Abstract
AIM: To investigate the hepatoprotective activity of tea polyphenols (TP) and its relation with cytochrome P450 (CYP450) expression in mice.
METHODS: Hepatic CYP450 and CYPb5 levels were measured by UV-spectrophotometry in mice 2 d after intraperitoneal TP (25, 50 and 100 mg/kg per day). Then the mice were intragastricly pre-treated with TP (100, 200 and 400 mg/kg per day) for six days before paracetamol (1000 mg/kg) was given. Their acute mortality was compared with that of control mice. The mice were pre-treated with TP (100, 200, and 400 mg/kg per day) for five days before paracetamol (500 mg/kg) was given. Hepatic CYP2E1 and CYP1A2 protein and mRNA expression levels were evaluated by Western blotting, immunohistochemical staining and transcriptase-polymerase chain reaction.
RESULTS: The hepatic CYP450 and CYPb5 levels in mice of TP-treated groups (100, 200 and 400 mg/kg per day) were decreased in a dose-dependent manner compared with those in the negative control mice. TP significantly attenuated the paracetamol-induced hepatic injury and dramatically reduced the mortality of paracetamol-treated mice. Furthermore, TP reduced CYP2E1 and CYP1A2 expression at both protein and mRNA levels in a dose-dependent manner.
CONCLUSION: TP possess potential hepatoprotective properties and can suppress CYP450 expression.
Keywords: Tea polyphenols, Cytochrome P450, Paracetamol-induced hepatotoxicity
INTRODUCTION
Tea has been consumed in China to promote health and longevity since 3000 B.C. It is now a popular beverage all over the world. Tea polyphenols (TP) are a large and diverse class of compounds extracted from tea. The major compounds of TP are picatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG) and epigallocatechin-3-gallate (EGCG). Recent studies indicate that TP can prevent oxidative stress-related diseases, including cancer, cardiovascular and degenerative diseases and have other bioactive properties[1–4].
In recent years, the interest in understanding the metabolic benefits of TP has been increasing. Liver is the main organ responsible for the metabolism of TP. Liu et al[5] reported that TP can markedly increase cytochrome P450 (CYP450) activity in rats. However, the effect of TP on CYP450 activity remains controversial[6,7]. Until now, no one could give a clear explanation of the different results. CYP450 enzymes play a pivotal role not only in the metabolism of xenobiotics, but also in the biosynthesis and catabolism of endogenous substrates, such as vitamins, fatty acids, hormones and prostaglandins[8]. Alteration in hepatic CYP450 enzyme expression would affect the pharmacokinetic profiles of clinically used drugs. Furthermore, both induction and suppression of several CYP450s may lead to cellular oxidative stress and tissue injury in response to xenobiotics[9].
Paracetamol, one of the most widely used hepatotoxic drugs, is safe at therapeutic doses, but causes liver failure in overdoses[10,11]. When a normal dose is used, paracetamol is extensively metabolized by conjugation with sulphate and glucuronic acid. A small fraction of the drug is subjected to oxidation reactions catalyzed by CYP450 enzymes in the liver, resulting in generation of N-acetyl-p-benzo-quinoneimine (NAPQI), a highly electrophilic metabolite that triggers ensuing liver damage. Exposure to high doses of paracetamol increases the NAPQI level. Normally, toxic oxidation metabolites generated in the liver are converted into non-toxic metabolites excreted in urine via conjugation with glutathione (GSH) containing sulphydryl groups. However, high doses of paracetamol limit the ability of GSH to detoxify NAPQI, and result in the consumption of liver GSH stores[12,13]. It was reported that oxidative stress constitutes a major mechanism underlying the pathogenesis of paracetamol-induced liver damage[14,15].
There are three families of CYP450 isoforms, CYP1, CYP2, and CYP3, which are mainly involved in the biotransformation of xenobiotics[16]. It has been shown that CYP2E1, CYP1A2, and intracellular GSH play an important role in the hepatotoxicity induced by paracetamol[17–19]. The present study was to investigate the hepatopro-tective activity of TP and its relation with CYP450 expression in mice.
MATERIALS AND METHODS
Animal treatment
TP obtained from Wuyuan Tea Plantation (Jiangxi Province, China) with a purity of > 98% containing 63.84% of EGCG and 7.30% of ECG were decaffeinized. Kunming male mice, weighing 18-22 g, provided by Experimental Animal Center of Dalian Medical University, were used in the study. The mice were randomly divided into high, medium, low TP dose groups, positive and negative control groups (n = 10). The mice in low, medium and high dose TP groups were intraperitoneally injected with 25, 50 and 100 mg/kg of TP per day for two days. The mice in positive control group were given 50 mg/kg chloramphenicol one hour before they were killed. The mice in the negative control group were given the same volume of saline solution for two days. On day 3, all the mice were killed by decapitation, with their livers removed immediately and cleaned with a cold saline solution. Specimens were stored at -80°C for preparation of liver microsomes.
Male mice in each group received intragastric TP at the dose of 100, 200, and 400 mg/kg, once a day for six days. The mice in positive control group were given 900 mg/kg N-acetylcysteine, once a day. The mice in negative control group were given the same volume of a saline solution. All the mice were given 1000 mg/kg paracetamol one hour later and the acute mortality of mice in both groups was recorded 72 h after paracetamol was given.
Male mice in each group received intragastric TP at the dose of 100, 200, and 400 mg/kg, once a day for five days. The mice in model and negative control groups were given the same volume of a saline solution. The mice were given 500 mg/kg paracetamol one hour later, except for the mice in negative control group. All the mice were killed by decapitation 24 h later with their livers removed immediately and cleaned with a cold saline solution. Specimens were fixed in formalin for histological examination or snap-frozen in liquid nitrogen and stored at -80°C for protein and RNA extraction.
Preparation of liver microsomes
Liver microsomes extracted from frozen samples were prepared as previously described[20]. Microsomal protein level was measured as previously described[21] with bovine serum albumin as a standard.
Measurement of microsome enzyme levels
The levels of CYP450 and CYPb5 were measured as previously described[22].
Western blot assay
Aliquots (80 μg) of liver microsomes from each sample were loaded onto each lane of 10% SDS-PAGE gel electrophoresis and electroblotted onto nitrocellulose membranes (Millipore, Bedford, MA). The membranes were probed with rabbit antibody against mice CYP2E1 (Boster Biological Technology Co., Ltd, Wuhan, China) at a dilution of 1:400 or CYP1A2 (Santa Cruz Biotechnology, Inc., California, USA) at a dilution of 1:500 and peroxidase-conjugated affinipure goat anti-rabbit IgG (Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China) according to the manufacturer’s constructions. The signals were visualized with a DAB assay kit (Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China) and analyzed with Quantitiy One software.
Histology and immunohistochemical assay
All specimens embedded in paraffin were cut into 4-μm thick serial sections. The specimens were mounted on slides and deparaffinized with graded concentrations of xylene and ethanol, and stained with hematoxylin and eosin (HE). For immunohistochemical staining, all sections were immersed in 3% H2O2 for 15 min at room temperature to block the endogenous peroxidase activity. To stain with CYP2E1 and CYP1A2, the sections were predigested with 0.4% proteinase K for 5 min at 37°C. The sections were then incubated with 10% normal goat serum for 15 min to reduce the nonspecific background staining and reacted with polyclonal rabbit anti-mouse antibody (Ab) at 4°C overnight. The working dilution of Abs used for examining mouse CYP2E1 and CYP1A2 in the liver was diluted at 1:100 and 1:50. After rinsing with phosphate-buffered saline (pH 7.4), the sections were incubated with biotinylated anti-rabbit immunoglobulin secondary Abs at room temperature for 30 min. Horseradish peroxidase labeled streptomycin-avidin complex was used to detect the second antibody. Finally, the sections were counterstained with hematoxylin before examination under a light microscope. The brown or dark brown stained cells were considered positive cells.
Reverse transcription-polymerase chain reaction (RT-PCR) assay
Total RNA was extracted from liver tissue with the TRIquick reagent (Solarbio Science & Technology CO., Ltd, Beijing, China). RNA purity was assessed by the optical densities at 260 nm and 280 nm, and the integrity was verified by electrophoresis on a 1% agrose gel containing 0.5 μg/mL of ethidium bromide. Reverse transcription was conducted for 30 min at 42°C from 500 ng of purified RNA in 10 μL of reserve transcriptions system reaction mixture using AMV reverse transcriptase (TaKaRa, Japan), followed by 30 cycles of PCR (denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 1 min). The amplified products were electrophoresed on a 1.5% agarose gel using 100 bp DNA ladder markers (TaKaRa, Japan) as a standard to determine the molecular size and visualized with ethidium bromide staining. Images of the ethidium bromide-stained agarose gel were acquired with a Gel Doc EQ system and quantified using Quantity One software. The sequences of oligonucleotide primers are provided in Table 1.
Table 1.
Sequences of oligonucleotide primers
| Primer | Sequence |
| CYP2E1 F | 5’-CACCGTTGCCTTGCTTGTCTG-3’ |
| CYP2E1 R | 5’-CTCATGAGCTCCAGACACTTC-3’ |
| CYP1A2 F | 5’-AGTGTTCTGGATGGTCAGAGC-3’ |
| CYP1A2 R | 5’-GCAAGAGGATGCTGACGTCG-3’ |
| GAPDH F | 5’-ATGGTGAAGGTCGGTGTGAAC-3’ |
| GAPGH R | 5’-GTCTTCTGGGTGGCAGTGATG-3’ |
Statistical analysis
Data were calculated separately and expressed as mean ± SD. Statistical significances were analyzed by one-way ANOVA followed by Student Newman-Keuls test using SPSS Version 11.5. The difference was considered significant at two-tailed. P < 0.05 was considered statistically significant.
RESULTS
Effect of TP on CYP450 and CYPb5 levels
Two days after peritoneal injection of TP, the CYP450 and CYPb5 levels in livers of mice were measured. The CYP450 and CYPb5 levels were significantly lower in the high, medium and low dose TP groups than in the negative control group (P < 0.01), indicating that TP can reduce the CYP450 and CYPb5 levels in liver of mice. The CYP450 level was higher in the medium and low dose groups than in the positive control group (P < 0.01); but, it was not significantly different between the high dose and positive control groups (P > 0.05). The CYPb5 level was markedly different in the low dose group (P < 0.01); but it, was not significantly different in the high and medium dose groups (P > 0.05) compared the positive control group, indicating that TP can suppress both CYP450 and CYPb5 in mice in a dose-dependent manner (Table 2).
Table 2.
Effects of TP on CYP450 and CYPb5 levels (mean ± SD)
| Group (n = 10) | CYP450 (nmol/g) | CYPb5 (nmol/g) |
| Negative control | 0.354 ± 0.024b | 0.160 ± 0.011b |
| Low dose | 0.279 ± 0.014ab | 0.108 ± 0.004ab |
| Medium dose | 0.204 ± 0.016ab | 0.077 ± 0.006a |
| High dose | 0.130 ± 0.016a | 0.048 ± 0.006a |
| Positive control | 0.147 ± 0.019a | 0.061 ± 0.009a |
P < 0.01 vs negative control group (NS group);
P < 0.01 vs positive control group (chloramphenicol group).
Effect of TP on mortality of paracetamol-treated mice
We examined the effect of TP on mortality of paracetamol-treated mice. The mortality rate of mice in the negative control group, low, medium and high dose TP groups was 90%, 70%, 40% and 20%, respectively, indicating that TP can significantly reduce the mortality of paracetamol-treated mice. The effect of high dose TP on mortality of paracetamol-treated mice was not different from that of mice in the positive control group (Table 3).
Table 3.
Effect of TP on mortality induced by paracetamol in mice
| Group (n = 10) | Dose (mg/kg) | Death (n) | Death rate (%) | Protection rate (%) |
| Negative control | 9 | 90 | 10 | |
| Positive control | 2 | 20 | 80 | |
| TP | ||||
| Low | 100 | 7 | 70 | 30 |
| Medium | 200 | 4 | 40 | 60 |
| High | 400 | 2 | 20 | 80 |
Western blot analysis
Western blot showed strong positive CYP2E1 signals in the control and model groups. The expression of CYP2E1 in the low dose group was not significantly different from that in the control and model groups. However, TP significantly decreased the CYP2E1 expression level in the medium and high dose groups in a dose-dependent manner (Figure 1). The expression of CYP1A2 and CYP2E1 protein was similar in the liver microsomes (Data not shown).
Figure 1.
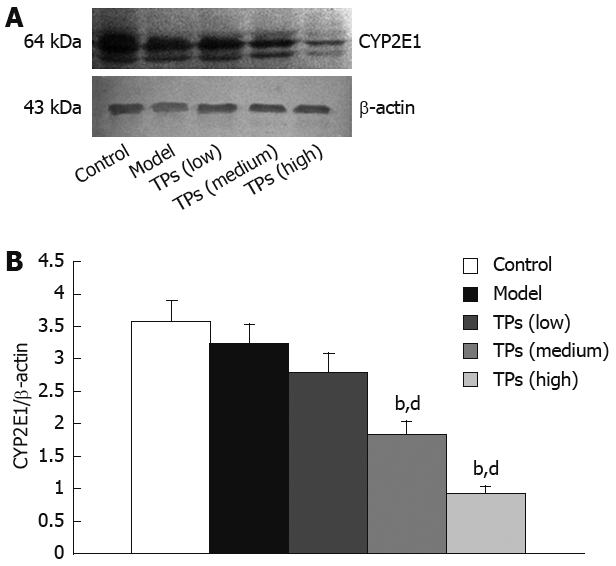
Western blot analysis of protein for CYP2E1 in liver tissue of mice showing representative bands of each group (A) and normalized densitometic ratios of CYP2E to β-actin (B). bP < 0.01 vs control group; dP < 0.01 vs model group.
Histology analysis
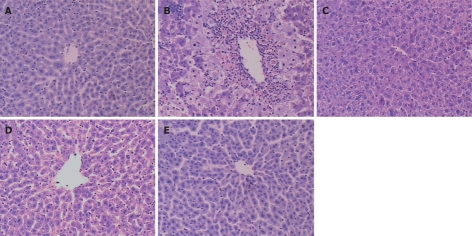
Liver tissues from the control group showed a normal lobular architecture with central veins and radiating hepatic cords (Figure 2A). However, liver tissues from the model group showed formation of more fibrous tissues extending into the hepatic lobules, leading to their complete separation. A large number of inflammatory cells infiltrated the intralobular and interlobular regions. The liver structure was in disorder and there were more necrotic and fatty degenerated liver cells than in the controls (Figure 2B). In the three TP treatment groups, however, hepatocyte degeneration, necrosis and infiltration of inflammatory cells were all apparently ameliorated (Figure 2C-E). Compared with the model group, the liver condition in TP treatment groups was significantly improved in a dose-dependent manner.
Figure 2.
Changes of HE staining in liver tissue of mice 24 h after paracetamol administration (× 100) in control group with a normal central lobular region (A), model group with some central lobular hepatocyte necrosis and microvesicular fatty change (B), low TP dose group (C), medium TP dose group (D) and high TP dose group (E). The pathological change of liver was much milder in different TP dose groups than in model group.
Immunohistochemical analysis of liver CYP2E1 and CYP1A2
The expression of CYP2E1 showed a deep brown immunostaining. No difference was found in the expression of CYP2E1 between the control and model groups. However, in medium and high dose groups, TP significantly suppressed the CYP2E1 expression in a dose-dependent manner (Figure 3A).
Figure 3.
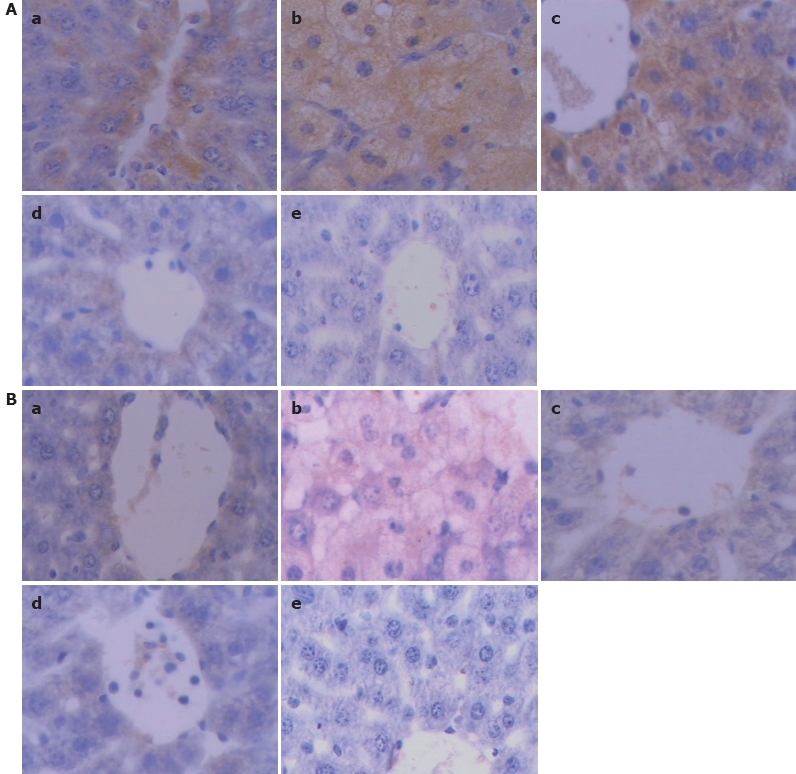
Immunohistochemical staining showing expression of CYP2E1 (A) and CYP1A2 (B) in liver tissue of mice 24 h after paracetamol administration (× 400) in control group (a), model group (b), low TP dose group (c), medium TP dose group (d) and high TP dose group (e). The brown or dark brown stained cells were considered positive.
The expression level of CYP1A2 (showing a deep brown immunostaining) was markedly lower in the low, medium and high dose TP groups than in the control and model groups (Figure 3B).
Expression levels of CYP2E1 and CYP1A2 mRNA
The expression levels of CYP2E1, CYP1A2 and GAPDH cDNAs in the mouse liver were assayed by RT-PCR (Figure 4A), and the ratio of CYP2E1 and CYP1A2/ GAPDH cDNAs was calculated (Figure 4B). TP significantly inhibited the CYP2E1 mRNA expression in medium and high dose groups (P < 0.01), compared to the control and model groups (P > 0.05). The expression of CYP1A2 mRNA was reduced in all TP groups compared with the control and model groups (P < 0.05), indicating that TP can suppress the expression of CYP2E1 and CYP1A2 mRNA in a dose-dependent manner.
Figure 4.
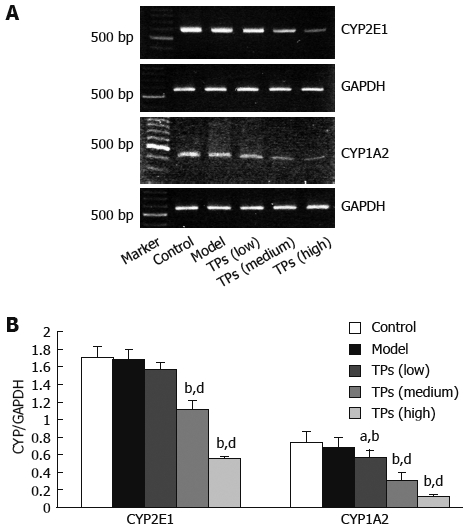
RT-PCR analysis of mRNA for CYP2E1 and CYP1A2 expression in liver tissue of mice showing representative bands of each group (A) and normalized densitometic ratio of CYP2E and CYP1A2 to GAPDH (B). aP < 0.05 vs model group; bP < 0.01 vs control group; dP < 0.01 vs model group.
DISCUSSION
Hepatic drug metabolizing enzyme is called mixed-function oxidase or monooxygenase containing many enzymes including phase I enzymes such as CYP450 and CYPb5, etc, and plays a prominent role in the metabolism of many pharmaceutical agents and activation or deactivation of potential carcinogens. Acquiring metabolic information and determining the effect of chemicals on hepatic drug metabolizing enzymes are important in developing clinically safe and efficient medications[23].
TP have various pharmacological effects on cancer, cardiovascular and degenerative diseases and have other bioactive properties[1–4]. In this experiment, TP significantly suppressed the CYP450 and CYPb5 levels in liver of mice, suggesting that TP can restrain the activity of drug metabolizing enzymes.
In the present study, 100, 200 and 400 mg/kg TP could reduce the mortality of mice after treatment with paracetamol (Table 2). The immunohistochemical study showed that TP markedly alleviated paracetamol- induced hepatotoxicity, thus improving alterations in liver tissue pathology. Especially marked hepatoprotective effects of TP were observed in the high dose TP group with its diffuse hemorrhage and necrosis changed into focal hemorrhage and necrosis (Figure 2) compared to the model group that received a single dose of 500 mg/kg paracetamol, indicating that TP can protect liver of mice against paracetamol injury, which is consistent with the reported data[24].
It was reported that many mechanisms are involved in paracetamol hepatotoxicity[25], showing that the toxicity is mediated by CYP450 metabolism of paracetamol to NAPQI which covalently binds to critical proteins leading to inactivation of these proteins, especially after GSH depletion. CYP2E1 is usually assumed to be the most active CYP450 in catalyzing the metabolism of paracetamol to hepatotoxic NAPQI[15,18]. Studies with knockout mice showed that CYP2E1 and CYP1A2 play an important role in the metabolism of paracetamol[26,27]. To confirm whether the protective effects of TP on liver are correlated with the inhibition of CYP2E1 and CYP1A2, we investigated the effect of TP on the expression of CYP2E1 and CYP1A2 mRNA and protein.
In the present study, 200 mg/kg and 400 mg/kg TP significantly down-regulated the CYP2E1 expression either at gene level (Figure 4) or at protein level (Figures 1 and 3) as shown by RT-PCR, Western blot and immunohistochemicstry. The effects of TP on the expression of CYP1A2 and CYP2E1 gene and protein were similar. It was reported that the CYP1A2 level in increased in rats after drinking a 2% solution of green or black tea[28]. Caffeine, a component of tea, is responsible for the induction of CYP1A2[29]. In our study, however, different doses of TP (100, 200 and 400 mg/kg) significantly suppressed the expression of CYP1A2 protein and mRNA, partly because TP used in our experiment did not contain caffeine.
The above findings indicate that the dose-dependent liver-protective effects of TP are likely related to the suppression of CYP2E1 and CYP1A2 expression, which reduces NAPQI associated with the paracetamol hepatotoxicity, and effectively protects liver against paracetamol hepatotoxicity.
In conclusion, TP restrain the activity of drug metabolizing enzymes, CYP450 and CYPb5, and protect liver against paracetamol-induced injury. The protective effects of TP are associated with the expression of CYP2E1 and CYP1A2 gene and protein.
COMMENTS
Background
Paracetamol is one of the most widely used hepatotoxic drugs, which is safe at therapeutic doses, but causes liver failure at overdoses. High doses of paracetamol limit the ability of glutathione (GSH) to detoxify N-acetyl-p-benzoquinonimine (NAPQI), and results in consumption of liver GSH stores. There are three families of cytochrome P450 (CYP450) isoforms, CYP1, CYP2, and CYP3, which are mainly involved in the biotransformation of xenobiotics. It has been shown that CYP2E1, CYP1A2 and intracellular GSH play an important role in paracetamol-induced hepatotoxicity. Tea polyphenols (TP) are a large and diverse class of compounds extracted from tea. Liver is the main organ responsible for the metabolism of TP. Some work has been done in the field of TP modulation or interaction with drug metabolizing enzymes. However, until now, no one could give a clear explanation for this.
Research frontiers
TP are a large and diverse class of compounds extracted from tea. Liver is the main organ responsible for the metabolism of TP. Some work has been done in the field of TP modulation or interaction with drug metabolizing enzymes. However, until now, no one could give a clear explanation for this. The research hotspot is to investigate the effects of TP on CYP450 and CYPb5 expression in livers of male mice. Whether the protective effect of TP on paracetamol-induced hepatotoxicity in mice is related with their effect on CYP450 was studied.
Innovations and breakthroughs
The protective effect of TP on paracetamol-induced hepatotoxicity was found to be related with their effect on CYP450.
Applications
TP possess potential hepatoprotective properties, which are closely related with their suppressive effect on CYP450 expression.
Terminology
Paracetamol-induced hepatotoxicity: When a normal dose is used, paracetamol is metabolized extensively by conjugation with sulphate and glucuronic acid. A small fraction of paracetamol is subjected to oxidation reactions catalyzed by CYP450 enzymes in the liver, resulting in generation of NAPQI, a highly electrophilic metabolite that triggers the ensuing liver damage.
Peer review
This is a good descriptive study in which the authors gave a clear explanation of the effect of TP on the expression of CYP450, CYP2E1 and CYP1A2 in liver of mice at both protein and mRNA levels. The interesting results suggest that TP can significantly attenuate paracetamol-induced hepatic injury, which is closely related with their suppressive effect on CYP450 expression.
Acknowledgments
The authors thank Professor Zhong-Gun Yan (Center for Molecular Medicine, Karolinska Institute, Stockholm, Sweden) and Jiang-Lin Fan (Department of Pathology, University of Yamanashi, Japan) for their technical assistance.
Supported by Grant from the Science Foundation of Educational Department of Liaoning Province, 05L117 and Dalian Science & Technology Bureau, 2007J22JH012
Peer reviewer: Vincent Lai, PhD, Derby NHS Foundation Trust, Utooxeter Road, Derby DE22 3NE, United Kingdom
S- Editor Li LF L- Editor Wang XL E- Editor Lin YP
References
- 1.Trevisanato SI, Kim YI. Tea and health. Nutr Rev. 2000;58:1–10. doi: 10.1111/j.1753-4887.2000.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 2.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 3.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 4.Naasani I, Oh-Hashi F, Oh-Hara T, Feng WY, Johnston J, Chan K, Tsuruo T. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003;63:824–830. [PubMed] [Google Scholar]
- 5.Liu TT, Liang NS, Li Y, Yang F, Lu Y, Meng ZQ, Zhang LS. Effects of long-term tea polyphenols consumption on hepatic microsomal drug-metabolizing enzymes and liver function in Wistar rats. World J Gastroenterol. 2003;9:2742–2744. doi: 10.3748/wjg.v9.i12.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan H, Wu J, Zheng S. [Inhibition of colorectal carcinoma induced by 1, 2-dimethylhydrazine in mice with tea polyphenols] Zhonghua Yufang Yixue Zazhi. 1995;29:356–359. [PubMed] [Google Scholar]
- 7.Mukhtar H, Wang ZY, Katiyar SK, Agarwal R. Tea components: antimutagenic and anticarcinogenic effects. Prev Med. 1992;21:351–360. doi: 10.1016/0091-7435(92)90042-g. [DOI] [PubMed] [Google Scholar]
- 8.Guengerich FP. Cytochrome P450 enzymes. Am Sci. 1993;81:440–447. [Google Scholar]
- 9.Cho MK, Kim YG, Lee MG, Kim SG. Suppression of rat hepatic cytochrome P450s by protein-calorie malnutrition: complete or partial restoration by cysteine or methionine supplementation. Arch Biochem Biophys. 1999;372:150–158. doi: 10.1006/abbi.1999.1482. [DOI] [PubMed] [Google Scholar]
- 10.McCloskey P, Edwards RJ, Tootle R, Selden C, Roberts E, Hodgson HJ. Resistance of three immortalized human hepatocyte cell lines to acetaminophen and N-acetyl-p-benzoquinoneimine toxicity. J Hepatol. 1999;31:841–851. doi: 10.1016/s0168-8278(99)80285-2. [DOI] [PubMed] [Google Scholar]
- 11.Lewerenz V, Hanelt S, Nastevska C, El-Bahay C, Röhrdanz E, Kahl R. Antioxidants protect primary rat hepatocyte cultures against acetaminophen-induced DNA strand breaks but not against acetaminophen-induced cytotoxicity. Toxicology. 2003;191:179–187. doi: 10.1016/s0300-483x(03)00256-7. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- 13.Savides MC, Oehme FW. Acetaminophen and its toxicity. J Appl Toxicol. 1983;3:96–111. doi: 10.1002/jat.2550030209. [DOI] [PubMed] [Google Scholar]
- 14.Ozdemirler G, Aykaç G, Uysal M, Oz H. Liver lipid peroxidation and glutathione-related defence enzyme systems in mice treated with paracetamol. J Appl Toxicol. 1994;14:297–299. doi: 10.1002/jat.2550140410. [DOI] [PubMed] [Google Scholar]
- 15.Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 16.Nedelcheva V, Gut I. P450 in the rat and man: methods of investigation, substrate specificities and relevance to cancer. Xenobiotica. 1994;24:1151–1175. doi: 10.3109/00498259409038673. [DOI] [PubMed] [Google Scholar]
- 17.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, Gonzalez FJ. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005;33:449–457. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- 18.Raucy JL, Lasker JM, Lieber CS, Black M. Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch Biochem Biophys. 1989;271:270–283. doi: 10.1016/0003-9861(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- 20.Souidi M, Parquet M, Férézou J, Lutton C. Modulation of cholesterol 7alpha-hydroxylase and sterol 27-hydroxylase activities by steroids and physiological conditions in hamster. Life Sci. 1999;64:1585–1593. doi: 10.1016/s0024-3205(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Liu GT. Empirical method of enzymology. In: SY Xu, RL Bian, X Chen., editors. Pharmacological empirical methodology. Beijing: Renmin Hygiene Publishing Company; 2001. p. 513. [Google Scholar]
- 23.Hu YZ, Yao TW. In vitro metabolism and inductive or inhibitive effect of DL111 on rat cytochrome P4501A enzyme. Chem Biol Interact. 2004;147:109–117. doi: 10.1016/j.cbi.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Oz HS, McClain CJ, Nagasawa HT, Ray MB, de Villiers WJ, Chen TS. Diverse antioxidants protect against acetaminophen hepatotoxicity. J Biochem Mol Toxicol. 2004;18:361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- 25.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 27.Genter MB, Liang HC, Gu J, Ding X, Negishi M, McKinnon RA, Nebert DW. Role of CYP2A5 and 2G1 in acetaminophen metabolism and toxicity in the olfactory mucosa of the Cyp1a2(-/-) mouse. Biochem Pharmacol. 1998;55:1819–1826. doi: 10.1016/s0006-2952(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 28.Sohn OS, Surace A, Fiala ES, Richie JP Jr, Colosimo S, Zang E, Weisburger JH. Effects of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica. 1994;24:119–127. doi: 10.3109/00498259409043226. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Bondoc FY, Lee MJ, Hussin AH, Thomas PE, Yang CS. Caffeine induces cytochrome P4501A2: induction of CYP1A2 by tea in rats. Drug Metab Dispos. 1996;24:529–533. [PubMed] [Google Scholar]