of novel molecular targets.
Acknowledgments
We thank the Medical Administration Service of the Veterans Health Services and Research Administration for providing the data on which this study is based and David Campbell and Eric Boyd, Information Management Services, Silver Spring, MD, for assistance with data preparation. We also thank David Check for assistance with the creation of Figure 1. Jill Koshiol had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This research was supported by the Intramural Research Program of the NIH, NCI.
References
- 1.Berger F, Isaacson PG, Piris MA, et al. Lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 132–134. [Google Scholar]
- 2.Owen RG, Treon SP, Al Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca R, Hayman S. Waldenstrom macroglobulinaemia. Br J Haematol. 2007;138:700–720. doi: 10.1111/j.1365-2141.2007.06724.x. [DOI] [PubMed] [Google Scholar]
- 4.Vijay A, Gertz MA. Waldenstrom macroglobulinemia. Blood. 2007;109:5096–5103. doi: 10.1182/blood-2006-11-055012. [DOI] [PubMed] [Google Scholar]
- 5.Groves FD, Travis LB, Devesa SS, Ries LA, Fraumeni JF., Jr Waldenstrom’s macroglobulinemia: incidence patterns in the United States, 1988–1994. Cancer. 1998;82:1078–1081. [PubMed] [Google Scholar]
- 6.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–3764. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 8.Kyle RA, Rajkumar SV, Therneau TM, Larson DR, Plevak MF, Melton LJ., III Prognostic factors and predictors of outcome of immunoglobulin M monoclonal gammopathy of undetermined significance. Clin Lymphoma. 2005;5:257–260. doi: 10.3816/clm.2005.n.011. [DOI] [PubMed] [Google Scholar]
- 9.Blattner WA, Garber JE, Mann DL, et al. Waldenstrom’s macroglobulinemia and autoimmune disease in a family. Ann Intern Med. 1980;93:830–832. doi: 10.7326/0003-4819-93-6-830. [DOI] [PubMed] [Google Scholar]
- 10.Brown AK, Elves MW, Gunson HH, Pell-Ilderton R. Waldenstroms macroglobulinaemia. A family study. Acta Haematol. 1967;38:184–192. doi: 10.1159/000209015. [DOI] [PubMed] [Google Scholar]
- 11.Elves MW, Brown AK. Cytogenetic studies in a family with Waldenstrom’s macroglobulinaemia. J Med Genet. 1968;5:118–122. doi: 10.1136/jmg.5.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Getaz EP, Staples WG. Familial Waldenstrom’s macroglobulinaemia: a case report. S Afr Med J. 1977;51:891–892. [PubMed] [Google Scholar]
- 13.McMaster ML, Giambarresi T, Vasquez L, Goldstein AM, Tucker MA. Cytogenetics of familial Waldenstrom’s macroglobulinemia: in pursuit of an understanding of genetic predisposition. Clin Lymphoma. 2005;5:230–234. doi: 10.3816/clm.2005.n.005. [DOI] [PubMed] [Google Scholar]
- 14.Ogmundsdottir HM, Sveinsdottir S, Sigfusson A, Skaftadottir I, Jonasson JG, Agnarsson BA. Enhanced B cell survival in familial macroglobulinaemia is associated with increased expression of Bcl-2. Clin Exp Immunol. 1999;117:252–260. doi: 10.1046/j.1365-2249.1999.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renier G, Ifrah N, Chevailler A, Saint-Andre JP, Boasson M, Hurez D. Four brothers with Waldenstrom’s macroglobulinemia. Cancer. 1989;64:1554–1559. doi: 10.1002/1097-0142(19891001)64:7<1554::aid-cncr2820640734>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Treon SP, Hunter ZR, Aggarwal A, et al. Characterization of familial Waldenstrom’s macroglobulinemia. Ann Oncol. 2006;17:488–494. doi: 10.1093/annonc/mdj111. [DOI] [PubMed] [Google Scholar]
- 17.McMaster ML, Goldin LR, Bai Y, et al. Genomewide linkage screen for Waldenstrom macroglobulinemia susceptibility loci in high-risk families. Am J Hum Genet. 2006;79:695–701. doi: 10.1086/507687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki H, Takishita M, Kosaka M, Saito S. Frequent somatic mutations in D and/or JH segments of Ig gene in Waldenstrom’s macroglobulinemia and chronic lymphocytic leukemia (CLL) with Richter’s syndrome but not in common CLL. Blood. 1995;85:1913–1919. [PubMed] [Google Scholar]
- 19.Wagner SD, Martinelli V, Luzzatto L. Similar patterns of V kappa gene usage but different degrees of somatic mutation in hairy cell leukemia, prolymphocytic leukemia, Waldenstrom’s macroglobulinemia, and myeloma. Blood. 1994;83:3647–3653. [PubMed] [Google Scholar]
- 20.Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 21.Boren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. Autoimmun Rev. 2004;3:401–406. doi: 10.1016/j.autrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landgren O, Engels EA, Pfeiffer RM, et al. Autoimmunity and susceptibility to Hodgkin lymphoma: a population-based case-control study in Scandinavia. J Natl Cancer Inst. 2006;98:1321–1330. doi: 10.1093/jnci/djj361. [DOI] [PubMed] [Google Scholar]
- 24.Mellemkjaer L, Pfeiffer RM, Engels EA, et al. Autoimmune disease in individuals and close family members and susceptibility to non-Hodgkin’s lymphoma. Arthritis Rheum. 2008;58:657–666. doi: 10.1002/art.23267. [DOI] [PubMed] [Google Scholar]
- 25.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165:2337–2344. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 26.Linet MS, Humphrey RL, Mehl ES, et al. A case-control and family study of Waldenstrom’s macroglobulinemia. Leukemia. 1993;7:1363–1369. [PubMed] [Google Scholar]
- 27.Richardson C, Waldrop J. In: Veterans: 2000 Census 2000 Brief. Bureau UC, editor. Washington DC: US Department of Commerce; 2003. [Google Scholar]
- 28.Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ. 1987:1–406. [PubMed] [Google Scholar]
- 29.Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjogren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65:796–803. doi: 10.1136/ard.2005.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander DD, Mink PJ, Adami HO, et al. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. 1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 31.Walker DH. Rickettsiae and rickettsial infections: the current state of knowledge. Clin Infect Dis. 2007;45(Suppl 1):S39–S44. doi: 10.1086/518145. [DOI] [PubMed] [Google Scholar]
- 32.Hutchings A, Purcell WM, Benfield MR. Peripheral blood antigen-presenting cells from African-Americans exhibit increased CD80 and CD86 expression. Clin Exp Immunol. 1999;118:247–252. doi: 10.1046/j.1365-2249.1999.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerman RH, Kimball PM, Van Buren CT, Lewis RM, Kahan BD. Possible contribution of pretransplant immune responder status to renal allograft survival differences of black versus white recipients. Transplantation. 1991;51:338–342. doi: 10.1097/00007890-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Chen QY, Jackson N, Vargas A, et al. Identification of three genomic haplotypes 5′ to the human CD1D gene and their distribution in four ethnic groups. Tissue Antigens. 2003;62:442–448. doi: 10.1034/j.1399-0039.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 35.Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom’s macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;23:4662–4668. doi: 10.1200/JCO.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 36.Lee R, Rose MS, Harmer CL. Vinblastine-loaded platelets--their effect in a patient with immune thrombocytopenia associated with Waldenstrom’s macroglobulinaemia. Clin Lab Haematol. 1982;4:61–65. doi: 10.1111/j.1365-2257.1982.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 37.Owen RG, Lubenko A, Savage J, Parapia LA, Jack AS, Morgan GJ. Autoimmune thrombocytopenia in Waldenstrom’s macroglobulinemia. Am J Hematol. 2001;66:116–119. doi: 10.1002/1096-8652(200102)66:2<116::AID-AJH1026>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 38.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:401–404. doi: 10.1158/1055-9965.EPI-06-1056. [DOI] [PubMed] [Google Scholar]
- 39.Fisher SG, Fisher RI. The emerging concept of antigen-driven lymphomas: epidemiology and treatment implications. Curr Opin Oncol. 2006;18:417–424. doi: 10.1097/01.cco.0000239878.31463.0b. [DOI] [PubMed] [Google Scholar]
- 40.Percy C, Van Holten V, Muir C. International classification of diseases for oncology. 2. Geneva: World Health Organization; 1990. [Google Scholar]
- 41.Smedby KE, Baecklund E, Askling J. Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev. 2006;15:2069–2077. doi: 10.1158/1055-9965.EPI-06-0300. [DOI] [PubMed] [Google Scholar]