Abstract
AIM: To prospectively evaluate the short and long term clinical impact of selective transarterial chemoembolization (TACE) on liver function in patients with hepatocellular carcinoma (HCC). To assess side effects in relation to treatments. To analyze the overall survival and HCC progression free survival probability.
METHODS: One hundred and seventeen cirrhotic patients with HCC were enrolled. Baseline liver function included Child-Pugh score and serum levels of alanine-aminotransferase (ALT), prothrombin time (PT) and bilirubin. According to Cancer Liver Italian Program (CLIP) and Barcelona Clinic Liver Cancer (BCLC) staging systems, 71 patients were eligible for TACE; 32 had previously received treatment for HCC. No significant differences in liver function were observed between previously treated and not treated patients. TACE was performed by selective catheterization of the arteries nourishing the lesions. While hospitalized, patients underwent clinical, hematologic and ultrasonographic assessments. One month after TACE a CT scan was performed to assess tumor response. A second TACE was performed “on demand”. Liver function tests were checked in all patients every four months.
RESULTS: After first TACE, the mean Child-Pugh score increased from a mean baseline 5.62 ± 1.12 to 6.11 ± 1.57 at discharge time (P < 0.0001), decreasing after four months to 5.81 ± 0.73 (not significant). ALT, PT and bilirubin significantly (P < 0.0001) increased 24 h after TACE and progressively decreased until discharge. After the second TACE, variations in Child-Pugh score, ALT, PT and bilirubin were comparable to that described after the first TACE. No major complications were observed. The mean follow-up was 14.7 ± 6.3 mo (median: 16 mo). Only one patient died. No other patient experienced important long term worsening of clinical status. The overall survival probability at twenty-four months was 98.18% with a correspondent HCC progression free survival probability of 69%.
CONCLUSION: Selective TACE may produce significant, but transitory increases in ALT values, with no major impact on liver function and Child-Pugh score. Preservation of liver function is achievable also in patients previously treated with other therapeutic modalities and in patients undergoing multiple TACE cycles. Liver function can remain stable in the long-term, with optimal medium term survival. This result can be achieved through rigorous patient selection on the basis of tumour characteristics and clinical conditions.
Keywords: Hepatocellular carcinoma, Transarterial chemoembolization, Liver function, Liver cirrhosis, Child-Pugh score
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm in the world[1] and its incidence is increasing worldwide[2]. Overall, HCC is associated with liver cirrhosis in 80% of cases and it is the leading cause of death among cirrhotic patients[3].
The treatment of patients with HCC has evolved in the last few years. However, curative treatments such as liver resection, liver transplantation or percutaneous ablation [percutaneous ethanol injection (PEI) and radiofrequency ablation (RF)] are applicable in only 30%-40% of cases[4]. Since transarterial chemoembolization (TACE) was introduced as a palliative treatment in patients with unresectable HCC, it has become one of the most common forms of interventional therapy[5]. Recently, it has been demonstrated that TACE improves survival compared with best supportive care in meta-analyses of randomized trials[6,7] and in two individual clinical trials[8,9].
Nowadays TACE is often performed by selective catheterization of the hepatic segmental arteries nourishing the HCC lesions, to limit as much as possible the injury to the surrounding non tumorous liver as reported in previous studies[10–12]. However, although selective TACE is currently widely used, to our knowledge there are no reported extensive data from large series on both short and long term effects of this treatment on liver function. Because the optimal number of sessions is not known[13], it is debatable if repeated courses of selective TACE may progressively impair liver function and if they are well tolerated or are limited by major side effects.
On this basis, in the present study, we prospectively evaluated the short and long term impact of selective TACE on liver function in a consecutive series of patients with liver cirrhosis and HCC. Side effects in relation to treatments were also assessed. Furthermore, we analyzed the overall survival and HCC progression free survival probability.
MATERIALS AND METHODS
Patients and HCC diagnosis
From September 1st 2006 to August 31st 2008, at a single center, we prospectively evaluated 117 consecutive patients with liver cirrhosis and HCC. Patients’ demographic characteristics and etiology of liver cirrhosis are reported in Table 1.
Table 1.
Patients’ demographic characteristics and etiology of liver cirrhosis n (%)
| Patients | |
| Patients number | 117 |
| Gender | |
| Male | 81 (69.2) |
| Female | 36 (30.8) |
| Age (yr) | |
| mean ± SD | 69 ± 7.8 |
| Range | 43-85 |
| Causes of cirrhosis | |
| HBV (hepatitis B) | 9 (7.7) |
| HBV and NAFLD | 5 (4.3) |
| HBV and alcohol abuse | 1 (0.09) |
| HCV (hepatitis C) | 59 (50.4) |
| HCV and NAFLD | 16 (13.7) |
| HCV and alcohol abuse | 10 (8.5) |
| Alcohol abuse | 8 (6.8) |
| Alcohol abuse and overweight | 8 (6.8) |
| Sarcoidosis | 1 (0.09) |
NAFLD: Non-alcoholic fatty liver disease.
HCC diagnosis was established by means of alpha-fetoprotein (AFP) assay, abdominal ultrasound examination and cross-sectional imaging, including at least one multiphase contrast-enhanced spiral or multidetector CT (CT; Hi Speed CT/I or light speed plus, GE Medical Systems, Milwaukee, USA). Extrahepatic metastases were ruled out by chest X-ray and bone scintigraphy. The HCC Tumor-Node-Metastasis (TNM) stages for our patients were: 44 (37.6%) patients T1 N0 M0, 40 (34.1%) patients T2 N0 M0, 32 (27.3%) patients T4 N0 M0, 1 (0.8%) patient T4 N0 M1.
Baseline evaluation of liver function included determination of the Child-Pugh score and serum levels of alanine-aminotransferase (ALT), prothrombin time (PT) and bilirubin.
The Child-Pugh class of disease was A (score 5-6) in 71 patients, B (score 7-9) in 26, and C (score 11-15) in 20.
We also stratified patients according to the staging system for HCC of Cancer Liver Italian Program (CLIP)[14]: it combines Child-Pugh staging with tumor criteria (tumor morphology, portal invasion and AFP levels). The CLIP score stratification of our patients is described in Figure 1.
Figure 1.
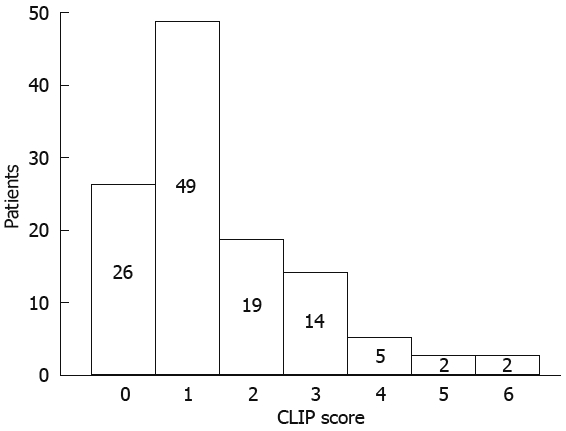
Stratification of HCC patients according to CLIP score.
According to the Barcelona Clinic Liver Cancer (BCLC) staging system[15], which assesses tumor characteristics and liver function to generate a treatment algorithm, 13 patients were suitable for curative treatments (resection, liver transplant, PEI/RF) and 33 patients had advanced (i.e. portal invasion) or terminal stage HCC suitable only for symptomatic treatments. The remaining 71 patients were considered eligible for TACE. In this group mean HCC size was 39.8 ± 14.1 mm.
We further stratified the patients suitable for TACE in two subgroups according to whether or not they had positive anamnesis for previous treatments for HCC. Thirty-two out of 71 patients (45%) had received treatment for HCC (TACE in 15, liver resection in 4, PEI/RF in 3 cases) before coming to our center. In these pretreated patients, the mean time between the first diagnosis of HCC and inclusion in our study was 16.7 ± 22.4 mo. No significant differences in Child-Pugh and CLIP scores were observed between previously and not previously treated patients (Table 2).
Table 2.
Child-Pugh and CLIP score in 71 patients eligible for TACE at enrollment (mean ± SD)
| Not pretreated patients | Previously treated patients | |
| Number of patients | 39 | 32 |
| Child-Pugh score | 5.82 ± 0.82 | 5.4 ± 1.2 |
| CLIP score | 1.12 ± 0.82 | 0.86 ± 0.83 |
TACE protocol
Informed consent was obtained from all patients after the nature and the purpose of TACE had been fully explained. Digital subtraction angiography (DSA; Multistar, Siemens, Erlangen, Germany) was performed in all patients immediately before TACE.
TACE was performed by selective catheterization of the hepatic segmental arteries nourishing the lesions, using either 5-F catheters (Simmons 1 and Cobra; Mallinckrodt, St Louis, USA or Hydrophilic Simmons 1 and Cobra; Terumo, Tokyo, Japan) or 3-F coaxial microcatheters (Tracker 18; Vascular Access System, Target, St José, USA; SP Catheter; Terumo). A mixture of iodised oil (Lipiodol UltraFluid; Laboratories Guerbet, Aulnay-sous-Bois, France) and epirubicin hydrochloride (Farmorubicina; Farmitalia Carlo Erba, Milan, Italy) was injected, followed by selective arterial embolization using gelatin sponge particles (Spongostan Standard; Johnson and Johnson Medical Limited, Gargrave, Skipton, UK).
The amount of administered Lipiodol (10-30 mL) and anticancer drug (40-100 mg) was decided on the basis of number, location and diameter of lesions.
Follow-up
After TACE procedure, the patients recovered with about 20 h bed rest. After this time, compression of the femoral artery, used as an access for administration of medication, was removed. Every 2 h during the first 6 h patients underwent clinical examination (abdominal evaluation and measurements of pulse rate, arterial blood pressure and body temperature).
A routine hematologic check was performed in all patients the morning after TACE and at discharge time, usually 5-7 d after the procedure. If severe abdominal pain or fever persisted during hospitalization, patients underwent abdominal ultrasonography to help exclude complications. One month after TACE a CT scan of the liver was performed to assess tumor response. TACE was repeated “on demand”, if there was no tumor response, insufficient tumor response or progressive disease.
Tumor response to TACE was evaluated according to World Health Organization (WHO) criteria (complete response (CR): complete disappearance of all known disease and no new lesions; partial response (PR): at least 50% reduction in total tumor load of all measurable lesions; stable disease (ST): does not qualify for CR/PR or progressive disease; progressive disease (PD): at least 25% increase in size of one or more measurable lesions or the appearance of new lesions) modified according to the EASL amendments that take into account the reduction in viable tumor volume due to TACE-induced necrosis[16].
Liver function tests were checked in all patients every four months in order to evaluate hepatic functional reserve.
Statistical analysis
Descriptive statistics (mean ± SD) were provided when appropriate. Parametric data were tested with Student’s paired t-test. Overall survival and HCC progression free survival curves were determined by the Kaplan-Meier method. A two sided P value of less 0.05 was considered statistically significant. Statistical analysis was performed with SAS software (SAS Institute, Cary, NC).
RESULTS
Ninety-eight TACE procedures were performed in 71 patients (mean number of treatments per patient 1.4 ± 0.61). After first TACE, the mean Child-Pugh score increased from a mean baseline 5.62 ± 1.12 to 6.11 ± 1.57 at discharge time (P < 0.0001), decreasing after four months to 5.81 ± 0.73 (not significant).
ALT, PT and bilirubin significantly (P < 0.0001) increased 24 h after TACE and then progressively decreased until discharge (Figure 2). After the second TACE cycle, variation of Child-Pugh scores (mean procedural value of 5.71 ± 0.78, discharge value to 5.9 ± 1.07, P = 0.056 not significant) and of ALT, PT and bilirubin were comparable to that described after the first TACE cycle.
Figure 2.
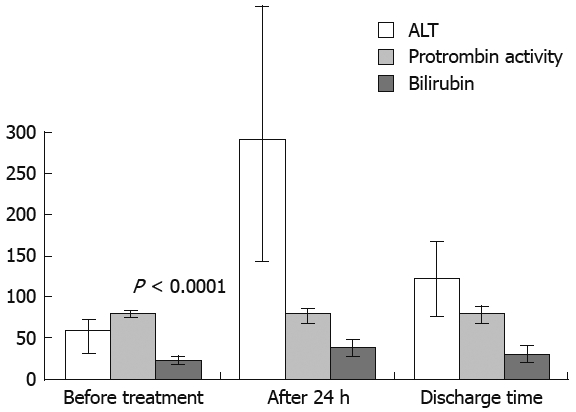
Variations of ALT, PT and bilirubin after the first TACE (71 patients).
No major complications were observed in our series of patients after TACE. After the first TACE cycle, post chemoembolization syndrome occurred in 23 (32.4%) patients, whereas 4 (5.6%) patients developed acute cholecystitis and 2 (2.8%) patients presented with hematomas at the site of the femoral artery used as an access for administration of medication.
Following a second TACE cycle (22 patients), 5 (22.7%) patients experienced post chemoembolization syndrome, 1 (4.5%) patient presented with acute cholecystitis and 1 (4.5%) case showed mild ascites. After both the first or second TACE, side effects were successfully managed with medical therapy. When comparing patients according to whether or not they had positive anamnesis for previous treatments for HCC, we did not find significant differences in the modifications of pre- and post-TACE Child-Pugh scores.
Six months after chemoembolization in 57 (80%) out of 71 patients, the mean tumor size had not changed. In the remaining 14 (20%) patients, mean tumor size was 44.8 ± 17.7 mm. The mean follow-up was 14.7 ± 6.3 mo (median 16 mo). At the end of the follow-up, 49 (69%) of the treated patients presented with a CR; 21 (29.57%) patients had a PR; only 1 (1.4%) patient had progression of the tumor that involved more than 50% of the liver, with rapidly progressive worsening of clinical condition and appearance of bone metastasis. No other patient with class A disease was reclassified as having a class B disease, and no other patient with class B disease was reclassified as having class C disease after either the first or the second TACE.
On follow-up, despite slight fluctuations in Child-Pugh score, no other patient experienced important long term worsening of clinical status and there was no progression of HCC that could modify CLIP or BCLC.
The overall survival probability at 24 mo was 98.18% (Figure 3) with a correspondent HCC progression free survival probability of 69% (Figure 4).
Figure 3.
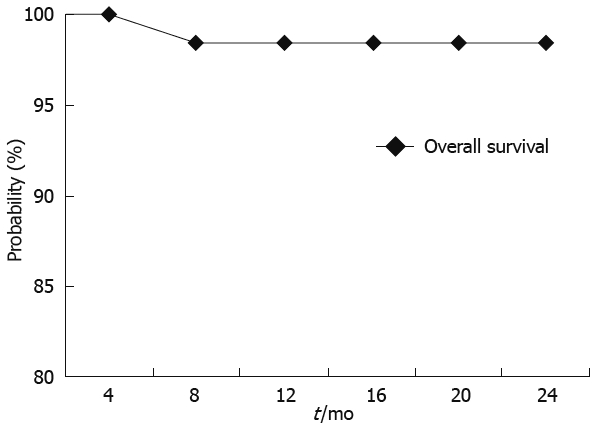
Overall survival probability according to Kaplan-Meier analysis.
Figure 4.
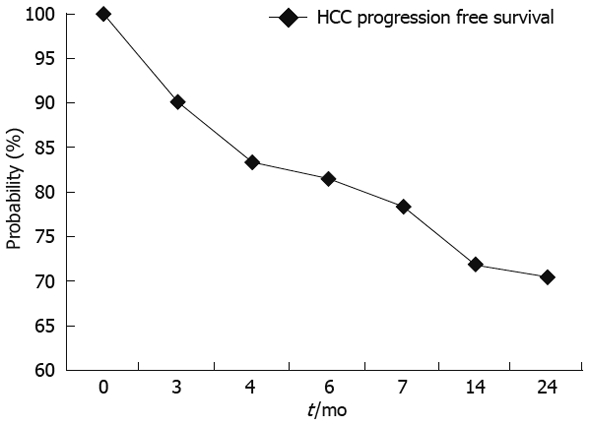
HCC progression free survival probability according to Kaplan-Meier analysis.
DISCUSSION
Our single center study prospectively evaluated a large cohort of patients with liver cirrhosis and HCC to assess the short and long term clinical impact of selective TACE on liver function.
TACE is the most widely used treatment in patients with HCC who are considered unsuitable candidates for surgery and/or ablative therapies[17,18] and, recently, it has been shown to improve survival compared with best supportive care. The proper selection of candidates for TACE appears to be a key point. Even if a consensus has not yet been reached, the best candidates for TACE seem to be asymptomatic patients with preserved liver functions without vascular invasion or extrahepatic tumor spread[19]. In fact, the benefits of the procedure should not be offset by treatment-induced liver damage. Thus, to minimize the injury to nontumoral liver tissue, TACE is often performed by selective (or superselective) catheterization of the hepatic segmental or subsegmental arteries nourishing the tumor.
Although the possible impairment of liver function is a critical point when assessing TACE feasibility, only a few studies (which are not recent) were adequately reported, evaluating the effects of non-selective TACE on hepatic function[20–23]. Furthermore, to the best of our knowledge, there are no extensive published data on the short and long term clinical impact of selective TACE in HCC patients.
Attempts to improve the classification and prognostic prediction of HCC are still evolving and there is no agreement on the best staging system that can be recommended worldwide[24]. For this reason, in our study patients suitable for TACE were selected using Child-Pugh, TNM, CLIP and BCLC staging systems. In this series, we had not only naive HCC patients: at the time of enrollment, about half of our patients had previously been treated for HCC. In our patients, after either first or second TACE, at discharge time, the increase in Child-Pugh score approached significance in comparison with baseline scores, without important variations in the patients’ clinical status.
We observed a statistically significant increase in ALT, PT and bilirubin 24 h after treatment. Interestingly, ALT values increased the most, when compared to PT and bilirubin. This phenomenon could be the expression of selective treatment that enables sufficient tumor necrosis without impairing liver function.
TACE was well tolerated by all patients, with no major complication observed in this series. As already known, the most frequent side effects were post-chemoembolization syndrome often associated with temporarily increased liver enzymes. The post-chemoembolization syndrome, which can have widely variable manifestations, consists usually of fever, abdominal pain, nausea, vomiting and leucocytosis: often it requires only patient monitoring and pain control, but it can prolong the hospitalization period[25].
As an institutional policy, we usually discharged patients 5-7 d after the procedure even if no major side effects occurred. This is in contrast with other authors who discharged patients 48-72 h after TACE[26,27]. Indeed we believe that this approach enables early diagnosis and treatment of possible clinical complications that could become symptomatic even a few days after treatment.
Considering the long-term follow-up, only slight and clinically negligible impairment of liver function was observed in our patients, with only one case of rapid HCC progression.
Liver function remained substantially stable independent of whether patients had undergone previous treatments or were treated by repeated TACE. In our study TACE was repeated “on demand”, when there was evidence of insufficient tumor response, tumor recurrence or disease progression. According to our data, this type of treatment schedule may help preserving liver function and can be well tolerated in elderly patients, often suffering from co-morbidities, as well as in patients previously treated with other therapeutic techniques. TACE repeated at a fixed time, until the planned number of courses has been reached, may cause progressive liver atrophy and vascular damage[28]. The decision to repeat TACE should be based not only on tumor response or progression, but also on patients’ clinical conditions and tolerance, which have to be assessed before each new course.
Recently, it has been demonstrated that TACE improves survival compared with best supportive care in patients with unresectable HCC. Indeed, our 2 years cumulative survival probability was 98%, which compares favorably with previous studies. We believe that this good clinical result may be related to the strict criteria applied in the selection of patients who may benefit from TACE. To this purpose, different staging systems should be used, to determine tumor characteristics and underlying liver disease, as these factors impact patient survival and treatment options.
In conclusion, our prospective study demonstrates that TACE may produce a significant, but transitory increase in ALT values, with no major impact on liver function and Child-Pugh scores, as an expression of treatment selectivity and tolerability. Preservation of liver function is achievable in patients previously treated with other therapeutic modalities as well as in patients undergoing multiple TACE cycles. Moreover, liver function can remain stable in the long-term, with optimal medium term survival. However, this result can be achieved only through rigorous patient selection, on the basis of tumor characteristics and clinical conditions.
At this time, further studies are warranted to consider the clinical impact of new methods of chemoembolization using drug-eluting particles, which could allow larger tumor necrosis with reduced systemic effects.
COMMENTS
Background
Since transarterial chemoembolization (TACE) was introduced as a palliative treatment in patients with unresectable hepatocellular carcinoma (HCC), it has become one of the most common forms of interventional therapy. However, although selective TACE is currently widely used, up to now there are no reported extensive data from large series on both short and long term effects of this treatment on liver function. It is debatable whether repeated courses of selective TACE progressively impair liver function and if they are well tolerated or are limited by major side effects.
Research frontiers
In the area of treatment of HCC, selective TACE is one of the most common forms of interventional therapy. In this field, the research hotspot is how to reduce TACE-related morbidity and how to improve overall survival and HCC progression free survival.
Applications
The study results suggest the proper selection of candidates for TACE using different staging systems and careful clinical management after the procedure. TACE repeated “on demand” may help preserving liver function and can be well tolerated in elderly patients as well as in patients previously treated with other therapeutic techniques.
Terminology
Selective TACE: it is a transarterial chemoembolization peformed by a selective catheterization of the hepatic segmental arteries nourishing the HCC lesions, to limit as far as possible the injury to the surrounding non tumorous liver; TACE “on demand”: it is a transarterial chemoembolization repeated not at a fixed time but in cases of absent or insufficient tumor response or progressive disease.
Peer review
This is a very interesting manuscript that describes the efficacy of selective transarterial chemoembolization in patients with non-operable hepatocellular carcinoma. The procedure was associated with minimal complications or alteration of liver function. The excellent long-term results, when considering tumor progression and survival, could be achieved only through rigorous patient selection, on the basis of tumor characteristics and clinical conditions.
Peer reviewers: R Todd Stravitz, MD, FACP, FACG, Associate Professor of Medicine, Section of Hepatology, Hume-Lee Transplant Center, Virginia Commonwealth University, Richmond, VA 23298-0341, United States; Tsianos Epameinondas, MD, PhD, Professor, 1st Division Of Internal Medicine & Hepato-Gastroenterology Unit, Medical school University of Ioannina, PO Box 1186 Ioannina 45110, Greece
S- Editor Li LF L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 5.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 9.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511–512. doi: 10.3748/wjg.v4.i6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, Miyayama S, Takashima T, Unoura M, Kogayashi K. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83. doi: 10.1148/radiology.188.1.8390073. [DOI] [PubMed] [Google Scholar]
- 12.Miraglia R, Pietrosi G, Maruzzelli L, Petridis I, Caruso S, Marrone G, Mamone G, Vizzini G, Luca A, Gridelli B. Efficacy of transcatheter embolization/chemoembolization (TAE/TACE) for the treatment of single hepatocellular carcinoma. World J Gastroenterol. 2007;13:2952–2955. doi: 10.3748/wjg.v13.i21.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 14.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 17.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18:1517–1526; quiz 1527. doi: 10.1016/j.jvir.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Khan KN, Nakata K, Kusumoto Y, Shima M, Ishii N, Koji T, Nagataki S. Evaluation of nontumorous tissue damage by transcatheter arterial embolization for hepatocellular carcinoma. Cancer Res. 1991;51:5667–5671. [PubMed] [Google Scholar]
- 22.Liaw YF, Lin DY. Transcatheter hepatic arterial embolization in the treatment of hepatocellular carcinoma. Hepatogastroenterology. 1990;37:484–488. [PubMed] [Google Scholar]
- 23.Bronowicki JP, Vetter D, Dumas F, Boudjema K, Bader R, Weiss AM, Wenger JJ, Boissel P, Bigard MA, Doffoel M. Transcatheter oily chemoembolization for hepatocellular carcinoma. A 4-year study of 127 French patients. Cancer. 1994;74:16–24. doi: 10.1002/1097-0142(19940701)74:1<16::aid-cncr2820740105>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buijs M, Vossen JA, Frangakis C, Hong K, Georgiades CS, Chen Y, Liapi E, Geschwind JF. Nonresectable hepatocellular carcinoma: long-term toxicity in patients treated with transarterial chemoembolization--single-center experience. Radiology. 2008;249:346–354. doi: 10.1148/radiol.2483071902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, Balzano S, Florio F. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000;215:123–128. doi: 10.1148/radiology.215.1.r00ap21123. [DOI] [PubMed] [Google Scholar]
- 27.Molinari M, Kachura JR, Dixon E, Rajan DK, Hayeems EB, Asch MR, Benjamin MS, Sherman M, Gallinger S, Burnett B, et al. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol) 2006;18:684–692. doi: 10.1016/j.clon.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita Y, Torashima M, Oguni T, Yamamoto A, Harada M, Miyazaki T, Takahashi M. Liver parenchymal changes after transcatheter arterial embolization therapy for hepatoma: CT evaluation. Abdom Imaging. 1993;18:352–356. doi: 10.1007/BF00201780. [DOI] [PubMed] [Google Scholar]


